Abstract
Free fatty acid (FFA) transport was measured in 11 and glycerol turnover in 5 newborns with continuous tracer infusion of [1-13C]palmitate or [2-13C]glycerol, respectively. In addition, simultaneous determination of glucose production in the latter group with [6,6-2H2]glucose tracer and measurement of the appearance rate of [13C]glucose derived from [13C]glycerol allowed calculation of gluconeogenesis from glycerol.
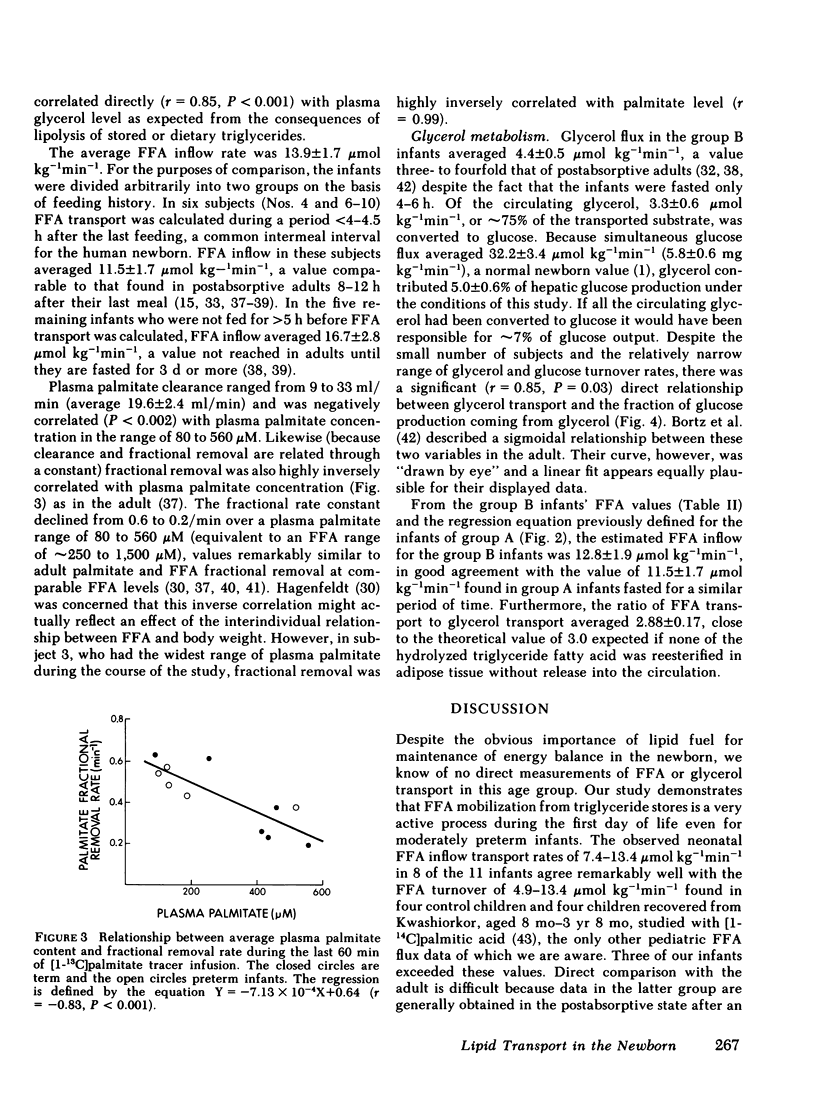
The average FFA inflow rate was 11.5±1.7 μmol kg−1min−1, 2.5-4.5 h after the last feeding, and 16.7±2.8 μmol kg−1min−1, 5-12 h after the last meal. These rates are comparable to those found in adults only after 8-16 h and ∼72 h of fasting, respectively. FFA inflow in the newborn was directly correlated with time of fasting, plasma FFA level, and plasma glycerol level. Palmitate clearance and fractional removal were inversely related to palmitate level.
