Abstract
Background: Little is known about the association between eating patterns and type 2 diabetes (T2D) risk in women.
Objective: The objective was to examine prospectively associations between regular breakfast consumption, eating frequency, and T2D risk in women.
Design: Eating pattern was assessed in 2002 in a cohort of 46,289 US women in the Nurses’ Health Study who were free of T2D, cardiovascular disease, or cancer and were followed for 6 y. We used Cox proportional hazards analysis to evaluate associations with incident T2D.
Results: We documented 1560 T2D cases during follow-up. After adjustment for known risk factors for T2D—except for body mass index (BMI), a potential mediator—women who consumed breakfast irregularly (0–6 times/wk) were at higher risk of T2D than were women who consumed breakfast daily (RR: 1.28; 95% CI: 1.14, 1.44). This association was moderately attenuated after adjustment for BMI (RR: 1.20; 95% CI: 1.07, 1.35). In comparison with women who ate 3 times/d, the RRs were 1.09 (0.84, 1.41) for women who ate 1–2 times/d, 1.13 (1.00, 1.27) for women who ate 4–5 times/d, and 0.99 (0.81, 1.21) for women who ate ≥6 times/d. Among irregular breakfast consumers, women with a higher eating frequency (≥4 times/d) had a significantly greater T2D risk (RR: 1.47; 95% CI: 1.23, 1.75) than did women who consumed breakfast daily and ate 1–3 times/d. Adjustment for BMI attenuated this association (RR: 1.24; 95% CI: 1.04, 1.48).
Conclusion: Irregular breakfast consumption was associated with a higher T2D risk in women, which was partially but not entirely mediated by BMI.
INTRODUCTION
The prevalence of type 2 diabetes (T2D)4 has been escalating worldwide. Among adults (20–79 y of age), an increase in prevalence from 6.4% (285 million) in 2010 to 7.7% (439 million) in 2030 has been estimated (1).T2D has become a major public health concern given the effect of diabetes on morbidity and premature mortality (2). In parallel, the proportion of people who report regularly consuming breakfast has been dropping over past decades among children, adolescents (3), and adults (4), perhaps due in part to the popular misconception that skipping breakfast could help with weight control. Increasing evidence indicates that skipping breakfast is directly associated with weight gain and other adverse health outcomes (5), including insulin resistance and T2D (6). Furthermore, most previous studies of breakfast frequency and type in the etiology of obesity and chronic diseases were small, and the results are conflicting (5, 7). In a recent cross-sectional study among US adults, consumption of ready-to-eat cereal breakfast was associated with a better cardiometabolic risk profile than was consumption of other types of breakfast (8), probably because of more favorable diet quality among consumers of ready-to-eat cereal breakfast (9).
Similarly, eating frequency or snacking may also influence body weight and risk of metabolic diseases (5, 7); however, studies on this topic have yielded inconsistent results. Studies in mice (10, 11) showed an improvement in glucose tolerance and glycemic response with reduced eating frequency, independent of total calorie intake. However, some trials observed metabolic advantages associated with increased eating frequency, while keeping energy constant, among patients with T2D (5) and among healthy populations (12). Earlier trials showed no effect on glucose metabolism in a comparison of subjects with T2D on low or high eating frequency regimes (13, 14).
Therefore, we prospectively examined in the Nurses’ Health Study whether regular breakfast consumption and eating frequency were associated with T2D risk and whether these associations were mediated through BMI (in kg/m2). We also examined potential modification of associations by BMI, healthy eating index, dietary glycemic load, and cereal fiber.
SUBJECTS AND METHODS
Subjects
The Nurses’ Health Study, established in 1976, is a prospective cohort study of 121,700 registered female nurses (30–55 y of age at baseline) residing in 11 states. Participants were mailed questionnaires at baseline and every second year to repeatedly assess lifestyle practice and chronic disease occurrence. The Institutional Review Boards of the Brigham and Women's Hospital and the Harvard School of Public Health, Boston, MA, approved the study protocol, and written consent was obtained from all subjects.
Dietary assessment
In 1984, dietary information was collected by using a 116-item food-frequency questionnaire (FFQ). During 1986 through 2006, participants were asked to update their diet information every 4 y by using a similar but expanded 131-item semiquantitative FFQ that was previously validated (15, 16). Participants were asked to select their usual intake of a standard portion of each food item. Nine responses were possible, ranging from “never or less than once/month” to “≥6 times/d.” Daily nutrient and energy intakes were calculated by multiplying the frequency of intake of each food by the nutrient and energy content estimated by using food-composition tables from the Harvard University food-composition database, which was derived from US Department of Agriculture sources (17), and summing across all items.
In 2002, the questionnaire included a question about eating frequency “How many times per day do you eat? Include meals and snacks. (For snacks, count juice and non-diet soda, but exclude coffee and diet soda): 1 or 2 times/day, 3/day, 4/day, 5/day, 6/day, 7/day. 8/day, 9+/day” and a question about breakfast consumption “How many days per week do you have breakfast (more than coffee or tea)? Never, 1/week, 2/week, 3/week, 4/week, 5/week, 6/week, 7/week.”
