Abstract
Abnormalities of the incretin axis have been implicated in the pathogenesis of type 2 diabetes mellitus. Glucagon-like peptide-1 (GLP-1) and gastroinhibitory intestinal peptide constitutes >90% of all the incretin function. Augmentation of GLP-1 results in improvement of beta cell health in a glucose-dependant manner (post-prandial hyperglycemia) and suppression of glucagon (fasting hyperglycemia), amongst other beneficial pleiotropic effects. Native GLP-1 has a very short plasma half-life and novel methods have been developed to augment its half life, such that its anti-hyperglycemic effects can be exploited. They can be broadly classified as exendin-based therapies (exenatide, exenatide once weekly), DPP-4-resistant analogues (lixisenatide, albiglutide), and analogues of human GLP-1 (liraglutide, taspoglutide). Currently, commercially available analogues are exenatide, exenatide once weekly, and liraglutide. This review aims to provide an overview of most GLP-1 analogues.
Keywords: Exenatide, glucagon-like-peptide, GLP-analogues, incretins, incretin-mimetics, liraglutide
INTRODUCTION
Abnormalities of the incretin axis have been implicated in the pathogenesis of type 2 diabetes mellitus (T2DM), contributing variably to as much as 11%. It has been labeled as the sixth member (quintessential quintet) of the pathogenetic “ominous octet,” as proposed by Ralf Defranzo. It is recognized that glucagon-like peptide-1 (GLP-1) and gastroinhibitory intestinal peptide (GIP) constitutes >90% of all the incretin function. Patients with T2DM have a markedly blunted incretin secretory response which has been proposed as the cause for an impaired postprandial insulin secretion by up to 60%. It is further recognized that by augmenting the incretin system, the alfa-cells in the pancreas downregulate their glucagon secretion. When taken together in the context of a paralyzed incretin axis, patients with T2DM experience a high post-prandial and fasting hyperglycemic response.[1,2,3,4,5,6,7]
GLUCAGON-LIKE PEPTIDE-1 SYSTEM
GLP-1 is a member of the “glucagon peptide family” and is derived from the expression of preproglucagon gene located on chromosome 17. The gene product is acted upon by a specific propeptide convertase (PC) that cleaves propeptide and proprotein substrates at the C-terminus to generate biologically active peptides. PC1/3 is particularly involved in the generation of GLP-1 and GLP-2 in the intestinal L cells. GLP-1 comprises of 30 amino acids. It further undergoes “amidation” at the carboxyl-terminus. This C-terminal amidation along with the histidine at position 7 of GLP-1 (representing the free N-terminal amino acid) is very important for GLP-1's insulinotropic and probably glucagon-inhibiting activity. Amidation in addition has shown to prolong the survival of GLP-1 in the blood stream[8,9,10,11] [Figure 1].
Figure 1.
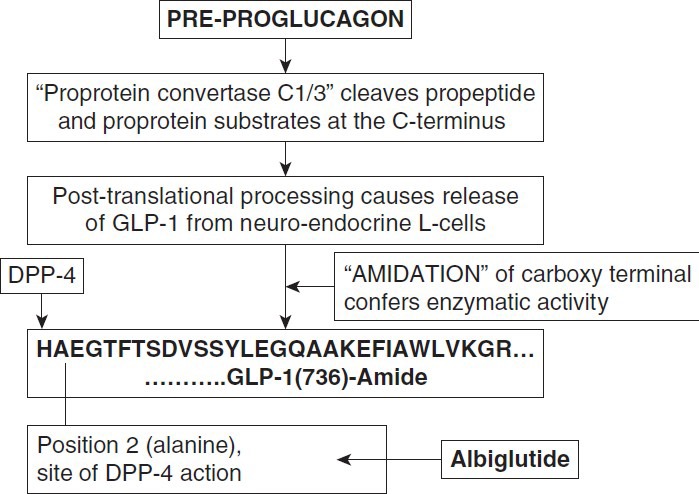
Production of GLP-1 (adapted from Holst, Jens J. The physiology and pharmacology of incretins in type 2 diabetes mellitus. Diabetes, Obesity and Metabolism. 10 Supplement 3:14-21, August 2008)
GLP-1 is released from the neuroendocrine L-cells in two forms: GLP-1 (7–36) amide {80% of circulating GLP-1} and GLP-1 (7–37) amide. It has been recognized that the posttranslational processing of preproglucagon differs in a tissue-specific manner. GLP-1 (1–36 amide) is predominantly secreted in the pancreas, whereas GLP-1 (1–37) is secreted in the ileum and hypothalamus.
GLP-1 is susceptible to cleavage at position 2 (alanine) by the ubiquitous dipeptidyl peptidase (DPP)-4, which occurs almost immediately upon secretion of GLP-1, rendering it a short half-life (<2 minutes).
GLP-1 mediates its effects through receptors belonging to the G protein-coupled receptor family, which are ubiquitously present. The GLP-1 receptor (GLP-1R) consists of 463 amino acids, which contains eight hydrophobic domains. The N-terminal extracellular hydrophobic domain is probably the signal sequence. It is remarkably preserved in homology in organs such as brain, lung, pancreatic islets, stomach, hypothalamus, heart, intestine, and kidney. The domain of GLP-1 (7–36) amide that is responsible for binding to its receptor is within amino acids 7 to 21. The free amino acid at the N-terminal end of GLP-1 at position 7 (histidine), has an imidazole side chain and free alfa-amino group. The positively charged imidazole side chain is the site of GLP-1R/GLP-1 action, whereas the free amino group is the site of DPP-4-mediated inactivation. Other amino acids in the N-terminus at positions 10 (glycine), 12 (phenylalanine), 13 (threonine), 15 (aspartate), and at positions 28 and 29 in the C-terminus are also directly involved in the receptor interaction.[12,13,14] Alteration of these sites should theoretically lead to modification of GLP-1 resistant to DPP-4 inhibition, thereby prolonging its half-life and efficiency.
Augmentation of GLP-1 results in improvement of mainly:
Beta cell health in a glucose-dependant manner (post-prandial hyperglycemia)
Suppression of glucagon (fasting hyperglycemia)amongst other beneficial pleiotropic effects.
