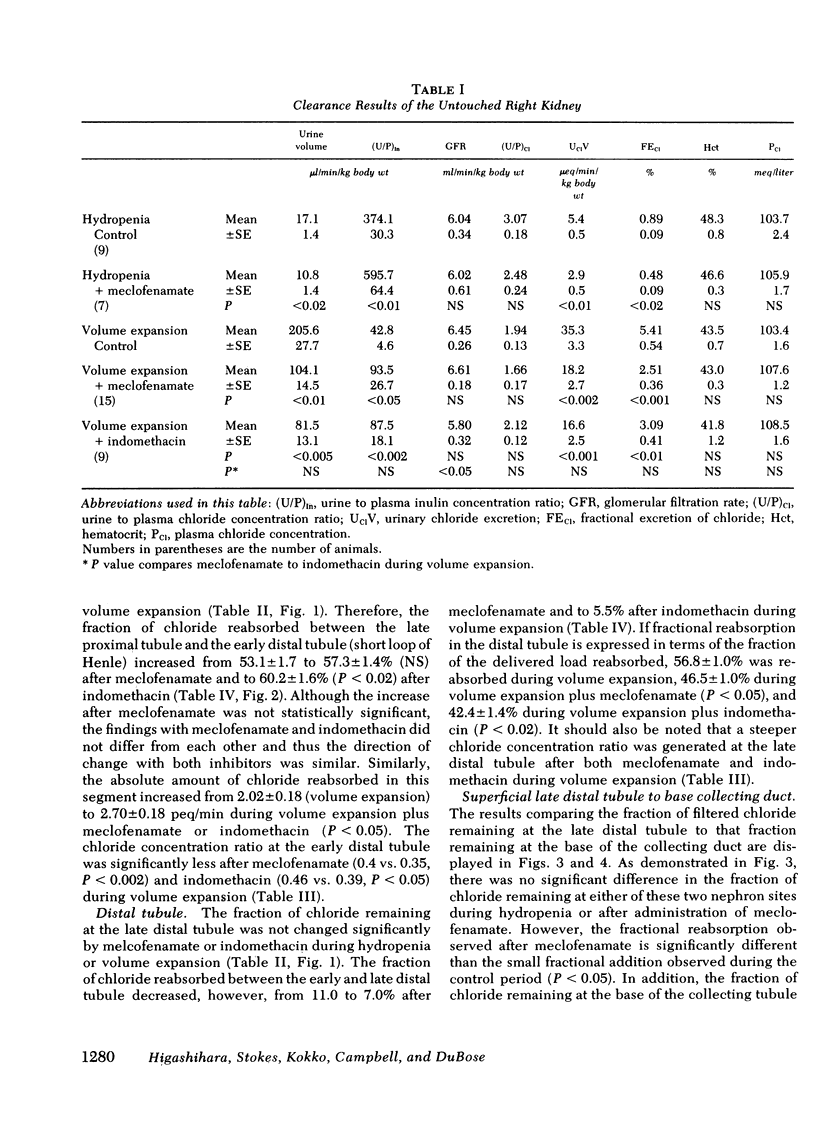
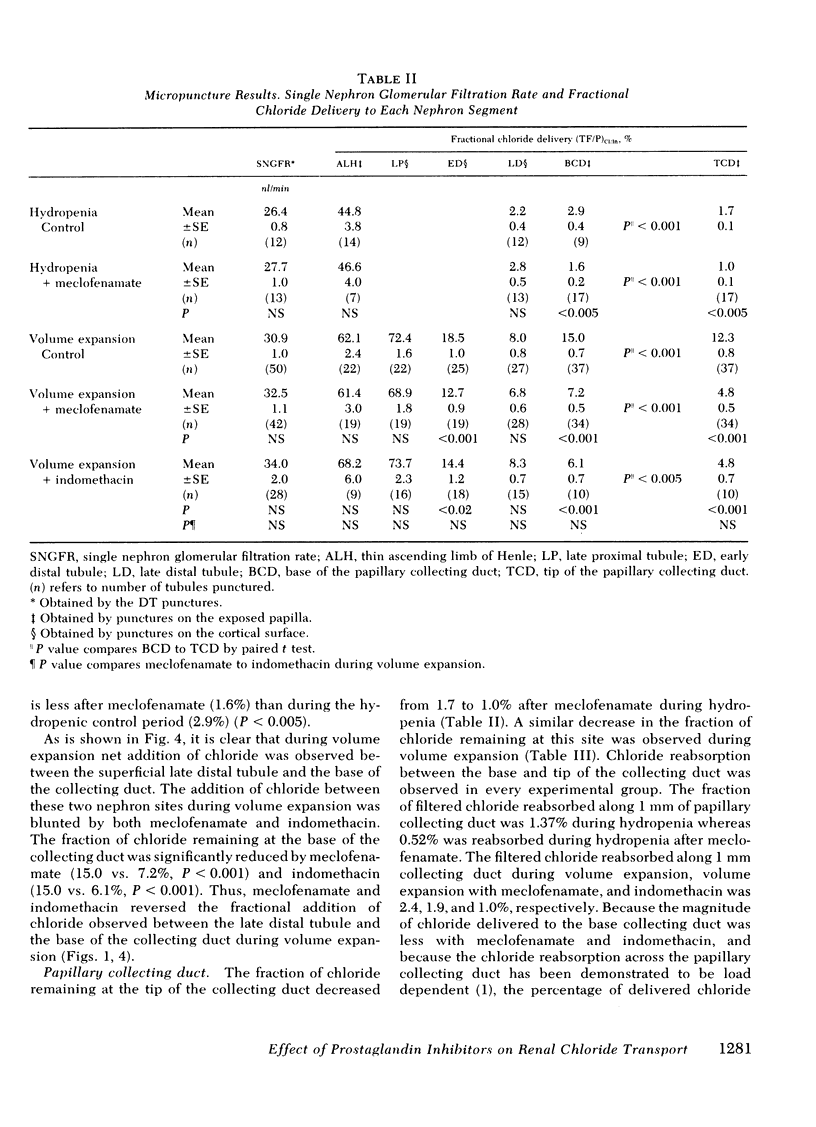
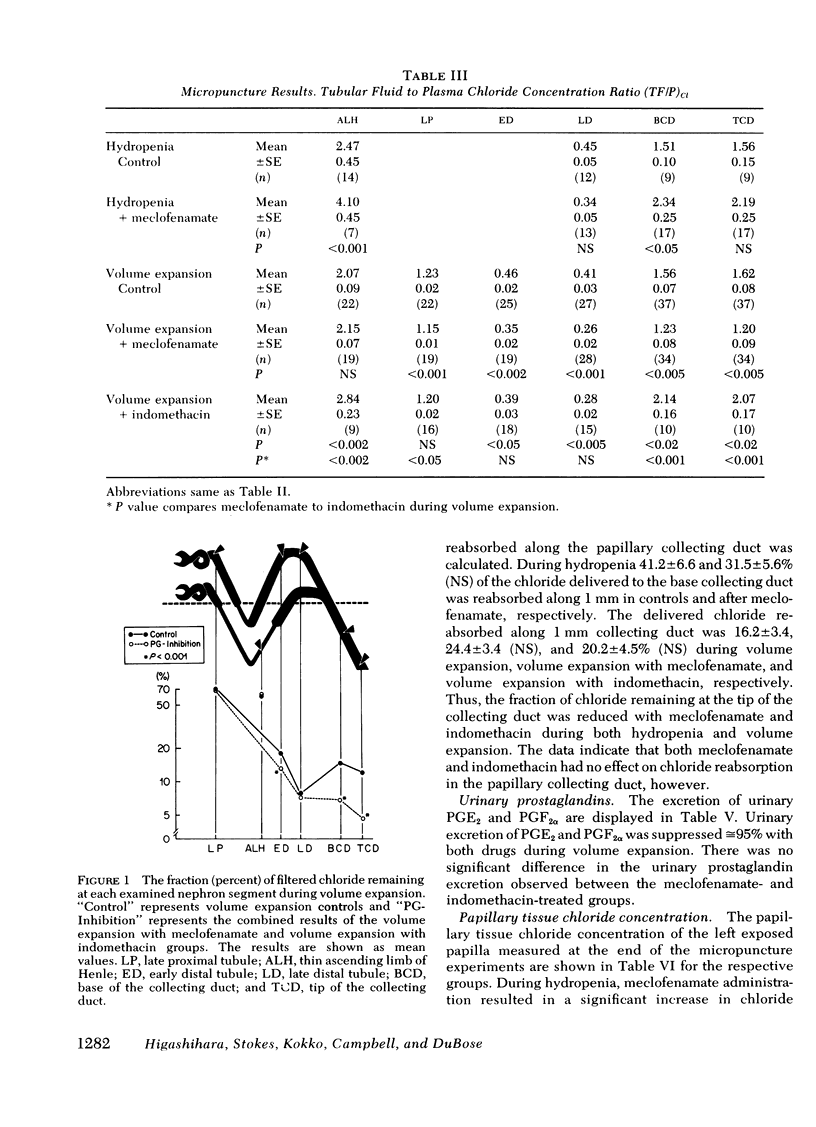
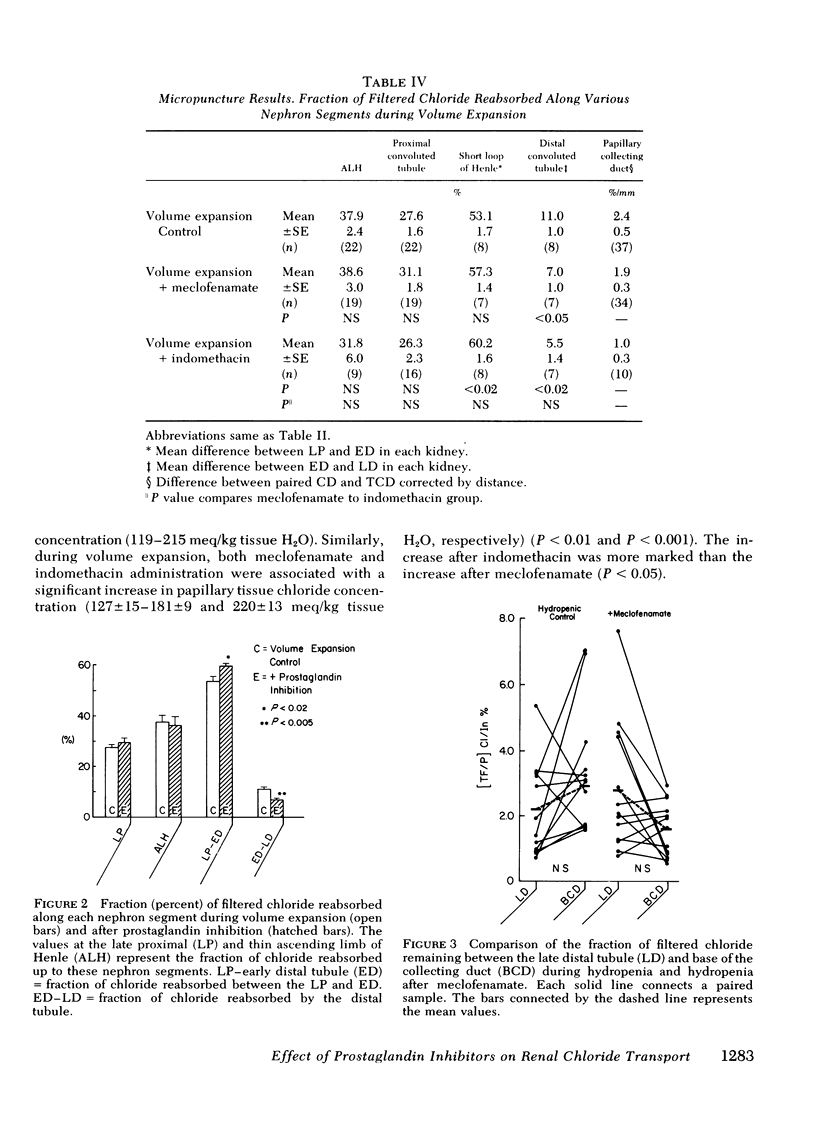
Abstract
Prostaglandins have been postulated to participate in the regulation of salt excretion during acute volume expansion. The present papillary and cortical micropuncture studies were designed to examine the effect of prostaglandin synthesis inhibitors on segmental chloride transport during hydropenia (with and without meclofenamate) and 10% volume expansion (with and without both meclofenamate and indomethacin). Both inhibitors significantly decreased the urinary excretion rate of prostaglandins E2 and F2α. Clearance studies on the intact right kidney demonstrated no effect of either agent on glomerular filtration rate, but a significant reduction in chloride excretion during hydropenia and volume expansion was observed. To assess the specific site(s) of enhanced chloride reabsorption, absolute and fractional chloride delivery was measured in the late proximal tubule, thin descending limb of Henle, and the early and late distal tubules. In addition, the fraction of filtered chloride remaining at the base and tip of the papillary collecting duct was compared to that fraction remaining at the superficial late distal tubule. During hydropenia, meclofenamate had no effect on fractional chloride delivery out of the superficial late distal tubule or the juxtamedullary thin descending limb of Henle, but significantly reduced the fraction of chloride