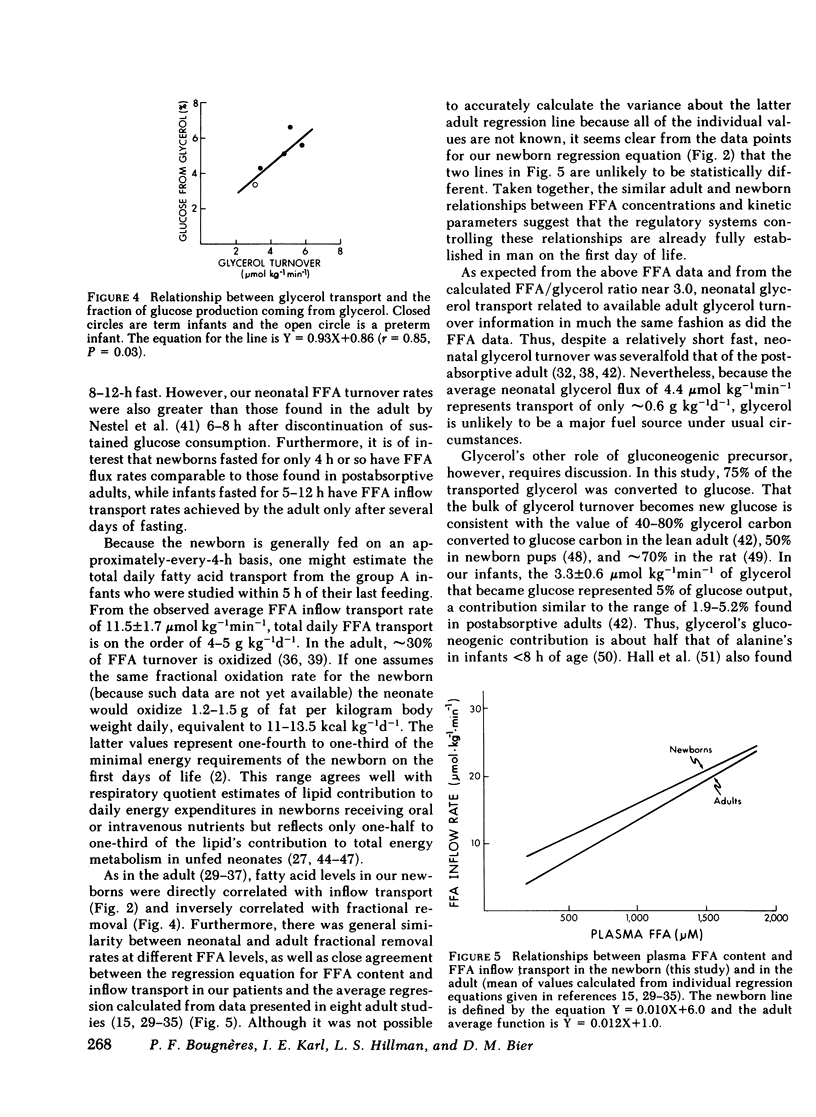
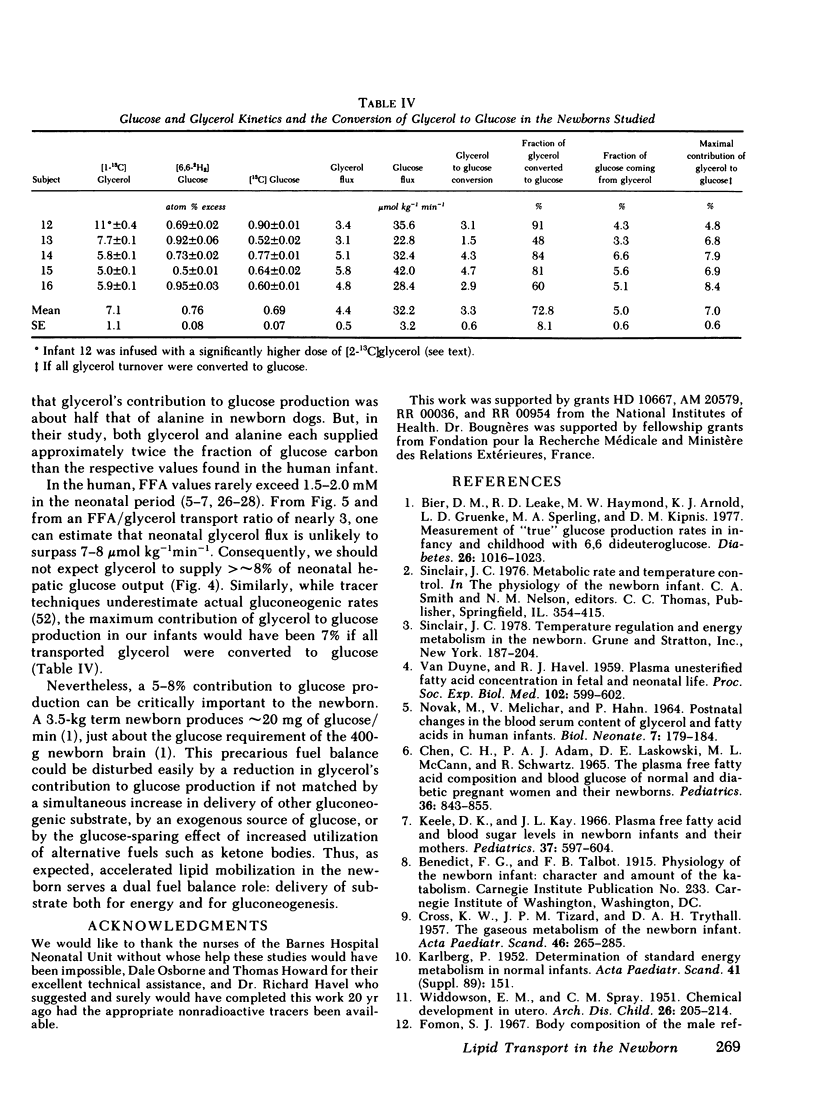
Glycerol flux averaged 4.4±0.5 μmol kg−1min−1, a value three- to fourfold that of the postabsorptive adult. Approximately 75% of transported glycerol was converted to glucose and represented 5.0±0.6% of hepatic glucose production. Furthermore, there was a direct relationship between glycerol turnover and the fraction of glucose coming from glycerol.