It therefore only makes sense that modern medicine has attempted to exploit this very function of GLP-1, as for the first time anti-diabetes therapy has been able to address the “original pathogenesis” (triumvirate) of T2DM (beta cell failure and insulin resistance).
GLUCAGON-LIKE PEPTIDE-1 ANALOGUES
What is the need for a Glucagon-like peptide-1 analogue?
With suboptimal diabetes control world over and failure of modern medicine to target the real pathogenesis of T2DM until today, it only makes sense that this class of drugs be encouraged as it represents a true class of “anti-hyperglycmics,” addressing 5/most of the proposed 8 pathogenetic components of T2DM (beta-cell failure; brain; alfa-cell; gastrointestinal tract/incretin; insulin resistance [mild]).[1]
Development of Glucagon-like peptide-1 analogues
Native GLP-1 has a very short plasma half-life. Key points that favor rapid metabolism are:
Native GLP-1 is cleaved at position 8 by DPP-4 (alanine)
Peptide bonds adjacent to Arg or Lys residues are often subject to serine protease cleavage and it is believed that Lys residue at position 34 might be susceptible to such proteases.
Modifying these sites may help prolong the half-life of GLP-1. GLP-1 analogues can be classified as follows:
Exendin-based therapies: Exendin-4 is a naturally occurring peptide of 39-amino acids obtained from the venom of “Heloderma lizard venom,” bearing a 53% homology to human GLP-1. It is resistant to DPP-IV-mediated inactivation. The second amino acid residue in N-terminal region, which is the site of DPP-mediated inactivation (alanine for human GLP-1), is replaced by glycine in exendin-4, e.g., Exenatide and Exenatide LAR (sustained release). LAR (once a week or once a month) preparations are made by microsphere-based technology, which incorporates drug molecules into a matrix of poly-D, L-lactide-co-glycolide, a common biodegradable medical polymer, allowing for slow release over time following subcutaneous injection.[15,16,17]
-
DPP-IV-resistant GLP-1 analogs: Potential sites for modifying the half-life of the GLP-1 molecules are [Figure 2]:
- Free amino acid side chain of the histidine residue (position 7)
- Amino acid side chains in positions 7 (histidine), 10 (glycine), 12 (phenylalanine), 13 (threonine), and 15 (aspartate) of the N-terminus of GLP-1 (involved in receptor interaction).
- Amino acid in position 28 (phenylalanine) and 29 of the carboxy terminus of GLP-1 (important for GLP-1 to adapt the conformation recognized by the receptor).
An example of this is Lixisenatide (44 amino acids), a novel human GLP-1R agonist based on the structure of exendin-4, being developed by Sanofi Aventis under license from Zealand Pharma. The C-terminus is modified with six lysine residues, which enables the molecule to withstand physiological degradation by DPP-4.[18]
Albiglutide is a novel GLP-1 mimetic generated by genetic fusion of a GLP-1 dimer to human albumin. It is under investigation by Glaxo-Smith Kline. It is resistant to DPP-4 degradation due to an amino acid substitution (alanine at position 2 to glutamic acid).[18]
All exendin-based therapies are also DPP-IV resistant as pointed out earlier.
-
Analogues of human GLP-1: GLP-1 can be conjugated to substances such as fatty acids, albumin, etc. in order to slow down its renal excretion. Fatty acid conjugation of GLP-1 facilitates its binding to serum albumin and has been used to produce long-lasting peptide analogs.[19]
- Acylation: This is a process that prolongs the half-life of GLP-1 via several mechanisms (slow release from the injection site; extensive binding to albumin, which protects it from degradation by DPP-4 and reduced renal clearance). E.g., NN2211 (liraglutide; [(Arg (34) Lys (26)-(N-epsilon-(gamma-Glu (N-alpha-hexadecanoyl))]). Liraglutide bears only one amino acid substitution (Lys34Arg) with the addition of a C-16 acyl group (palmitoyl) attached to Lys26 via a glutamate linker and thus retains 97% sequence homology to human GLP-1. These characteristics contribute to extending the plasma half-life to 10 to 12 hours in human beings.[20]
- Alpha-aminoisobutyric acid (Aib) conjugation: Aib is an [alpha]-dimethyl-substituted non-natural amino acid that has d-amino acid structural characteristics which protects the surrounding peptide bonds against proteolysis. It has the potential of augmenting [alpha]-helicity of a peptide, which potentially enhances the bioactivity of a peptide that requires [alpha]-helical structure for its receptor recognition. Aib substitution at position 8 and 35 as occurs in taspoglutide enhances enzymatic stability and potency of the molecule without any major alteration of the structure of native peptide, with the exception of a slight increase in C-terminal [alpha]-helicity that may enhance receptor binding (e.g., [Aib8,35]hGLP-1(7-36) NH2 (BIM-51077, taspoglutide).[23]
Figure 2.
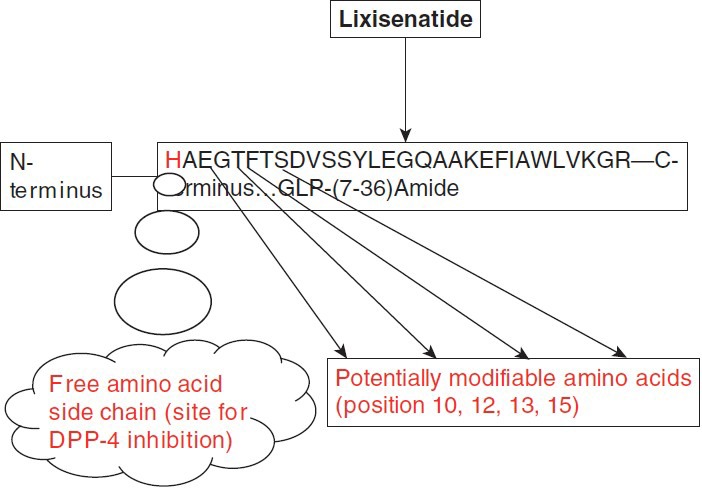
Potential amino acid modifications that can lead to a DPP-4-resistant GLP-1 molecule analogue with prolonged half-life
Dulaglutide (LY2189265) is a GLP-1 analogue covalently linked to a constant fragment (Fc) of a human immunoglobulin class 4 (IgG4) which not only renders it less susceptible to DPP-IV hydrolysis but also reduces renal clearance because of the size of the resultant fusion protein (molecular weight = 59.7 kDa).