delivered to the base of the papillary collecting duct. During volume expansion, neither meclofenamate nor indomethacin had an effect on absolute chloride delivery out of the proximal tubule or the thin descending limb of Henle. However, absolute chloride delivery to the early distal tubule was significantly reduced, and was associated with a decrease in fractional chloride reabsorption in this segment. Furthermore, the fraction of chloride delivered to the base of the collecting duct was significantly reduced. Fractional reabsorption along the terminal 1 mm of the collecting duct was not altered by either meclofenamate or indomethacin. These results suggest that inhibitors of prostaglandin synthesis result in an increase in chloride reabsorption in the superficial loop of Henle, and in segments between the superficial late distal tubule and the base of the collecting duct. The results are consistent with the view that prostaglandins inhibit chloride transport in the thick ascending limb of Henle, and/or the cortical and outer medullary collecting tubule.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chuang E. L., Reineck H. J., Osgood R. W., Kunau R. T., Jr, Stein J. H. Studies on the mechanism of reduced urinary osmolality after exposure of renal papilla. J Clin Invest. 1978 Mar;61(3):633–639. doi: 10.1172/JCI108974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowshaw K., Szlyk J. Z. Distribution of prostaglandins in rabbit kidney. Biochem J. 1970 Feb;116(3):421–424. doi: 10.1042/bj1160421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray F., Charbonnel B., Maclouf J. Radioimmunoassay of prostaglandins Falpha, E1 and E2 in human plasma. Eur J Clin Invest. 