Abstract
The electrophoretic translocation of polynucleotides through nanopores may permit direct single-molecule nucleic acid sequencing. Here we describe the translocation of ssRNA heteropolymers (91 to 6083 bases) through the α-hemolysin nanopore. Translocation of these long ssRNAs is characterized by surprisingly long, almost complete ionic current blockades with durations averaging milliseconds per base (at +180 mV). The event durations decrease exponentially with increased trans-membrane potential, but are largely unaffected by the presence of urea. When the ssRNA is coupled at the 3′ end to streptavidin, which cannot translocate through the pore, permanent blockades are observed, supporting our conclusion that the transient blockades arise from ssRNA translocation.
Keywords: α-hemolysin, RNA, translocation, nanopore, heteropolymer, single-molecule
INTRODUCTION
Single-molecule techniques offer the prospect of ultra-rapid, low-cost polynucleotide sequencing. In one approach, the electrophoretic (voltage-driven) or enzyme-driven translocation of DNA or RNA through a nanopore interrupts ionic flow through the pore in a base-specific manner, potentially allowing label-free sequencing without a need for amplification or nucleobase modification.1–7
The heptameric α-hemolysin (αHL) pore from Staphylococcus aureus has been extensively characterized as a potential platform for the single-molecule sequencing of DNA and RNA. The αHL pore contains a ~1.5 nm constriction which prevents the passage of double stranded (ds) nucleic acids, but permits the passage of single stranded (ss) oligonucleotides.8–10 The αHL pore is amenable to genetic engineering and its well defined geometry permits the introduction of chemical functionality with atomic precision.11–14 Both wild-type and mutant forms of the αHL pore insert readily into lipid bilayers, and maintain structural integrity under elevated temperatures,15 at various salt concentrations, and in the presence of detergents and chemical denaturants.16
The translocation of short (<450 nt) ssDNA and ssRNA through the αHL pore has been extensively characterized in terms of the polymer composition, strand length and the applied voltage.8, 17, 18 Nucleobase identification in immobilized short DNA and RNA homo- and hetero-oligomers has also been achieved.19, 20 Protein nanopores from other organisms have also been investigated for nucleotide sequencing applications.7, 21 Porin A from Mycobacterium smegmatis (MspA), is particularly advantageous, thanks to the large differences in the extent to which each base blocks the ionic current through the pore.5, 22, 23
Findings from the translocation of short (homo-oligomeric) nucleic acids cannot be simply extrapolated to longer polymers, due to the variety and complexity of the 3-dimensional structures that can be adopted. Notably, ss-heteropolymers can form ‘blob’-type structures,24 which may be stabilized by either short duplex regions or non-specific interactions, and which cannot translocate the pore without being unraveled. To date, most studies regarding the translocation of long DNA and RNA polymers have used comparatively large solid-state nanopores (typically 4 to 10 nm in diameter), and have focused on ds polynucleotides.25–28 Studies into the translocation of single stranded nucleic acids through solid-state nanopores have sought to minimize structural complexity either by using homopolymeric RNA,27, 29 or by using a double-stranded leader-sequence to drive the translocation of heteropolymeric ssDNA.24
Work from this laboratory has characterized interactions of hetero-oligomeric nucleic acids with the αHL pore,12, 19, 20 including an examination of the translocation of a 95-base ssRNA hetero-oligomer through the wild-type pore.16 In the present study, we sought to determine whether RNA polynucleotides significantly longer than those previously studied can be electrophoretically driven through the αHL pore.
RESULTS
In an initial experiment, a 1 kb ssRNA heteropolymer (see Supporting Information for methods) was added to the cis (ground) side of a recording chamber in which the two compartments were separated by a 1,2-diphytanoyl-sn-glycero-3-phosphocholine (DPhPC) lipid bilayer containing a single pore formed by the homo-heptameric form of the M113R mutant of αHL, which exhibits a higher frequency of polynucleotide translocation events relative to the wild-type pore.13 The application of a positive transmembrane potential (+180 mV) led to numerous blockades of the ionic current with a residual current (I%RES) typically < 1% (Figure S1). The majority of the blockade events ranged in duration from a few tens of s through to seconds. A minority of the events led to ‘permanent’ current blockades (>30 s) requiring the application of a negative potential (−150 mV) to unblock the pore.
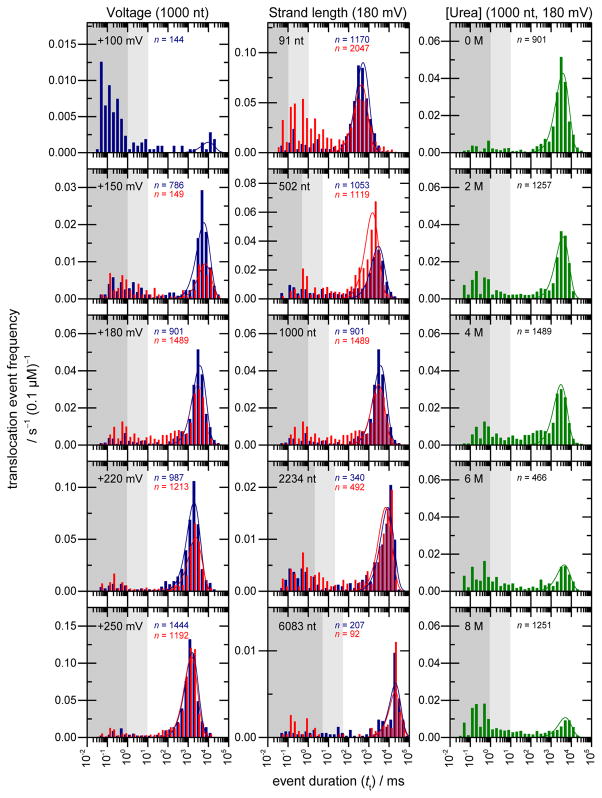
We hypothesized that the transient current blockades of millisecond to second duration were caused by translocation of ssRNA through the nanopore. To test this, the effects of varying the transmembrane potential and RNA strand length were examined (Figure 1). At +100 mV, few events were observed. The majority of the events at +100 mV had durations (tt) < 101 ms. However, a small number of events formed a population with much longer durations (tt ~104 ms). On increasing the transmembrane potential to +150 mV, the fraction of events with tt > 102 ms increased from 14% of all observed events to 71% of all observed events. From +180 mV to +250 mV, the fraction of events with tt > 102 ms increased further (Figures 1 and 2). For each voltage, the well-defined population of long events was fitted to a probability distribution function (incorporating a single exponential term, see Supporting Information), yielding the most probable event duration (t̄t).30, 31 Values of t̄t decrease exponentially with increasing voltage (Figure 2A), consistent with RNA translocation.14, 26, 32–34
Figure 1.
Blockade durations (tt) showing the interactions of ssRNAs with the M113R HL nanopore at 0 M (blue) or 4 M (red) urea. The y-axes show the translocation event frequency (ft = (t̄ON + t̄t) −1); note different graph panes have different scales. Histograms of the inter-event intervals (tON) as a function of voltage, strand length and urea concentration are shown in Figure S2. The effects of varying the applied voltage (left), the strand length (centre) and the urea concentration in the electrolyte (right) are shown. Peaks are fitted to a single-exponential probability density function (see Supporting Information). Experimental conditions: 1 M KCl, 20 mM Tris.HCl, pH 7.50, with 0.1 μM RNA in the cis compartment. Data are compiled from several experiments (N ≥ 3 in all cases). Dark grey shading indicates event durations corresponding to <1 μs/base; light grey shading corresponds to 1–10 μs/base.
Figure 2.
Characteristics of long (>10 ms) blockades, as functions of the applied voltage (left), strand length (centre) and urea concentration (right). Data are obtained from peak fitting of the histograms shown in Figure 1 and Figure S2. In all cases, the data points represent the weighted average (see Supporting Information) from N ≥3 experiments with the error bars showing the weighted error. Voltage dependencies are fitted to exponential functions. The strand-length – dependence data in Figure 2A are fitted to linear functions. The experimental conditions are given in Figure 1.
To test the effect of varying the RNA strand length, single-stranded heteropolymers from 91 to 6083 nt were synthesized and their interactions with the αHL pore were examined at +180 mV. In all cases, a broad range of blockade durations was observed, with a well defined major population of long events (Figure 1, centre). An approximately linear (R2 = 0.995) increase in t̄t was observed with increasing strand length (Figure 2A), again consistent with RNA translocation.8, 33, 35 For all experimental conditions of voltage and strand length, the residual current recorded during long blockade events (I%RES = 100 x IB/IO; IB = current in blocked state, IO = current at open pore) was less than 1% (Figure S3).
For both voltage – and strand-length – dependence experiments, mean inter-event (open-pore) dwell times (t̄ON) were determined, allowing the calculation of both capture frequencies (fc = 1/t̄ON) and translocation event frequencies (ft = 1/(t̄ON + t̄t)). For all experimental conditions, open-pore dwell times formed a single population (Figure S2). Values of t̄ON decreased exponentially with increasing voltage and also decreased with increasing strand length (Figure 2B), although in the latter case the experimental error in the values obtained is too great to determine whether t̄ON decreases in a linear or an exponential manner. Increasing the voltage resulted in an exponential increase in the values of both fc and ft, consistent with RNA translocation (Figure 2C and D).13, 36, 37 Increasing the strand length led to an increase in fc from 0.7 s −1(0.1 μM) −1 for 91 nt ssRNA to 5.1 s −1(0.1 μM) −1 for 6083 nt ssRNA (Figure 2C), (although ft decreased (Figure 2D) due to the linear increase in t̄t with increasing strand length, Figure 2A).
To prove that the long blockades (tt = 103 to 104 ms) arise from the translocation of ssRNA, the inability of the protein streptavidin to pass through the αHL nanopore was exploited.19, 20, 38 A 502 nt ssRNA was biotinylated at the 3′ end and, after purification, incubated with streptavidin, which forms an extremely tight non-covalent complex with the biotin ligand. The biotin-streptavidin–coupled RNA polymers were then introduced into the cis compartment, under an applied voltage of +180 mV (Figure S4). Under these conditions, 52% (s.d.= 14%) of the events were ‘permanent’ blocks (I%RES ≈ 6%; this increase in I%RES is consistent with findings from previous experiments, see Discussion) requiring the voltage polarity to be reversed in order to unblock the pore; only 48% of events were transient (<60 s). Control experiments showed that in the absence of streptavidin, only 10% (s.d. = 2%) of the events were ‘permanent’ blocks, with 90% being transient blockades (I%RES ≈1%). As streptavidin-coupled RNA is incapable of translocating through the pore, and as the introduction of the preformed complex into the cis compartment does not generate a substantial number of long transient blockades, this finding clearly demonstrates that the long transient events are caused by the translocation of RNA polymers.
Experiments were performed to determine whether the chemical denaturant urea alters t̄t. The voltage- and strand-length experiments described above were repeated in the presence of 4 M urea (higher urea concentrations led to frequent rupture of the DPhPC bilayer, hindering data acquisition, especially at higher voltages), and a further set of experiments at constant voltage (+180 mV) and constant strand length (1000 nt) were conducted in the presence of 0 to 8 M urea (Figures 1, 2, and S2–S3). In all cases, values of t̄t in the presence of urea were similar to values obtained at 0 M urea (Figure 1 and 2A). Values of t̄ON were slightly increased by the presence of 4 M urea (Figure 2B), causing a decrease in fc (Figure 2C). (As t̄t > t̄ON, values of ft showed little variation with varying urea concentration, Figure 2D).
Increasing the urea concentration from 0 M to 4 M urea increased the fraction of blockade events with tt corresponding to 10–100 μs/base. This effect was most pronounced for the shortest RNA strands (Figure 1, centre). For the 91 nt ssRNA at 0M urea, ~8% of events satisfying tt > 1 ms were in the range tt = 1 to 10 ms. At 4 M urea, ~25% of events satisfying tt > 1 ms were in the range tt = 1 to 10 ms. To investigate this, CD spectroscopy was used to assess the ability of urea to alter the bulk structural properties of the RNA strands in solution. CD spectroscopy has been previously used to examine urea/nucleic acid interactions including the urea-mediated melting of well-defined RNA duplexes39–41 and the urea-mediated thermal destabilization of RNAs with more complex tertiary structures,40 such as the aptamer domain of the adenine riboswitch.41 Increasing the urea concentration from 0 M to 8 M urea led to a decrease in the CD signal at 260 nm and a red shift peak in the CD signal between 260 and 270 nm (Figure 3A). Increasing the ssRNA strand length from 91 to 6083 nt reduced both the urea-dependent decrease in the CD signal at 260 nm and the extent of the red shift (Figure 3B), indicating that elevated urea concentrations have a smaller effect on the bulk structures of the longer ssRNAs than of the shorter strands.