Currently available Glucagon-like peptide-1 analogues
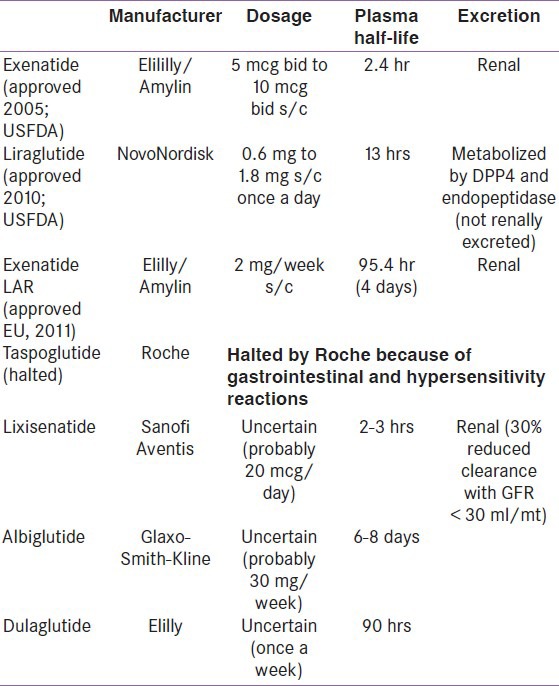
Exenatide (approved by USFDA, 2005; available for use in India marketed by Eli Lilly; brand name Byetta)
Liraglutide (approved by EU, Japan, 2009, USFDA, 2010; available for use in India marketed by NovoNordisk, brand name Victosa)
Exenatide LAR (sustained release; once weekly) (approved by USFDA, June 2011)
Possible future GLP-1 analogues
Taspoglutide (trials halted due to hypersensitivity and gastrointestinal complications)
Lixisenatide
Albiglutide
Dulaglutide (LY2189265)
For a brief overview, please refer to Table 1 for understanding the pharmacokinetic profile of various GLP-1 analogues.
Table 1.
Pharmacokinetics of Glucagon-like peptide-1 analogues
Exenatide: It is a synthetic exendin-4 analogue obtained from the saliva of the Heloderma suspectum (lizard). Exenatide was approved by the US Food and Drug Administration (FDA) in April 2005 and by the European Union in November 2006 for the treatment of T2DM. The half-life of exenatide after subcutaneous administration is about 2.4 hours, and is therefore given twice daily starting at a dose of 5 mcg twice a day titrating it upwards to 10 mcg twice a day within 1 hour following a meal after month of initiation. It is renally excreted and is associated with a reduction in HbA1c of approximately 0.8–1.1% affecting both fasting (-15 – 25 mg%) and post-prandial plasma glucose (-15 – 30 mg%). Weight loss of approximately 1.0 to 2.5 kg over 30 weeks and 3 to 6 kg over 52 weeks is observed. Nausea and vomiting are the most important adverse effects resulting in 7 to 15% withdrawal from therapy. There is a warning issued toward the rare complication of pancreatitis, and it can be strongly argued by most authors that the risk is more theoretical than practical.[15,16,23,24]
Liraglutide: Is an acylated analogue of GLP-1 that self-associates into a heptameric structure that delays absorption from the subcutaneous injection site. It was approved by for use by EU and Japan in 2009, and the USFDA, in 2010. It shares a 97% homology to native GLP-1. It has a plasma half-life of 9 to 14 hours and is metabolized by DPP-4 and neutral endopeptidases with an elimination half-life of 10 to 18 hours. It is initiated in a dose of 0.6 mg/day titrated on a weekly basis to a maximum of 1.8 mg/day. It is virtually free of renal and gastrointestinal excretion being slightly affected (retained) with moderate and severe liver impairment although its clinical importance is not known. Average HbA1c reduction seen is up to 1.6% and weight loss of up to 2.5 kg over 30 weeks. Nausea and vomiting are the commonest side effects which are the cause for withdrawal of therapy in approximately 8% of patients. There is a warning issued toward the rare complication of pancreatitis.[17,25,26,27]
Exenatide vs liraglutide
The only study that compares Liraglutide with Exenatide is the LEAD-6, until the DURATION-6 becomes available, which compares Exenatide LAR with Liraglutide. From the LEAD-6, it is clear that the reduction in HbA1c is 0.33% greater with liraglutide compared with exenatide. Further benefits are obtained with fasting plasma glucose (0.9 mmol/l), body weight (0.9 kg), and systolic blood pressure (3.8 mmHg) with minimal minor hypoglycemia (1.30 episodes/patient-year) or nausea (3.2%) in these patients switched onto Liraglutide from Exenatide. In LEAD 6, pancreatic [beta]-cell function (proinsulin: insulin ratio and HOMA-beta) was improved, and triglycerides and free fatty acids were reduced to a greater extent with liraglutide than exenatide. The gastrointestinal side effects were most pronounced with exenatide BID (28%-nausea, 9.9%-vomiting) compared to liraglutide (25.5%-nausea, 6.0%-vomiting). Liraglutide was found to be less immunogenic than exenatide, and fewer than 10% of liraglutide-treated patients developed antibodies to liraglutide[28,29,30] [Table 2].
Table 2.