Despite the absolutely elevated neonatal FFA and glycerol transport rates, these were quantitatively similar to values found in adults with comparable elevated substrate levels. Furthermore, other similarities with the adult in the relationships between inflow transport and substrate values, and between transport and fractional removal suggest that the regulatory aspects of lipid transport in man are already well developed by the first day of life.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bier D. M., Arnold K. J., Sherman W. R., Holland W. H., Holmes W. F., Kipnis D. M. In-vivo measurement of glucose and alanine metabolism with stable isotopic tracers. Diabetes. 1977 Nov;26(11):1005–1015. doi: 10.2337/diab.26.11.1005. [DOI] [PubMed] [Google Scholar]
- Bier D. M., Leake R. D., Haymond M. W., Arnold K. J., Gruenke L. D., Sperling M. A., Kipnis D. M. Measurement of "true" glucose production rates in infancy and childhood with 6,6-dideuteroglucose. Diabetes. 1977 Nov;26(11):1016–1023. doi: 10.2337/diab.26.11.1016. [DOI] [PubMed] [Google Scholar]
- Björntorp P., Bergman H., Varnauskas E., Lindholm B. Lipid mobilization in relation to body composition in man. Metabolism. 1969 Oct;18(10):840–851. doi: 10.1016/0026-0495(69)90059-6. [DOI] [PubMed] [Google Scholar]
- Bortz W. M., Paul P., Haff A. C., Holmes W. L. Glycerol turnover and oxidation in man. J Clin Invest. 1972 Jun;51(6):1537–1546. doi: 10.1172/JCI106950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougnères P. F., Bier D. M. Stable isotope dilution method for measurement of palmitate content and labeled palmitate tracer enrichment in microliter plasma samples. J Lipid Res. 1982 Mar;23(3):502–507. [PubMed] [Google Scholar]
- CROSS K. W., TIZARD J. P., TRYTHALL D. A. The gaseous metabolism of the newborn infant. Acta Paediatr. 1957 May;46(3):265–285. doi: 10.1111/j.1651-2227.1957.tb16152.x. [DOI] [PubMed] [Google Scholar]
- Cassady G. Plasma volume studies in low birth weight infants. Pediatrics. 1966 Dec;38(6):1020–1027. [PubMed] [Google Scholar]
- Chen C. H., Adam P. A., Laskowski D. E., McCann M. L., Schwartz R. The plasma free fatty acid composition and blood glucose of normal and diabetic pregnant women and of their newborns. Pediatrics. 1965 Dec;36(6):843–855. [PubMed] [Google Scholar]
- Chiasson J. L., Liljenquist J. E., Lacy W. W., Jennings A. S., Cherrington A. D. Gluconeogenesis: methodological approaches in vivo. Fed Proc. 1977 Feb;36(2):229–235. [PubMed] [Google Scholar]
- Eaton R. P., Berman M., Steinberg D. Kinetic studies of plasma free fatty acid and triglyceride metabolism in man. J Clin Invest. 1969 Aug;48(8):1560–1579. doi: 10.1172/JCI106122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomon S. J. Body composition of the male reference infant during the first year of life. Pediatrics. 1967 Nov;40(5):863–870. [PubMed] [Google Scholar]
- Frazer T. E., Karl I. E., Hillman L. S., Bier D. M. Direct measurement of gluconeogenesis from [2,3]13C2]alanine in the human neonate. Am J Physiol. 1981 Jun;240(6):E615–E621. doi: 10.1152/ajpendo.1981.240.6.E615. [DOI] [PubMed] [Google Scholar]
- Friedman Z., Danon A., Lamberth E. L., Jr, Mann W. J. Cord blood fatty acid composition in infants and in their mothers during the third trimester. J Pediatr. 1978 Mar;92(3):461–466. doi: 10.1016/s0022-3476(78)80450-8. [DOI] [PubMed] [Google Scholar]
