Abstract
Mechanical strain or stretch of collagen has been shown to be protective of fibrils against both thermal and enzymatic degradation. The details of this mechanochemical relationship could change our understanding of load-bearing tissue formation, growth, maintenance and disease in vertebrate animals. However, extracting a quantitative relationship between strain and the rate of enzymatic degradation is extremely difficult in bulk tissue due to confounding diffusion effects. In this investigation, we develop a dynamic, enzyme-induced creep assay and diffusion/reaction rate scaling arguments to extract a lower bound on the relationship between strain and the cutting rate of bacterial collagenase (BC) at low strains. The assay method permits continuous, forced probing of enzyme-induced strain which is very sensitive to degradation rate differences between specimens at low initial strain. The results, obtained on uniaxially-loaded strips of bovine corneal tissue (0.1, 0.25 or 0.5 N), demonstrate that small differences in strain alter the enzymatic cutting rate of the BC substantially. It was estimated that a change in tissue elongation of only 1.5% (at ~5% strain) reduces the maximum cutting-rate of the enzyme by more than half. Estimation of the average load per monomer in the tissue strips indicates that this protective “cutoff” occurs when the collagen monomers are transitioning from an entropic to an energetic mechanical regime. The continuous tracking of the enzymatic cleavage rate as a function of strain during the initial creep response indicates that the decrease in the cleavage rate of the BC is non-linear (initially-steep between 4.5 and 6.5% then flattens out from 6.5–9.5%). The high sensitivity to strain at low strain implies that even lightly-loaded collagenous tissue may exhibit significant strain-protection. The dynamic, enzyme-induced creep assay described herein has the potential to permit the rapid characterization of collagen/enzyme mechanochemistry in many different tissue types.
Keywords: Collagen, Bacterial Collagenase, Mechanical Load, Strain, Creep, Degradation, Mechanochemistry
Introduction
Collagen is the structural protein and load-bearing molecule of choice in vertebrate animals. It seems that wherever there is a difficult mechanical environment (e.g. joints, tendons, ligaments etc), collagen provides the critical link between force and motion. It is thus reasonable to suggest that mechanical forces or strains play a critical role in directing the placement and retention of collagen. This idea has been around in various forms since the late 1800s 1–7. However, rather than assume that the connective tissue fibroblastic cells direct the removal and placement of individual collagen monomers, we have suggested that collagen incorporation, removal and retention in fibrils is controlled directly by the state of mechanical strain in the tissue 8. Fundamental to this “smart matrix” hypothesis is the idea that collagen molecular stability is influenced by mechanical strain. Direct strain-stabilization of collagen could explain why this material appears to “seek out” high tensile forces and how it persists in their path.
Collagen type I can be found in over 80% of collagenous structures in vertebrate ECMs 9. Collagen molecules are adapted to carry tensile loads and are often anisotropically-arranged into aligned hierarchical structures (fibrils, fibers or filaments) which correspond to the direction of tensile load in load-bearing extracellular matrices. In spite of the importance of the role of collagen in a number of critical biological events (development, growth, repair of connective tissue), irreversible pathologies (osteoarthritis, osteoporosis, intervertebral disk degeneration) and difficult to manage injuries (ACL rupture) there has been little progress in determining precisely how collagenous load-bearing tissue is initially organized, load-optimized, grown and maintained 4, 5. The manner by which load guides matrix retention and remodeling remains unclear, but the existence of a collagen/enzyme mechanochemical relationship might provide insight into this important problem.
A series of in vitro experiments on collagen mechanochemistry have demonstrated that collagen gains stability against thermal denaturation 10, 11 and enzymatic cleavage 8, 12–15 when it is under applied mechanical load or strain. In a recent investigation 15, excised rat tail tendon fibers held at constant strain (stretch) and exposed to bacterial collagenase relaxed more slowly at higher strain. These results suggest that the strain is protective of the collagen, but there are some experimental concerns including the possibility that the 4% strain difference between samples in the dense tendon tissue could have affected diffusion rates and the use of a room temperature apparatus. More critically, the initial transient data was not used in the experiments because of a concern regarding osmotic loading. As we will demonstrate, the initial transient is where one can (and should) extract quantitative enzymatic cleavage rate information before diffusion dominates the kinetics.
There has also recently been one in vivo study which has demonstrated that the application of tension to intentionally-damaged intervertebral disk reduces the rate of degradation of collagen in anulus fibrosus in a rodent injury model 16. The latter study suggests a potentially important clinical route to the prevention of tissue loss following injury via the application of mechanical tensile strain. Such a possibility was predicted in 2005 14. However, a connection to strain-altered enzyme/collagen cleavage rates was not established.
Taken together, there is a growing preponderance of evidence which suggests that collagen may possess mechanochemical stability enhancement properties. Strain protection against enzymatic cleavage potentially permits collagen to preferentially remain in the path of applied loads which could help explain collagen’s dominance as a load-bearing molecule in animals. In this investigation, an attempt is made to extract a lower bound for the relationship between strain and the cutting rate of loaded-collagen by bacterial collagenase at low (near physiological) strains. As will be demonstrated through diffusion/reaction rate scaling arguments applied at the surface of the sample, this can be done by examining the difference in the initial strain rates of native collagenous tissue exposed to a degrading enzyme under constant load (enzymatically-induced creep experiments). The enzymatic creep assay is well-suited to examine bulk tissue mechanochemical behavior principally because the probe value (strain) is externally driven. Unlike stress-relaxation experiments in tissue, where the probe value (load) decays, the energy to induce creep is externally applied and under user control. As will be seen, this assay method permits a reasonable estimation of the number of loaded collagen molecules being cut per second by the enzyme.
Experimental Section
Specimen Preparation
The corneal stroma is a relatively acellular, hydrated network of fibrils comprising heterotypic type I/V collagen. In addition to their high collagen content, corneas were chosen for their transparency and their unique fibril architecture comprising aligned, alternating arrays of monodisperse diameter collagen fibrils. Corneas were obtained from right or left eyes of two week old cows (40–100 pounds) from Research 87 Inc (Boylston, MA). After cleaning the globes, the corneas were excised followed by epithelial and endothelial debridement with a razor blade. The bare stromal tissue was stored at −80°C until use. Prior to testing, specimens were devitalized by freezing and thawing (to −80°C). A custom made cutting die was used to generate accurate and repeatable test strips from the tissue. The central region of each dissected cornea was used to produce one vertically oriented (superior-to-inferior) tensile specimen approximately 0.75±0.1 mm thick × 17.5± 2.5 mm length × 6 mm wide. During excision and mounting into the test chamber, all tensile specimens were kept moistened with 37°C DMEM- Dulbecco's Modified Eagle's Medium- (Mediatech Inc., Manassas, VA).
Mechanical Loading Apparatus
To investigate the effect of mechanical forces/strains on the rate of degradation of tissue strips, an environmentally controlled, miniature uniaxial testing device was designed and constructed (see acknowledgements, supplementary material and the thesis of K.P. Church 17). The device comprises a load cell (Honeywell Sensotec, Model 31; Max - 5 lb; Resolution 0.0002 lb, Columbus, OH) and a uniaxial motor (Zaber Technologies T-LA60; Resolution: 16 µm; Max Speed: 4 mm/s, Speed resolution 0.001 mm/s, Vancouver, BC, Canada) which are under the control of a custom LabView program. The combined system load accuracy with PID controller is ±0.01N. The specimen chamber’s quartz glass allows optical access for polarization microscopy of the specimen and is equipped with an integrated PID driven temperature control system. Each corneal strip was positioned between two cam grips inside the chamber, immersed in 37°C DMEM and uniaxially stretched. To prevent slippage of the tissue, following clamping, cyanoacrylate glue was applied to the sample grip interface and permitted to dry (briefly). In addition, the load on the specimen was monitored for sudden relaxation (an indicator of slip) and movies were taken during at least two experiments from each load value to verify reported strains (see supplemental data). In the experiment, strain, ε, was calculated directly from the tissue grip displacement, l, using the initial specimen gage length, lo, as the reference: ε = (l−lo)/lo.