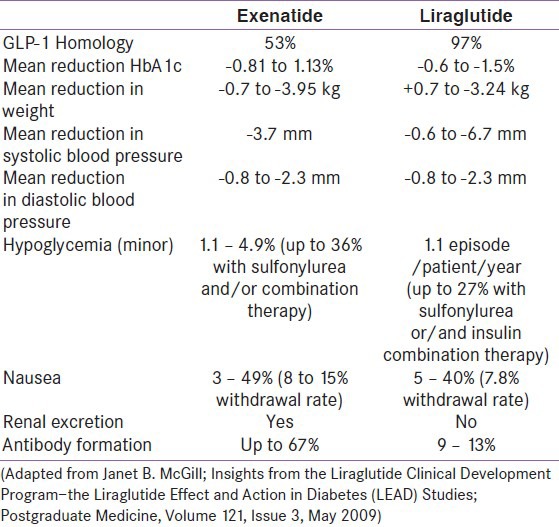
Some studies have showed that liraglutide may be cost-effective and associated with benefits in life expectancy, quality of life, and reduced complication rates compared to exenatide.[31]
Exenatide LAR: Approved for use by the European Medical Association in 2011, it represent the first once a week injectable anti-hyperglycemia. It is licensed for use at 2 mg/week and has a plasma half-life of 4 days. It is renally excreted and shares much of the same characteristics of exenatide with a convenient once a week dosing and a safer side-effect profile (reduced upper gastrointestinal symptoms). The incidence of nausea and vomiting is lower in once a week preparation compared to twice a day exenatide (26 vs 35%) and vomiting (11 vs 19%). It is slightly more effective in reducing HbA1c and fasting plasma glucose compared to regular exenatide.[17,32]
Taspoglutide: In development by Roche, it shares a 93% homology with the native GLP-1 and is fully resistant to DPP-4 degradation. Limited data from two published trials suggest that is has an HbA1c-lowering capability of approximately 1.1%. When compared to exenatide in the T-emerge 2 study, there was a higher incidence of gastrointestinal side effects and hypersensitivity reactions observed following which Roceh decided to halt all further studies.[17,33,34,35]
Lixisenatide: Lixisenatide is a novel human GLP-1R agonist undergoing phase 3 trials being developed by Sanofi Aventis under license from Zealand Pharma. It is a very potent and selective GLP-1R agonist (four-times more potent than human GLP-1). The GetGoal-S trial reveals much of its efficacy with an HbA1c -lowering capacity of approximately -0.74% without any significant risk of symptomatic hypoglycemia (1.7%). It causes significant weight loss; however, mild nausea can occur in up to 22% of patients. A 20 μg once-daily dose of lixisenatide demonstrates the best efficacy-to-tolerability ratio.[18,36]
Albiglutide: It is a long-acting GLP-1 mimetic, resistant to DPP-4 degradation under investigation by Glaxo-Smith Kline. It is also currently in phase 3 trials. It may provide a more patient-friendly dosing profile (once-weekly or less frequent dosing) compared to currently-available GLP-1 analogues. Efficacy data as shown in 40 Japanese patients suggests a mean HbA1c lowering of -0.58% (15 mg/week), -0.57% (30 mg/week), -0.63% (50 mg/biweekly), and -0.51% (100 mg/month). Common side effects included nausea (11.8–54.3%), vomiting (0.41–2%), headache (5.9–23.5%), dizziness (5.7–14.3%), nasopharyngitis (5.7–11.4%), back pain (0–14.3%), upper respiratory tract infections (0–15.2%), and local skin reactions (2.9–28.6%).[18,37]
Dulaglutide: Under investigation by Eli Lilly, it is a long-acting GLP-1 analogue projected to have a convenient once a week dosing. In a randomized placebo-controlled double-blinded study (262 obese type 2 patients), an HbA1c reduction of approximately -1.28 –1.52% was seen along with weight loss of -1.40 and -2.51 kg. Upper gastrointestinal symptoms of nausea (13%), diarrhea (9%), and abdominal distension (8%) were the most frequently reported adverse events.[18,37,38]
GLUCAGON-LIKE PEPTIDE ANALOGUE MONOTHERAPY
All the currently available GLP-1 analogues are more or less equally efficacious in their anti-hyperglycemic effects. They have proved to be non-inferior to maximum dose metformin, pioglitazone, sulfonylurea, and insulin (biphasic and long-acting). From the LEAD-6, as shown in the previous chapter, it was seen that liraglutide might have an edge over exenatide with regards to A1c lowering by 0.33% in favor of liraglutide, with higher percentage of patients achieving HbA1c of <7.0% compared to exenatide (54% vs 43%). With the DURATION-6 due for completion in the near future, preliminary reports comparing sustained release exenatide and liraglutide suggests that the glycemic efficacy of liraglutide might see an edge over sustained release exenatide, with the later having a safer side-effect profile. Exenatide LAR might have a greater benefit compared to exenatide with regards to A1c and fasting blood glucose control; however, daily exenatide has shown a greater post-prandial glucose-lowering effect. Weight loss for all the three GLP-1 analogues (exenatide, exenatide LAR, and liraglutide) seems to be more or less similar.[15,17,21,23,25,28,32,39] An overview of their efficacy is mentioned in Table 3.
Table 3.