- Galster A. D., Clutter W. E., Cryer P. E., Collins J. A., Bier D. M. Epinephrine plasma thresholds for lipolytic effects in man: measurements of fatty acid transport with [l-13C]palmitic acid. J Clin Invest. 1981 Jun;67(6):1729–1738. doi: 10.1172/JCI110211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenfeldt L. A gas chromatographic method for the determination of individual free fatty acids in plasma. Clin Chim Acta. 1966 Feb;13(2):266–268. doi: 10.1016/0009-8981(66)90304-4. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L. Turnover of individual free fatty acids in man. Fed Proc. 1975 Dec;34(13):2246–2249. [PubMed] [Google Scholar]
- Hagenfeldt L., Wahren J., Pernow B., Räf L. Uptake of individual free fatty acids by skeletal muscle and liver in man. J Clin Invest. 1972 Sep;51(9):2324–2330. doi: 10.1172/JCI107043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenfeldt L., Wahren J. Turnover of plasma-free arachidonic and oleic acids in resting and exercising human subjects. Metabolism. 1975 Jul;24(7):799–806. doi: 10.1016/0026-0495(75)90126-2. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L., Wennlung A., Felig P., Wahren J. Turnover and splanchnic metabolism of free fatty acids in hyperthyroid patients. J Clin Invest. 1981 Jun;67(6):1672–1677. doi: 10.1172/JCI110204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S. E., Goebel R., Barnes I., Hetenyi G., Jr, Berman M. The turnover and conversion to glucose of alanine in newborn and grown dogs. Fed Proc. 1977 Feb;36(2):239–244. [PubMed] [Google Scholar]
- Hall S. E., Hall A. J., Layberry R. A., Berman M., Hetenyi G., Jr Effects of age and fasting on gluconeogenesis from glycerol in dogs. Am J Physiol. 1976 Feb;230(2):362–367. doi: 10.1152/ajplegacy.1976.230.2.362. [DOI] [PubMed] [Google Scholar]
- Hall S. E., Saunders J., Sönksen P. H. Glucose and free fatty acid turnover in normal subjects and in diabetic patients before and after insulin treatment. Diabetologia. 1979 May;16(5):297–306. doi: 10.1007/BF01223618. [DOI] [PubMed] [Google Scholar]
- Havel R. J. Caloric homeostasis and disorders of fuel transport. N Engl J Med. 1972 Dec 7;287(23):1186–1192. doi: 10.1056/NEJM197212072872307. [DOI] [PubMed] [Google Scholar]
- Havel R. J., Kane J. P., Balasse E. O., Segel N., Basso L. V. Splanchnic metabolism of free fatty acids and production of triglycerides of very low density lipoproteins in normotriglyceridemic and hypertriglyceridemic humans. J Clin Invest. 1970 Nov;49(11):2017–2035. doi: 10.1172/JCI106422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim T. Thermogenesis in the newborn infant. Clin Obstet Gynecol. 1971 Sep;14(3):790–820. doi: 10.1097/00003081-197109000-00009. [DOI] [PubMed] [Google Scholar]
- Hetenyi G., Jr Correction for the metabolic exchange of 14C for 12C atoms in the pathway of gluconeogenesis in vivo. Fed Proc. 1982 Jan;41(1):104–109. [PubMed] [Google Scholar]
- Issekutz B., Jr, Bortz W. M., Miller H. I., Paul P. Turnover rate of plasma FFA in humans and in dogs. Metabolism. 1967 Nov;16(11):1001–1009. doi: 10.1016/0026-0495(67)90093-5. [DOI] [PubMed] [Google Scholar]
- KARLBERG P. [Determinations of standard energy metabolism (basal metabolism) in normal infants]. Acta Paediatr Suppl. 1952 Oct;41(89):1–151. [PubMed] [Google Scholar]
- Keele D. K., Kay J. L. Plasma free fatty acid and blood sugar levels in newborn infants and their mothers. Pediatrics. 1966 Apr;37(4):597–604. [PubMed] [Google Scholar]