Digestion Protocol
Crude bacterial collagenase (BC) from Clostridium Histolyticum (Clostridiopeptidase A, Sigma-Aldrich-No.C0130) with a molecular weight range from 68kDa to 125kDA was used. In order to activate the solution, each mole of collagenase requires four moles of calcium [Ca++]. DMEM provides enough calcium to support the enzyme at the concentrations used in our system. Loaded tissue specimens were “crept-in” for 15 minutes in DMEM. Control experiments without digestion exhibited slight additional material creep (max 2% over 1.25 hours) which was much less than the enzymatically induced creep (see Figure 4 and supplementary material). The DMEM was replaced with solution containing BC (DMEM and 0.05mM BC) for the duration of the experiment. The concentration far exceeds the Michaelis-Menten constant, Km, for the collagen/BC interaction and the surfeit of enzyme relative to available monomer should ensure maximum reaction rate kinetics. However, it should be stated that because this is a diffusive, erosion problem, strict Michaelis-Menten kinetics cannot be applied without some modification. Nonetheless, it is possible to estimate the rate of loaded monomer cleavage from this data directly. For consistency, collagenase solutions were made previously and stored in individual vials at −80°C until use for each experiment. Before injection into the chamber to initiate the degradation process, collagenase solution was preheated in a water bath (37°C) for 30 minutes. Collagenase activity can vary from batch-to-batch and can decline with time, however, the repeatability of our experimental series indicated minimal batch-to-batch variation and the short duration of our experiments (< 2 hrs) substantially mitigates concerns regarding reduced activity.
Figure 4.
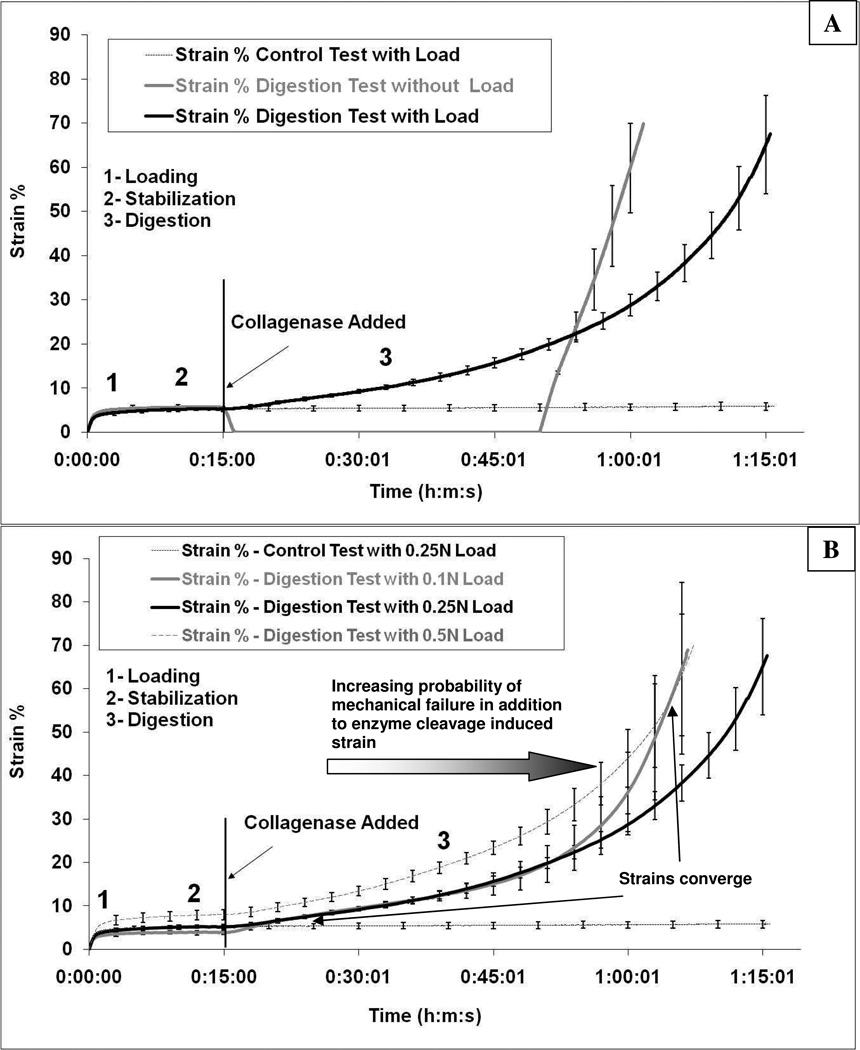
Strain comparison of enzymatically-induced creep response for different protocols. A) Comparison of 0.25N continuous load to 0.25N load-unload-reload specimen. B) Comparison of continuously loaded specimens at 0.5N, 0.25N and 0.1N. The 0.25 N control experiment is included for contrast in each figure. The strains for both the 0.1N and the 0.25N samples converge rapidly within the first 5–7 minutes of the experiment (see supplementary movie as well) while the strains for the 0.1N and 0.5N samples converge at the end of the experiment. The gradient arrow in B indicates that as the strain increases, the probability of mechanical failure of tissue components increases, thus the strain increase at longer times is not purely enzymatically driven.
Polarization Microscopy (Birefringence) - Dynamic Imaging
One of the reasons the corneal stroma was chosen as a test specimen is because it comprises a transparent array of aligned collagen fibrils which alternate in direction 18. Corneal collagen fibrils are also effectively mechanically isolated (with regard to tension) as there are only weak proteoglycan interactions between individual fibrils 19. Thus, uniaxial strain preferentially loads fibrils oriented in the direction of the load while others remain relatively unloaded. One of our goals was to use polarization to discern which fibrils are degrading faster in real time (loaded or unloaded). Polarization lens (43 mm Diameter, Prinz, Japan) axes were set to 45°–135° to select corneal fibrils aligned with the load axis (90°) in our set-up. To select arrays of unloaded corneal fibrils (i.e. oblique to the load axis) the polarization lens axes were set to 0°–90°. The specimens were then digitally imaged (Prosilica, Model CV640, Black and white, Frame rate 120 fps, Resolution 9.9×9.9 µm per pixel, Newburyport, MA) every 10 seconds for up to 90 minutes through crossed polarizers during each experiment. Movies were generated from these pictures to monitor the degradation rate on the set of illuminated fibrils and permit examination of the specimen for anomalous behavior (premature tearing or evidence of grip slippage – see movie in supplementary material).
Loading protocols
All experiments in the protocols were performed in load-control mode (fixed load with feedback) while both strain and load were recorded. After a specimen was positioned inside the chamber, the sample was stretched until a load of 0.01N (our threshold for resolution) was detected and the resulting strain was our reference or “zero strain”. The experimental loads of (0.1N, 0.25N and 0.5N) were chosen based on an estimate that the initial, per monomer loads in the tensile specimens are close to the molecular transition from an entropic to an energetic mechanical state 20. In load-control, the creep becomes dynamic permitting the specimens to “sample” multiple strains in a single experiment. The specific experimental loading protocols were:
Control test with load
After preloading 0.01N, specimens (N=9) were loaded at 0.1N, 0.25N or 0.5N for 75 minutes while both strain and load were captured from samples. This test was designed to determine the expected normal creep response and to demonstrate that the grip design was adequate at the various applied loads.
Digestion test with load
After Preloading 0.01N, specimens (N=15) were loaded at 0.1N, 0.25N or 0.5N for 15 minutes (creep-in). Then the DMEM was replaced by preheated active BC solution (0.05mM) for an additional 75 minutes or until failure.