1975 Jul 29;5(4):311–318. doi: 10.1111/j.1365-2362.1975.tb00459.x. [DOI] [PubMed] [Google Scholar]
- DuBose T. D., Jr, Seldin D. W., Kokko J. P. Segmental chloride reabsorption in the rat nephron as a function of load. Am J Physiol. 1978 Feb;234(2):F97–105. doi: 10.1152/ajprenal.1978.234.2.F97. [DOI] [PubMed] [Google Scholar]
- Düsing R., Opitz W. D., Kramer H. J. The role of prostaglandins in the natriuresis of acutely salt-loaded rats. Nephron. 1977;18(4):212–219. doi: 10.1159/000180831. [DOI] [PubMed] [Google Scholar]
- Frölich J. C., Hollifield J. W., Dormois J. C., Frölich B. L., Seyberth H., Michelakis A. M., Oates J. A. Suppression of plasma renin activity by indomethacin in man. Circ Res. 1976 Sep;39(3):447–452. doi: 10.1161/01.res.39.3.447. [DOI] [PubMed] [Google Scholar]
- Fülgraff G., Meiforth A. Effects of prostaglandin E 2 on excretion and reabsorption of sodium and fluid in rat kidneys (micropuncture studies). Pflugers Arch. 1971;330(3):243–256. doi: 10.1007/BF00588615. [DOI] [PubMed] [Google Scholar]
- Ganguli M., Tobian L., Azar S., O'Donnell M. Evidence that prostaglandin synthesis inhibitors increase the concentration of sodium and chloride in rat renal medulla. Circ Res. 1977 May;40(5 Suppl 1):I135–I139. [PubMed] [Google Scholar]
