Abstract
Sensory and endoneurocrine tissues as diverse as the lens, the olfactory epithelium, the inner ear, the cranial sensory ganglia, and the anterior pituitary arise from a common pool of progenitors in the preplacodal ectoderm (PPE). Around late gastrulation, the PPE forms at the border surrounding the anterior neural plate, and expresses a unique set of evolutionarily conserved transcription regulators including Six1, Eya 1 and Eya2. Here, we describe the first report to generate and characterize the SIX1+ PPE cells from human ES cells by adherent differentiation. Before forming PPE cells, differentiating cultures first expressed the non-neural ectoderm specific transcriptional factors TFAP2A, GATA2, GATA3, DLX3, and DLX5, which are crucial in establishing the PPE competence. We demonstrated that bone morphogenetic protein (BMP) activity plays a transient but essential role in inducing expression of these PPE competence factors and eventually the PPE cells. Interestingly, we found that attenuating BMP signaling after establishing the competence state induces anterior placode precursors. By manipulating BMP and hedgehog signaling pathways, we further differentiate these precursors into restricted lineages including the lens placode and the oral ectoderm (pituitary precursor) cells. Finally, we also show that sensory neurons can be generated from human PPE cells, demonstrating the multipotency of the human ES-derived PPE cells.
Keywords: differentiation, stem cells, preplacodal ectoderm, cranial placodes, multipotency, lens, adherent cultures
Introduction
The preplacodal ectoderm (PPE) together with the neural ectoderm, the epidermis and the neural crest constitute the earliest ectoderm lineages. The PPE harbors precursors for mature sensory and neuroendocrine tissues within the vertebrate head, including the lens, the olfactory epithelium, the anterior pituitary, the paired cranial ganglia, and the inner ear epithelium (Baker and Bronner-Fraser, 2001; Schlosser, 2006). Embryological experiments suggest that the cranial placode precursors or the PPE cells are derived from the anterior neural plate border (Bhattacharyya et al., 2004; Couly and Le Douarin, 1985; Kozlowski et al., 1997; Pieper et al., 2012; Streit, 2002; Whitlock and Westerfield, 2000; Xu et al., 2008). PPE cells can be identified by expression of the evolutionarily conserved transcription regulators such as Six1, Eya1, and Eya2 (Bessarab et al., 2004; Chen et al., 2009; David et al., 2001; Pandur and Moody, 2000; Sahly et al., 1999; Sato et al., 2010). In vivo studies in animal models have demonstrated that the transcription factors Tfap2a, Gata2, Gata3, Dlx3, and Dlx5 are expressed in the prospective PPE and they form a genetic network to impart PPE competence to the non-neural ectoderm (Bhat et al., 2013; Esterberg and Fritz, 2009; Kwon et al., 2010; McLarren et al., 2003; Pieper et al., 2012; Woda et al., 2003). Genetic studies have demonstrated that these PPE competence factors are both necessary and sufficient for PPE specification in a cell autonomous fashion (Kwon et al., 2010; Pieper et al., 2012). As development proceeds, the definitive PPE cells express Six1 and Eya1/2, which work synergistically to suppress neural/neural crest fates, promote preplacodal identity, and regulate subsequent differentiation of the cranial placodes (Brugmann et al., 2004; Christophorou et al., 2009; Grocott et al., 2012; Ikeda et al., 2002; Li et al., 2003; Schlosser et al., 2008).
It has been shown that an intermediate level of BMP activity is required for specification of neural plate border including PPE cells in frog and zebrafish embryos (Glavic et al., 2004; Nguyen et al., 1998; Tribulo et al., 2003). Furthermore, it has been demonstrated that BMP signal is required for PPE specification by initiating the expression of the PPE competence factors (Kwon et al., 2010; Nguyen et al., 1998; Pieper et al., 2012). Interestingly, other studies have shown that BMP signaling has to be blocked to allow formation of the definitive PPE (Ahrens and Schlosser, 2005; Brugmann et al., 2004; Kwon et al., 2010; Litsiou et al., 2005). These findings suggest that there are distinct requirements of BMPs during the development of PPE cells.
Human ES cells are pluripotent cell types and possess developmental potentials to differentiate into various cell lineages in the body (Amit et al., 2000; Thomson et al., 1998). BMP signaling is capable of inducing formation of diverse early cell types from human ES cells (Pera et al., 2004; Teo et al., 2012; Xu et al., 2002; Yu et al., 2011; Zhang et al., 2008). In agreement with the in vivo function of BMP in ectodermal differentiation, activating and blocking BMP activity respectively promotes epidermal and neural differentiation in human ES cultures (Chambers et al., 2009; Metallo et al., 2008). There are reports describing culture protocols for human ES cells to generate placode cell types such as lens fiber cells (Yang et al., 2010), sensory hair cells (Chen et al., 2012), and ganglion neurons (Chen et al., 2012; Shi et al., 2007). To our knowledge, characterization of the formation of PPE cells from human ES cells has not been previously reported. Generation of multipotent PPE cells would be of importance for generation of sensory cells and studies of the signaling cues that control their generation and differentiation.
In this study, we aim to determine the condition to generate PPE-like cells and their derivatives from human ES cells. Using SIX1 and other embryonic PPE markers, we attempted to identify PPE-like cells in differentiating human ES cell cultures using adherent cultures with serum free media, which are known to allow generation of non-neural or neural plate border cell types under certain culture parameters (Chambers et al., 2009; Ying et al., 2003). We also investigated the signaling requirements, particularly that of BMP, for PPE formation and differentiation. To study the developmental potential of these PPE cells, we tested a number of differentiation conditions to promote generation of differentiating placode cell types and tissues including lens cells, trigeminal precursors, early anterior pituitary cells, and placode-specific sensory neurons.
Materials and Methods
Human ES cell culture
H9 human ES cell cultures were maintained as originally described (Thomson et al., 1998). Briefly, human ES cells were cultured on mouse embryonic fibroblasts and maintained in Dulbecco’s modified eagle medium/F12 medium supplemented with 20% KnockOut™ serum replacement, 1 × non-essential amino acid, 1 × Glutamax™, β-mercaptoethanol (100 μM) and 8 ng/ml basic FGF. Human ES cells were passaged mechanically every 5 to 7 days onto mouse embryonic fibroblast feeders or matrigel-coated surfaces. All chemicals and medium components were purchased from Life Technologies unless otherwise stated. Human ES cell experiments were approved by the Embryonic Stem Cell Research Oversight Committees of the University of Connecticut.
Preplacodal ectoderm, neural and epidermal differentiation
Human ES cells cultured on matrigel (Xu et al., 2001) were dissociated into single cells with Accutase (Innovative Cell Technologies, Inc.), resuspended in mouse embryonic fibroblast-conditioned medium containing 8 ng/ml basis FGF and 10 μM Rho kinase inhibitor, Y27632 (Calbiochem, Inc. or Tocris) at the desired cell density, and were seeded onto matrigel-coated surfaces. Y27632 was removed from conditioned medium 24 hours after passage. Differentiation was started at 48 hours (2-day) or 72 hours (3-day) post-seeding (designated as day 0) by changing to serum-free (SF) media containing 1% (v/v) N2 supplement, 2% (v/v) B27 supplement, 1 × non-essential amino acid, 1 × Glutamax, 100 μM β-mercaptoethanol in Dulbecco’s modified eagle medium/F12 (Yao et al., 2006). Medium was changed daily for the first 6 days and every other day thereafter.
BMP4 (20 to 150 ng/ml, R&D systems), Noggin (300 or 500 ng/ml, R&D systems), BIO (0.05–2 μM, Calbiochem Inc.), dorsomorphin (2 μM, Stemgent), LDN193189 (100 nM, Stemgent), cyclopamine (1 μM, Calbiochem), purmorphamine (2 μM, Stemgent), or retinoic acid (100–300 ng/ml, Sigma) were tested at the range stated and the effective concentrations were indicated in the text.
For neural differentiation, 300 ng/ml NOGGIN plus 10 μM SB431542 were added into SF medium for 7 days (Chambers et al., 2009). For epidermal differentiation, 100–300 ng/ml retinoic acid and/or 20 ng/ml BMP4 proteins were added into SF medium for 7 days (Metallo et al., 2008). For oral ectoderm induction, LDN193189 (100 nM) and purmorphamine (2 μM) were added from differentiation day 6 to day 13.
Differentiation of lens and corneal epithelial cells
Fresh whole bovine eyes obtained from local slaughterhouse were collected and dissected on ice with procedures as previously described (Schulz et al., 1993). Vitreous was mixed with M199 medium (Gibco) (1:1 ratio) plus antibiotic for cultures of human ES cell-derived lens placode cells for 7 to 14 days. Lens primordia were lifted up using forceps or 30G needles and cultured in the same medium for another 3 to 5 days.
Differentiation of peripheral sensory neurons
SF-derived monolayer culture at day 7 to 10 was briefly digested with Accutase at room temperature and triturated into small tissue pieces with diameters around 50–100 μm. Cell clumps were then transferred to medium composed of SF supplemented with 10 ng/ml basis FGF, 50 ng/ml insulin growth factor-1 (IGF1, Sigma), 10 μM Y27632, cAMP, and 5 μg/ml heparan sulfate. Tissue clumps were cultured in suspension with medium changed every other day. Spheres were collected on day 5 and cultured in the SF medium containing 10 ng/ml FGF2, 20 ng/ml NT3 (Peprotech), 20 ng/ml BDNF (Peprotech) and 100 ng/ml FGF1 (Peprotech) for 5 to 14 days.
Immunocytochemistry
For immunocytochemistry, cells were fixed in 4% paraformaldehyde with/or without 10% fetal bovine serum. Fixed cells were then permeabilized in phosphate-buffered saline containing 0.1–0.4% Triton X-100. Primary antibodies used in this study were: mouse anti-TFAP2A (5E4 and 3B5), mouse anti-PAX3, mouse anti-PAX6, mouse anti-PAX7, rat anti-cytokeratin 8 (TROMA-I) from Developmental Studies Hybridoma Bank, Iowa, USA; goat anti-DLX5, mouse anti-GATA3 and mouse anti-OCT3/4 (Santa Cruz biotechnology), rat anti-E-cadherin (Zymed), rabbit anti-NANOG from BD Biosciences and rabbit anti-SIX1 (Sigma). Alexa Fluor® secondary antibodies (Invitrogen) were used. Nuclei were counterstained with Hoechst-33342 (Invitrogen). Slides were mounted with Fluromount-G (SouthernBiotech). Images were taken using a Zeiss Image M1 microscope.
Reverse transcription and polymerase chain reaction
Total RNAs were extracted using TRIZOL reagent (Invitrogen) and first strand cDNAs were generated using a mixture of oligo-dT and random hexamer primers and Superscript® Reverse Transcriptase II (Invitrogen) according to manufacturer’s instructions. Primer sequences were designed based on exon sequences downloaded from Ensemble database (http://useast.ensembl.org/index.html) using the Primer3 software (http://frodo.wi.mit.edu/primer3/). For semi-quantitative PCR, amplified products were separated on 1 to 1.5% agarose gels stained with ethidium bromide. Quantitative (q)PCR was performed using MESA green qPCR master mix with ROX reference (Eurogentec) in an ABI7900 HT machine or StepOne™ Real-Time PCR system (Life Technologies). Approximately 1.25 ng/μl cDNAs were used for each qPCR reaction. Primer concentration used was 160 nM. Melting curves were performed to check for absence of non-specific PCR products. Different housekeeping genes (e.g. ACTB, AASDH and CPNE2) were tested in qPCR and ACTB was selected as the control based on its stable expression level across different treatment conditions and GAPDH was used as internal control for semi-quantitative RT-PCR. Primer sequences are available upon request.
Statistics
Results were expressed as means ± standard errors. Each dataset was generated from at least three independent experiments. For cell counting, the number of cell counts in 8 to 10 random fields was determined by Nucleus Counter plug-in for ImageJ using the following setting: particle sizes ranged from 25 to 700 arbitrary units, threshold set with Otsu method, background subtracted and watershed filtered. Double-labeled cells were counted with at least 100 cells/nuclei for each image, and a total of 800 to 1200 cells were counted for each marker. In experiments with two conditions, Student’s t-tests were carried out using Microsoft excel. For multiple time points/conditions, statistics were inferred using the ezANOVA software by carrying out ANOVA tests followed by Tukey pairwise comparisons.
Tissue preparation and sectioning
Embryonic day 10.5 embryos (day 0 designated as 12 am on the day of plug) or putative lentoid were fixed in 4% PFA solution. Tissues were embedded in the freezing reagent Optical Cutting Temperature (O.C.T.) Compound (Tissue-Tek®, manufactured by Sakura Finetek, U.S.A.). Frozen sections were prepared at 8 μm in thickness using the Research Cryostat (CM3050 S, LEICA, Microsystems Inc.) and mounted on precleaned Fisherbrand® Colorfrost® plus microscope slides. All animal procedures were approved by the Animal Care Committee, University of Connecticut Health Center.
Results
Generation of PPE cells by adherent differentiation of human ES cells
Previous studies demonstrated that adherent cultures allow efficient generation of neural ectoderm and neural crest precursors from human ES cells (Chambers et al., 2009; Menendez et al., 2011). By manipulating the basic adherent culture parameters and removing neural inducing molecules Noggin (NOG) and SB431542 from the neural differentiation medium, we examined if adherent cultures of human ES cells would allow generation of non-neural ectoderm cells such as PPE cells. Undifferentiated human ES cells were dissociated into single cells with Accutase and cultured on matrigel-coated surfaces in human ES cell medium containing the ROCK inhibitor Y-27632 (Bajpai et al., 2008). After 48 hours (2 days) or 72 hours (3 days), human ES cell medium was replaced with various differentiation media. Using a serum-free medium (SF) (see Materials and Methods), we observed significant induction of PPE marker gene SIX1 by one week of differentiation (Fig 1A). Remarkably, SIX1 transcription was only detected in SF cultures, but not under conditions for neural or epidermal differentiation as described previously (Chambers et al., 2009; Metallo et al., 2008) (Fig 1A). Conversely, the neural precursor marker HES5 (Chambers et al., 2009) was detected in neural differentiation condition but not the SF or epidermal differentiation conditions (Fig 1A). DeltaNp63, a specific TP63 isoform that marks the ES cell-derived epidermal precursors (Metallo et al., 2008), was moderately induced in SF and strongly expressed in epidermal cultures (Fig 1A). ECAD (E-cadherin), normally expressed in the human ES cells and the non-neural ectoderm (Choi and Gumbiner, 1989; Nose and Takeichi, 1986; Weston et al., 2004), was maintained in day-8 SF and epidermal but not in the neural differentiation cultures (Fig 1A). OCT4, a pluripotency marker, was dramatically down-regulated in SF and was not found in neural and epidermal cultures (Fig 1A). In mouse embryos, Six1 is also expressed in mesodermal tissues, such as the head mesenchyme, the cardiac, and the somatic mesoderm (Ghanbari et al., 2001; Sato et al., 2010). To rule out that SIX1 expression might be derived from mesodermal cells in our cultures, we examined BRACHYURY (T), an early pan-mesoderm marker. No T transcripts were detected by RT-PCR (data not shown), indicating that mesoderm differentiation was suppressed in human ES cells cultured in the SF medium. These findings suggest that adherent differentiation in the SF medium promotes formation of epidermal precursors and PPE cells from human ES cells.
Figure 1. Derivation of preplacodal and non-neural ectoderm from human ES cells.
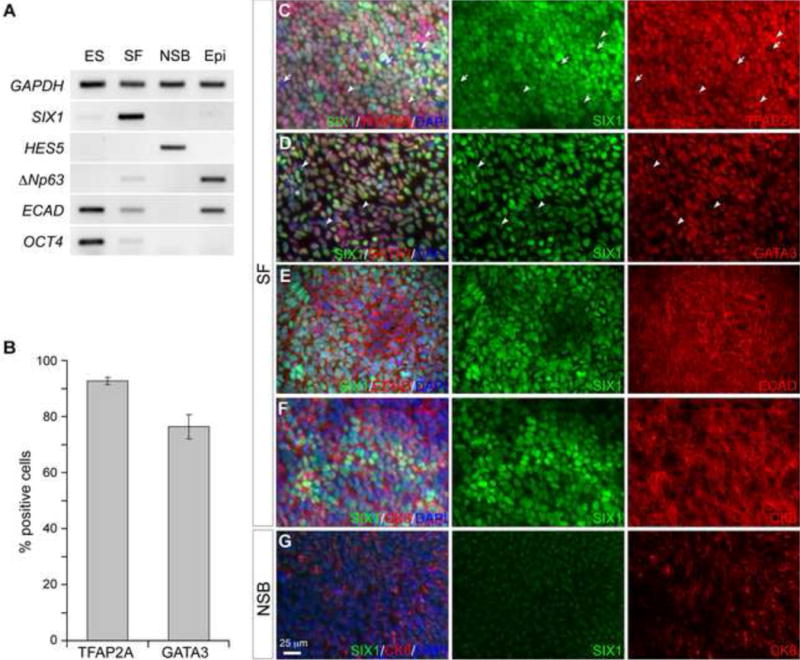
(A) Semi-quantitative RT-PCR of undifferentiated human ES cells (ES) and differentiation day-7 cultures under SF, neural (NSB) and epidermal (Epi) differentiation conditions. (B) Histogram presentation of the percentage of TFAP2A+ and GATA3+ cells in human ES cells cultured in the SF medium at day 8. Error bars are standard errors. (C–G) Immunofluorescence analyses of adherent human ES cells cultured in the SF medium (C–F) and neural differentiation medium (NSB)(G) on day 8 with the antibodies indicated. Arrows indicate cells negative for both SIX1 and TFAP2A; arrowheads show SIX1−/TFAP2A+ and SIX1+/GATA3− cells.
In agreement with the assessment of PPE induction by RT-PCR analyses, immunocytochemistry revealed that after 8 day culture in SF medium, human ES cells produced high percentage of cells expressing TFAP2A and GATA3 (92.6% ± 1.3% and 76.3% ± 4.3%, respectively; Fig 1B–D), which are markers for the non-neural ectoderm (Li and Cornell, 2007; Luo et al., 2002; Neave et al., 1995; Sheng and Stern, 1999). In the day 8 cultures, the majority of the SIX1+ cells co-expressed TFAP2A, GA1 A3, and ECAD (Fig 1C–E). Cytokeratin-8 (CK8) is expressed in placodal ectoderm (see below) and non-neural ectoderm derived epithelium (Page, 1989). CK8 expression is also present in pluripotent human ES cells but down-regulated at the onset of neural differentiation (Maurer et al., 2008). In human ES cells cultured in the SF medium at day 8, SIX1+ cells co-expressed CK8 (Fig 1F). Culturing human ES cells in neural induction medium abolished the induction of SIX1+ cells and greatly reduced the expression of CK8 (Fig 1F, G). Altogether, our results strongly suggest that PPE cells can be generated from human ES cells under this adherent culture condition.
Cell density at the onset of differentiation is important for the optimal generation of PPE cells
Previous studies suggest that cell density significantly affects differentiation outcomes of ES cells (Chambers et al., 2009). We thus sought to examine how cell density at the onset of differentiation might affect the generation of PPE cells in adherent cultures by varying the initial seeding density and the length of the pre-differentiation period (Fig 2A). At the lowest seeding density tested (1.0 × 104 cells/cm2) and a pre-differentiation period of 2 days, although the majority of differentiating cells expressed TFAP2A at day 8, less than 20% of them expressed SIX1 (18.1 ± 4.7%; Fig 2B). Increasing the density to 2.3 or 3.4 × 104 cells/cm2 significantly promoted the percentage of SIX1+ cells to 59.9 ± 5.2% and 70.1 ± 2.2% of the total cell populations respectively (Fig 2B). As expected, extending the pre-differentiation period to 3 days also resulted in increased percentages of SIX1+ cells in cultures with low seeding density of 1.0 or 1.5 × 104 cells/cm2 compared with those with 2-day pre-differentiation (Fig 2B). Importantly, the majority of SIX1+ cells were positive for TFAP2A, validating the PPE identity of these SIX1+ cells.
Figure 2. Cell density affects the differentiation of distinct ectodermal linages in adherent human ES cell culture.
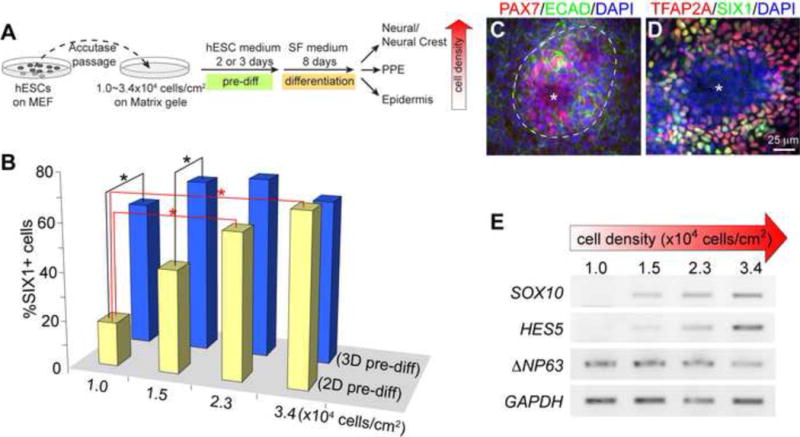
(A) Schematic diagram for generating placodal ectoderm cells from human ES cells, and the change of ectodermal differentiation in relation to the increase of cell density. (B) Histogram presentation of the percentage of SIX1+ cells on differentiation day 8 cultures started with different seeding density or different pre-differentiation periods as indicated in (A). Asterisks indicate significant differences (p < 0.05, ANOVA test). (C and D) Immunofluorescence of day-8 cultures with 3.4 × 104 cells/cm2 seeding density and 3-day pre-differentiation. Antibodies are indicated on the top. Asterisks indicate cells lacking ECAD, SIX1, and TFAP2A; dashed lines demarcate area containing PAX7+ cells. (E) Semi-quantitative RT-PCR of day-8 cultures with the indicated seeding densities and a 3-day pre-differentiation period.
Interestingly, some ECAD-negative (ECAD−) areas started to appear in cultures with high seeding density (2.3 or 3.4 × 104 cells/cm2) and 3-day pre-differentiation (Fig 2C). In these ECAD− areas, the expression of TFAP2A and SIX1 was greatly reduced or absent (Fig 2D and data not shown). Interestingly, the neural crest marker PAX7 was specifically detected within and along the outer periphery of these ECAD− areas (Fig 2C). These observations suggest that increasing the density with 3-day pre-differentiation may predispose the generation of neural crest cells or other neural lineages. Indeed, as the seeding density was changed from 1.0 to 3.4 × 104 cells/cm2, the expression level of neural and neural crest markers HES5 and SOX10 (Betters et al., 2010; Curchoe et al., 2010) steadily increased, whereas the expression of non-neural ectoderm marker —NP63 gradually decreased (Fig 2E). Collectively, these results show that the cell density of human ES cells at the onset of differentiation alters the cellular differentiation of different ectodermal lineages. Although moderately high seeding density (> 1.5 × 104 cells/cm2) is important for the generation of PPE cells from human ES cells, exceedingly high seeding density (> 2.2 × 104 cells/cm2) together with a prolonged pre-differentiation period promotes production of neural and neural crest cells. For the rest of the study, we used an intermediate seeding density (1.7–2.0 × 104 cells/cm2) with a 3-day pre-differentiation to reliably produce about 70% of SIX1+ cells in the cultures.
Formation of a competent non-neural ectoderm state precedes the appearance of PPE identity in differentiating human ES cell cultures
In vivo studies in animal models have shown that Tfap2a, Gata2, Gata3, Dlx3, and Dlx5 are broadly expressed in the non-neural ectoderm including the prospective PPE, and they act in concert to impart PPE competence to the non-neural ectoderm (Esterberg and Fritz, 2009; Kwon et al., 2010; McLarren et al., 2003; Pieper et al., 2012; Woda et al., 2003). To investigate the molecular mechanism underlying human ES cell differentiation into PPE cells, we performed detailed analyses of markers for pluripotency, PPE competence, and PPE itself. Semi-quantitative RT-PCR revealed that the PPE competence genes (TFAP2A, GATA3 and DLX5), and the PPE genes (SIX1) were hardly detectable in undifferentiated human ES cells (Fig 3A). In human ES cells cultured in SF medium, the expression of TFAP2A, GATA3 and DLX5 was greatly increased at day 5 and was maintained at high levels through day 8 (Fig 3A). In contrast to the early induction of PPE competence genes, robust SIX1 expression was detected only at day 8 (Fig 3A).
Figure 3. Development of the PPE competence and definitive PPE identity during human ES cell differentiation.
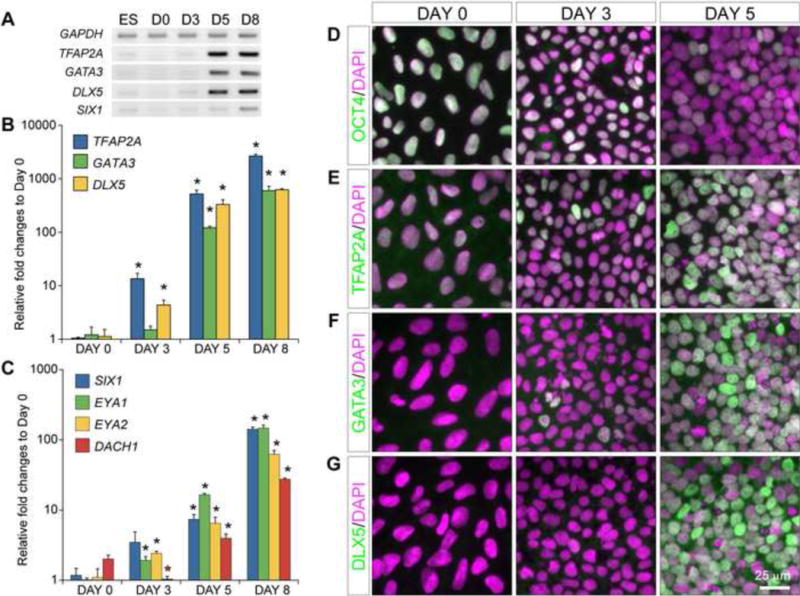
(A) Semi-quantitative PCR of human ES cells cultured under SF condition at day (D) 0, 3, 5, and 8. (B and C) Histogram of relative expression of PPE competence and definitive PPE transcripts using qPCR. Expression data were presented in log scales with three independent biological replicates. Asterisks indicate significant differences (p < 0.05, ANOVA test) from that of day 0 cultures. Note that DACH1 expression was significantly down-regulated from day 0 to day 3 before increasing until day 8. (D–G) Immunofluorescence analyses on day (D) 0, 3, and 5 with the indicated antibodies. Note the progressive decrease of OCT4 and increase of TFAP2A, GATA3 and DLX5 signals (green) from day 0 to day 5.
We next performed qPCR to quantify the dynamic change of gene expression during differentiation. As implicated from RT-PCR, the most dramatic increases in the transcription of TFAP2A, GATA3, and DLX5 were found between day 3 and day 5, and their strong expression persisted until day 8 (Fig 3B). By contrast, although low levels of SIX1, EYA1, and EYA2 transcripts were detected at day 5, expression of these genes exponentially increased between day 5 and day 8 (Fig 3C). DACH1, which is broadly expressed across the surface ectoderm including the placode progenitors (Purcell et al., 2005), has been shown to synergize with SIX/EYA factors to regulate target gene expression (Ikeda et al., 2002; Li et al., 2003). Its expression level was progressively increased from day 3 to 8 (Fig 3C). Therefore, the PPE competence genes are strongly expressed at day 5 preceding the induction of definitive PPE markers, and the latter peak at day 8 during human ES cell differentiation.
Finally, we performed immunocytochemistry to gain insight into the gene expression in situ. As expected, the level of expression and the number of positive cells for the pluripotency marker OCT4 and NANOG decreased precipitously from day 0 to day 5 (Fig 3D and data not shown). In agreement with the RT-PCR results, scattered TFAP2A+ cells were initially detected in day-3 culture but strong expression of TFAP2A was found in most of the cells by day 5 (93.4 ± 5.0%; Fig 3E). Cells positive for GATA3 and DLX5 were rarely detected at day 3, but they became abundant at day 5 (69.7 ± 7.0% and 53.5 ± 6.7% respectively; Fig 3F and G). Co-localization analyses revealed that almost all the DLX5+ cells co-expressed TFAP2A and GATA3 (100% and 96.6%, respectively; Fig 4D).
Figure 4. Endogenous BMP signaling is essential for the production of PPE cells in adherent culture of human ES cells.
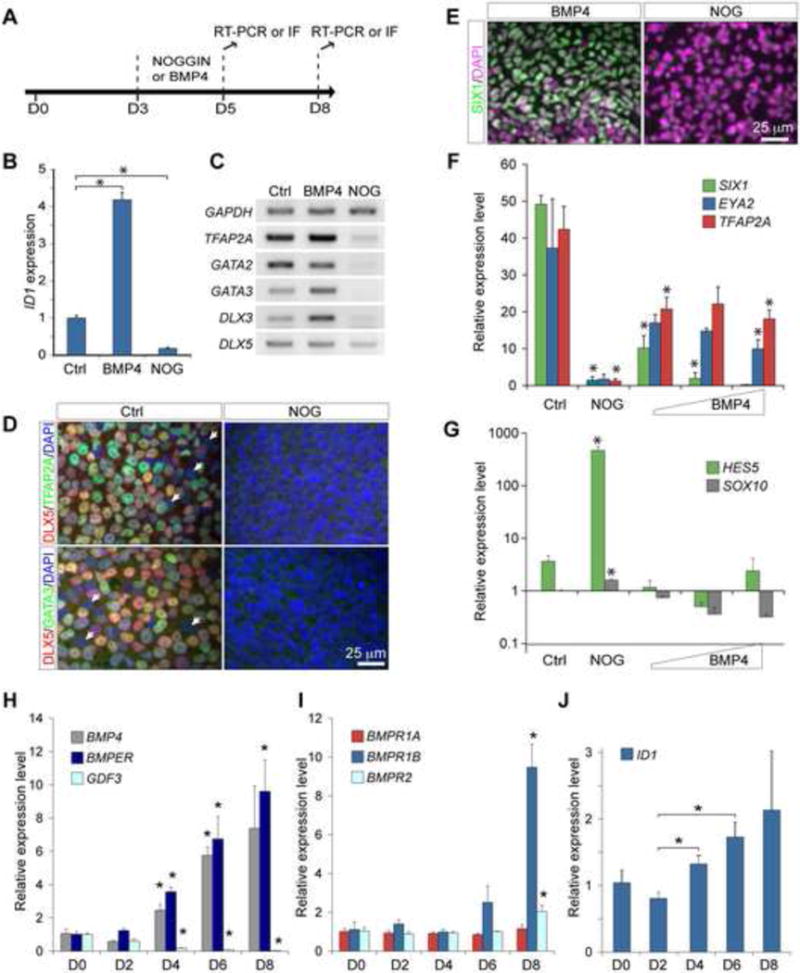
(A) Schematic representation of the manipulations of BMP signaling during human ES cell differentiation. (B) qPCR analysis ID1 expression in day-5 cultures. (C) Semi-quantitative RT-PCR of selected genes in day-5 cultures. (D) Immunofluorescence of day-5 cultures without (Ctrl) or with 300 ng/ml NOGGIN (NOG) treatment. (E) Immunofluorescence for SIX1 in day-8 cultures treated with 20 ng/ml BMP4 or 300 ng/ml NOGGIN. (F–J) Relative expression levels of PPE (F), neural/neural crest (G), extracellular BMP ligand/regulator (H), BMP receptor (I), and the BMP target ID1 (J) transcripts examined by qPCR. Expression data were presented in log scales, and generated from three independent biological replicates. Error bars are S.E. Increasing amount of BMP4 ligands (20, 50, 150 ng/ml) as indicated by the wedged shaped bar in F and G. Asterisks indicate significant differences (p < 0.05, ANOVA test).
In summary, our results show that the differentiating human ES cells first acquire the PPE competence state before establishing the definite PPE identity similar to the in vivo development of the PPE.
BMP signaling is transiently required for establishing the PPE-competent non-neural ectoderm
Genetic studies in zebrafish and frog embryos demonstrate that BMP signaling plays instructive roles in the induction of the PPE competence genes (Kwon et al., 2010; Pieper et al., 2012). Based on the gene expression profile, we postulate that the differentiating human ES cells acquire the PPE competence between day 3 and day 5. To determine whether the formation of human ES cell-derived PPE cells are also dependent on the BMP signaling pathway, we added recombinant BMP4 ligand or NOGGIN (NOG) protein, an extracellular antagonist of BMP ligands (Zimmerman et al., 1996) between day 3 and day 5 (Fig 4A). qPCR analyses showed that the addition of BMP4 (20ng/ml) significantly induced, whereas NOG (300ng/ml) markedly suppressed, the transcription of ID1, a readout gene of BMP signaling (Hollnagel et al., 1999) (Fig 4B). Adding BMP4 had marginal or no effect on the transcription of TFAP2A, GATA2, GATA3, DLX3, and DLX5 at day 5 (Fig 4C). By contrast, adding NOG almost completely abolished the transcription of all the PPE competence genes tested (Fig 4C). Moreover, few TFAP2A+, DLX5+, or GATA3+ cells were found in NOG-treated cultures at day 5 (Fig 4D). Finally, the NOG treatment resulted in a great reduction in the number of SIX1+ cells and the levels of SIX1, EYA2, and TFAP2A transcripts at day 8 (Fig 4E and F). These results collectively demonstrate that BMP signaling is essential for the development of the PPE competence and ultimately PPE cells. Compared with the control, the NOG treatment led to significant increase in the level of HES5, and moderate elevation of SOX10 (Fig 4G), indicating that blocking BMP between day 3 and day 5 induces neural and neural crest cells in our cultures.
Interestingly, treatment with BMP4 at different dosages (20, 50, and 150 ng/ml) from day 3 to day 5 reduced the levels of SIX1, EYA2, and TFAP2A expression, as well as the number of SIX1+ cells at day 8 (Fig 4E, F, and data not shown, compared with Fig 1C–F). As expected, addition of BMP4 inhibited the expression of neural and neural crest markers (Fig 4G). Therefore, although BMP signaling is essential for PPE induction, excess BMP activity has negative effect on the production of PPE cells in human ES cells cultures. We thus hypothesized that PPE differentiation of human ES cells in the SF medium may be regulated by endogenous BMP signaling. To test this hypothesis, we examined the expression of various components in the BMP signaling pathway during differentiation. None of the BMP inhibitors, NOG, CHORDIN, and FOLLISTATIN, was detected in the culture from day 0 to day 8 (data not shown). For BMP ligands, we detected BMP4, but not BMP2 and BMP7 using RT-PCR (data not shown). qPCR analyses showed that the level of BMP4 transcripts was gradually increased from day 4 through day 8 (Fig 4H). Interestingly, the bimodal BMP regulator BMPER, which acts as a feedback regulator to control placodal ectoderm development (Esterberg and Fritz, 2009), was found significantly up-regulated from day 4 to day 8 coincident with the increase of BMP4 (Fig 4H). GDF3, which encodes an antagonist of BMP4 and is essential for human ES cell pluripotency (Levine and Brivanlou, 2006), was highly expressed in undifferentiated human ES cells and progressively reduced from day 4 to day 8 (Fig 4H). Robust expression of BMP receptor genes BMPR1A, BMPR1B, and BMPR2 was detected in the cultures from day 0 to day 8 by RT-PCR (data not shown). qPCR analyses revealed that expression of selected BMP receptors, BMPR1B and BMPR2, were up-regulated at day 8 (Fig 4I). Coinciding with the gradual increase of BMP agonist (BMP4) and reduction of BMP antagonist (GDF3), the expression of ID1 was gradually increased from day 2 to day 8 (Fig 4J). This data suggests that there is endogenous BMP signaling in the differentiating human ES cells, and that a progressive increase of endogenous BMP activity largely coincides with the establishment of the PPE competence state.
In summery, we demonstrate that endogenous BMP signaling is essential for the induction of PPE cells from human ES cells using adherent cultures under SF condition.
Late BMP inhibition induces anterior placodal ectoderm
Manipulations of BMP signaling level by genetic and pharmacological approaches suggest that formation of the PPE requires an inhibition of BMP signaling (Ahrens and Schlosser, 2005; Esterberg and Fritz, 2009; Kwon et al., 2010; Litsiou et al., 2005). We therefore explored conditions for directed differentiation of the human ES cell-derived PPE by changing BMP signaling at late stages. We first tested the effect of blocking BMP signaling by adding NOG (300 ng/ml) from day 6 to day 9 when the cultures clearly established PPE competence. In contrast to the inhibition of the BMP at the earlier stage, adding NOG after day 5 did not abolish SIX1+ cells (data not shown). To further examine the effect of BMP blockade in our cultures, we explored the use of the small molecule inhibitor LDN193189 (LDN), which suppresses BMP signaling at the receptor level and is used as a cost-effective alternative of BMP inhibitors (Kriks et al., 2011). Addition of LDN, similar to NOG, significantly reduced the expression of ID1 (Fig 5A compared to Fig 4B). Applying LDN from day 6 to day 9 had little effect on the percentage of SIX1+ cells in the cultures at day 9 compared with cultures receiving only DMSO (Fig 5C). Interestingly, qPCR analyses revealed that adding LDN caused a slight overall reduction of the SIX1 transcription (Fig 5B), suggesting that BMP inhibition reduces SIX1 transcription per cells, but not the number of SIX+ cells. To test whether inhibition of BMP might cause PPE cells to acquire regional identity, we examined the expression of markers for the anterior and posterior placodal ectoderm. Pax6 is expressed in the anterior placodal ectoderm that gives rise to the lens, the olfactory and the anterior pituitary placodes (Aota et al., 2003; Christophorou et al., 2009; Donner et al., 2007), while Pax3 is expressed in the trigeminal placode precursors (Baker et al., 1999). Compared with the control, LDN treatment resulted in significant increase in the transcript expression of PAX6 and PAX3, but not the posterior placode marker PAX2 (Fig 5B). Immunocytochemistry confirmed that adding LDN significantly enhanced the percentage of PAX6+ (46.8% vs. 6.9% in controls) and PAX3+ cells (18.8% vs. 1.3% in controls) (Fig 5C). Importantly, in the presence of LDN, the majority of PAX6+ and PAX3+ cells co-expressed SIX1 (90% and 70% respectively; Fig 5D–G), indicating that they were PPE derivatives. To confirm that the SIX1+/PAX6+ cells indeed represented the anterior placodal ectoderm (APE), we performed immunocytochemistry for DLX5, which is co-expressed with PAX6 in the lens and olfactory progenitors (Bhattacharyya et al., 2004; Bhattacharyya and Bronner-Fraser, 2008). We found that the majority of PAX6+ cells co-expressed DLX5 in the cultures with LDN treatment (Fig 5H). Finally, we showed that these PAX6+ cells also expressed ECAD confirming that they were not derived from cells of the neural plate (Fig 5I). Altogether, our results demonstrate that although BMP signaling is essential for the PPE competence, blocking BMP signaling after the establishment of PPE competence promotes differentiation of PPE cells into APE cells.
Figure 5. Attenuation of BMP is essential for differentiation of the anterior placodal ectoderm.
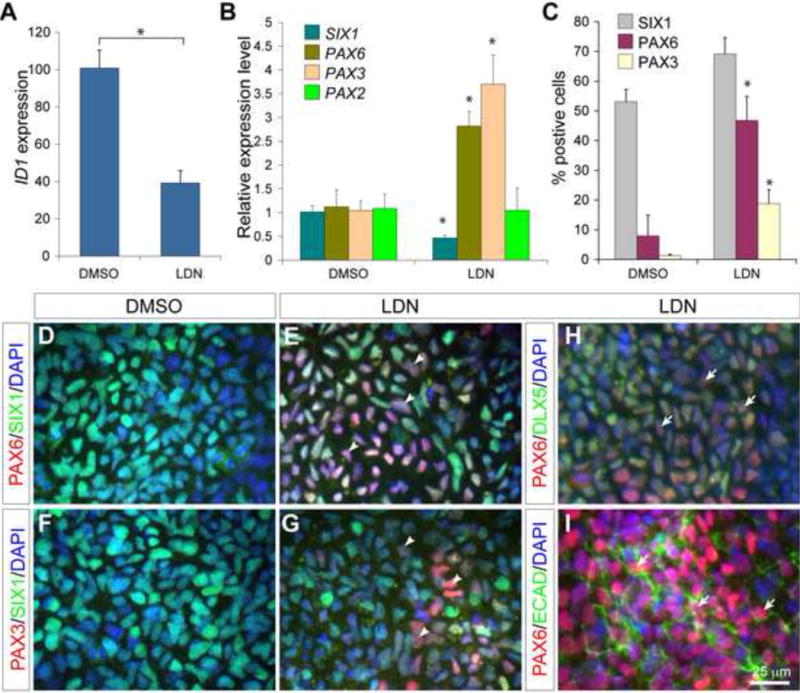
(A and B) qPCR analyses of ID1 (A) and placodal ectoderm genes (B) in day-9 cultures treated with DMSO or LDN193189 from day 6 to day 9. (C) Histogram of the percentage of cells positive for SIX1, PAX6, or PAX3 in day-9 cultures with DMSO or LDN193189 treatment from day 6 to day 9. (D–I) Immunofluorescence of day-9 cultures with the indicated antibodies. Arrowheads show induction of PAX6 or PAX3 in SIX1+ cells; arrows denote the colocalization of PAX6 with DLX5 or ECAD; asterisks indicate significant differences (p < 0.05, t-test).
BMP signaling is required to induce lens placodes
We next explored conditions to direct differentiation of the human ES cell-derived PPE cells. It has been shown that, regardless of their anterior or posterior positions, PPE explants from chick embryos produce lens precursor cells under neutral differentiation conditions (Bailey et al., 2006). We tested if prolonged cultures of human ES cells in the SF media could result in the induction of lens cells. Under the SF condition, human ES cells could survive up to 18 days before massive cell death occurred (data not shown). Remarkably, prolonged culture of the human ES cell-derived PPE cells in the SF media resulted in high levels of FOXE3 and PAX6, which are normally expressed in the lens placode (Blixt et al., 2000; Donner et al., 2007), at differentiation day 18 (Fig 6A), suggesting that the human ES cell-derived PPE cells are able to spontaneously generate lens cells. However, no induction of alpha-A crystalline (CRYAA), a more mature and definitive lens placode marker, was detected (data not shown). By contrast, continued culture of the putative APE cells that were generated by transient LDN treatment consistently resulted in expression of FOXE3 and CRYAA as early as day 13 (see below). These results suggest that the human ES cell-derived APE cells may be more suitable than the uncommitted PPE cells to generate lens cells.
Figure 6. Differentiation of APE cells by manipulating BMP and hedgehog signaling.
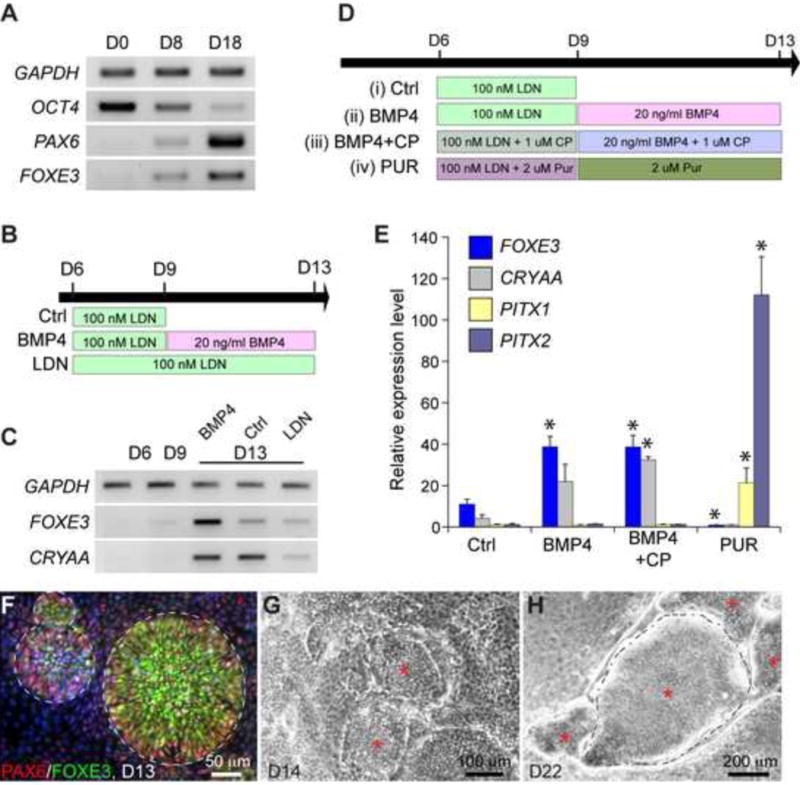
(A) Semi-quantitative RT-PCR of day 0, 8, and 18 cultures under SF condition. (B) Conditions to examine the role of BMP signaling in the induction of lens placode. (C) RT-PCR analysis of markers for lens placode. (D) Schematic presentation of conditions to examine the role of BMP and hedgehog signaling in the differentiation of APE cells. (E) qPCR analysis of selected genes in day-13 cultures. Asterisks indicate statistical significant difference (p < 0.05, ANOVA test) from the control (Ctrl). Abbreviations: LDN, LDN193189; CP, cyclopamine; Pur, purmorphamine. (F) Immunofluorescence for FOXE3 and PAX6 on day-13 cultures. Note that cells co-expressing FOXE3 and PAX6 were clustered together to form rosette like structures demarcated by dashed lines. (G and H) Bright-field images of lens placode cell structures (asterisks) observed at day 14 (G) and day 22 (H). Dashed lines in panel H highlight the separation of the lens-like structures from the surrounding cell monolayer.
It has been demonstrated that BMP4 activity is required for lens induction in mouse and chick embryos (Furuta and Hogan, 1998; Gunhaga, 2011). We thus postulated that addition of exogenous BMP4 proteins after formation of APE cells would promote the production of the lens cells, whereas continuous blockade of BMP signaling with LDN would suppress lens cell production (Fig 6B). Indeed, adding BMP4 to the putative APE cells led to higher levels of FOXE3 and CRYAA expression than those in the control without BMP4 at day 13 (Fig 6C). On the other hand, continuous LDN treatment from day 6 greatly inhibited the induction of FOXE3 and CRYAA (Fig 6C). In the presence of BMP4, APE cells produced spherical and elevated clusters with a diameter of 100–200 μm by day 13 (Fig 6F–G). Remarkably, these clusters were exclusively composed of cells that were positive for both FOXE3 and PAX6 cells (Fig 6F), reminiscent of the embryonic lens placode cells (Blixt et al., 2000; Donner et al., 2007; Purcell et al., 2005).
To further differentiate the lens primordia induced from APE cells, we cultured them in medium containing bovine vitreous humor (Schulz et al., 1993) and WNT / FGF proteins (Yang et al., 2010), which are known to promote lens differentiation (Fig S1A). In the presence of vitreous humor, lens primordia developed into elevated structures with clear separations from the adjacent monolayer (Fig 6H). To test whether these lens primordia can form 3-dimensional structures similar to the in vivo lens, we detached and cultured them in suspension. Within 24 hours, the original concaved sheet of tissues folded and transformed into spheres frequently containing multiple internal vesicles (Fig S1B). Histological analyses revealed that the outer epithelial layers co-expressed PAX6 and CRYAA (Fig S1C) and were enclosed by a thick layer of extracellular matrix displaying laminin immunoreactivity (Fig S1D), resembling the organization of the lens epithelium and the lens capsule in vivo. Pax6 expression is normally down-regulated during lens fiber differentiation and Cryaa is continuously expressed in lens fiber cells. However, only few PAX6−/CRYAA+ cells were detected in the spheres, suggesting that our culture system did not allow efficient lens fiber differentiation. As expected, expression of lens differentiation markers CRYGC, BSFP2, and GJA8 was not induced (Fig S1E). Interestingly, markers for corneal precursors (KRT15) and corneal epithelium (KRT3) were consistently detected (Fig S1E), suggesting induction of the corneal tissues.
We conclude that that the BMP signaling is sufficient and essential for the induction of lens placodes from human ES cell-derived APE cells.
Hedgehog signaling regulates the differentiation of the lens placode and oral ectoderm from human ES cell-derived APE cells
During embryogenesis, the APE further differentiates into the lens, the olfactory and the anterior pituitary (Baker and Bronner-Fraser, 2001; Grocott et al., 2012; Schlosser, 2006). The development of the APE is regulated by hedgehog signaling. It has been shown that elevated hedgehog signaling suppresses lens formation and enhances pituitary induction in zebrafish and amphibian embryos (Cornesse et al., 2005; Treier et al., 2001). Conversely, reduced hedgehog signaling in embryos can lead to expansion of the lens territory (Zilinski et al., 2005). To examine if the human ES cell-derived APE cells are multipotent and their differentiation can be manipulated by altering hedgehog signaling, we challenged them with cyclopamine or purmorphamine, which blocks and activates hedgehog signaling respectively (Sinha and Chen, 2006; Taipale et al., 2000) (Fig 6D). In the presence of cyclopamine (1 μM), there was progressive increase in the expression of FOXE3 and CRYAA from day 9 to day 13 (data not shown). Although BMP4 alone was sufficient to promote FOXE3, combined treatment of BMP4 with cyclopamine led to more consistent increase in FOXE3 and CRYAA expression as measured by qPCR (Fig 6D, E). Strikingly, addition of purmorphamine (2 μM) abolished expression of lens markers FOXE3 and CRYAA, but resulted in 20–100 fold increases in the expression of PITX1 and PITX2, markers for the oral ectoderm (Fig 6E). Therefore, in agreement with the in vivo studies, we found that hedgehog signaling plays an important role in the differentiation of the human ES cell-derived APE cells. Inhibition of hedgehog facilitates the differentiation of lens placodes, while activation of hedgehog signaling promotes oral ectoderm at the expense of the lens cells. These results demonstrate the multipotency of the PPE cells in vitro.
Differentiation of sensory neurons from human PPE cells
The PPE contains progenitors for sensory neurons populating multiple cranial ganglia. We investigated whether the human ES cell-derived PPE cells have the potential to generate peripheral sensory tissues. Using a novel method combining suspension and adherent cultures, we coerced the human ES cell-derived PPE cells to generate TuJ1+ bipolar cells that also expressed BRN3A and peripherin, which are used as markers for peripheral sensory neurons (Mizuseki et al., 2003) (Fig 7A and B). Sensory neurons derived from neural crest and placode display many similar molecular characters and both express Brn3a and peripherin. It has been shown that human olfactory/otic placode-derived neurons display cytokeratin immunoreactivity (Okabe et al., 1997), suggesting that CK8 can be used as a marker to distinguish these two types of sensory neurons. Indeed, immunofluorescence analyses showed that CK8 was specifically expressed in placode-derived sensory neurons in the olfactory, the trigeminal, and the otic placodes, but not in neural crest-derived neurons in the dorsal root ganglia of mouse embryos (Fig 7D–K). To confirm the ES cell-derived sensory neurons were originated from PPE, we performed co-localization analysis for TuJ1, SIX1 and CK8. Significantly, the human ES cell-derived TuJ1+/SIX1+ bipolar neurons displayed CK8 immunoreactivity (Fig 7C), demonstrating that these sensory neurons are derived from PPE, but not from neural crest cells. Altogether, we develop a novel method to generate peripheral sensory neurons from human ES cells. Furthermore, our results demonstrate that the human ES cell-derived PPE cells possess neurogenic potential.
Figure 7. Generation of placode derived sensory neurons from human ES cells.
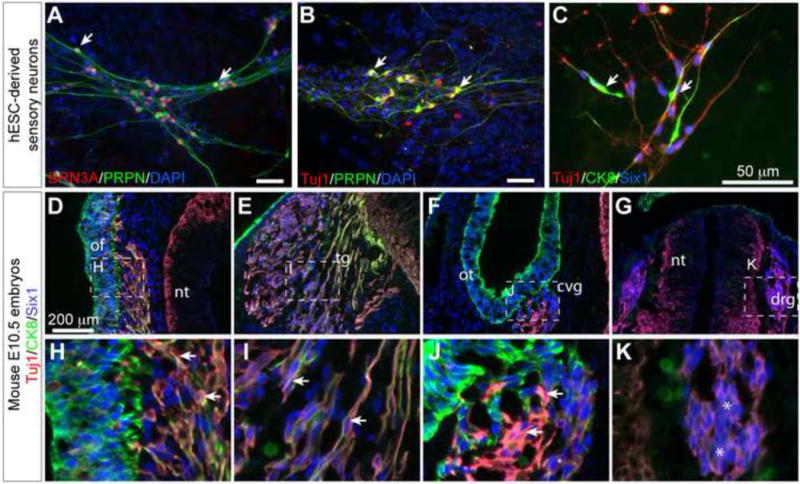
(A–C) Immunofluorescence analysis of placode-derived sensory neurons. Antibodies used are indicated in the images. Note that the ES-derived TuJ1+ neurons (arrows) express markers for peripheral sensory neuron markers BRN3A and peripherin as well as markers characteristic of placode-derived neurons, such as SIX1 and CK8. (D–K) Immunofluorescence on sections of E10.5 mouse embryo. Note that neurons (TuJ1+) (arrows) in the olfactory (D, H), the trigeminal (E, I), and the otic (F, J) placode expressed both Six1 and Ck8, whereas neural crest derived sensory neurons (asterisks) in the dorsal root ganglia (G, K) only expressed Six1 but not CK8. Abbreviations: of, olfactory epithelium; tg, trigeminal ganglion; ot, otic vesicle, cvg, cochleovestibular ganglion; nt, neural tube; drg, dorsal root ganglion.
Discussion
In this study, we presented a differentiation protocol that allows consistent and high yield (~70%) production of human SIX1+ PPE cells. To our knowledge, this study is the first to characterize the formation of PPE cells from human ES cells. We found that the duration of the pre-differentiation period and the initial seeding cell density significantly impacted the cell yield and the outcome of the differentiation, in line with previous findings (Chambers et al., 2009). Our characterization of these pre-differentiation parameters and the associated differentiation outcomes may facilitate future studies using adherent cultures to achieve higher efficiency and consistency of cell differentiation. Future studies should determine the molecular, cellular, or mechanical basis for these basic and critical parameters. This knowledge would help devise novel methodology for high-efficiency human ES cell differentiation into specific cell lineages.
We found a striking similarity in the changes of developmental competence and molecular progression between the differentiating human ES cells and their in vivo orthologs in animal models for placodal ectoderm. For instance, BMP is required in both embryos (Kwon et al., 2010; Pieper et al., 2012) and human ES cells (this study) to establish the PPE competence (see below). Moreover, the human PPE competence state expresses the same set of factors featured in other embryos (Kwon et al., 2010; Pieper et al., 2012). These similarities may be rooted in the correct choice of the in vitro differentiation method for PPE, the intrinsic potential of human ES cells to respond and follow their endogenous differentiation pathways, and the evolutionary conservation in developmental routes across vertebrate species. Therefore, the cell model may be useful in discovering new pathways and factors for PPE development.
BMP signaling has been shown to induce formation of different early cell types from human ES cells, including the extraembryonic endoderm (Pera et al., 2004), the trophoblasts (Xu et al., 2002; Yu et al., 2011), the mesoderm (Zhang et al., 2008), and the endoderm (Teo et al., 2012). The diverse inductive function of BMPs may result from the interplays between BMP and other extracellular signaling. For instance, in the presence of FGF signals, BMP activation promotes trophoblast induction rather than mesoderm induction (Yu et al., 2011), whereas Activin signals cooperate with BMP activation to promote endoderm induction (Teo et al., 2012). Cellular competence is known to change rapidly in days during ES cell differentiation (Harvey et al., 2010). BMP activation at a later differentiation time point may have allowed efficient induction of non-neural ectoderm precursors instead of other lineages. Also, we found that there are changing requirements of BMP signaling during the formation and the subsequent differentiation of human ES-derived PPE cells. Although blocking BMP activity abolishes expression of PPE competence factors and PPE generation, applying BMP antagonists after competence gene induction did not affect SIX1 and other PPE markers. This suggests that BMP works within a narrow window to induce the PPE. Different from the previous findings (Ahrens and Schlosser, 2005; Brugmann et al., 2004; Kwon et al., 2010; Litsiou et al., 2005), we found that robust expression of BMP4 and ID1 persisted in day-8 cultures when SIX1 and other PPE markers were induced (Figs 1 and 4). There are complex regulations of BMP signaling besides the expression of BMP ligands, and ID1 expression may not necessarily represent the actual BMP activity. Nevertheless, our findings raise the possibility that BMP may be active during human PPE cell formation. Interestingly, we found that inhibition of BMP signaling led to APE formation following by the formation of PPE, while subsequent activation of BMP4 promoted the APE to differentiate into lens placode. We therefore conclude that during human PPE cell formation and differentiation, there are changing requirements for BMP signaling.
An interesting finding of our study is the default differentiation bias of human ES cells into the non-neural ectoderm lineage including the PPE, under the “neutral” differentiation conditions using a serum free differentiation medium (Yao et al., 2006). This observation is in stark contrast to findings in the mouse ES cells, which undergo neural differentiation in similar serum free differentiation conditions (Ying et al., 2003). Although expressing the same set of the core pluripotent factors, the signaling requirements for pluripotency maintenance and differentiation are entirely different in ES cells for mouse and human (Pera and Tam, 2010). It is conceivable that intrinsic differences in mouse and human ES cells may have contributed to the decision of neural versus non-neural lineages under ‘neutral’ differentiation conditions. On the other hand, environmental factors such as cell-matrix interactions may play an instructive role in driving specific differentiation events. For example, upon differentiation, specific extracellular matrix-cell surface receptor interactions could affect the magnitude, the duration, and the interpretation of the growth factor mediated signaling. Interestingly, Smith and colleagues noted that mouse ES cells differentiating on laminin, a matrix that is present with high content (50–60%, BD Bioscience website) in Matrigel (the matrix used for human ES cells adherent differentiation in this study), adopted non-neural fibroblastic cell morphologies (Ying et al., 2003).
Concluding remarks
Traditionally, ES cells have been used as a model for developmental studies in areas related to early embryonic stages when tissues are difficult to access and obtain in large quantities (Coucouvanis and Martin, 1999). Because of the ethical and practical considerations of human embryo studies, development of human ES cells as a tool to study differentiation of early human cell types has become an attractive alternative. The fact that most of the major ectoderm cell types can be derived from human ES cells using a feeder-free adherent differentiation method suggests that this method may be particularly suitable for ectoderm differentiation. We propose that this in vitro cell model will be useful for future research in the area of early human ectoderm cell type development.
Supplementary Material
Highlights
Generation of preplacodal ectoderm (PPE) from human embryonic stem cells
Production of placodal sensory neurons and lens from hESC-derived PPE
Characterization of cell density and endogenous BMP signals in hESC differentiation
Novel roles of BMP activities in generation and differentiation of human PPE cells
Acknowledgments
We would like to thank members of the Li and the Morest laboratories for their support, comments, and critical discussion for the current work, Dr. Xu Maisano and Mr. Alexander Dee for critical readings of the manuscript. We also thank Dr. Peter Carlsson (Göteborg University, Sweden) for providing the rabbit FOXE3 antibody, the excellent services and technical helps from the Stem Cell Core Facility, and the Molecular Core of the University of Connecticut Health Center. The monoclonal antibodies against TFAP2A (5E4 and 3B5), PAX3, PAX6, PAX7, cytokeratin 8 (TROMA-I) were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biology, Iowa City, IA 52242. This work is supported by the State of Connecticut under the Connecticut Stem Cell Research Grant Program (O8SCBUCHC016 to D.K.M. and 10SCB30-UCHC to J.Y.L.) and the National Institute of Health (5R01DC000127 to D.K.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahrens K, Schlosser G. Tissues and signals involved in the induction of placodal Six1 expression in Xenopus laevis. Developmental biology. 2005;288:40–59. doi: 10.1016/j.ydbio.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Developmental biology. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- Aota S, Nakajima N, Sakamoto R, Watanabe S, Ibaraki N, Okazaki K. Pax6 autoregulation mediated by direct interaction of Pax6 protein with the head surface ectoderm-specific enhancer of the mouse Pax6 gene. Developmental biology. 2003;257:1–13. doi: 10.1016/s0012-1606(03)00058-7. [DOI] [PubMed] [Google Scholar]
- Bailey AP, Bhattacharyya S, Bronner-Fraser M, Streit A. Lens specification is the ground state of all sensory placodes, from which FGF promotes olfactory identity. Dev Cell. 2006;11:505–517. doi: 10.1016/j.devcel.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Bajpai R, Lesperance J, Kim M, Terskikh AV. Efficient propagation of single cells Accutase-dissociated human embryonic stem cells. Molecular reproduction and development. 2008;75:818–827. doi: 10.1002/mrd.20809. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Developmental biology. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Baker CV, Stark MR, Marcelle C, Bronner-Fraser M. Competence, specification and induction of Pax-3 in the trigeminal placode. Development. 1999;126:147–156. doi: 10.1242/dev.126.1.147. [DOI] [PubMed] [Google Scholar]
- Bessarab DA, Chong SW, Korzh V. Expression of zebrafish six1 during sensory organ development and myogenesis. Developmental dynamics: an official publication of the American Association of Anatomists. 2004;230:781–786. doi: 10.1002/dvdy.20093. [DOI] [PubMed] [Google Scholar]
- Betters E, Liu Y, Kjaeldgaard A, Sundstrom E, Garcia-Castro MI. Analysis of early human neural crest development. Developmental biology. 2010;344:578–592. doi: 10.1016/j.ydbio.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat N, Kwon HJ, Riley BB. A gene network that coordinates preplacodal competence and neural crest specification in zebrafish. Developmental biology. 2013;373:107–117. doi: 10.1016/j.ydbio.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Bailey AP, Bronner-Fraser M, Streit A. Segregation of lens and olfactory precursors from a common territory: cell sorting and reciprocity of Dlx5 and Pax6 expression. Developmental biology. 2004;271:403–414. doi: 10.1016/j.ydbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Bronner-Fraser M. Competence, specification and commitment to an olfactory placode fate. Development. 2008;135:4165–4177. doi: 10.1242/dev.026633. [DOI] [PubMed] [Google Scholar]
- Blixt A, Mahlapuu M, Aitola M, Pelto-Huikko M, Enerback S, Carlsson P. A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle. Gene Dev. 2000;14:245–254. [PMC free article] [PubMed] [Google Scholar]
- Brugmann SA, Pandur PD, Kenyon KL, Pignoni F, Moody SA. Six1 promotes a placodal fate within the lateral neurogenic ectoderm by functioning as both a transcriptional activator and repressor. Development. 2004;131:5871–5881. doi: 10.1242/dev.01516. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nature biotechnology. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Kim EH, Xu PX. Initiation of olfactory placode development and neurogenesis is blocked in mice lacking both Six1 and Six4. Developmental biology. 2009;326:75–85. doi: 10.1016/j.ydbio.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jongkamonwiwat N, Abbas L, Eshtan SJ, Johnson SL, Kuhn S, Milo M, Thurlow JK, Andrews PW, Marcotti W, Moore HD, Rivolta MN. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature. 2012;490:278–282. doi: 10.1038/nature11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Gumbiner B. Expression of Cell-Adhesion Molecule E-Cadherin in Xenopus Embryos Begins at Gastrulation and Predominates in the Ectoderm. J Cell Biol. 1989;108:2449–2466. doi: 10.1083/jcb.108.6.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophorou NAD, Bailey AP, Hanson S, Streit A. Activation of Six1 target genes is required for sensory placode formation. Developmental biology. 2009;336:327–336. doi: 10.1016/j.ydbio.2009.09.025. [DOI] [PubMed] [Google Scholar]
- Cornesse Y, Pieler T, Hollemann T. Olfactory and lens placode formation is controlled by the hedgehog-interacting protein (Xhip) in Xenopus. Developmental biology. 2005;277:296–315. doi: 10.1016/j.ydbio.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Coucouvanis E, Martin GR. BMP signaling plays a role in visceral endoderm differentiation and cavitation in the early mouse embryo. Development. 1999;126:535–546. doi: 10.1242/dev.126.3.535. [DOI] [PubMed] [Google Scholar]
- Couly GF, Le Douarin NM. Mapping of the early neural primordium in quail-chick chimeras. I. Developmental relationships between placodes, facial ectoderm, and prosencephalon. Dev Biol. 1985;110:422–439. doi: 10.1016/0012-1606(85)90101-0. [DOI] [PubMed] [Google Scholar]
- Curchoe CL, Maurer J, McKeown SJ, Cattarossi G, Cimadamore F, Nilbratt M, Snyder EY, Bronner-Fraser M, Terskikh AV. Early acquisition of neural crest competence during hESCs neuralization. PloS one. 2010;5:e13890. doi: 10.1371/journal.pone.0013890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David R, Ahrens K, Wedlich D, Schlosser G. Xenopus Eya1 demarcates all neurogenic placodes as well as migrating hypaxial muscle precursors. Mechanisms of development. 2001;103:189–192. doi: 10.1016/s0925-4773(01)00355-0. [DOI] [PubMed] [Google Scholar]
- Donner AL, Episkopou V, Maas RL. Sox2 and Pou2f1 interact to control lens and olfactory placode development. Developmental biology. 2007;303:784–799. doi: 10.1016/j.ydbio.2006.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterberg R, Fritz A. dlx3b/4b are required for the formation of the preplacodal region and otic placode through local modulation of BMP activity. Developmental biology. 2009;325:189–199. doi: 10.1016/j.ydbio.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Hogan BL. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanbari H, Seo HC, Fjose A, Brandli AW. Molecular cloning and embryonic expression of Xenopus Six homeobox genes. Mechanisms of development. 2001;101:271–277. doi: 10.1016/s0925-4773(00)00572-4. [DOI] [PubMed] [Google Scholar]
- Glavic A, Maris Honore S, Gloria Feijoo C, Bastidas F, Allende ML, Mayor R. Role of BMP signaling and the homeoprotein Iroquois in the specification of the cranial placodal field. Developmental biology. 2004;272:89–103. doi: 10.1016/j.ydbio.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Grocott T, Tambalo M, Streit A. The peripheral sensory nervous system in the vertebrate head: a gene regulatory perspective. Developmental biology. 2012;370:3–23. doi: 10.1016/j.ydbio.2012.06.028. [DOI] [PubMed] [Google Scholar]
- Gunhaga L. The lens: a classical model of embryonic induction providing new insights into cell determination in early development. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2011;366:1193–1203. doi: 10.1098/rstb.2010.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey NT, Hughes JN, Lonic A, Yap C, Long C, Rathjen PD, Rathjen J. Response to BMP4 signalling during ES cell differentiation defines intermediates of the ectoderm lineage. Journal of cell science. 2010;123:1796–1804. doi: 10.1242/jcs.047530. [DOI] [PubMed] [Google Scholar]
- Hollnagel A, Oehlmann V, Heymer J, Ruther U, Nordheim A. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. The Journal of biological chemistry. 1999;274:19838–19845. doi: 10.1074/jbc.274.28.19838. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Watanabe Y, Ohto H, Kawakami K. Molecular interaction and synergistic activation of a promoter by Six, Eya, and Dach proteins mediated through CREB binding protein. Mol Cell Biol. 2002;22:6759–6766. doi: 10.1128/MCB.22.19.6759-6766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski DJ, Murakami T, Ho RK, Weinberg ES. Regional cell movement and tissue patterning in the zebrafish embryo revealed by fate mapping with caged fluorescein. Biochemistry and cell biology = Biochimie et biologie cellulaire. 1997;75:551–562. [PubMed] [Google Scholar]
- Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HJ, Bhat N, Sweet EM, Cornell RA, Riley BB. Identification of early requirements for preplacodal ectoderm and sensory organ development. PLoS genetics. 2010;6 doi: 10.1371/journal.pgen.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Brivanlou AH. GDF3, a BMP inhibitor, regulates cell fate in stem cells and early embryos. Development. 2006;133:209–216. doi: 10.1242/dev.02192. [DOI] [PubMed] [Google Scholar]
- Li W, Cornell RA. Redundant activities of Tfap2a and Tfap2c are required for neural crest induction and development of other non-neural ectoderm derivatives in zebrafish embryos. Developmental biology. 2007;304:338–354. doi: 10.1016/j.ydbio.2006.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Oghi KA, Zhang J, Krones A, Bush KT, Glass CK, Nigam SK, Aggarwal AK, Maas R, Rose DW, Rosenfeld MG. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426:247–254. doi: 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- Litsiou A, Hanson S, Streit A. A balance of FGF, BMP and WNT signalling positions the future placode territory in the head. Development. 2005;132:4051–4062. doi: 10.1242/dev.01964. [DOI] [PubMed] [Google Scholar]
- Luo T, Matsuo-Takasaki M, Thomas ML, Weeks DL, Sargent TD. Transcription factor AP-2 is an essential and direct regulator of epidermal development in Xenopus. Developmental biology. 2002;245:136–144. doi: 10.1006/dbio.2002.0621. [DOI] [PubMed] [Google Scholar]
- Maurer J, Nelson B, Cecena G, Bajpai R, Mercola M, Terskikh A, Oshima RG. Contrasting expression of keratins in mouse and human embryonic stem cells. PloS one. 2008;3:e3451. doi: 10.1371/journal.pone.0003451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLarren KW, Litsiou A, Streit A. DLX5 positions the neural crest and preplacode region at the border of the neural plate. Developmental biology. 2003;259:34–47. doi: 10.1016/s0012-1606(03)00177-5. [DOI] [PubMed] [Google Scholar]
- Menendez L, Yatskievych TA, Antin PB, Dalton S. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19240–19245. doi: 10.1073/pnas.1113746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metallo CM, Ji L, de Pablo JJ, Palecek SP. Retinoic acid and bone morphogenetic protein signaling synergize to efficiently direct epithelial differentiation of human embryonic stem cells. Stem Cells. 2008;26:372–380. doi: 10.1634/stemcells.2007-0501. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Sakamoto T, Watanabe K, Muguruma K, Ikeya M, Nishiyama A, Arakawa A, Suemori H, Nakatsuji N, Kawasaki H, Murakami F, Sasai Y. Generation of neural crest-derived peripheral neurons and floor plate cells from mouse and primate embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:5828–5833. doi: 10.1073/pnas.1037282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neave B, Rodaway A, Wilson SW, Patient R, Holder N. Expression of zebrafish GATA 3 (gta3) during gastrulation and neurulation suggests a role in the specification of cell fate. Mechanisms of development. 1995;51:169–182. doi: 10.1016/0925-4773(95)00351-7. [DOI] [PubMed] [Google Scholar]
- Nguyen VH, Schmid B, Trout J, Connors SA, Ekker M, Mullins MC. Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Developmental biology. 1998;199:93–110. doi: 10.1006/dbio.1998.8927. [DOI] [PubMed] [Google Scholar]
- Nose A, Takeichi M. A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J Cell Biol. 1986;103:2649–2658. doi: 10.1083/jcb.103.6.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe H, Okubo T, Adachi H, Ishikawa T, Ochi Y. Immunohistochemical demonstration of cytokeratin in human embryonic neurons arising from placodes. Brain & development. 1997;19:347–352. doi: 10.1016/s0387-7604(97)00034-x. [DOI] [PubMed] [Google Scholar]
- Page M. Changing patterns of cytokeratins and vimentin in the early chick embryo. Development. 1989;105:97–107. doi: 10.1242/dev.105.1.97. [DOI] [PubMed] [Google Scholar]
- Pandur PD, Moody SA. Xenopus Six1 gene is expressed in neurogenic cranial placodes and maintained in the differentiating lateral lines. Mech Dev. 2000;96:253–257. doi: 10.1016/s0925-4773(00)00396-8. [DOI] [PubMed] [Google Scholar]
- Pera MF, Andrade J, Houssami S, Reubinoff B, Trounson A, Stanley EG, Ward-van Oostwaard D, Mummery C. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. Journal of cell science. 2004;117:1269–1280. doi: 10.1242/jcs.00970. [DOI] [PubMed] [Google Scholar]
- Pera MF, Tam PP. Extrinsic regulation of pluripotent stem cells. Nature. 2010;465:713–720. doi: 10.1038/nature09228. [DOI] [PubMed] [Google Scholar]
- Pieper M, Ahrens K, Rink E, Peter A, Schlosser G. Differential distribution of competence for panplacodal and neural crest induction to non-neural and neural ectoderm. Development. 2012;139:1175–1187. doi: 10.1242/dev.074468. [DOI] [PubMed] [Google Scholar]
- Purcell P, Oliver G, Mardon G, Donner AL, Maas RL. Pax6-dependence of Six3, Eya1 and Dach1 expression during lens and nasal placode induction. Gene expression patterns: GEP. 2005;6:110–118. doi: 10.1016/j.modgep.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Sahly I, Andermann P, Petit C. The zebrafish eya1 gene and its expression pattern during embryogenesis. Development genes and evolution. 1999;209:399–410. doi: 10.1007/s004270050270. [DOI] [PubMed] [Google Scholar]
- Sato S, Ikeda K, Shioi G, Ochi H, Ogino H, Yajima H, Kawakami K. Conserved expression of mouse Six1 in the pre-placodal region (PPR) and identification of an enhancer for the rostral PPR. Developmental biology. 2010;344:158–171. doi: 10.1016/j.ydbio.2010.04.029. [DOI] [PubMed] [Google Scholar]
- Schlosser G. Induction and specification of cranial placodes. Developmental biology. 2006;294:303–351. doi: 10.1016/j.ydbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Schlosser G, Awtry T, Brugmann SA, Jensen ED, Neilson K, Ruan G, Stammler A, Voelker D, Yan B, Zhang C, Klymkowsky MW, Moody SA. Eya1 and Six1 promote neurogenesis in the cranial placodes in a SoxB1-dependent fashion. Developmental biology. 2008;320:199–214. doi: 10.1016/j.ydbio.2008.05.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz MW, Chamberlain CG, de Iongh RU, McAvoy JW. Acidic and basic FGF in ocular media and lens: implications for lens polarity and growth patterns. Development. 1993;118:117–126. doi: 10.1242/dev.118.1.117. [DOI] [PubMed] [Google Scholar]
- Sheng G, Stern CD. Gata2 and Gata3: novel markers for early embryonic polarity and for non-neural ectoderm in the chick embryo. Mechanisms of development. 1999;87:213–216. doi: 10.1016/s0925-4773(99)00150-1. [DOI] [PubMed] [Google Scholar]
- Shi F, Corrales CE, Liberman MC, Edge AS. BMP4 induction of sensory neurons from human embryonic stem cells and reinnervation of sensory epithelium. The European journal of neuroscience. 2007;26:3016–3023. doi: 10.1111/j.1460-9568.2007.05909.x. [DOI] [PubMed] [Google Scholar]
- Sinha S, Chen JK. Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nature chemical biology. 2006;2:29–30. doi: 10.1038/nchembio753. [DOI] [PubMed] [Google Scholar]
- Streit A. Extensive cell movements accompany formation of the otic placode. Developmental biology. 2002;249:237–254. doi: 10.1006/dbio.2002.0739. [DOI] [PubMed] [Google Scholar]
- Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, Beachy PA. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- Teo AK, Ali Y, Wong KY, Chipperfield H, Sadasivam A, Poobalan Y, Tan EK, Wang ST, Abraham S, Tsuneyoshi N, Stanton LW, Dunn NR. Activin and BMP4 synergistically promote formation of definitive endoderm in human embryonic stem cells. Stem Cells. 2012;30:631–642. doi: 10.1002/stem.1022. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Treier M, O’Connell S, Gleiberman A, Price J, Szeto DP, Burgess R, Chuang PT, McMahon AP, Rosenfeld MG. Hedgehog signaling is required for pituitary gland development. Development. 2001;128:377–386. doi: 10.1242/dev.128.3.377. [DOI] [PubMed] [Google Scholar]
- Tribulo C, Aybar MJ, Nguyen VH, Mullins MC, Mayor R. Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development. 2003;130:6441–6452. doi: 10.1242/dev.00878. [DOI] [PubMed] [Google Scholar]
- Weston JA, Yoshida H, Robinson V, Nishikawa S, Fraser ST, Nishikawa S. Neural crest and the origin of ectomesenchyme: neural fold heterogeneity suggests an alternative hypothesis. Developmental dynamics: an official publication of the American Association of Anatomists. 2004;229:118–130. doi: 10.1002/dvdy.10478. [DOI] [PubMed] [Google Scholar]
- Whitlock KE, Westerfield M. The olfactory placodes of the zebrafish form by convergence of cellular fields at the edge of the neural plate. Development. 2000;127:3645–3653. doi: 10.1242/dev.127.17.3645. [DOI] [PubMed] [Google Scholar]
- Woda JM, Pastagia J, Mercola M, Artinger KB. Dlx proteins position the neural plate border and determine adjacent cell fates. Development. 2003;130:331–342. doi: 10.1242/dev.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nature biotechnology. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- Xu H, Dude CM, Baker CV. Fine-grained fate maps for the ophthalmic and maxillomandibular trigeminal placodes in the chick embryo. Developmental biology. 2008;317:174–186. doi: 10.1016/j.ydbio.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Xu RH, Chen X, Li DS, Li R, Addicks GC, Glennon C, Zwaka TP, Thomson JA. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nature biotechnology. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- Yang C, Yang Y, Brennan L, Bouhassira EE, Kantorow M, Cvekl A. Efficient generation of lens progenitor cells and lentoid bodies from human embryonic stem cells in chemically defined conditions. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2010;24:3274–3283. doi: 10.1096/fj.10-157255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Chen S, Clark J, Hao E, Beattie GM, Hayek A, Ding S. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6907–6912. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nature biotechnology. 2003;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- Yu P, Pan G, Yu J, Thomson JA. FGF2 sustains NANOG and switches the outcome of BMP4-induced human embryonic stem cell differentiation. Cell Stem Cell. 2011;8:326–334. doi: 10.1016/j.stem.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Li J, Tan Z, Wang C, Liu T, Chen L, Yong J, Jiang W, Sun X, Du L, Ding M, Deng H. Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood. 2008;111:1933–1941. doi: 10.1182/blood-2007-02-074120. [DOI] [PubMed] [Google Scholar]
- Zilinski CA, Shah R, Lane ME, Jamrich M. Modulation of zebrafish pitx3 expression in the primordia of the pituitary, lens, olfactory epithelium and cranial ganglia by hedgehog and nodal signaling. Genesis. 2005;41:33–40. doi: 10.1002/gene.20094. [DOI] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