Digestion test without load (probed with sudden reload)
After preloading 0.01N, specimens (N=5) were loaded at 0.25N for 15 minutes (creep-in). Specimens were then unloaded and DMEM replaced by 0.05mM preheated, active BC solution for an additional 35 minutes. Specimens were then reloaded to probe the mechanical integrity of the specimen at 0.25N loading until failure.
Mechanical characterization of tissue
In general, the cornea is, like most collagenous connective tissue, a non-linear, viscoelastic material with an increasing equilibrium tensile modulus as a function of increasing force/strain. Its uniaxial mechanical response is generally more complex than tissues which possess uniformly aligned collagen due to the distributed orientation of collagen lamellae 18, 21 in the plane of the tissue. Control tensile tests on the cornea (see supplementary material) indicate that the tissue has a viscoelastic time constant which is O(10 min). The initial tensile modulus for the tissue strips for each experiment was determined just prior to the addition of collagenase. For comparison, the equilibrium (long-time) modulus was also obtained for two of the three experimental loads (0.1N and 0.25N).
Time scale ratio estimate
BC is an aggressive binder and cleaver of collagen, thus the time scale for collagen cleavage is likely to be shorter than the time scale for diffusion into the whole tissue strip. Given data obtained from our own assay (see supplementary information) for the rate limiting step in collagen fibril catalysis for our specific enzyme, kcat (~0.033 sec−1) and an estimated enzyme diffusion coefficient in the cornea, De of ~1×10−7cm2/sec (based on the diffusion coefficient of a similar sized molecule – albumin 22), an initial reaction-diffusion time scale ratio can be calculated from the following equation:
| (1) |
Where Cc is the concentration of collagen in the corneal strip, Ce is the concentration of enzyme in the chamber, h is the half-width of the tissue strip. Ts calculated in this way is ~0.1. The time scale ratio is a reflection of the balance between the time to digest the available collagen in the tissue (numerator) and the time for the enzyme to diffuse to the center of the sample (denominator). The value of Ts less than one indicates that for corneal tissue (h~375 microns), the diffusion delay will dominate the cutting rate. However, at the beginning of the experiment (i.e. for short enzymatic penetration distances – or small h), the reaction speed of the enzyme will be the limiting factor and will dominate the physics. This is because diffusion is quite fast over short distances. Thus, it is possible to extract good estimates for the reaction speeds for the enzyme if we examine the initial few minutes of the induced-creep response. Using equation 1, we can calculate the estimated cross-over point, where the reaction-diffusion time scale ratio is approximately 1.0. The cross-over occurs at ~6 minutes. We use this argument to justify extracting enzymatic cleavage rates during the initial reaction rate limited period (up to 6 minutes). There is clearly some limitation of the validity of this assumption due to fibril packing, proteoglycan presence and the actual activity of the BC on corneal collagen packed into fibrils. Nonetheless, for a well-designed enzymatic creep experiment, there will be an initial time period where the degradation reaction is enzyme activity-rate limited. During this period, estimates of enzyme reaction rates on loaded tissue may be extracted.
Estimate of lower bound on enzymatic cleavage rate
Figure 1 demonstrates the expected progression of the experiment where the outer surface layers of the tissue are cleaved first by the diffusing enzyme. During enzyme-induced creep, the enzymatic cleavage rate of loaded material can be obtained by determining the kinetics of loaded area loss. Area loss may be estimated with reasonable accuracy by assuming that as the enzyme removes loaded fibrils from the outside of the tissue, load is transferred to uncut fibrils (deeper in the sample – see Figure 1). The resulting increase in strain should reflect the amount of loaded area which has been “lost” to the enzyme activity. Neglecting the viscoelastic contribution (as we do) will result in an underestimate of the area loss in this experiment (as viscous dissipation will retard the strain rate). Likewise, by holding the tensile modulus constant for each force, E≠ f(t), the area lost will be further underestimated. This is due to the fact that there is a non-linear relationship between stress and strain in the corneal tissue in the regime examined. Our simplified equation for calculating the instantaneous loaded area, A(t), is
| (2) |
Where F is the force (known and fixed), E is the initial modulus of the tissue (specific and fixed for each applied load), ε(t) is the instantaneous strain (measured). Given the arguments above, this estimate of the load-bearing area “consumed” by the enzyme will be a lower bound. Samples which undergo larger strains or strain-rates will be more affected by the underestimate created by equation 2. This is an important point. To obtain the rate of load bearing area loss (which is reflective of the enzymatic activity on the load-bearing monomers), the first derivative of equation 2 can be taken. To do this a five point centered difference was used (Figure 6 data).
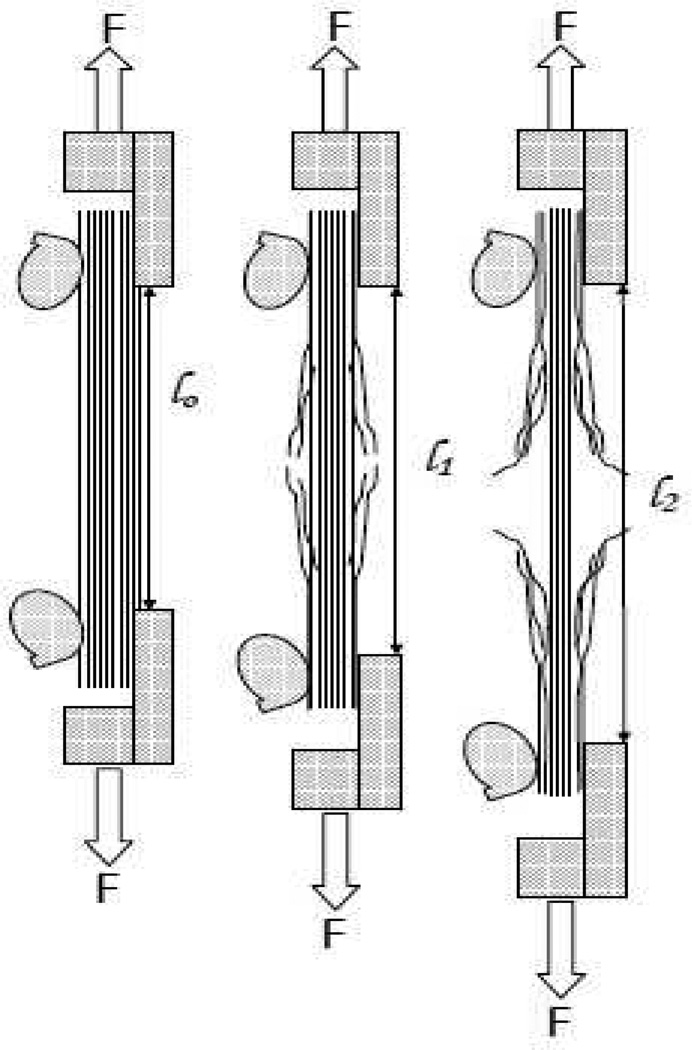
Figure 1.
Side view of anticipated degradation progression as a function of time. In the dynamic enzyme-induced creep experiment, the tissue will degrade from the external surfaces transferring load to the interior fibrils. Left frame (pre-exposure), the corneal tissue is intact and holding the load securely in the cam grips, the gage length is l0. Center frame (short exposure time). The tissue, just having been exposed to enzyme, has begun to degrade, since the load is fixed, the loss of load bearing area results in extension of the strip to a new gage length, l1. Right frame (long exposure), the tissue has been exposed to the enzyme for a significant amount of time and has strained considerably. The combination of strain and diffusion limitations should begin to slow the degradation rate.