A comparison of metabolic parameters for various Glucagon-like peptide-1 analogues (monotherapy data)
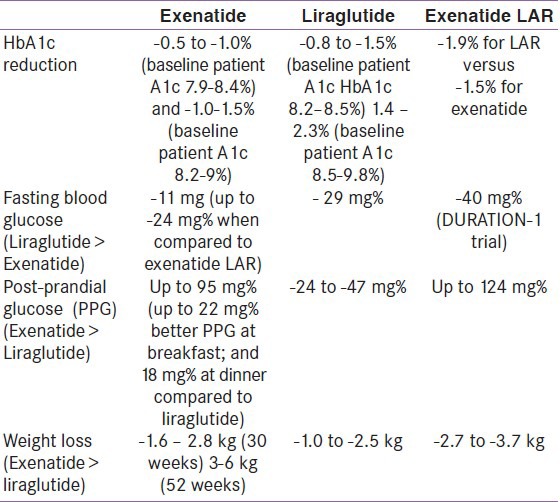
A meta-analysis that was conducted on the efficacy of GLP-1 analogues (exenatide and liraglutide) suggested an A1c reduction of between -0.81 to -1.13%; fasting blood glucose reduction of 21 to 33 mg%, and post-prandial glucose reduction of 16 to 41 mg%. Average weight loss ranged from -0.78 to 3.95 kg, more for exenatide compared to liraglutide.[40]
The commonest observed adverse effects with use of GLP-1 analogues are upper gastrointestinal symptoms (nausea, vomiting, abdominal fullness, and uncommonly diarrhea). This leads to a withdrawal rate of approximately 4%, according to a meta-analysis, more for exenatide compared to liraglutide. Exenatide LAR has shown to have the least side effects when compared to exenatide (26% vs 35% for nausea and 110% vs 19% for vomiting) with greatest therapy.[17,32,39]
Non-glycemic benefits: The GLP-1R has a wide tissue distribution being present virtually in organ system (pancreas, lung, heart, vascular smooth muscle cells, endothelial cells, macrophages and monocytes, kidney, gastrointestinal tract [stomach and intestine], central nervous system [brain], and peripheral nervous system). In the brain, the GLP-1R is found only on mainly large output neurons (pyramidal neurons, dentate granule neurons, and Purkinje cells) in and around synapses (suggesting that they directly modulate synaptic activity and plasticity), caudal part of nucleus tractus solitarius in brainstem, dorsomedial (DMN), paraventricular (PVN), ventromedial (VMN), lateral and arcuate (ARC), and supraoptic nuclei of hypothalamus, sensory circumventricular organs such as the subfornical organ, organum vasculosum, laminae terminus, and the area postrema. The ARC nucleus which is located at the base of the hypothalamus lies outside the blood-brain barrier. It is postulated that circulating levels of incretins may gain access to the central nervous system via the ARC nucleus.[41]
The brain and cardiac tissue expresses the same GLP-1R as is expressed on pancreatic tissue. In the liver, however, stimulation of the GLP-1R causes anabolic effect instead of neoglucogenesis and glycogenolysis as would be expected on stimulating it via glucagon, suggesting that the GLP-1R on hepatocyte must be different arising from an unidentified gene locus encoding a second GLP-1R or an alternatively spliced receptor related to the superfamily of glucagon-related peptide receptors.[41]
GLP-Analogues and Cardiovascular System: GLP-1 analogues have shown to exert a positive effect on myocardial contractility, hypertension (natriuretic/diuretic effect), endothelium (anti-atherosclerotic), and lipid profile. An absolute benefit in the lipid parameters (improvement in HDL cholesterol, fasting triglycerides) has been ascribed directly to weight loss with the exception of post-prandial hypertriglyceridemia which seems to lower following a GLP-1 analogue, the mechanism of which is currently speculative.
GLP-Analogues and Neurological system: GLP-1 analogues have a role in neuronal protection, resulting in an improvement in cognition, memory, and spatial learning. It modifies eating behavior by inducing satiety, thereby reducing energy intake by approximately 12%. Via interaction with the peripheral nervous system (vagus) central, GLP-1 augmentation causes gastric slowing, inducing a post-prandial satiety.[41]
GLP-Analogues and Obesity: Weight loss induced by GLP-1 analogues is dose dependent and progressive. Liraglutide has shown to induce a mean weight loss of approximately 6.0 kg with >35% of the subjects achieving >= 10% reduction of weight. Similarly, long-acting exenatide (exenatide-LAR) has shown to improve bodyweight with an average weight reduction of 5.8 lbs.[41,42,43]
GLP-Analogues and Insulin Resistance: GLP-1 has shown to reduce insulin sensitivity through restoration of insulin signaling and by reduction of hepatic gluconeogenesis. Enhanced insulin secretion causes increased uptake of glucose in the muscle and adipocyte and reduced outpouring of glucose from the liver. By promoting weight loss, it has further shown to improve peripheral insulin-mediated glucose uptake. It has shown to reduce insulin resistance locally at the level of beta-cell and fat cell (reduced release of free fatty acids) and systemically (down-gradation of markers of inflammation).[41,44]
GLP-Analogues and Gastrointestinal/Hepatobiliary system: All GLP-1 analogues are associated with an improved HOMA-beta score and proinsulin/insulin ratio (beta-cell mass and health); however, it is believed that the improvement is better with liraglutide over exenatide (32.1% vs 2.7%).[27,41] It suppresses glucagon (improved fasting hyperglycemia), delays gastric emptying via “ileal-break mechanism,” and improves hepatosteatosis.[41]
GLP-1 Analogues and Renal System: It has shown to reduce albuminuria independent of the presence of other poor prognostic markers (poor diabetes control, hypertension, etc).[41]
GLP-1 Analogues and Bone protection: Via its antagonistic action on neuropeptide Y, it has shown to improve bone mass.[41]
GLP-1 Analogues and skin protection: Independent reports are emerging suggesting a potential beneficial role of liraglutide and exenatide in psoriasis via its influence on natural killer cells (implicated in pathogenesis of psoriasis).[45]
GLUCAGON-LIKE PEPTIDE COMBINATION THERAPY
GLP-1 analogues are currently approved for treatment of T2DM as “monotherapy” and as “add-on therapy” to existing anti-diabetes medication (mono, dual, or triple therapy). They have shown to improve HbA1c by 0.6 to 1.5% with sustained benefit of >1% over a three-year study period.
From the LEAD meta-analysis, it was clear that when liraglutide is added onto existing oral therapy, there results a benefit in HbA1c of approximately 1.5%. Similarly, exenatide and exenatide LAR have shown a benefit of up to–1.1% reduction in A1c.
In combination with metformin (LEAD-2; Defranzo)[25,46,47,48]
HbA1c: -1.0% for Liraglutide versus -0.8% for exenatide, with 66% and 46% of patients respectively achieving target A1c of <7%
Fasting glucose: -18 –27 mg%
Post-prandial glucose: average -47 mg%
This was shown to be non-inferior the combination therapy of glimepiride and metformin combination.
In combination with sulfonylurea (SU) (Buse JB; LEAD-1)[25,46,47,48,49]
HbA1c: -1.1% for Liraglutide versus -0.86% for exenatide, with 66% and 41% of patients respectively achieving target A1c of <7%
In combination with dual oral therapy (LEAD-1 SU+TZD; LEAD-4 Metformin+TZD; Kendall DM).[25,46,47,50,51,52]
HbA1c: up to -1.5% for Liraglutide versus -1.0% for exenatide, with 54% and 34% of patients respectively achieving target A1c of <7%
In the HELLA study, although the use of exenatide on the background of dual or tripple oral therapy was shown to be non-inferior to insulin glargine in terms of HbA1c reduction, a far greater proportion of patients achieved their A1c goal of <7% with exenatide (53.4%) compared to insulin glargine (19.8%).[52]
A systematic review of exenatide use in clinical practice revealed a significant reduction in HbA1c (-0.4 to -0.9%), fasting blood glucose (-10 mg/dl), weight (-2 to -11 kg), and systolic blood pressure (-2 to -11 mmHg). Use of exenatide resulted in the reduction of concomitantly used anti-diabetes medication dosages, by up to 75% in sulfonylureas, 22% in metformin, and 66% in TZDs. The review also stated that the use of exenatide was associated with significant lower rates of all-cause and cardiovascular-related hospitalization and mortality.[53]
GLUCAGON-LIKE PEPTIDE COMBINED WITH INSULIN
It is well known that in most patients, exogenous insulin therapy represents the final link between an exhausted beta cell and plasma glucose. Most studies that have employed the use of GLP-1 analogues in patients with existing insulin therapy have done so with the rationale that clinical improvement might be seen because of weight loss (improved insulin resistance) and possibly from an insulin-sparing effect (improved beta-cell health/insulin secretion). Several small studies have demonstrated clear benefits in terms of improvements in HbA1c, weight (3–5 kg), and reduction of total insulin dose by as much as 30 to 50%[54] in patients with T2DM.
“The Association of British Clinical Diabetologists” studied 4874 patients using exenatide as part of a national UK audit, of which 1921 patients (39.6%) used exenatide with insulin. The addition of exenatide to existing insulin therapy resulted in a mean HbA1c reduction of 0.51 ± 0.06%; weight reduction of 5.8 ± 0.2 kg; and total insulin dose reduction of 44 ± 4 Units/day. 17.1% ended up discontinuing insulin therapy, 23.1% discontinued sulfonylurea therapy, and 54.3% discontinued TZD therapy. 34.2% of the patients who continued insulin achieved an HbA1c reduction >= 1%. However, the same population had a higher rate of exenatide discontinuation (31.0 vs 13.9%), hypoglycemia (8.9 vs 6.1%), gastrointestinal side effects (28.4 vs 25.0%), and treatment dissatisfaction (20.8 vs 5.7%).[54]
Most of the studies employing GLP-1 analogues have selected patients with a high body mass index (BMI) and have also demonstrated significant weight reduction. The question therefore remains whether the benefits obtained in terms of HbA1c reduction are because of weight loss (improvement in insulin resistance) or due to improvement in beta-cell mass/health or both. This question cannot be answered better by a study that used liraglutide in patients with type 1 diabetes mellitus (T1DM). Although the number of patients studied were only 14, for the first time the use of liraglutide showed a reduction of mean fasting glucose (130 +/- 10 to 110 +/- 8 mg/dl) and mean weekly glucose (137.5 +/- 20 to 115 +/- 12 mg/dl) in just 1 week. Basal insulin requirements decreased from 24.5 +/- 6 to 16.5 +/- 6 units and bolus insulin from 22.5 +/- 4 to 15.5 +/- 4 units. Both HbA1c and body weight reduced significantly from 6.5 to 6.1% and 4.5 +/- 1.5 kg, respectively.[55] Liraglutide treatment provided an additional strategy for improving glycemic control in T1DM and clearly demonstrated improvements in beta cell health as was seen in the LEAD-meta-analysis (improvements in HOMA-beta scores and proinsulin/insulin ratios).
CONCLUSION
GLP-1 analogues have shown that they are useful not only in patients with T2DM but also in patients with T1DM. They have shown to significantly reduce fasting and post-prandial blood glucose, HbA1c, weight, and daily insulin requirements. They also have a substantial beneficial pleiotropic effect, extending to virtually every organ system. They have revolutionized the way we look at diabetes therapy, providing us with the first anti-hyperglycemic (hypoglycemia free) weight-loosing injectable.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.DeFronzo RA. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–95. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deacon CF. DPPIV and diabetes. Clin Chem Lab Med. 2008;46:A18. [Google Scholar]
- 3.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–65. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Drucker DJ, Nauck MA. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 5.Meier JJ, Nauck MA. Incretins and the development of type 2 diabetes. Curr Diab Rep. 2006;6:194–201. doi: 10.1007/s11892-006-0034-7. [DOI] [PubMed] [Google Scholar]
- 6.Holst JJ. The physiology and pharmacology of incretins in type 2 diabetes mellitus. Diabetes Obes Metab. 2008;10(Suppl 3):S14–21. [Google Scholar]
- 7.Gallwitz B. Glucagon-like peptide-1-based therapies for the treatment of type 2 diabetes mellitus. Treat Endocrinol. 2005;4:361–70. doi: 10.2165/00024677-200504060-00005. [DOI] [PubMed] [Google Scholar]
- 8.Hui H, Zhao X, Perfetti R. Structure and function studies of glucagon-like peptide-1 (GLP-1): The designing of a novel pharmacological agent for the treatment of diabetes. Diabetes Metab Res Rev. 2005;21:313–31. doi: 10.1002/dmrr.553. [DOI] [PubMed] [Google Scholar]