- Higashihara E., DuBose T. D., Jr, Kokko J. P. Direct examination of chloride transport across papillary collecting duct of the rat. Am J Physiol. 1978 Sep;235(3):F219–F226. doi: 10.1152/ajprenal.1978.235.3.F219. [DOI] [PubMed] [Google Scholar]
- Iino Y., Imai M. Effects of prostaglandins on Na transport in isolated collecting tubules. Pflugers Arch. 1978 Feb 22;373(2):125–132. doi: 10.1007/BF00584850. [DOI] [PubMed] [Google Scholar]
- Kokko J. P. Sodium chloride and water transport in the descending limb of Henle. J Clin Invest. 1970 Oct;49(10):1838–1846. doi: 10.1172/JCI106401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyssac P. P., Christensen P., Hill R., Skinner S. L. Indomethacin blockade of renal PGE-synthesis: effect on total renal and tubular function and plasma renin concentration in hydropenic rats and on their response to isotonic saline. Acta Physiol Scand. 1975 Aug;94(4):484–496. doi: 10.1111/j.1748-1716.1975.tb05908.x. [DOI] [PubMed] [Google Scholar]
- Roman R. J., Kauker M. L. Renal effect of prostaglandin synthetase inhibition in rats: micropuncture studies. Am J Physiol. 1978 Aug;235(2):F111–F118. doi: 10.1152/ajprenal.1978.235.2.F111. [DOI] [PubMed] [Google Scholar]
- Stein J. H., Osgood R. W., Kunau R. T., Jr Direct measurement of papillary collecting duct sodium transport in the rat. Evidence for heterogeneity of nephron function during Ringer loading. J Clin Invest. 1976 Oct;58(4):767–773. doi: 10.1172/JCI108527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes J. B. Effect of prostaglandin E2 on chloride transport across the rabbit thick ascending limb of Henle. