Abstract
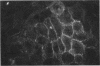
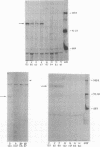
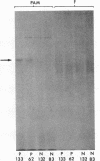
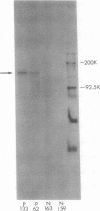
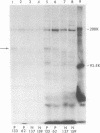
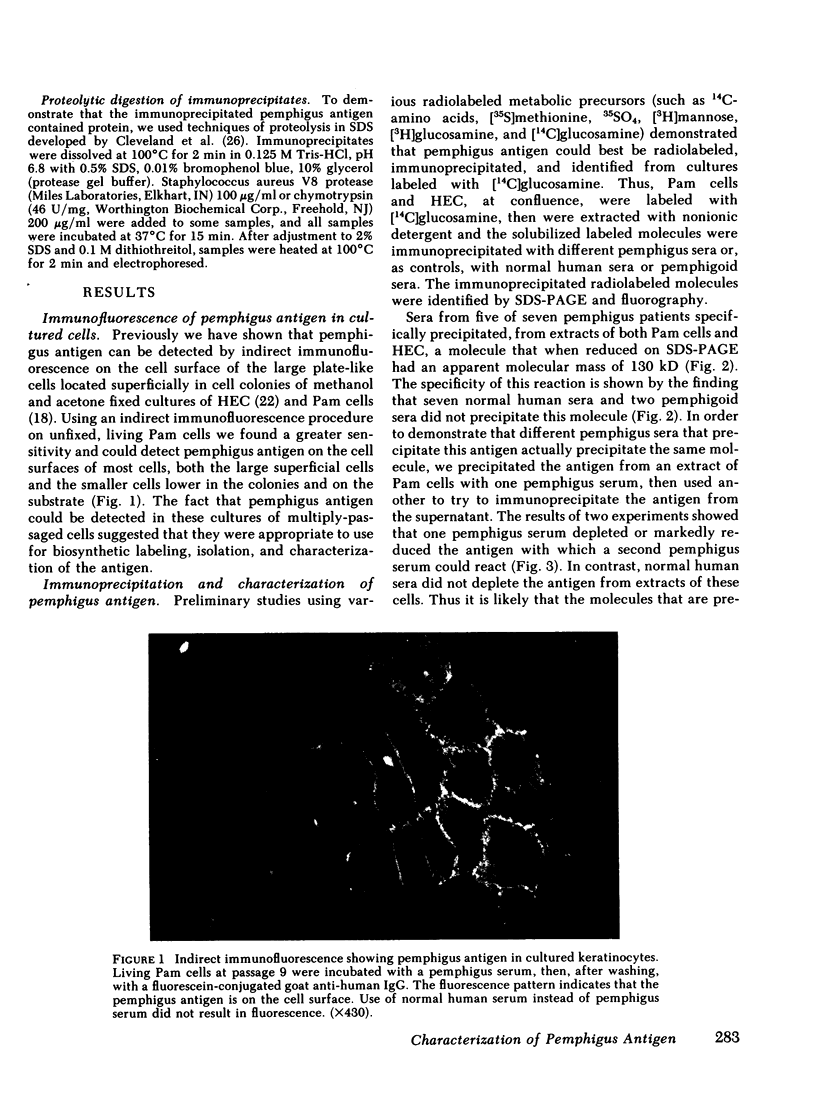
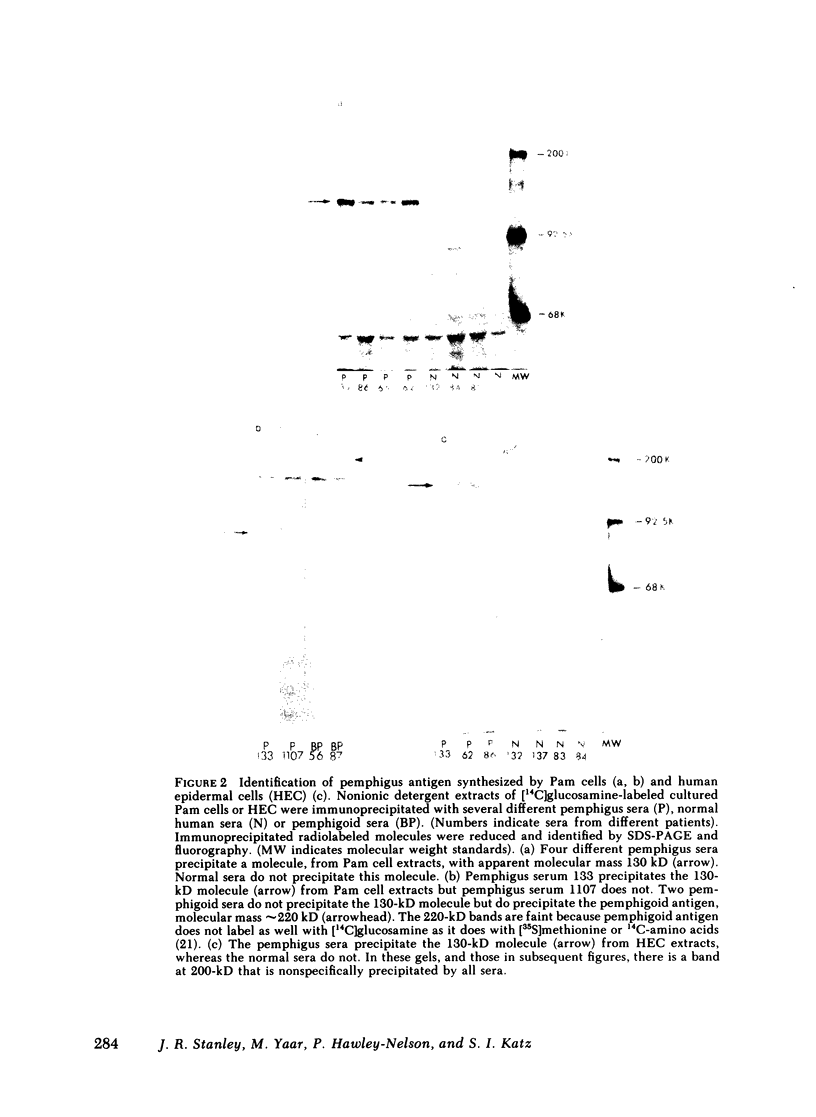
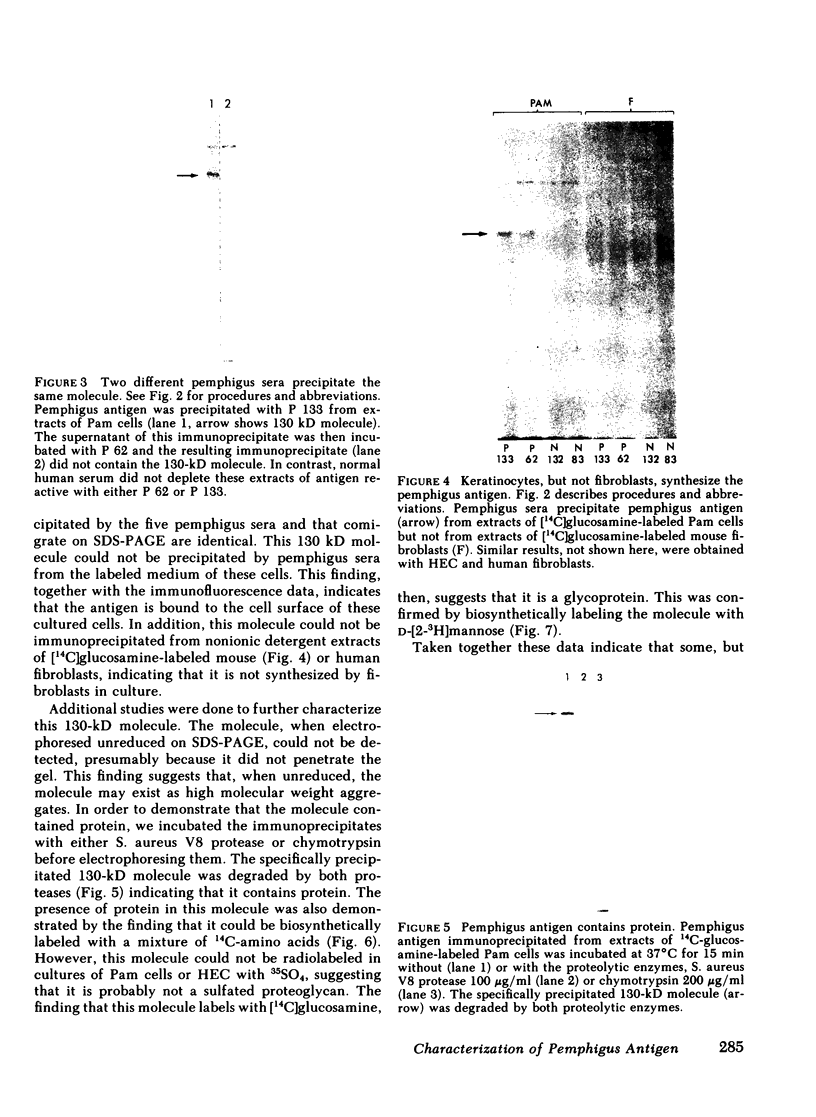
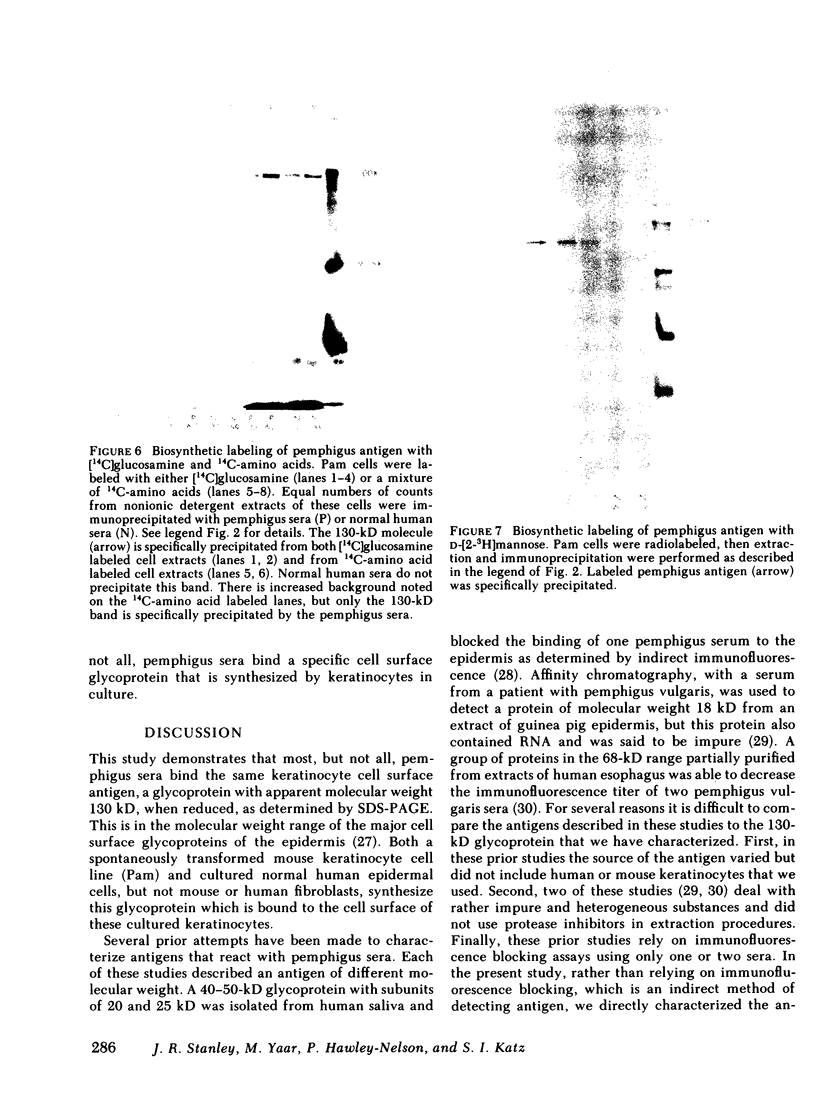
Pemphigus is an antibody-mediated autoimmune skin disease in which loss of cell-to-cell contacts in the epidermis results in blister formation. Patients with pemphigus develop antibodies that bind to the keratinocyte cell surface, the site of primary pathology. The purpose of this study was to characterize the antigen(s) to which pemphigus antibodies bind. Because we could detect pemphigus antigen by indirect immunofluorescence on the surface of multiply-passaged cells in cultures of both a spontaneously transformed mouse keratinocyte cell line (Pam) and normal human epidermal cells, we used these cells as a source of antigen. In order to demonstrate biosynthesis of antigen and to characterize the antigen(s), we radiolabeled cell cultures with [14C]glucosamine or d-[2-3H]mannose and used different pemphigus sera to immunoprecipitate antigen from nonionic detergent extracts of these labeled cells. Specifically precipitated radiolabeled molecules were identified using sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE) and fluorography. Sera from five of seven pemphigus patients specifically precipitated (from extracts of both Pam cells and human epidermal cells) a molecule that, when reduced, was ∼130 kD, whereas seven normal human sera and two pemphigoid sera did not precipitate this molecule. The findings that (a) these precipitated molecules comigrated on SDS-PAGE and that (b) the 130-kD molecule could no longer be precipitated from cell extracts that had been previously reacted with a pemphigus serum, indicate that reactive pemphigus sera bind the same molecule. The molecule was not detected in the culture medium of these cells. This finding, along with the cell surface immunofluorescence pattern, suggests that the antigen is bound to the cell surface. Cultured mouse and human fibroblasts do not synthesize the antigen. The antigen contains protein because it was degraded by V8 protease and chymotrypsin, and it could also be labeled with [14C]amino acids. It is probably not a sulfated proteoglycan because it did not label with 35SO4. Taken together, these data indicate that some, but not all, pemphigus sera bind a specific cell surface glycoprotein that is synthesized by keratinocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutner E. H., Jordon R. E., Chorzelski T. P. The immunopathology of pemphigus and bullous pemphigoid. J Invest Dermatol. 1968 Aug;51(2):63–80. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brigden W. D., Amos H. E. The differential binding of antibody from the sera of patients with pemphigus and pemphigoid to isolated guinea-pig epidermal cells. Br J Dermatol. 1975 Oct;93(4):425–430. doi: 10.1111/j.1365-2133.1975.tb06516.x. [DOI] [PubMed] [Google Scholar]
- Bystryn J. C., Abel E., DeFeo C. Pemphigus foliaceus. Subcorneal intercellular antibodies of unique specificity. Arch Dermatol. 1974 Dec;110(6):857–861. doi: 10.1001/archderm.110.6.857. [DOI] [PubMed] [Google Scholar]
- Bystryn J. C., Rodriguez J. Absence of intercellular antigens in the deep layers of the epidermis in pemphigus foliaceus. J Clin Invest. 1978 Feb;61(2):339–348. doi: 10.1172/JCI108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Diaz L. A., Marcelo C. L. Pemphigoid and pemphigus antigens in cultured epidermal cells. Br J Dermatol. 1978 Jun;98(6):631–637. doi: 10.1111/j.1365-2133.1978.tb03581.x. [DOI] [PubMed] [Google Scholar]
- Diaz L. A., Patel H., Calvanico N. J. Isolation of pemphigus antigen from human saliva. J Immunol. 1980 Feb;124(2):760–765. [PubMed] [Google Scholar]
- Diaz L. A., Weiss H. J., Calvanico N. J. Phylogenetic studies with pemphigus and pemphigoid antibodies. Acta Derm Venereol. 1978;58(6):537–540. [PubMed] [Google Scholar]
- Farb R. M., Dykes R., Lazarus G. S. Anti-epidermal-cell-surface pemphigus antibody detaches viable epidermal cells from culture plates by activation of proteinase. Proc Natl Acad Sci U S A. 1978 Jan;75(1):459–463. doi: 10.1073/pnas.75.1.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick R. E., Newcomer V. D. The correlation of disease activity and antibody titers in pemphigus. Arch Dermatol. 1980 Mar;116(3):285–290. [PubMed] [Google Scholar]
- Hawley-Nelson P., Sullivan J. E., Kung M., Hennings H., Yuspa S. H. Optimized conditions for the growth of human epidermal cells in culture. J Invest Dermatol. 1980 Aug;75(2):176–182. doi: 10.1111/1523-1747.ep12522602. [DOI] [PubMed] [Google Scholar]
- Judd K. P., Lever W. F. Correlation of antibodies in skin and serum with disease severity in pemphigus. Arch Dermatol. 1979 Apr;115(4):428–432. [PubMed] [Google Scholar]