- 9.Suda K, Takahashi H, Fukase N, Manaka H, Tominaga M, Sasaki H. Distribution and molecular forms of glucagon-like peptide in the dog. Life Sci. 1989;45:1793–8. doi: 10.1016/0024-3205(89)90519-5. [DOI] [PubMed] [Google Scholar]
- 10.Wettergren A, Pridal L, Wojdemann M, Holst JJ. Amidated and non-amidated glucagon-like peptide-1 (GLP-1): Non-pancreatic effects (cephalic phase acid secretion) and stability in plasma in humans. Regul Pept. 1998;77:83–7. doi: 10.1016/s0167-0115(98)00044-5. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki S, Kawai K, Ohashi S, Mukai H, Yamashita K. Comparison of the effects of various C-terminal and N-terminal fragment peptides of glucagon-like peptide-1 on insulin and glucagon release from the isolated perfused rat pancreas. Endocrinology. 1989;125:3109–14. doi: 10.1210/endo-125-6-3109. [DOI] [PubMed] [Google Scholar]
- 12.Adelhorst K, Hedegaard BB, Knudsen LB, Kirk O. Structure-activity studies of glucagon-like peptide-1. J Biol Chem. 1994;269:6275–8. [PubMed] [Google Scholar]
- 13.Ohneda A, Ohneda K, Ohneda M, Koizumi F, Ohashi S, Kawai K, et al. The structure-function relationship of GLP-1 related peptides in the endocrine function of the canine pancreas. Tohoku J Exp Med. 1991;165:209–21. doi: 10.1620/tjem.165.209. [DOI] [PubMed] [Google Scholar]
- 14.Hareter A, Hoffmann E, Bode HP, Göke B, Göke R. The positive charge of the imidazole side chain of histidine7 is crucial for GLP-1 action. Endocr J. 1997;44:701–5. doi: 10.1507/endocrj.44.701. [DOI] [PubMed] [Google Scholar]
- 15.Sennik D, Ahmed F, Russell-Jones D. Exenatide, a GLP-1 agonist in the treatment of type 2 diabetes. Expert Rev Endocrinol Metab. 2012;7:15–26. doi: 10.1586/eem.11.79. [DOI] [PubMed] [Google Scholar]
- 16.Exendin 4: AC 2993, AC 2993 LAR. BioDrugs. 2002;16:220–2. doi: 10.2165/00063030-200216030-00008. [DOI] [PubMed] [Google Scholar]
- 17.Madsbad S, Kielgast U, Asmar M, Deacon CF, Torekov SS, Holst JJ. An overview of once-weekly glucagon-like peptide-1 receptor agonists—Available efficacy and safety data and perspectives for the future. Diabetes Obes Metab. 2011;13:394–407. doi: 10.1111/j.1463-1326.2011.01357.x. [DOI] [PubMed] [Google Scholar]
- 18.Seewoodhary J. Novel GLP-1 mimetics in diabetes: Lixisenatide and albiglutide. Future Prescriber. 2011;12:20–2. [Google Scholar]
- 19.Heinemann L, Sinha K, Weyer C, Loftager M, Hirschberger S, Heise T. Time-action profile of the soluble, fatty acid acylated, long-acting insulin analogue NN304. Diabet Med. 1999;16:332–8. doi: 10.1046/j.1464-5491.1999.00081.x. [DOI] [PubMed] [Google Scholar]
- 20.Juhl CB, Hollingdal M, Sturis J, Jakobsen G, Agersø H, Veldhuis J, et al. Bedtime administration of NN2211, a long-acting GLP-1 derivative, substantially reduces fasting and postprandial glycemia in type 2 diabetes. Diabetes. 2002;51:424–9. doi: 10.2337/diabetes.51.2.424. [DOI] [PubMed] [Google Scholar]
- 21.Holz GG, Chepurny OG. Glucagon-like peptide-1 synthetic analogs: New therapeutic agents for use in the treatment of diabetes mellitus. Curr Med Chem. 2003;10:2471–83. doi: 10.2174/0929867033456648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JG, Baggio LL, Bridon DP, Castaigne JP, Robitaille MF, Jetté L, et al. Development and characterization of a glucagon-like peptide 1-albumin conjugate: The ability to activate the glucagon-like peptide 1 receptor in vivo. Diabetes. 2003;52:751–9. doi: 10.2337/diabetes.52.3.751. [DOI] [PubMed] [Google Scholar]
- 23.Norris SL, Lee N, Thakurta S, Chan BKS. Exenatide efficacy and safety: A systematic review. Diabet Med. 2009;26:837–46. doi: 10.1111/j.1464-5491.2009.02790.x. [DOI] [PubMed] [Google Scholar]
- 24.Dore DD, Bloomgren GL, Wenten M, Hoffman C, Clifford CR, Quinn SG, et al. A cohort study of acute pancreatitis in relation to exenatide use. Diabetes Obes Metab. 2011;13:559–66. doi: 10.1111/j.1463-1326.2011.01376.x. [DOI] [PubMed] [Google Scholar]
- 25.McGill JB. Insights from the Liraglutide Clinical Development Program—The Liraglutide Effect and Action in Diabetes (LEAD) Studies. Postgrad Med. 2009;121:16–25. doi: 10.3810/pgm.2009.05.1998. [DOI] [PubMed] [Google Scholar]
- 26.Davies MJ, Kela R, Khunti K. Liraglutide - overview of the preclinical and clinical data and its role in the treatment of type 2 diabetes. Diabetes Obes Metab. 2011;13:207–20. doi: 10.1111/j.1463-1326.2010.01330.x. [DOI] [PubMed] [Google Scholar]
- 27.Croom KF, McCormack PL. Liraglutide: A review of its use in type 2 diabetes mellitus. Drugs. 2009;69:1985–2004. doi: 10.2165/11201060-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Polster M, Zanutto E, McDonald S, Conner C, Hammer M. A comparison of preferences for two GLP-1 products-liraglutide and exenatide—For the treatment of type 2 diabetes. J Med Econ. 2010;13:655–61. doi: 10.3111/13696998.2010.529377. [DOI] [PubMed] [Google Scholar]
- 29.Pinkney J, Fox T, Ranganath L. Selecting GLP-1 agonists in the management of type 2 diabetes: Differential pharmacology and therapeutic benefits of liraglutide and exenatide. Ther Clin Risk Manag. 2010;6:401–11. doi: 10.2147/tcrm.s7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: A 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 31.Valentine WJ, Palmer AJ, Lammert M, Langer J, Brändle M. Evaluating the long-term cost-effectiveness of liraglutide versus exenatide BID in patients with type 2 diabetes who fail to improve with oral antidiabetic agents. Clin Ther. 2011;33:1698–712. doi: 10.1016/j.clinthera.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 32.Seewoodhary J, Bain S. New once-weekly formulation of exenatide for type 2 diabetes. Future Prescriber. 2010;11:18–21. [Google Scholar]
- 33.Dong JZ, Shen Y, Zhang J, Tsomaia N, Mierke DF, Taylor JE. Discovery and characterization of taspoglutide, a novel analogue of human glucagon-like peptide-1, engineered for sustained therapeutic activity in type 2 diabetes. Diabetes Obes Metab. 2011;13:19–25. doi: 10.1111/j.1463-1326.2010.01313.x. [DOI] [PubMed] [Google Scholar]