The Alternate Healthy Eating Index 2010 (AHEI-2010) (18), a score created based on foods and nutrients predictive of chronic disease risk (19), was used to reflect the overall diet quality of the participants. Cereal fiber intake and alcohol consumption were defined and calculated as described previously (20, 21). In summary, whole grain intake from breakfast cereal was derived from >250 brand-name cereals by using information provided by product labels and breakfast cereal manufacturers. Total alcohol intake was the sum of the values for beer, wine, and spirits. The glycemic index is a measure of the postprandial blood glucose response per gram of carbohydrate of a food as compared with a reference food such as white bread or glucose. The dietary glycemic load was calculated by multiplying the carbohydrate content of each food by its glycemic index and then multiplying this value by the frequency of consumption and summing these values for all foods. Hence, glycemic load represents both the quality and the quantity of the carbohydrate consumed.
Measurement of nondietary factors
Family history of T2D was assessed in 1982 and 1988. Lifestyle factors such as physical activity, BMI, and cigarette smoking were assessed biannually. Physical activity was expressed as hours per week and converted to metabolic equivalent hours per week by using a validated questionnaire (22). BMI was calculated as weight (kg) divided the square of height (m). Self-reported weights correlated highly with measured weights (r = 0.96) (23). Menopausal status and hormone use were assessed in 1976 and every 2 y thereafter. Women were classified as postmenopausal at the first report of natural menopause or surgery with bilateral oophorectomy.
Exclusion of participants at baseline
Because the question on eating frequency and breakfast consumption was first asked in 2002, we used 2002 as the baseline for the current analysis. Participants who did not complete the original 1980 FFQ, had implausible energy intakes (<500 or >3500 kcal/d), or did not answer the eating frequency or breakfast consumption questions in 2002 were excluded from the study. We also excluded participants with a diagnosis of diabetes, cardiovascular disease, or cancer (except for nonmelanoma skin cancer) by 2002. Thus, 46,289 women remained for follow-up from 2002 to 2008.
Ascertainment of T2D cases
The outcome was T2D incidence. Women reporting a diagnosis of T2D in the biennial follow-up questionnaire were sent a supplementary questionnaire to confirm the diagnosis. The American Diabetes Association criteria were used to confirm self-reported diagnosis of T2D (24). Cases of type 1 diabetes were excluded. In a validation study of the supplementary questionnaire for diabetes diagnosis, medical record review confirmed 98% (61 of 62) of self-reported T2D cases (25).
Statistical analysis
Participants contributed follow-up time from the date they returned their baseline questionnaire to the date of diagnosis of T2D, death, loss to follow-up, or end of the study period (30 June 2008), whichever came first. To examine associations between eating frequency or breakfast-consumption pattern and T2D risk, we estimated RRs and 95% CIs by using the Cox proportional hazards regression model with age (in mo) as the time scale, with calendar year as a stratification variable, and with time-varying covariates. We analyzed breakfast consumption as a categorical variable (7 times/wk; 0–6 times/wk) alone and in conjunction with the number of eating occasions (1–3 times/d; 4–9 times/d) in relation to T2D risk.
In the basic multivariate model 1, in addition to stratifying by age and time period, we adjusted for known and suspected risk factors of T2D, including family history of T2D in 1982 and 1988, alcohol intake, physical activity, menopausal status and hormone use, and smoking status. In multivariate model 2, we further adjusted for the dietary variables, including energy intake, cereal fiber intake (26), and AHEI-2010 (18), because these variables could mediate the breakfast-T2D association. In multivariate model 3, we further adjusted for BMI, which was updated every 2 y to assess whether the association between breakfast consumption and T2D risk is mediated via BMI. In additional multivariate models, we further adjusted for other potential confounders, such as coffee consumption (27) and glycemic load (28). In models in which breakfast consumption was the main exposure, we also adjusted for the number of eating occasions (1–2, 3, 4–5, or ≥6–9 times). Cumulative averages of dietary covariates were calculated at each time point, starting in 1984, to better represent long-term diet and to minimize within-person variation (16). Because our baseline was 2002, we used the cumulatively averaged dietary variables starting in 2002. The residual method was used to adjust AHEI-2010, intakes of cereal fiber, and glycemic load for total energy intake (29).We stopped updating diet when the participants first reported a chronic disease diagnosis (eg, cancer, cardiovascular disease, high blood pressure, and hypercholesterolemia), because these are risk factors for T2D and those with any of these conditions may change their diet. All other covariates were updated for each 2-y follow-up period. Values for smoking, physical activity, and dietary variables were carried forward from previous years if missing and were coded as “missing” if absent at baseline. BMI was used in categories, and the missing value (n = 1) was assigned to the median category. Tests for trend were calculated by including the median category of eating frequency as an ordinal variable.
To examine whether the association between eating occasions and the risk of T2D is mediated by the consumption of juice and soft drinks, we conducted a sensitivity analysis in which we further adjusted for total sweetened beverages.
To assess whether the relation between breakfast consumption and T2D changed across the different strata of the main T2D risk factors, we conducted interaction tests between breakfast-consumption pattern (7 times/wk or 0–6 times/wk) and known risk factors for T2D such as BMI (deciles), cereal fiber intake, glycemic load, AHEI-2010 (all quintiles), physical activity (<median or ≥median value), age group (<65 or ≥65 y), and working status (full-time: yes or no). Because it is difficult to define which meal constitutes breakfast among shift workers, and because the metabolic consequences could be different from those of nonshift workers, we stratified by working rotating shifts in the past (yes or no). A likelihood ratio test was used to compare the model including the cross-product terms [eg, breakfast consumption (binary) × median of deciles of BMI (continuous)] with a model including only main effects. A joint analysis was conducted for breakfast consumption and AHEI. SAS version 9.1 (SAS Institute) was used for all analyses, and a 2-sided P value <0.05 was considered statistically significant.
RESULTS
In this cohort of 46,289 women, we documented 1560 incident T2D cases during 6 y of follow-up (260,188 person-years). Most of the women (76%) consumed breakfast daily. Daily breakfast consumers were older, had a slightly lower BMI, smoked less, were more physically active, consumed more calories, consumed less alcohol, consumed more cereal fiber, drank less coffee, and had a healthier overall diet compared with women who consumed breakfast irregularly (0–6 times/wk) (all P < 0.01) (Table 1). Among the irregular breakfast consumers (n = 11,229), 4158 women (37%) consumed breakfast 0–2 times/wk, and 7071 women (63%) consumed breakfast 3–6 times/wk. Interestingly, women who consumed breakfast 0–2 times/wk smoked more, exercised less, drank more alcohol, consumed less cereal fiber, and had a poorer overall diet quality but had a slightly lower mean BMI (26.6) as compared with women who consumed breakfast 3–6 times/wk (mean BMI: 27.1) (data not shown).
TABLE 1.
Age-standardized characteristics of participants at baseline, by breakfast consumption among 46,289 US women from the Nurses’ Health Study1
| Irregular breakfast consumers (0–6 times/wk) (n = 11,229) | Regular breakfast consumers (7 times/wk) (n = 35,060) | |
| Age (y) | 64.7 ± 6.52 | 67.8 ± 7.0 |
| BMI (kg/m2) | 26.8 ± 5.3 | 26.2 ± 4.9 |
| Family history of diabetes, 1988 [n (%)] | 2583 (23) | 8064 (23) |
| Current postmenopausal hormone users [n (%)] | 4155 (37) | 15,076 (43) |
| Current smoking [n (%)] | 1797 (16) | 1753 (5) |
| Physical activity (MET-h3/wk) | 15.9 ± 21.0 | 19.0 ± 22.6 |
| Alcohol intake (g/d) | 6.8 ± 9.6 | 5.7 ± 8.1 |
| Energy intake (kcal/d) | 1649 ± 428 | 1736 ± 404 |
| Cereal fiber intake (g/d)4 | 4.2 ± 1.6 | 5.2 ± 1.8 |
| Coffee intake (servings/d)5 | 2.3 ± 1.5 | 2.1 ± 1.4 |
| Dietary glycemic load | 104 ± 33 | 115 ± 31 |
| Dietary glycemic index | 52.3 ± 2.9 | 52.6 ± 2.5 |
| Alternative Healthy Eating Index 20106 | 50.7 ± 8.8 | 52.7 ± 9.2 |
| Eating times (times/d) | 3.7 ± 1.3 | 4.2 ± 1.0 |
Variables were assessed at baseline, 2002 (simple update or cumulative average for dietary variables) unless otherwise indicated. Compared with regular breakfast consumers, women who skipped breakfast ≥1 time/wk had a slightly higher BMI, smoked more, exercised less, consumed more alcohol and less cereal fiber, and drank more coffee. They tended to have poorer diet quality as reflected by a lower Alternative Healthy Eating Index (P < 0.05 for all).
Mean ± SD (all such values).
MET-h, metabolic equivalent hours.
Energy-adjusted.
Includes decaffeinated coffee
This index is a new measure of diet quality that incorporates current scientific evidence on diet and health. In this analysis, higher indexes indicate better diet quality.
After adjustment for age, there was a 39% higher risk of T2D among women who consumed breakfast irregularly (≤6 times/wk) as compared with those who consumed breakfast 7 times/wk (Table 2). This direct association remained statistically significant after adjustment for standard and dietary risk factors for T2D (28% higher risk) and even after further adjustment for BMI (20% higher risk)—a potential mediator. On stratification by BMI (<25 or ≥25), the results were similar between overweight/obese women (multivariate RR: 1.24; 1.09, 1.40; BMI ≥25) and lean women (multivariate RR: 1.25; 95% CI: 0.91, 1.70; BMI <25). Even after further adjustment for BMI (as a continuous variable) to take care for any residual confounding, the results did not materially change. When we further categorized women who consumed breakfast ≤6 times/wk into 2 groups (0–2 times/wk or 3–6 times/wk), the direct association with T2D risk remained as high for the 2 groups, even after adjustment for BMI (multivariate RR: 1.20; 95% CI: 1.05, 1.37; multivariate RR: 1.21; 95% CI: 1.02, 1.44, respectively)—a potential mediator.
TABLE 2.
RRs of type 2 diabetes for 2 categories of breakfast consumption1
| Regular breakfast consumers: 7 times/wk (n = 1074) | Irregular breakfast consumers: 0–6 times/wk (n = 486) | P value | |
| Person-years | 197,294 | 62,894 | |
| Age RR (95% CI) | 1.00 (Reference) | 1.39 (1.25, 1.55) | <0.001 |
| Multivariate RR (95% CI)2 | 1.00 (Reference) | 1.34 (1.20, 1.50) | <0.001 |
| Multivariate RR (95% CI)3 | 1.00 (Reference) | 1.28 (1.14, 1.44) | <0.001 |
| Multivariate RR (95% CI) + BMI4 | 1.00 (Reference) | 1.20 (1.07, 1.35) | <0.01 |
RRs (95% CIs) were derived from Cox proportional hazards models.
Adjusted for age (in mo), family history of type 2 diabetes (yes or no), alcohol intake (0 to <5, 5 to <15, or ≥15 g/d), physical activity (1 to <3, 3 to <9, 9 to <18, 18 to <27, or ≥27 metabolic equivalent h/wk or missing category), menopausal status and hormone use (premenopausal, postmenopausal and never used hormones, postmenopausal and current hormone users, or postmenopausal and past hormone users), and smoking status (never, past, current 1–14 cigarettes/d, current 15–24 cigarettes/d, current ≥25 cigarettes/d, or missing category).
Adjusted as for the previous multivariate model plus energy intake (kcal/d, continuous), cereal fiber intake (quintiles, g/d), and the Alternative Healthy Eating Index 2010 (quintiles or missing category).
Adjusted as for the previous multivariate model plus BMI (in kg/m2; <21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–32.9, 33–34.9, 35–39.9, or ≥40); the percentages of observations assigned to missing categories were as follows (baseline/follow-up): 0.25%/0.35% for smoking, 6.4%/7.6% for physical activity, and 0.37%/0.35% for the Alternative Healthy Eating Index 2010.
No association was observed between eating frequency and risk of T2D (Table 3). For instance, compared with women who ate 3 times/d, women who ate 1–2 times/d had a multivariate RR (95% CI) of 1.09 (0.84, 1.41), whereas women who ate 4–5 times/d or ≥6 times/d had RRs of 1.13 (1.00, 1.27) and 0.99 (0.81, 1.21), respectively—all nonsignificant associations even before adjustment for BMI. The results did not materially change when we further adjusted for BMI or breakfast consumption or when we stratified by breakfast consumption. The mean BMI for women who ate 1–2 times/d (mean BMI: 25.7) and for women who ate 3 times/d (25.6) was lower than the BMI for women who ate 4–5 times/d (26.4) or who ate ≥6 times/d (26.6) (P < 0.05). Most of the women who ate ≥3 times/d consumed breakfast regularly (3 times/d: 72%; 4–5 times/d: 80.2%; ≥6 times/d: 82%) as compared with women who ate 1–2 times/d, as only 28% consumed breakfast regularly. When we classified participants according to their breakfast consumption combined with their eating frequency, the ideal eating pattern appeared to be daily breakfast consumption plus 1–3 eating occasions (Table 4). Women in this category had the lowest BMI (mean BMI: 25.3), whereas women who ate 4–9 times/d and consumed breakfast 0–6 times/wk (mean BMI: 27.4) had the highest BMI. Additional eating occasions did not seem to compensate for skipping breakfast. In fact, eating 4–9 times/d was associated with an increased T2D risk for those who consumed breakfast 0–6 times/wk (multivariate RR: 1.47; 95% CI: 1.23, 1.75). These associations were attenuated but remained significant after adjustment for BMI (multivariate RR: 1.24; 95% CI: 1.04, 1.48).
TABLE 3.
RRs of type 2 diabetes for eating frequency (meals + snacks)1
| Eating frequency |
|||||
| 1–2 times/d(n = 71) | 3 times/d(n = 366) | 4–5 times/d(n = 984) | ≥6 times/d(n = 139) | P-trend | |
| Person-years | 11,058 | 69,373 | 155,693 | 24,064 | |
| Age RR (95% CI) | 1.21 (0.94, 1.56) | 1.00 (Reference) | 1.18 (1.04, 1.33) | 1.07 (0.88, 1.30) | 0.76 |
| Multivariate RR (95% CI)2 | 1.12 (0.87, 1.45) | 1.00 (Reference) | 1.12 (0.99, 1.27) | 0.99 (0.81, 1.21) | 0.83 |
| Multivariate RR (95% CI)3 | 1.09 (0.84, 1.41) | 1.00 (Reference) | 1.13 (1.00, 1.27) | 0.99 (0.81, 1.21) | 0.89 |
| Multivariate RR (95% CI) + BMI4 | 1.13 (0.87, 1.46) | 1.00 (Reference) | 1.04 (0.92, 1.17) | 0.89 (0.73, 1.08) | 0.15 |
RRs (95% CIs) were derived from Cox proportional hazards models.
Adjusted for age (in mo), family history of type 2 diabetes (yes or no), alcohol intake (0 to <5, 5 to <15, or ≥15 g/d), physical activity (1 to <3, 3 to <9, 9 to <18, 18 to <27, or ≥27 metabolic equivalent h/wk or missing category), menopausal status and hormone use (premenopausal, postmenopausal and never used hormones, postmenopausal and current hormone users, or postmenopausal and past hormone users), and smoking status (never, past, current 1–14 cigarettes/d, current 15–24 cigarettes/d, current ≥25 cigarettes/d, or missing category).
Adjusted as for the previous multivariate model plus energy intake (kcal/d, continuous), cereal fiber intake (quintiles, g/d), and the Alternative Healthy Eating Index 2010 (quintiles or missing category).
Adjusted as for the previous multivariate model plus BMI (in kg/m2; <21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–32.9, 33–34.9, 35–39.9, or ≥40).
TABLE 4.
RRs of type 2 diabetes for combinations of breakfast and eating frequency1
| Regular breakfast consumers: 7 times/wk |
Irregular breakfast consumers: 0-6 times/wk |
|||
| Eating frequency: 1–3 times/d (n = 248) | Eating frequency: 4–9 times/d (n = 826) | Eating frequency: 1–3 times/d (n = 189) | Eating frequency: 4–9 times/d (n = 297) | |
| Person-years | 52,727 | 144,567 | 27,705 | 35,189 |
| Age RR (95% CI) | 1.00 (Reference) | 1.19 (1.03, 1.37) | 1.41 (1.16, 1.71) | 1.74 (1.46, 2.06) |
| Multivariate RR (95% CI)2 | 1.00 (Reference) | 1.13 (0.98, 1.30) | 1.33 (1.10, 1.62) | 1.56 (1.31, 1.86) |
| Multivariate RR (95% CI)3 | 1.00 (Reference) | 1.11 (0.96, 1.29) | 1.26 (1.04, 1.54) | 1.47 (1.23, 1.75) |
| Multivariate RR (95% CI) + BMI4 | 1.00 (Reference) | 1.03 (0.89, 1.19) | 1.21 (0.99, 1.47) | 1.24 (1.04, 1.48) |
RRs (95% CIs) were derived from Cox proportional hazards models. P-interaction between breakfast consumption and eating frequency was not significant (P = 0.97).
Adjusted for age (in mo), family history of type 2 diabetes (yes or no), alcohol intake (0 to <5, 5 to <15, or ≥15 g/d), physical activity (1 to <3, 3 to <9, 9 to <18, 18 to <27, or ≥27 metabolic equivalent h/wk or missing category), menopausal status and hormone use (premenopausal, postmenopausal and never used hormones, postmenopausal and current hormone users, or postmenopausal and past hormone users), and smoking status (never, past, current 1–14 cigarettes/d, current 15–24 cigarettes/d, current ≥25 cigarettes/d, or missing category).
Adjusted as for the previous multivariate model plus energy intake (kcal/d, continuous), cereal fiber intake (quintiles, g/d), and the Alternative Healthy Eating Index 2010 (quintiles or missing category).
Adjusted as for the previous multivariate model plus BMI (in kg/m2; <21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–32.9, 33–34.9, 35–39.9, or ≥40).
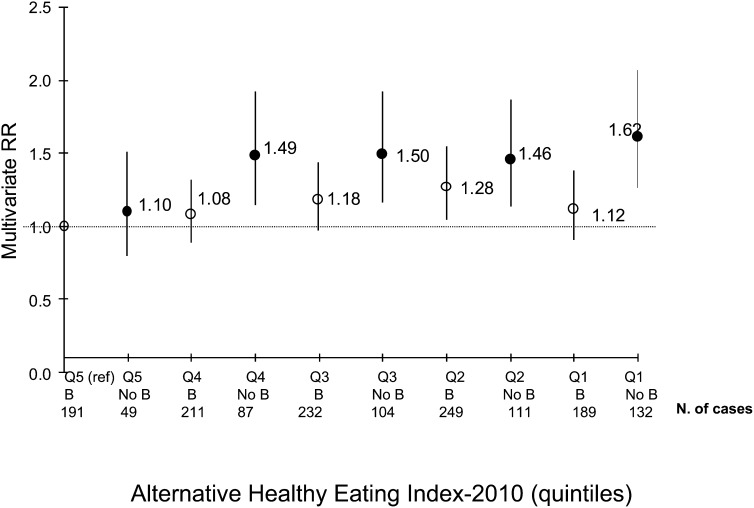
We also examined interactions with potential effect modifiers. No significant interaction was found between breakfast consumption and BMI (P-interaction = 0.83), working rotating shift (P-interaction = 0.42), cereal fiber intake (P-interaction = 0.74), glycemic load (P-interaction = 0.47), or AHEI-2010 (P-interaction = 0.51). The only significant interactions were between breakfast consumption and each of age (<65 or ≥65 y; P-interaction < 0.01) and working status (part-time or full-time; P-interaction = 0.02); both remained significant even after BMI was added to the model (data not shown). In our sample, 69% of women were ≥65 y of age, of whom 5% worked full time, and 31% were <65 y of age, of whom 38% worked full time. Among women <65 y of age, those who did not consume breakfast daily had a 50% higher risk of T2D than did women who did (multivariate RR: 1.50; 95% CI: 1.24, 1.81); however, this association was not significant among older women (multivariate RR: 1.14; 95% CI: 0.98, 1.33). The addition of BMI to the model did not alter the significance and strength of these associations (data not shown). Moreover, among women who worked full time, those who did not consume breakfast regularly had a 54% higher risk of T2D (multivariate RR: 1.54; 95% CI: 1.19, 1.99) compared with women who ate breakfast daily. This association was weaker among women who did not work full time (multivariate RR for the same comparison: 1.20; 95% CI: 1.05, 1.37) and lost significance when we added BMI to the model (multivariate RR: 1.12; 95% CI: 0.98, 1.28). Although the interaction between breakfast consumption and AHEI-2010 was not significant, the highest risk of T2D was seen for the combination of not eating breakfast regularly and having the lowest AHEI (Figure 1). This value also reflects that eating breakfast and having a lower-quality diet was associated with a T2D risk similar to that of skipping breakfast and having a good-quality diet. In all of the analyses mentioned, further adjustment for intakes of coffee, eating times, glycemic load, and total sweetened beverages did not appreciably alter the RRs.
FIGURE 1.
Breakfast consumption and Alternative Healthy Eating Index 2010 in relation to risk of type 2 diabetes in the B and No B groups. Values are RRs derived from Cox proportional hazards models (P-interaction = 0.51). All multivariate models were adjusted for age (in mo), family history of type 2 diabetes (yes or no), alcohol intake (0 to <5, 5 to <15, or ≥15 g/d), physical activity (1 to <3, 3 to <9, 9 to <18, 18 to <27, or ≥27 metabolic equivalent h/wk or missing category), menopausal status and hormone use (premenopausal, postmenopausal and never used hormones, postmenopausal and current hormone users, or postmenopausal and past hormone users), smoking status (never, past, current 1–14 cigarettes/d, current 15–24 cigarettes/d, current ≥25 cigarettes/d, or missing category), energy intake (kcal/d, continuous), cereal fiber intake (quintiles, g/d), and Alternative Healthy Eating Index 2010 (quintiles or missing category). B, regular breakfast consumers (7 times/wk); No B, irregular breakfast consumers (0–6 times/wk).