- King I. A., Tabiowo A., Williams R. H. Incorporation of l-[3H]fucose and d-[3H]glucosamine into cell-surface-associated glycoconjugates in epidermis of cultured pig skin slices. Biochem J. 1980 Jul 15;190(1):65–77. doi: 10.1042/bj1900065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Miyagawa S., Hojo T., Ishii H., Yoshioka J., Sakamoto K. Isolation and characterization of soluble epidermal antigens reactive with pemphigus antibodies. Acta Derm Venereol. 1977;57(1):7–13. [PubMed] [Google Scholar]
- Morioka S., Naito K., Ogawa H. The pathogenic role of pemphigus antibodies and proteinase in epidermal acantholysis. J Invest Dermatol. 1981 May;76(5):337–341. doi: 10.1111/1523-1747.ep12519988. [DOI] [PubMed] [Google Scholar]
- Sams W. M., Jr, Jordon R. E. Correlation of pemphigoid and pemphigus antibody titres with activity of disease. Br J Dermatol. 1971 Jan;84(1):7–13. doi: 10.1111/j.1365-2133.1971.tb14190.x. [DOI] [PubMed] [Google Scholar]
- Schiltz J. R., Michel B., Papay R. Appearance of "pemphigus acantholysis factor" in human skin cultured with pemphigus antibody. J Invest Dermatol. 1979 Dec;73(6):575–581. doi: 10.1111/1523-1747.ep12541618. [DOI] [PubMed] [Google Scholar]
- Schiltz J. R., Michel B., Papay R. Pemphigus antibody interaction with human epidermal cells in culture. J Clin Invest. 1978 Oct;62(4):778–788. doi: 10.1172/JCI109189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltz J. R., Michel B. Production of epidermal acantholysis in normal human skin in vitro by the IgG fraction from pemphigus serum. J Invest Dermatol. 1976 Aug;67(2):254–260. doi: 10.1111/1523-1747.ep12513454. [DOI] [PubMed] [Google Scholar]
- Shu S. Y., Beutner E. H. Isolation and characterization of antigens reactive with pemphigus antibodies. J Invest Dermatol. 1973 Nov;61(5):270–276. doi: 10.1111/1523-1747.ep12676492. [DOI] [PubMed] [Google Scholar]