- 34.Nauck MA, Ratner RE, Kapitza C, Berria R, Boldrin M, Balena R. Treatment with the human once-weekly glucagon-like peptide-1 analog taspoglutide in combination with metformin improves glycemic control and lowers body weight in patients with type 2 diabetes inadequately controlled with metformin alone: A double-blind placebo-controlled study. Diabetes Care. 2009;32:1237–43. doi: 10.2337/dc08-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ratner R, Nauck M, Kapitza C, Asnaghi V, Boldrin M, Balena R. Safety and tolerability of high doses of taspoglutide, a once-weekly human GLP-1 analogue, in diabetic patients treated with metformin: A randomized double-blind placebo-controlled study. Diabet Med. 2010;27:556–62. doi: 10.1111/j.1464-5491.2010.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christensen M, Knop FK, Vilsbøll T. The GetGoal clinical trial program of lixisenatide, a once-daily GLP-1 receptor agonist. Expert Rev Endocrinol Metab. 2011;6:513–25. [Google Scholar]
- 37.Matthews JE, Stewart MW, De Boever EH, Dobbins RL, Hodge RJ, Walker SE, et al. Pharmacodynamics, pharmacokinetics, safety, and tolerability of albiglutide, a long-acting glucagon- like peptide-1 mimetic, in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:4810–7. doi: 10.1210/jc.2008-1518. [DOI] [PubMed] [Google Scholar]
- 38.Barrington P, Chien JY, Showalter HD, Schneck K, Cui S, Tibaldi F, et al. A 5-week study of the pharmacokinetics and pharmacodynamics of LY2189265, a novel, long-acting glucagon-like peptide-1 analogue, in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13:426–33. doi: 10.1111/j.1463-1326.2011.01364.x. [DOI] [PubMed] [Google Scholar]
- 39.Aroda VR, DeYoung MB. Clinical implications of exenatide as a twice-daily or once-weekly therapy for type 2 diabetes. Postgrad Med. 2011;123:228–38. doi: 10.3810/pgm.2011.09.2479. [DOI] [PubMed] [Google Scholar]
- 40.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: Systematic review and meta-analysis. JAMA. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 41.Gupta V. Pleiotropic effects of incretins. Indian J Endocrinol Metab. 2012;16:47–56. doi: 10.4103/2230-8210.94259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Novo N. Significant weight loss sustained in obese people treated with liraglutide for one year. [Last accessed on 2012 Feb 01]. Available from: www.novonordisk.com/science/Pipeline/liraglutide-press-releases .
- 43.Amylin Pharmaceuticals, Inc. and Eli Lilly and Company. Exenatide once weekly provided sustained improvements in glycemic control with weight loss over two years: DURATION-1 interim longterm data presented at ADA. [Last cited in 2009]. Available from: www.amylin.com/assets/001/5097.pdf .
- 44.Lee YS, Shin S, Shigihara T, Hahm E, Liu MJ, Han J, et al. Glucagon-like peptide-1 gene therapy in obese diabetic mice results in long-term cure of diabetes by improving insulin sensitivity and reducing hepatic gluconeogenesis. Diabetes. 2007;56:1671–9. doi: 10.2337/db06-1182. [DOI] [PubMed] [Google Scholar]
- 45.Hogan AE, Tobin AM, Ahern T, Corrigan MA, Gaoatswe G, Jackson R. Glucagon-like peptide-1 (GLP-1) and the regulation of human invariant natural killer T cells: Lessons from obesity, diabetes and psoriasis. Diabetologia. 2011;54:2745–54. doi: 10.1007/s00125-011-2232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: An overview of the LEAD 1–5 studies. Diabetes Obes Metab. 2009;11(Suppl 3):S26–34. doi: 10.1111/j.1463-1326.2009.01075.x. [DOI] [PubMed] [Google Scholar]
- 47.Raskin P, Mora PF. Glycaemic control with liraglutide: The phase 3 trial programme. Int J Clin Pract. 2010;64(Suppl 167):S21–7. doi: 10.1111/j.1742-1241.2010.02496.x. [DOI] [PubMed] [Google Scholar]
- 48.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 49.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–35. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- 50.Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–91. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 51.Liutkus J, Rosas Guzman J, Norwood P, Pop L, Northrup J, Cao D, et al. A placebo-controlled trial of exenatide twice-daily added to thiazolidinediones alone or in combination with metformin. Diabetes Obes Metab. 2010;12:1058–65. doi: 10.1111/j.1463-1326.2010.01251.x. [DOI] [PubMed] [Google Scholar]
- 52.Davies MJ, Donnelly R, Barnett AH, Jones S, Nicolay C, Kilcoyne A. Exenatide compared with long-acting insulin to achieve glycaemic control with minimal weight gain in patients with type 2 diabetes: Results of the helping evaluate exenatide in patients with diabetes compared with long-acting insulin (HEELA) study. Diabetes Obes Metab. 2009;11:1153–62. doi: 10.1111/j.1463-1326.2009.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Best JH, Lavillotti K, Deyoung MB, Garrison LP. The effects of exenatide BID on metabolic control, medication use, and hospitalization in patients with type 2 diabetes mellitus in clinical practice: A systematic review. Diabetes Obes Metab. 2012;14:387–98. doi: 10.1111/j.1463-1326.2011.01533.x. [DOI] [PubMed] [Google Scholar]
- 54.Thong KY, Jose B, Sukumar N, Cull ML, Mills AP, Sathyapalan T, et al. Safety, efficacy and tolerability of exenatide in combination with insulin in the Association of British Clinical Diabetologists nationwide exenatide audit. Diabetes Obes Metab. 2011;13:703–10. doi: 10.1111/j.1463-1326.2011.01393.x. [DOI] [PubMed] [Google Scholar]
- 55.Varanasi A, Bellini N, Rawal D, Vora M, Makdissi A, Dhindsa S, et al. Liraglutide as additional treatment for type 1 diabetes. Eur J Endocrinol. 2011;165:77–84. doi: 10.1530/EJE-11-0330. [DOI] [PubMed] [Google Scholar]