DISCUSSION
In this large prospective cohort study, regular breakfast consumption was associated with a lower T2D risk in women. An increased eating frequency (>3 times/d) did not appear to attenuate the higher T2D risk associated with irregular breakfast consumption. Contrarily, for irregular breakfast consumption pattern, increased eating frequency was associated with a higher T2D risk; these associations were partially mediated by BMI.
Studies of breakfast consumption and chronic disease risk have yielded inconsistent results (5).Whereas several observational studies have shown an inverse association between breakfast consumption and BMI (30, 31), the limited evidence from randomized controlled trials does not support an effect of breakfast consumption on weight loss (32, 33). In one trial in 52 moderately obese women (32), no difference in weight loss was found between the groups with and without breakfast. In another randomized trial in 10 healthy lean women (33), no difference in body weight was found between the 2 groups (eating compared with skipping breakfast) during 14 d. In a randomized crossover trial, men who regularly consumed breakfast had better metabolic and endocrine responses in response to foods consumed later during the day than did those who skipped breakfast (34). Our findings on breakfast consumption and T2D risk in women are consistent with what was found in a cohort of US men (6).
In a pilot study conducted in healthy overweight adults (20–40 y of age) and in children (9–13 y of age), both breakfast frequency (daily consumption compared with plain water) and quality (breakfast meals with a low glycemic index) were independently associated with appetite control and glycemic control (35). Other cohort studies found that consumption of whole-grain, but not refined-grain, cereal is associated with a lower BMI and insulin concentrations (36, 37). In our previous analysis in men (6), diet quality as reflected by prudent dietary patterns, glycemic load, or cereal fiber intake did not modify the inverse association between breakfast consumption and T2D. In the current study, AHEI-2010, glycemic load, and cereal fiber intake did not modify that association either. These results suggest that breakfast consumption itself confers independent metabolic effects above and beyond the role of dietary quality, particularly because women who consumed breakfast regularly and had the worst AHEI-2010 score did not have a significantly higher risk of T2D. Nonetheless, our results suggest that the combination of poor diet quality (lowest quintile of AHEI-2010 score) with a poor meal pattern (irregular breakfast consumption) is particularly detrimental.
We did not observe an association between eating frequency and T2D risk. Furthermore, when we combined breakfast consumption and eating frequency into one variable, increasing eating occasions did not counteract the higher T2D risk emanating from irregular breakfast consumption, nor did it add any further benefit when accompanied with regular breakfast consumption. In fact, there was an even higher T2D risk associated with higher eating frequency and irregular breakfast consumption, which suggested that eating earlier in the day than later could be beneficial for T2D risk. In contrast, increased meal frequency was previously shown to be associated with a reduction in serum lipid and insulin concentrations in healthy subjects (12) and in subjects with T2D (38); however, such metabolic advantages occur only when total energy intake is held constant—a phenomenon highly unlikely to occur in free-living individuals. Even though we adjusted for total calorie intake in our analyses, this is still a crude adjustment different from that of a controlled feeding study in which total calorie intake could be predetermined. Moreover, none of the abovementioned studies (12, 38) examined the effect of omitting breakfast per se when comparing the high and the low meal frequency patterns. Hence, despite the observed metabolic advantages associated with higher eating frequency (12, 38), additional advice should be made on consuming breakfast regularly without increasing daily calorie intakes beyond those needed.
Interestingly, the direct association between irregular breakfast consumption and T2D risk was observed only among younger women (<65 y of age). A potential interpretation is that other age-related diseases could overshadow the potentially higher T2D risk associated with eating habits. Furthermore, women who worked full time and did not consume breakfast regularly were at higher risk of T2D than were women who worked part time and did not consume breakfast regularly. This finding could have been a result of the work-related stress and its association with elevated concentrations of glycated hemoglobin (39) and the metabolic syndrome (40) among persons without diabetes. We found no significant interaction between breakfast consumption and shift work, but our study may have been underpowered to examine this interaction. However, there is strong evidence of a direct association between night shift work and T2D (41), partially explained by a higher BMI and disturbed circadian rhythms (42). Our findings suggest that night shift work and skipping breakfast independently affect risk of T2D.
Breakfast is the first meal in the morning that breaks the fast. Prolonged periods of fasting such as omitting breakfast, even at a single occasion, can increase postprandial insulin resistance and hyperinsulinemia in response to foods consumed at the next meal (33, 34). In addition, skipping breakfast has been associated with an increased risk of obesity (43). Nevertheless, the positive association between skipping breakfast and T2D observed in our cohort remained elevated, although attenuated, after adjustment for BMI, which suggests that the association is not completely mediated via weight control.
The current study had several limitations. First, nondifferential measurement error in our assessment of breakfast consumption and eating frequency may have biased our results toward the null (44). Breakfast consumption was self-reported and subject to a subjective interpretation of what constitutes a breakfast; however, our question specified that drinking coffee or tea alone was not considered to be breakfast. Also, repeated assessment of the meal frequency over a 6-y period would have reduced random within-person error (16). Second, there was no information of the nutrient composition of the breakfast consumed. However, the AHEI-2010 was used to reflect overall dietary quality. Third, the question on eating frequency has not been validated and did not differentiate meals from snacks; however, our question clarified that beverages consumed without food such as juice and nondiet soda, but not coffee and diet soda, should be included in the eating frequency assessment. Fourth, it is well known that total energy intake is substantially underreported by FFQs; thus, our adjustment for energy intake is likely to be incomplete. Finally, residual confounding is a concern in observational studies because breakfast eaters tend to have a healthy lifestyle and diet, although we controlled for known and suspected risk factors of T2D.
Despite these limitations, our study had several strengths. To our knowledge, this was the first large prospective study to assess the relation between breakfast consumption and T2D risk among women. In addition, we examined combinations of eating patterns (eg, regular breakfast consumption and eating occasions) in relation to T2D risk. Finally, we controlled for a wide variety of potential confounders in our models. In conclusion, our findings suggest that regular breakfast consumption may decrease T2D risk in women. Further studies are needed to assess specific breakfast foods and the change in breakfast consumption patterns in relation to T2D risk.
Acknowledgments
The authors’ responsibilities were as follows—RAM and FBH: collected data and conceived of the design; RAM, FBH, RMvD, LC, WCW, and EG: provided statistical expertise; and RAM: analyzed the data and wrote the first draft of the manuscript. All authors contributed to the interpretation of the results and to the critical revision of the manuscript for important intellectual content and approved the final version of the manuscript. None of the authors declared a conflict of interest. The funding sources were not involved in the data collection, data analysis, or writing and publication of the manuscript.
Footnotes
Abbreviations used: AHEI, Alternate Healthy Eating Index; FFQ, food-frequency questionnaire; T2D, type 2 diabetes.
REFERENCES
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4–14. [DOI] [PubMed] [Google Scholar]
- 2.Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care 2003;26:917–32. [DOI] [PubMed] [Google Scholar]
- 3.Rampersaud GC, Pereira MA, Girard BL, Adams J, Metzl JD. Breakfast habits, nutritional status, body weight, and academic performance in children and adolescents. J Am Diet Assoc 2005;105:743–60, quiz 761–2. [DOI] [PubMed] [Google Scholar]
- 4.Haines PS, Guilkey DK, Popkin BM. Trends in breakfast consumption of US adults between 1965 and 1991. J Am Diet Assoc 1996;96:464–70. [DOI] [PubMed] [Google Scholar]
- 5.Timlin MT, Pereira MA. Breakfast frequency and quality in the etiology of adult obesity and chronic diseases. Nutr Rev 2007;65:268–81. [DOI] [PubMed] [Google Scholar]
- 6.Mekary RA, Giovannucci E, Willett WC, van Dam RM, Hu FB. Eating patterns and type 2 diabetes risk in men: breakfast omission, eating frequency, and snacking. Am J Clin Nutr 2012;95:1182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCrory MA, Campbell WW. Effects of eating frequency, snacking, and breakfast skipping on energy regulation: symposium overview. J Nutr 2011;141:144–7. [DOI] [PubMed] [Google Scholar]
- 8.Deshmukh-Taskar P, Nicklas TA, Radcliffe JD, O'Neil CE, Liu Y. The relationship of breakfast skipping and type of breakfast consumed with overweight/obesity, abdominal obesity, other cardiometabolic risk factors and the metabolic syndrome in young adults. The National Health and Nutrition Examination Survey (NHANES): 1999-2006. Public Health Nutr 2012;Oct 3 (Epub ahead of print; DOI:10.1017/S1368980012004296). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deshmukh-Taskar PR, Radcliffe JD, Liu Y, Nicklas TA. Do breakfast skipping and breakfast type affect energy intake, nutrient intake, nutrient adequacy, and diet quality in young adults? NHANES 1999-2002. J Am Coll Nutr 2010;29:407–18. [DOI] [PubMed] [Google Scholar]
- 10.Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci USA 2003;100:6216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang ZQ, Bell-Farrow AD, Sonntag W, Cefalu WT. Effect of age and caloric restriction on insulin receptor binding and glucose transporter levels in aging rats. Exp Gerontol 1997;32:671–84. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins DJ, Wolever TM, Vuksan V, Brighenti F, Cunnane SC, Rao AV, Jenkins AL, Buckley G, Patten R, Singer W, et al. Nibbling versus gorging: metabolic advantages of increased meal frequency. N Engl J Med 1989;321:929–34. [DOI] [PubMed] [Google Scholar]
- 13.Arnold L, Mann JI, Ball MJ. Metabolic effects of alterations in meal frequency in type 2 diabetes. Diabetes Care 1997;20:1651–4. [DOI] [PubMed] [Google Scholar]