Figure 3.
Circular dichroism (CD) study of the effect of urea on the RNAs used in this work. A: A red shift in the CD peak at ~260 nm is observed with increasing urea concentration for all the RNA strands. B: The ability of increasing the urea concentration to induce structural changes within the RNA in bulk solution correlates with the gradient of the traces in Panel A (i.e., dλ/d[urea], given in nm.M −1), which decreases with increasing strand length. Experimental conditions: 1 M KCl, 20 mM Tris.HCl, pH 7.50, at an RNA concentration of 24 μg mL −1.
DISCUSSION
The ability to electrophoretically translocate long RNA heteropolymers through nanopores might be used in approaches to sequence RNA at the single molecule level. However, to date, no detailed characterization of ssRNA translocation through the archetypal αHL nanopore has been reported.
The blockade durations (t̄t) described above, which are between 11.2 and 1.6 ms/base (these values for +100 and +250 mV, respectively), appear initially incompatible with previous data obtained with homo-oligomeric RNA, for which translocation velocities of 1 to 10 s/base have been reported.8, 17, 35 However, the voltage- and strand-length dependencies of the blockade durations and the abolition of the events when the RNA is complexed with streptavidin provides strong evidence that the observed blockades do indeed arise from RNA translocation. (Long transient events (tt > ~102 ms) observed in experiments using 502 nt RNA coupled with streptavidin are most likely due to residual unbiotinylated RNA polymers. However, the possibility that in some cases the streptavidin-conjugated RNA exits the pore from the cis side – i.e., against the electric field – cannot be excluded. Very short transient blockades (tt < 0.5 ms) were also observed and may arise from collisions of RNA molecules with the mouth of the nanopore.)
The precise origin of the surprisingly long values of t̄t observed for ssRNA translocation through the αHL pore is not known. However, a number of possibilities come to mind. Firstly, in contrast to previous studies, the RNA sequences used here (see Supporting Information) were not chosen to eliminate base-pairing. Complex secondary structures arising from both local and long-range base pairing may be possible (see Supporting Information for further analysis of RNA structures), and the values of t̄t may reflect the stability of these base-paired structures. Secondly, the ssRNA used is likely to form ‘blobs’ in solution (see Supporting Information), which would have to ‘unwind’ to permit translocation. Such a finding has previously been described for ssDNA by Dekker and coworkers.24 The formation of RNA blobs may also hinder the successful threading of RNA into the nanopore (particularly if the strand ends are partly buried within the blob), with the entropic barrier originating from the requirement to thread one end of the RNA strand into the nanopore prior to translocation contributing to the observed values of t̄t.42 It is even conceivable that long RNA heteropolymers may contain specific motifs that can transiently bind to the α-hemolysin pore.
Not all blockade events are of ms/base duration, with a significant number of events (especially at low voltages) shorter than 1 μs/base. Data falling within this range are highlighted in dark grey in Figure 1. These events cannot arise from RNA translocation as this would require the heteropolymer to translocate through the pore faster than short homopolymeric nucleic acids. Therefore, these events presumably arise from transient collisions of ssRNA with the mouth of the nanopore.8 Data that would imply translocation at 1 to 10 μs/base are highlighted in light grey. In this region, translocation remains improbable, as the structural complexity of heteropolymeric RNA would be anticipated to result in slower translocation than for short homo-oligomers.
In contrast to previous studies,13, 14, 17, 33, 43 the translocation of long RNA heteropolymers is associated with almost 100% current block (I%RES < 1%, Figure S3). Although the origin of this observation is not clear, there is potential for complex structures (such as RNA blobs) to form within the vestibule of the protein, generating an almost complete ionic current blockade. Consistent with previous findings,19 the blockade current when streptavidin-immobilized RNA is present in the pore (I%RES ≈ 6%) is higher than that recorded during translocation events (I%RES ≈ 1%); this observation is interpreted in terms of the polymer being stretched under the influence of the electric field when immobilized at the cis side of the pore.