- Stanley J. R., Hawley-Nelson P., Poirier M., Katz S. I., Yuspa S. H. Detection of pemphigoid antigen, pemphigus antigen, and keratin filaments by indirect immunofluorescence in cultured human epidermal cells. J Invest Dermatol. 1980 Aug;75(2):183–186. doi: 10.1111/1523-1747.ep12522615. [DOI] [PubMed] [Google Scholar]
- Stanley J. R., Hawley-Nelson P., Yuspa S. H., Shevach E. M., Katz S. I. Characterization of bullous pemphigoid antigen: a unique basement membrane protein of stratified squamous epithelia. Cell. 1981 Jun;24(3):897–903. doi: 10.1016/0092-8674(81)90115-x. [DOI] [PubMed] [Google Scholar]
- Takigawa M., Imamura S., Ofuji S. Surface distribution of pemphigus antibody-binding substances(s) on isolated guinea pig epidermal cells. An immunoferritin electron microscopic study. J Invest Dermatol. 1978 Sep;71(3):182–185. doi: 10.1111/1523-1747.ep12547113. [DOI] [PubMed] [Google Scholar]
- Wolff K., Schreiner E. Ultrastructural localization of pemphigus autoantibodies within e epidermis. Nature. 1971 Jan 1;229(5279):59–61. doi: 10.1038/229059a0. [DOI] [PubMed] [Google Scholar]
- Wood G. W., Beutner E. H. Blocking-immunofluorescence studies on the specificity of pemphigus autoantibodies. Clin Immunol Immunopathol. 1977 Mar;7(2):168–175. doi: 10.1016/0090-1229(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Hawley-Nelson P., Koehler B., Stanley J. R. A survey of transformation markers in differentiating epidermal cell lines in culture. Cancer Res. 1980 Dec;40(12):4694–4703. [PubMed] [Google Scholar]
- Yuspa S. H., Hawley-Nelson P., Stanley J. R., Hennings H. Epidermal cell culture. Transplant Proc. 1980 Sep;12(3 Suppl 1):114–122. [PubMed] [Google Scholar]