- 14.Thomsen C, Christiansen C, Rasmussen OW, Hermansen K. Comparison of the effects of two weeks’ intervention with different meal frequencies on glucose metabolism, insulin sensitivity and lipid levels in non-insulin-dependent diabetic patients. Ann Nutr Metab 1997;41:173–80. [DOI] [PubMed] [Google Scholar]
- 15.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 16.Willett WC. Nutritional epidemiology. New York, NY: Oxford University Press, 1998. [Google Scholar]
- 17.US Department of Agriculture. Composition of foods—raw, processed, and prepared, 1963-1992. Agricultural handbook no. 8 series. Washington, DC: US Department of Agriculture, Government Printing Office, 1993. [Google Scholar]
- 18.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Hunter DJ, Colditz GA, Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr 2002;76:1261–71. [DOI] [PubMed] [Google Scholar]
- 20.de Munter JS, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med 2007;4:e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuchs CS, Stampfer MJ, Colditz GA, Giovannucci EL, Manson JE, Kawachi I, Hunter DJ, Hankinson SE, Hennekens CH, Rosner B, et al. Alcohol consumption and mortality among women. N Engl J Med 1995;332:1245–50. [DOI] [PubMed] [Google Scholar]
- 22.Wolf A, Hunter D, Colditz GA, Manson JE, Stampfer MJ, Corsano K, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994;23:991–9. [DOI] [PubMed] [Google Scholar]
- 23.Willett WC, Hennekens CH, Bain C, Rosner B, Speizer FE. Cigarette smoking and non-fatal myocardial infarction in women. Am J Epidemiol 1981;113:575–82. [DOI] [PubMed] [Google Scholar]
- 24.Hagberg LA, Lindholm L. Cost-effectiveness of healthcare-based interventions aimed at improving physical activity. Scand J Public Health 2006;34:641–53. [DOI] [PubMed] [Google Scholar]
- 25.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med 2001;161:1542–8. [DOI] [PubMed] [Google Scholar]
- 26.Salmerón J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA 1997;277:472–7. [DOI] [PubMed] [Google Scholar]
- 27.Salazar-Martinez E, Willett WC, Ascherio A, Manson JE, Leitzmann MF, Stampfer MJ, Hu FB. Coffee consumption and risk for type 2 diabetes mellitus. Ann Intern Med 2004;140:1–8. [DOI] [PubMed] [Google Scholar]
- 28.Mekary RA, Rimm EB, Giovannucci E, Stampfer MJ, Willett WC, Ludwig DS, Hu FB. Joint association of glycemic load and alcohol intake with type 2 diabetes incidence in women. Am J Clin Nutr 2011;94:1525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65(suppl):1220S–8S. [DOI] [PubMed] [Google Scholar]
- 30.Song WO, Chun OK, Obayashi S, Cho S, Chung CE. Is consumption of breakfast associated with body mass index in US adults? J Am Diet Assoc 2005;105:1373–82. [DOI] [PubMed] [Google Scholar]
- 31.Ma Y, Bertone ER, Stanek EJ, 3rd, Reed GW, Hebert JR, Cohen NL, Merriam PA, Ockene IS. Association between eating patterns and obesity in a free-living US adult population. Am J Epidemiol 2003;158:85–92. [DOI] [PubMed] [Google Scholar]
- 32.Schlundt DG, Hill JO, Sbrocco T, Pope-Cordle J, Sharp T. The role of breakfast in the treatment of obesity: a randomized clinical trial. Am J Clin Nutr 1992;55:645–51. [DOI] [PubMed] [Google Scholar]
- 33.Farshchi HR, Taylor MA, Macdonald IA. Deleterious effects of omitting breakfast on insulin sensitivity and fasting lipid profiles in healthy lean women. Am J Clin Nutr 2005;81:388–96. [DOI] [PubMed] [Google Scholar]
- 34.Astbury NM, Taylor MA, Macdonald IA. Breakfast consumption affects appetite, energy intake, and the metabolic and endocrine responses to foods consumed later in the day in male habitual breakfast eaters. J Nutr 2011;141:1381–9. [DOI] [PubMed] [Google Scholar]
- 35.Pereira MA, Erickson E, McKee P, Schrankler K, Raatz SK, Lytle LA, Pellegrini AD. Breakfast frequency and quality may affect glycemia and appetite in adults and children. J Nutr 2011;141:163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKeown NM, Meigs JB, Liu S, Wilson PW, Jacques PF. Whole-grain intake is favorably associated with metabolic risk factors for type 2 diabetes and cardiovascular disease in the Framingham Offspring Study. Am J Clin Nutr 2002;76:390–8. [DOI] [PubMed] [Google Scholar]
- 37.Liese AD, Roach AK, Sparks KC, Marquart L, D'Agostino RB, Jr, Mayer-Davis EJ. Whole-grain intake and insulin sensitivity: the Insulin Resistance Atherosclerosis Study. Am J Clin Nutr 2003;78:965–71. [DOI] [PubMed] [Google Scholar]
- 38.Bertelsen J, Christiansen C, Thomsen C, Poulsen PL, Vestergaard S, Steinov A, Rasmussen LH, Rasmussen O, Hermansen K. Effect of meal frequency on blood glucose, insulin, and free fatty acids in NIDDM subjects. Diabetes Care 1993;16:4–7. [DOI] [PubMed] [Google Scholar]
- 39.Kawakami N, Araki S, Hayashi T, Masumoto T. Relationship between perceived job-stress and glycosylated hemoglobin in white-collar workers. Ind Health 1989;27:149–54. [DOI] [PubMed] [Google Scholar]
- 40.Chandola T, Brunner E, Marmot M. Chronic stress at work and the metabolic syndrome: prospective study. BMJ 2006;332:521–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med 2011;8:e1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 2009;106:4453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Timlin MT, Pereira MA, Story M, Neumark-Sztainer D. Breakfast eating and weight change in a 5-year prospective analysis of adolescents: Project EAT (Eating Among Teens). Pediatrics 2008;121:e638–45. [DOI] [PubMed] [Google Scholar]
- 44.Barron BA. The effects of misclassification on the estimation of relative risk. Biometrics 1977;33:414–8. [PubMed] [Google Scholar]