- Krauss A. N., Auld P. A. Metabolic requirements of low-birth-weight infants. J Pediatr. 1969 Dec;75(6):952–956. doi: 10.1016/s0022-3476(69)80331-8. [DOI] [PubMed] [Google Scholar]
- Lewis B., Wittmann W., Krut L. H., Hansen J. D., Brock J. F. Free fatty acid flux through plasma in protein malnutrition of infants. Clin Sci. 1966 Jun;30(3):371–375. [PubMed] [Google Scholar]
- Melichar V., Wolf H. Postnatal changes in the blood serum content of glycerol and free fatty acids in premature infants. Influence of hypothermia and of respiratory distress. Biol Neonat. 1967;11(1):50–60. doi: 10.1159/000240054. [DOI] [PubMed] [Google Scholar]
- Mestyán J., Rubecz I. The metabolic pattern of premature infants receiving aminosol-glucose infusion. Acta Paediatr Acad Sci Hung. 1973;14(3):319–328. [PubMed] [Google Scholar]
- NOVAK M., MELICHAR V., HAHN P., KOLDOVSKY O. RELEASE OF FREE FATTY ACIDS FROM ADIPOSE TISSUE OBTAINED FROM NEWBORN INFANTS. J Lipid Res. 1965 Jan;6:91–95. [PubMed] [Google Scholar]
- NOVAK M., MELICHAR V., HAHN P. POSTNATAL CHANGES IN THE BLOOD SERUM CONTENT OF GLYCEROL AND FATTY ACIDS IN HUMAN INFANTS. Biol Neonat. 1964;7:179–184. doi: 10.1159/000239922. [DOI] [PubMed] [Google Scholar]
- Nestel P. J., Ishikawa T., Goldrick R. B. Diminished plasma free fatty acid clearance in obese subjects. Metabolism. 1978 May;27(5):589–597. doi: 10.1016/0026-0495(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Okajima F., Chenoweth M., Rognstad R., Dunn A., Katz J. Metabolism of 3H- and 14C-labelled lactate in starved rats. Biochem J. 1981 Feb 15;194(2):525–540. doi: 10.1042/bj1940525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson B., Gentz J. The pattern of blood lipids, glycerol and ketone bodies during the neonatal period, infancy and childhood. Acta Paediatr Scand. 1966 Jul;55(4):353–362. doi: 10.1111/j.1651-2227.1966.tb08806.x. [DOI] [PubMed] [Google Scholar]
- Rubecz I., Mestyán J. Energy metabolism and intravenous nutrition of premature infants. I. The responses of oxygen consumption, respiratory quotient and substrate utilization to infusion of aminosol-glucose. Biol Neonate. 1973;23(1):45–58. doi: 10.1159/000240586. [DOI] [PubMed] [Google Scholar]
- Rubecz I., Mestyán J., Varga P., Klujber L. Energy metabolism, substrate utilization, and nitrogen balance in parenterally fed postoperative neonates and infants. The effect of glucose, glucose + amino acids, lipid + amino acids infused in isocaloric amounts. J Pediatr. 1981 Jan;98(1):42–46. doi: 10.1016/s0022-3476(81)80530-6. [DOI] [PubMed] [Google Scholar]
- Saunders J., Hall S. E., Sönksen P. H. Glucose and free fatty acid turnover in thyrotoxicosis and hypothyroidism, before and after treatment. Clin Endocrinol (Oxf) 1980 Jul;13(1):33–44. doi: 10.1111/j.1365-2265.1980.tb01020.x. [DOI] [PubMed] [Google Scholar]
- VAN DUYNE C. M., HAVEL R. J. Plasma unesterified fatty acid concentration in fetal and neonatal life. Proc Soc Exp Biol Med. 1959 Oct-Dec;102:599–602. doi: 10.3181/00379727-102-25330. [DOI] [PubMed] [Google Scholar]
- WIDDOWSON E. M. Chemical composition of newly born mammals. Nature. 1950 Oct 14;166(4224):626–628. doi: 10.1038/166626a0. [DOI] [PubMed] [Google Scholar]
- WIDDOWSON E. M., SPRAY C. M. Chemical development in utero. Arch Dis Child. 1951 Jun;26(127):205–214. doi: 10.1136/adc.26.127.205. [DOI] [PMC free article] [PubMed] [Google Scholar]