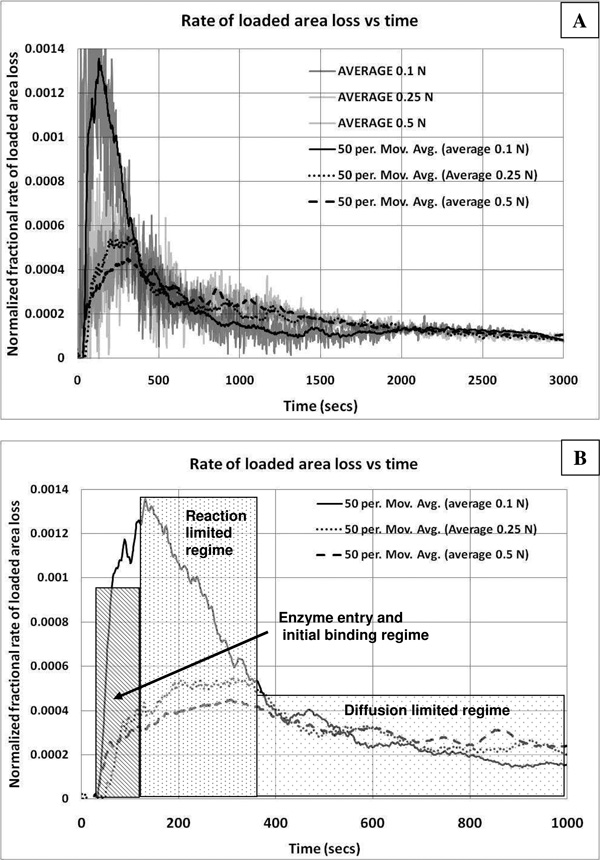
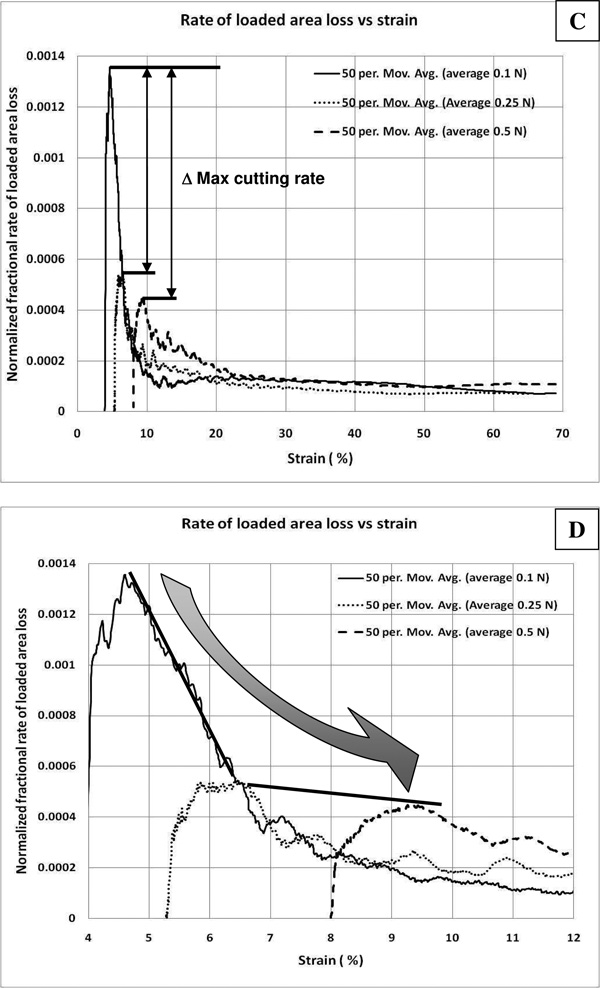
Figure 6.
Dynamics of enzyme induced creep experiment. Rate of loss of load-bearing area vs time and strain (i.e. proportional to enzyme cutting rate). For all graphs, the ordinate axes represent the normalized rate of load bearing area loss which may be interpreted as a measure of enzymatic activity on load-bearing tissue components (at low strain). The first derivative was calculated using a 5 point centered difference. A) Area loss rate versus time for the whole experiment (smooth line is a 50 point moving average trendline). The general temporal trend in the loss of loaded material begins with an initial climb to a maximum degradation rate, followed by a decline in the rate of material loss during the remainder of the experiment. B) Area loss vs time for the initial 1000 seconds after enzymatic exposure (trendline). In this plot the temporal dynamics of the initial enzyme induced creep is shown. Given our scaling argument, we assume that during the initial 300 seconds (after a short transient indicating enzyme arrival and binding), that the enzymatic reaction is not limited by diffusion. During this time (dotted box), for all three experiments, a peak in the cutting rate is reached. As the reaction proceeds, diffusion is likely to begin to dominate the reaction rate (gradient box). C) Rate of load-bearing area loss as a function of strain (trendlines shown only). The effect of strain on the cutting rate can be directly extracted. Remarkably, at the beginning of the experiment, it can be seen that ~1.5% increase in applied strain decreases the cutting rate by a factor of ~2.6. The difference in maximum cutting rates (peaks) was statistically significant between the 0.1 and the 0.25 and 0.5 N runs (p<0.05). There was no significant difference between the 0.5 and 0.25N runs. In support of the hypothesis that enzymatic activity is a function of strain, once the strains for the 0.1 and 0.25N samples converge, so does the cutting rate. D) Relationship between strain and enzyme cutting rate at low strain (trendline). For the sample under the lower load, the effect of strain (over a continuous strain regime from ~4.5–6.5%) depresses the ability of the enzyme to cut the load bearing collagen fibrils at a rate of approximately 30%/1% (found by linear regression of the raw data). However, we suspect that diffusion limitation prevents further extrapolation given the higher maximum cutting rate for 0.5N sample at similar strains. If we connect the maximum cutting rates, we get two lines which suggest that the relationship between strain and enzyme activity is sharply non-linear and decreasing with strain from about 4.5 to 9.5%
To estimate the modulus of the loaded fibrils in the tissue, the cutting rate of the enzyme and the average per monomer load, it was necessary to determine how many collagen monomers in a corneal strip cross-section are carrying the load. The initial load-bearing area in a representative tissue strip was calculated using the assumption that approximately 18% of the fibrils in the cross-sectional area of the tissue strip are oriented such that they directly support the load (i.e. run from grip-to-grip). This estimate is based on calculations performed on X-Ray data on bovine corneas from Professor Keith Meek’s laboratory (18, 21 and personal communication with Professor Meek). Calculations based on the hydration of the normal cornea, the tissue cross-sectional area, the molecular packing of the collagen monomers into fibrils and the number of fibrils aligned with the load suggests that there are approximately 9.0×1010 monomers in a given cross-section which are carrying the applied load. If the applied forces are divided by the number of fibrils in cross section, an average initial load of approximately 1.1 pN per monomer in the 0.1 N test, 2.75 pN per monomer in the 0.25N test and 5.5 pN per monomer in the 0.5N test is found. This load range occurs near the entropic to energetic transition in mechanical properties for type I collagen 20.
Statistical analysis
The single controlled-variable in the experiments was the applied load. Comparisons of the dependent parameters of interest (time to tissue failure, fraction of area remaining and enzyme cutting rate maximum) were made using a two-tailed Student’s T-test for unpaired experiments. These statistics were designed to reject our null hypothesis: Enzyme cutting rate is independent of applied load.