To investigate the origin of the extremely long duration of blockade events observed during ssRNA translocation, we investigated whether the incorporation of urea into the electrolyte solution altered t̄t. Urea is a commonly used denaturant for proteins, and has also been investigated in detail as a denaturant of nucleic acid structures.39–41 However, our results show that incorporating urea into the electrolyte solution does not decrease t̄t. For the shorter RNA polymers, however, urea has an effect on the fraction of blockade events with tt corresponding to 10 to 100 μs/base. For the 91 nt RNA at 0M urea, ~8% of events satisfying tt > 1 ms were in the range tt = 1 to 10 ms. At 4 M urea, ~25% of events satisfying tt > 1 ms were in the range tt = 1 to 10 ms, an increase of ~300%. These events may be assigned to the translocation of RNA molecules with a urea-induced decrease in structural complexity. Urea has a smaller effect on the longer ssRNAs, and for the 2036 and 6083 nt polymers the fraction of events with tt corresponding to 10 to 100 μs/base is not altered by 4 M urea.
At 0 M urea, values of fc increase with strand length, whereas at 4 M urea no such increase is observed (Figure 2C). The reason for this is not clear, however previous studies have shown that the capture frequency of dsDNA by solid-state nanopores is strand-length dependent, with the capture frequency increasing with strand length until a plateau is reached.37, 42 The authors interpret this finding by using a model in which DNA capture by the pore requires one end of the polymer to be threaded through the pore, with the spatial confinement of the polymer end creating a free energy barrier to capture. Successful penetration of this barrier is enhanced as the effective charge (which is proportional to the length of the DNA) increases. Below a threshold strand length (104 bp in reference 37), capture frequencies increase with increasing strand length, however above this threshold, diffusion to the nanopore limits the maximum capture frequency.37 An analogous explanation may apply to our results. In the absence of urea, an increase in values of fc with increasing strand length is observed, as described above. However, in the presence of 4 M urea, values of fc are unchanged by varying the strand length. As high urea concentrations significantly increase the viscosity of aqueous solutions,16 at 4 M urea the diffusion of the RNA strands through the bulk electrolyte may limit fc.
For the future direct nanopore sequencing of long nucleic acids, continuous polymer translocation through the pore at a controlled velocity is desirable. Technologies based on both the voltage-driven and enzyme-driven3, 5 translocation of nucleic acids through nanopores allow the speed of translocation to be varied (for example by varying the applied voltage (electrophoretic systems) or substrate concentrations (enzyme-driven systems)), however several challenges remain to be overcome in both cases.7 For example, with voltage-driven systems, translocation velocities are typically too high to allow single base resolution, whereas for enzyme-driven systems the issues of enzyme dissociation from the nucleic acid (limited processivity) and step size variation (enzyme step sizes are typically stochastic) arise. Our results demonstrate the possibility of kilobase ssRNA reads with a voltage driven system, but further experiments are required to determine whether translocation of long ssRNA occurs in a smooth or discontinuous manner.
Supplementary Material
Acknowledgments
JAC, DJ and HB gratefully acknowledge funding from the National Human Genome Research Institute (National Institutes of Health) and Oxford Nanopore Technologies. DJ was supported by graduate fellowships from the Royal Thai Government and the Thailand National Science and Technology Development Agency. We thank Giovanni Maglia and Murugappan Muthukumar for helpful comments.
Footnotes
Full experimental methods are provided in the Supporting Information, including detailed experimental methods, typical bilayer recording traces, open-pore dwell-time histograms, blockade current vs. dwell time scatter plots, CD spectra, RNA sequences and a discussion of RNA structural complexity. In summary, αHL heptamers (M113R) were produced by in vitro transcription/translation in the presence of rabbit red blood cell membranes.44 Single-stranded RNAs were produced by transcription from linearized DNA plasmids and purified to homogeneity prior to use. Planar bilayer recordings were carried out as described previously,45 with a single αHL pore in a DPhPC bilayer. The buffered electrolyte used throughout was 1 M KCl, 20 mM Tris.HCl, pH 7.50, containing 0 to 8 M urea. The RNA concentration was 0.1 M (in RNA strands) in the cis (ground) compartment of the chamber. All analyzed events had a residual current (I%RES) ≤ 25% of the unoccupied pore value. CD experiments were performed at 25°C at an RNA concentration of 24 μg mL −1 in planar bilayer recording buffer.
This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Bayley H. Phys Life Rev. 2012;9(2):161–163. doi: 10.1016/j.plrev.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Branton D, Deamer DW, Marziali A, Bayley H, Benner SA, Butler T, Di Ventra M, Garaj S, Hibbs A, Huang XH, Jovanovich SB, Krstic PS, Lindsay S, Ling XSS, Mastrangelo CH, Meller A, Oliver JS, Pershin YV, Ramsey JM, Riehn R, Soni GV, Tabard-Cossa V, Wanunu M, Wiggin M, Schloss JA. Nat Biotechnol. 2008;26(10):1146–1153. doi: 10.1038/nbt.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherf GM, Lieberman KR, Rashid H, Lam CE, Karplus K, Akeson M. Nat Biotechnol. 2012;30(4):344–348. doi: 10.1038/nbt.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howorka S, Siwy Z. Chem Soc Rev. 2009;38(8):2360–2384. doi: 10.1039/b813796j. [DOI] [PubMed] [Google Scholar]
- 5.Manrao EA, Derrington IM, Laszlo AH, Langford KW, Hopper MK, Gillgren N, Pavlenok M, Niederweis M, Gundlach JH. Nat Biotechnol. 2012;30(4):349–U174. doi: 10.1038/nbt.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkatesan BM, Bashir R. Nature Nanotech. 2011;6(10):615–624. doi: 10.1038/nnano.2011.129. [DOI] [PubMed] [Google Scholar]
- 7.Wanunu M. Phys Life Rev. 2012;9(2):125–158. doi: 10.1016/j.plrev.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasianowicz JJ, Brandin E, Branton D, Deamer DW. Proc Natl Acad Sci. 1996;93(24):13770–13773. doi: 10.1073/pnas.93.24.13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song LZ, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE. Science. 1996;274(5294):1859–1866. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- 10.Vercoutere W, Winters-Hilt S, Olsen H, Deamer D, Haussler D, Akeson M. Nat Biotechnol. 2001;19(3):248–252. doi: 10.1038/85696. [DOI] [PubMed] [Google Scholar]
- 11.Bayley H, Cremer PS. Nature. 2001;413(6852):226–230. doi: 10.1038/35093038. [DOI] [PubMed] [Google Scholar]
- 12.Maglia G, Henricus M, Wyss R, Li QH, Cheley S, Bayley H. Nano Lett. 2009;9(11):3831–3836. doi: 10.1021/nl9020232. [DOI] [PubMed] [Google Scholar]
- 13.Maglia G, Restrepo MR, Mikhailova E, Bayley H. Proc Natl Acad Sci. 2008;105(50):19720–19725. doi: 10.1073/pnas.0808296105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rincon-Restrepo M, Milthallova E, Bayley H, Maglia G. Nano Lett. 2011;11(2):746–750. doi: 10.1021/nl1038874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang XF, Gu LQ, Cheley S, Bayley H. Angew Chem Int Ed. 2005;44(10):1495–1499. doi: 10.1002/anie.200461885. [DOI] [PubMed] [Google Scholar]
- 16.Japrung D, Henricus M, Li QH, Maglia G, Bayley H. Biophys J. 2010;98(9):1856–1863. doi: 10.1016/j.bpj.2009.12.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akeson M, Branton D, Kasianowicz JJ, Brandin E, Deamer DW. Biophys J. 1999;77(6):3227–3233. doi: 10.1016/S0006-3495(99)77153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renner S, Geltinger S, Simmel FC. Small. 2010;6(2):190–194. doi: 10.1002/smll.200901435. [DOI] [PubMed] [Google Scholar]
- 19.Stoddart D, Heron AJ, Mikhailova E, Maglia G, Bayley H. Proc Natl Acad Sci. 2009;106(19):7702–7707. doi: 10.1073/pnas.0901054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayub M, Bayley H. Nano Lett. 2012;12(11):5637–5643. doi: 10.1021/nl3027873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wendell D, Jing P, Geng J, Subramaniam V, Lee TJ, Montemagno C, Guo P. Nature Nanotech. 2009;4(11):765–772. doi: 10.1038/nnano.2009.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler TZ, Pavlenok M, Derrington IM, Niederweis M, Gundlach JH. Proc Natl Acad Sci. 2008;105(52):20647–20652. doi: 10.1073/pnas.0807514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derrington IM, Butler TZ, Collins MD, Manrao E, Pavlenok M, Niederweis M, Gundlach JH. Proc Natl Acad Sci. 2010;107(37):16060–16065. doi: 10.1073/pnas.1001831107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kowalczyk SW, Tuijtel MW, Donkers SP, Dekker C. Nano Lett. 2010;10(4):1414–1420. doi: 10.1021/nl100271c. [DOI] [PubMed] [Google Scholar]
- 25.Storm AJ, Storm C, Chen JH, Zandbergen H, Joanny JF, Dekker C. Nano Lett. 2005;5(7):1193–1197. doi: 10.1021/nl048030d. [DOI] [PubMed] [Google Scholar]
- 26.Wanunu M, Sutin J, McNally B, Chow A, Meller A. Biophys J. 2008;95(10):4716–4725. doi: 10.1529/biophysj.108.140475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skinner GM, van den Hout M, Broekmans O, Dekker C, Dekker NH. Nano Lett. 2009;9(8):2953–2960. doi: 10.1021/nl901370w. [DOI] [PubMed] [Google Scholar]
- 28.van den Hout M, Vilfan ID, Hage S, Dekker NH. Nano Lett. 2010;10(2):701–707. doi: 10.1021/nl903925a. [DOI] [PubMed] [Google Scholar]
- 29.van den Hout M, Skinner GM, Klijnhout S, Krudde V, Dekker NH. Small. 2011;7(15):2217–2224. doi: 10.1002/smll.201100265. [DOI] [PubMed] [Google Scholar]
- 30.Cacciuto A, Luijten E. Phys Rev Lett. 2006;96:238104. doi: 10.1103/PhysRevLett.96.238104. [DOI] [PubMed] [Google Scholar]
- 31.Sakmann B, Neher E. Single-Channel Recording. 2. Plenum Press; New York: 1995. [Google Scholar]
- 32.Meller A. J Phys: Condens Matter. 2003;15(17):R581–R607. [Google Scholar]
- 33.Meller A, Nivon L, Branton D. Phys Rev Lett. 2001;86(15):3435–3438. doi: 10.1103/PhysRevLett.86.3435. [DOI] [PubMed] [Google Scholar]
- 34.de Zoysa RSS, Krishantha DMM, Zhao QT, Gupta J, Guan XY. Electrophoresis. 2011;32(21):3034–3041. doi: 10.1002/elps.201100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deamer DW, Branton D. Acc Chem Res. 2002;35(10):817–825. doi: 10.1021/ar000138m. [DOI] [PubMed] [Google Scholar]
- 36.Henrickson SE, Misakian M, Robertson B, Kasianowicz JJ. Phys Rev Lett. 2000;85(14):3057–3060. doi: 10.1103/PhysRevLett.85.3057. [DOI] [PubMed] [Google Scholar]
- 37.Wanunu M, Morrison W, Rabin Y, Grosberg AY, Meller A. Nature Nanotech. 2010;5(2):160–165. doi: 10.1038/nnano.2009.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Purnell R, Schmidt J. Methods Mol Biol. 2012;870:39–53. doi: 10.1007/978-1-61779-773-6_3. [DOI] [PubMed] [Google Scholar]
- 39.Priyakumar UD, Hyeon C, Thirumalai D, MacKerell AD. J Am Chem Soc. 2009;131(49):17759–17761. doi: 10.1021/ja905795v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shelton VM, Sosnick TR, Pan T. Biochem. 1999;38(51):16831–16839. doi: 10.1021/bi991699s. [DOI] [PubMed] [Google Scholar]
- 41.Lambert D, Draper DE. Biochem. 2012;51(44):9014–9026. doi: 10.1021/bi301103j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muthukumar M. J Chem Phys. 2010;(19):132. doi: 10.1063/1.3429882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butler TZ, Gundlach JH, Troll M. Biophys J. 2007;93(9):3229–3240. doi: 10.1529/biophysj.107.107003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheley S, Malghani MS, Song LZ, Hobaugh M, Gouaux JE, Yang J, Bayley H. Protein Eng. 1997;10(12):1433–1443. doi: 10.1093/protein/10.12.1433. [DOI] [PubMed] [Google Scholar]
- 45.Maglia G, Heron AJ, Stoddart D, Japrung D, Bayley H. Methods Enzymol. 2010;475:591–623. doi: 10.1016/S0076-6879(10)75022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.