Results
Polarization imaging
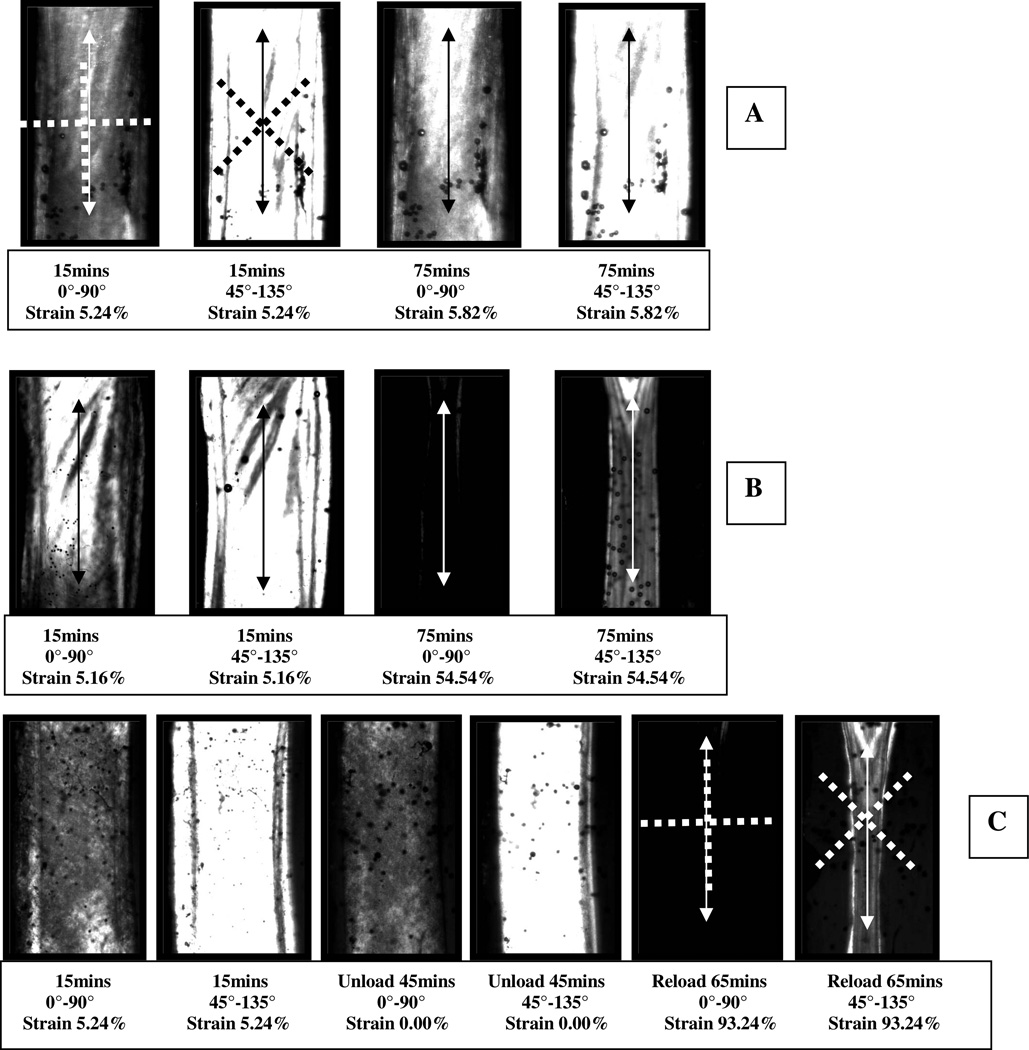
The dynamic polarization images confirmed the amount of creep in the samples and indicated that the grips held the tissue throughout the experiments. While there is significant birefringence signal change in the loaded/degraded samples, the rapidity of the experiments and the dynamic stretching limited our ability to extract a distinct preferential attack on the unloaded fibrils (which was the original goal). Figure 2 (A–C) displays the polarization images captured from specimens at 0°–90° and 45°–135° relative to load direction in control and degradation tests. Figure 2A (Control Test with Load 0.25N) demonstrates that the polarization signal does not change appreciably with time. Figure 2B (Digestion Test with 0.25N Load), demonstrate that the loaded specimens lose birefringence intensity in general over the course of the experiment but that the signal from off-axis aligned structures is nearly extinguished. Figure 2C demonstrates that when samples were unloaded, the degradation did not change the birefringence signal appreciably. However, upon reloading, the sample rapidly loses birefringence for fibrils off the load axis. This is likely because fibrils are degraded, but remain in place until loaded. In general, in these rapid degradation tests, the birefringence images do not provide clear and conclusive evidence of preferential degradation of unloaded fibrils. Nonetheless, the images are quite useful in support of the instrument readings by permitting direct examination of strain rates and load bearing tissue remaining. Examination of the last frames in Figure 2B, S4 and C corroborates the strain data readings and shows that the 0.25N continuously-loaded sample (2B; last frame) clearly has more loaded material left than either the 0.1N continuously-loaded sample (S4; last frame) or the 0.25N load/unloaded sample (2C; last frame) even after a longer exposure time (75 vs 65 minutes). See supplementary movie for dynamic view of experiment.
Figure 2.
Polarization imaging of tissue strips before collagen exposure and after sample failure. Polarization axes were oriented at either 0°–90° or 45°–135° (see first frames of (A) for polarization axis orientation example indicated by dashed lines). A) Control Test with 0.25N load no degradation; B) Digestion Test with 0.25N load; C) Digestion Test without Load. Figure S4 in supplementary information shows digestion test with 0.1N load
General strain versus time response of samples
Mechanical Response of the tissue
The initial modulus obtained from the mechanical characterization of the tissue strips was 3.0 MPa for the 0.1N load, 5.9 MPa for the 0.25N load and 6.3 MPa for the 0.5N load. This modulus is calculated by using the instantaneous values of stress and strain at the 15 minute time point. The long time or equilibrium modulus is generally appreciably smaller for each of the loaded tissues due to relaxation. The increase in tensile modulus from 0.1 to 0.5 N is due to the presence of the typical “knee” found in connective tissue stress-strain relationships and indicates that for the corneal strip in this experiment, the tissue is transitioning to a stiffer mechanical regime.
Controls
The strain in the 0.1N control is relatively stable after 15 minutes but the strain in the 0.25N control creeps steadily for one hour after the 15 minute initial creep-in period. The total creep during the post creep-in hour is below 2% in the 0.1, 0.25N and 0.5N test. Given the large rates of creep induced by exposure to enzyme in the experimental series, this small control creep rate is acceptable. In addition, the slightly increased steady creep in the more highly-loaded samples makes the final conclusions of this study even more conservative (a higher creep/stretch for lower loaded sample).
Experimental series – continuous load
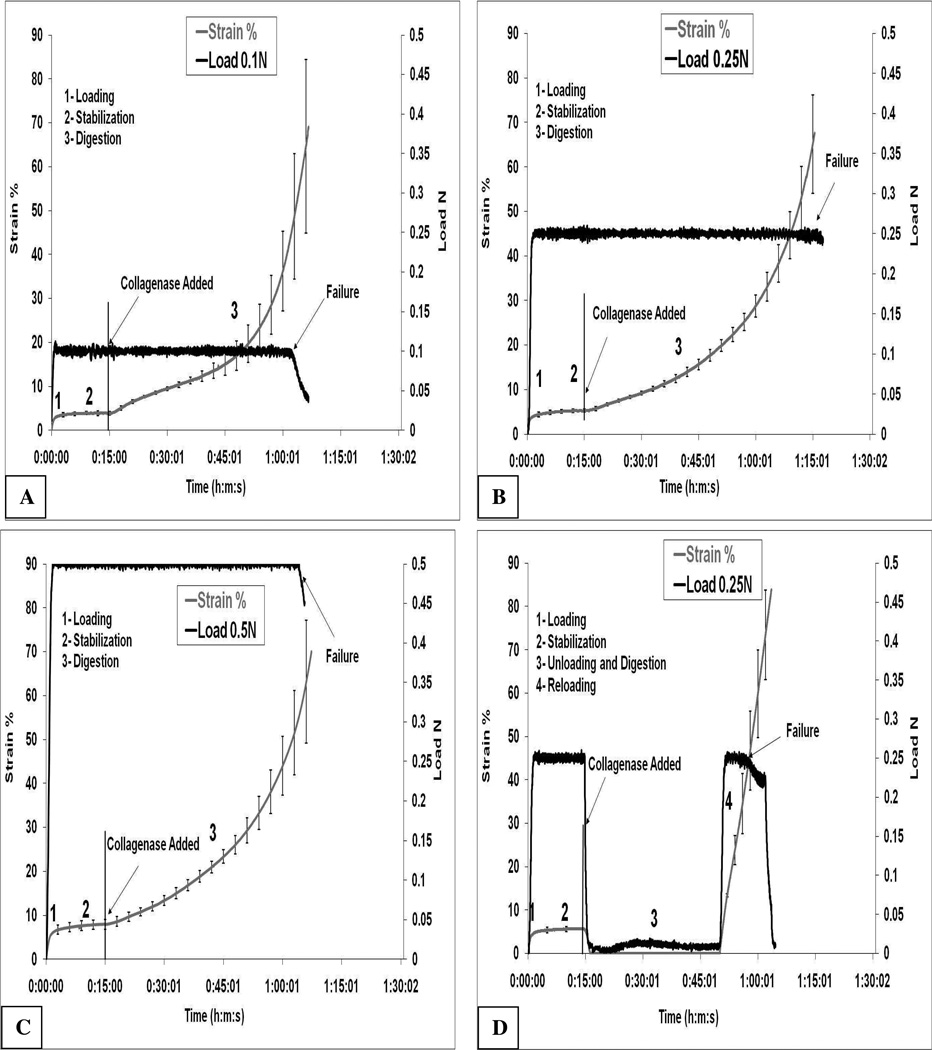
Figures 3A, 3B and 3C show the tissue response in the experimental series where the applied load is held continuously until specimen failure in the presence of collagenase. Failure is defined as the point where the load can no longer be sustained by the tissue at the maximum rate of strain (which is limited by the linear actuator maximum speed). What can be observed in the enzymatic creep response of the specimens is that degradation begins to weaken the tissue soon after introduction of the enzyme to the chamber. There is an initial increase in the strain for all of the continuously loaded specimens. Following the initial relatively-linear strain response (after about 30 minutes of exposure), there is a rapid and non-linear increase in the strain for both samples which culminates in failure. This phase of the creep response is more variable (see error bars) and likely indicates a more chaotic period where there is both enzymatic and overload-induced mechanical fibril failure.
Figure 3.
Strain and load vs. time for the four loading protocols. A) Load controlled to 0.1N B) Load controlled to 0.25N C) Load-controlled to 0.5N. D) Load initially controlled to 0.25N then unloaded at time of addition of collagenase; Load reapplied after 35 minutes to probe degradation of structural components. Load was the controlled variable and data is representative of one experiment. Arrows indicate time of addition of collagenase and point of failure of tissue (load can no longer be maintained).
Experimental series – load-unload-reload
This experiment was conducted because there was no simple way to continuously probe the tensile mechanical behavior of the entire sample at zero load. Instead, the tissue was exposed to enzyme under zero load conditions for a set period of time, then reloaded (at a judicious time point) to probe the mechanical state of the sample. In Figure 3D, it can be observed that specimens which were unloaded and exposed to 0.05 mM BC at 37°C for 35 minutes, then reloaded, experienced a sharp rise in strain and could not sustain the load (0.25N) for more than 5–10 minutes upon reloading.
Comparison of strain versus time response of samples
In Figure 4A, the 0.25N control and experimental runs are plotted on the same axes (strain vs. time) with error bars. The data in 4A indicate that completely unloading the tissue during exposure to collagenase results in a significant decrease in the time to failure (2550±88 sec vs. 3730±137 sec; p≪0.05). In Figure 4B, the three continuously loaded samples (0.1N, 0.25N and 0.5N) are plotted on the same axes (strain vs. time). It can be seen that the 0.25N and 0.1N curves appear to be different only at the very beginning of degradation and at the end. Further, the 0.1N load fails before the 0.25N loaded sample (2822±384 sec vs. 3730±137 sec; p≪0.05). It is important to note that if the degradation rates were equal, the strain should be different at all time points for all samples. The convergence of the strains for the 0.1 and 0.25N samples (early) and the convergence with of the 0.1 and 0.5N samples (late) is consistent with the hypothesis that strain (as opposed to load) is the relevant parameter for mechanochemical influence on enzymatic activity.
Area loss dynamics
In this investigation, we are trying to reject the null hypothesis which states that the degradation rate of the tissue is unaffected by small strain differences. Another goal is to determine the magnitude of the strain protection in terms of the enzymatic activity.
Effective load-bearing area loss
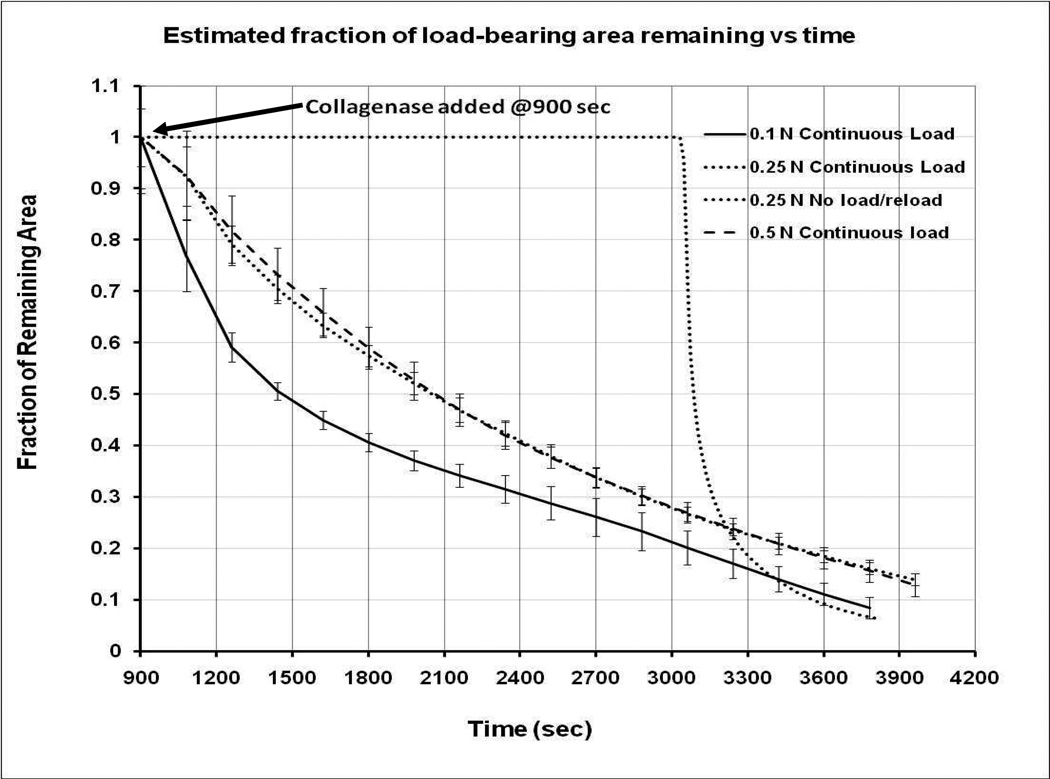
Figure 5 shows the decay in the effective load-bearing area for the entire time course calculated with equation 2. In spite of the conservative assumption of constant modulus, it can be readily observed that during this time there is a significant increase in the loss of loaded area from the sample which is under the 0.1N load (relative to both the 0.25 and 0.5N loaded samples). Within the first 10 minutes of enzyme exposure, the corneal strips loaded with 0.1N have lost more than 50% of their load bearing fibrils compared to ~30% for the 0.25N and 0.5N loaded specimens.
Figure 5.
Effective load-bearing area vs time. The sample loaded area plotted as a function of time for all three experimental protocols. There is a rapid loss in the area after the addition of collagenase for all three continuously loaded samples. No loss is shown for the unloaded sample as it was not possible to probe the sample without load. However, upon reloading, the fraction of area remaining converged on the data from the 0.1N sample. Vertical bars are standard deviations. Differences in remaining area were statistically significant for loading conditions: 0.1 N vs 0.25 N and 0.1 N vs 0.5 N. There were no statistically significant differences between the 0.25 and 0.5 N runs.
Rate of load- bearing area loss
Figure 5 shows that at the very beginning of the experiment, just after enzyme addition to the chamber, there appears to be a substantial difference in the loaded-tissue area loss rate between the 0.1N and 0.25N/0.5N samples. Taking the first time derivative of the load-bearing area and plotting it against time and then strain produces a set of curves which suggest the composition of multiple physical processes (Figure 6 series). In Figures 6A and 6B, the enzymatic cutting rate is plotted against time. The curves show an initial rise in cutting rate to a maximum followed by a decline. The initial rise represents the rate of cutting as the enzyme arrives at the specimen surface and begins cutting load bearing tissue. The peak of the curves represent the maximum cutting rate obtained. The decline in the cutting rate is likely due to diffusion limitations at longer times, but to strain-effects at shorter times (before diffusion limitations slow the reaction rate – i.e. up to 6 minutes). Examination of the data shows that the maximum rate of area loss (which is directly reflective of the enzymatic cleavage rate on loaded tissue) is more than 2 times larger (p<0.05) for the 0.1N sample relative to either the 0.25 or 0.5N samples during initial degradation. When we take into account the fact that as strain increases, the modulus increases in this region of the stress-strain relationship, the difference is even more dramatic, with the lower loaded sample degrading more than 3 times faster (data not shown). An estimate of the maximum cutting rates for each sample, in monomers per second and based on loaded area loss, can be obtained by converting the rate of load-bearing area loss using the estimated area per monomer in the corneal strip. For the sample under 0.1N load the maximum loaded monomer cutting rate is ~1.2×108 monomers/second, while the sample loaded with 0.25N had a maximum loaded monomer cutting rate of ~0.47×108 monomers/second (p<0.05). The sample at 0.5N was not significantly different from the 0.25N sample. The ratio of these rates is ~2.6. Figures 6C and 6D plot the collagen cleavage rate against strain. The data demonstrate that the sharp decline in the enzyme activity occurs over very small strain difference (~Δ1.5%). Further, the cutting rates converge rapidly as the strain values for the two samples converge. A linear regression of the data for the 0.1 N loaded sample indicates that the relationship between strain and enzyme activity on loaded fibrils is linear from 4.5–6.5% strain and declines at a rate of ~30%/1%. The maximum cutting rate for the 0.5N sample suggests that the strain protection effect does not continue to increase with strain after 6.5%, but rather, the relationship between strain and cutting rate flattens out as the strain (see Figure 6D). Thus protection appears to occur quickly and remain in effect up to at least 9.5% strain.
Note on diffusion and mechanical failure effects
Diffusion. Enzymatic erosion tests of differentially-loaded tissue samples are generally subject to diffusion differences caused by matrix compaction/extension. At the beginning of the load-control tests in this investigation (where the largest differential in enzymatic activity was found), the difference in the strain between the two samples which show the greatest strain effect is only ~1.5%. The sample strains then rapidly converge. The initial strain difference is so small that it is highly unlikely to retard diffusion enough to reduce enzyme activity appreciably. The hydraulic diameter of the network in a normal cornea is approximately 18 nm 23. Assuming a poisson’s ratio of 0.4–0.5 for the corneal stroma, the maximum difference in the hydraulic diameter between the two samples will be less than ~0.2 nm for the strain difference. Thus, with regard to diffusion of the enzyme, the specimens are virtually identical through the first 30 minutes. In more dense tissue (rabbit tendon), with a higher strain difference, Nabeshima et al. found little influence of strain on the ability of the enzyme to diffuse through and bind to the matrix 13. Mechanical Failure. The mechanical failure of the tissue will proceed with increasing probability as the strain on the matrix increases. Thus the effective cutting rate at larger values of strain should include both enzymatic cleavage and mechanical breaking/separation of monomers. The effective cutting rates as a function of strain for the samples are similar from their convergence at 20% to ~35% strain after which the lower loaded sample begins to fail rapidly. This is difficult to interpret but indicates that as the mechanical failure rate increases, the enzymatic cutting rate is decreasing further. The decrease could be mechanochemical or diffusion related at higher strains. These effects cannot be readily separated without further investigation.
Discussion
To prevent dissipation of their structure, metazoans necessarily place load-bearing material in the path of forces, where it must persist, even in the presence of catabolic enzymes. In vertebrates, this material is often collagen. Simple free-energy arguments would appear to favor an increase in the rate of collagen fibril catabolism with increasing strain energy density (to reduce stored energy in strained monomers). However, there is a mounting body of evidence indicating that collagen fibrils are generally stabilized by mechanical load, which is non-intuitive, but which makes practical sense given the load-bearing role of collagen as a material. Further, if collagen cleavage rates were to increase with applied strain, then the presence of catabolic enzyme in loaded tissue would constitute the formation of a dangerous and unstable situation, where increased cleavage of load-bearing structure could lead to increased strain on remaining tissue and still faster cleavage. The idea that collagen fibrils are made more stable by mechanical load appears to be consistent with what can be generally observed in vertebrate animals: applied nominal strains appear to cause the retention and organization rather than the removal of load-bearing structure 1, 3–5, 7, 24. In the current investigation, we have found that increasing the applied load increases the time to failure of corneal collagen strips. This is definitive and likely to be unrelated to diffusion effects as discussed above. We further determined that there appears to be a precipitous reduction in the maximum rate of load-bearing area loss (enzymatic cleavage rate) as a function of strain over a very small initial strain difference between the two samples (Δof ~1.5%). The magnitude of change in the enzyme activity rate (more than two-fold) is remarkable since it appears to occur over such a small change in tissue length. This large difference in the rate of enzymatic activity results in the strain of the 0.1N sample “catching up to” the 0.25N sample in the first few minutes of the experiment and then catching up to the 0.5N sample at the end of the experiment. The extraction of the decreasing linear relationship between strain and enzymatic activity (which was extracted from the 0.1 N loaded sample) depends on the scaling calculation to provide a reasonable estimate of when diffusion will begin to limit the reaction rate. If the diffusion limitation occurs much earlier than our calculation suggests, there may be some error in the data extracted from the linear regression. However, the fact that the more highly-loaded sample does not exhibit a similar decline during the same period supports our assertion that it is strain, not diffusion which is slowing the enzymatic activity (at least initially).
Conclusion
In this study, we use a dynamic, enzyme-induced creep experiment to demonstrate that small mechanical strain differences induce loaded specimens to degrade at substantially different rates (higher strain, lower rate of degradation). Further, we extract a continuous relationship between mechanical strain and the enzymatic cleavage rate of collagenous tissue at low strains. The experiment differs from other experiments in that it is performed at physiological temperature and it takes advantage of the physics of the diffusion/reaction time scale to probe the enzyme activity when diffusion is not expected to interfere with the results. The data provide a clear demonstration that the enzymatic cleavage rate depends on the mechanical state of the tissue independent of diffusion effects. By all measures, the samples under lower load (unloaded/reloaded and 0.1N load) fail faster than the more highly loaded samples (0.25N and 0.5N). Perhaps the most striking finding is the fact that the maximum enzymatic activity rate differs by more than 50% over strain difference of only ~1.5%. This highly-sensitive drop-off in the cleavage rate occurs where the tissue exhibits stiffening which is likely due to the transition from an entropic loading regime to an energetic one 20. In addition to demonstrating that strain is protective, the data also indicate that the protection is not absolute. All specimens will degrade to failure (at this concentration of enzyme – 0.05 mM) in spite of the fact that the strains are continually increasing throughout the experiment. Crude BC is a non-specific and aggressive enzyme capable of degrading collagen in addition to many other molecules. This enzyme attacks the collagen molecule at multiple binding sites and is not a particularly relevant probe for in vivo collagenase activity. However, the fact that mechanical strain strongly depresses BC activity is very encouraging for the strain-stabilization hypothesis in vertebrate animals. There are very few native enzymes (Matrix Metalloproteinase family and Cathepsins) and non-native enzymes (BC) capable of degrading the collagen triple helix directly. In general, these enzymes must first bind to and then cleave the collagen monomer. In the case of MMPs, it has been suggested that the enzyme must first orient and then unwind the triple helix to perform an effective cleavage event 25, 26. The manipulation takes place near the thermally labile domain which is devoid of stabilizing hydroxyproline 27 and is likely to preferentially strain relative to the remaining collagen monomer. Thus MMPs are potentially even more susceptible to strain-induced stability. The method and bioreactor developed in this manuscript for probing collagen/enzyme mechanochemistry (dynamic, enzyme-induced creep) should apply quite well to the investigation of collagen/MMP mechanochemistry.
Supplementary Material
Acknowledgement
This work was partially supported by NEI R01EY015500 and NIAMS R21AR057056. The authors would like to acknowledge the Northeastern University Mechanical and Industrial Engineering Undergraduate Capstone course which is responsible for supporting and providing the initial design of the bioreactor. We would like to thank Professor Keith Meek and Sally Hayes for their efforts in helping us estimate how many fibrils are oriented in the direction of the applied load in bovine corneas.
References
- 1.Carter D, Beaupre G. Skeletal form and function: Mechanobiology of skeletal development, aging and regeneration. Cambridge: Press Syndicate of the University of Cambridge; 2001. [Google Scholar]
- 2.Carter DR, Van Der Meulen MC, Beaupre GS. Mechanical factors in bone growth and development. Bone. 1996;18(1 Suppl):5S–10S. doi: 10.1016/8756-3282(95)00373-8. [DOI] [PubMed] [Google Scholar]
- 3.Chalmers J, Ray R. The growth of transplanted foetal bones in different immunological environments. J. Bone Joint Surg. 1962;44B:149–164. [Google Scholar]
- 4.Cowin SC. How is a tissue built? J Biomech Eng. 2000;122(6):553–569. doi: 10.1115/1.1324665. [DOI] [PubMed] [Google Scholar]
- 5.Cowin SC. Tissue growth and remodeling. Annu Rev Biomed Eng. 2004;6:77–107. doi: 10.1146/annurev.bioeng.6.040803.140250. [DOI] [PubMed] [Google Scholar]
- 6.Roux W. Gesammelte abhandulgen uber entwicklungsmechanics der organismen. Leipzig: Wilhem Engelmann; 1895. [Google Scholar]
- 7.Wolff J. Das Gesetz Der Transformation Der Knochen. Berlin: A Hirschwald; 1891. [Google Scholar]
- 8.Bhole AP, Flynn BP, Liles M, Saeidi N, Dimarzio CA, Ruberti JW. Mechanical strain enhances survivability of collagen micronetworks in the presence of collagenase: implications for load-bearing matrix growth and stability. Philos Transact A Math Phys Eng Sci. 2009;367(1902):3339–3362. doi: 10.1098/rsta.2009.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kadler KE, Holmes DF, Trotter JA, Chapman JA. Collagen fibril formation. Biochem J. 1996;316(Pt 1):1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bass EC, Wistrom EV, Diederich CJ, Nau WH, Pellegrino R, Ruberti J, Lotz JC. Heat-induced changes in porcine annulus fibrosus biomechanics. J Biomech. 2004;37(2):233–240. doi: 10.1016/j.jbiomech.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Miles CA, Ghelashvili M. Polymer-in-a-box mechanism for the thermal stabilization of collagen molecules in fibers. Biophys J. 1999;76(6):3243–3252. doi: 10.1016/S0006-3495(99)77476-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C, Yannas IV. Mechanochemical studies of enzymatic degradation of insoluble collagen fibers. J Biomed Mater Res. 1977;11(1):137–154. doi: 10.1002/jbm.820110113. [DOI] [PubMed] [Google Scholar]
- 13.Nabeshima Y, Grood ES, Sakurai A, Herman JH. Uniaxial tension inhibits tendon collagen degradation by collagenase in vitro. J Orthop Res. 1996;14(1):123–130. doi: 10.1002/jor.1100140120. [DOI] [PubMed] [Google Scholar]
- 14.Ruberti JW, Hallab NJ. Strain-controlled enzymatic cleavage of collagen in loaded matrix. Biochem Biophys Res Commun. 2005;336(2):483–489. doi: 10.1016/j.bbrc.2005.08.128. [DOI] [PubMed] [Google Scholar]
- 15.Wyatt KE, Bourne JW, Torzilli PA. Deformation-Dependent Enzyme Mechanokinetic Cleavage of Type I Collagen. J Biomech Eng. 2009;131(5):051004. doi: 10.1115/1.3078177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lotz JC, Hadi T, Bratton C, Reiser KM, Hsieh AH. Anulus fibrosus tension inhibits degenerative structural changes in lamellar collagen. Eur Spine J. 2008;17(9):1149–1159. doi: 10.1007/s00586-008-0721-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Church K. Influence of mechanical load on enzymatic degradation of type 1 collagen in a miniature bioreactor. Boston: Masters, Northeastern University; 2007. [Google Scholar]
- 18.Meek KM, Leonard DW. Ultrastructure of the corneal stroma: a comparative study. Biophys J. 1993;64(1):273–280. doi: 10.1016/S0006-3495(93)81364-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott JE. Proteoglycan: collagen interactions and corneal ultrastructure. Biochem Soc Trans. 1991;19(4):877–881. doi: 10.1042/bst0190877. [DOI] [PubMed] [Google Scholar]
- 20.Sun YL, Luo ZP, Fertala A, An KN. Direct quantification of the flexibility of type I collagen monomer. Biochem Biophys Res Commun. 2002;295(2):382–386. doi: 10.1016/s0006-291x(02)00685-x. [DOI] [PubMed] [Google Scholar]
- 21.Meek KM, Boote C. The organization of collagen in the corneal stroma. Exp Eye Res. 2004;78(3):503–512. doi: 10.1016/j.exer.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Maurice DM, Watson PG. The distribution and movement of serum albumin in the cornea. Exp Eye Res. 1965;4(4):355–363. doi: 10.1016/s0014-4835(65)80052-5. [DOI] [PubMed] [Google Scholar]
- 23.Overby D, Ruberti J, Gong H, Freddo TF, Johnson M. Specific hydraulic conductivity of corneal stroma as seen by quick-freeze/deep-etch. J Biomech Eng. 2001;123(2):154–161. doi: 10.1115/1.1351888. [DOI] [PubMed] [Google Scholar]
- 24.Cowin SC. The mechanical and stress adaptive properties of bone. Ann Biomed Eng. 1983;11(3–4):263–295. doi: 10.1007/BF02363288. [DOI] [PubMed] [Google Scholar]
- 25.Chung L, Dinakarpandian D, Yoshida N, Lauer-Fields JL, Fields GB, Visse R, Nagase H. Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis. Embo J. 2004;23(15):3020–3030. doi: 10.1038/sj.emboj.7600318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauer-Fields JL, Juska D, Fields GB. Matrix metalloproteinases and collagen catabolism. Biopolymers. 2002;66(1):19–32. doi: 10.1002/bip.10201. [DOI] [PubMed] [Google Scholar]
- 27.Miles CA, Burjanadze TV, Bailey AJ. The kinetics of the thermal denaturation of collagen in unrestrained rat tail tendon determined by differential scanning calorimetry. J Mol Biol. 1995;245(4):437–446. doi: 10.1006/jmbi.1994.0035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.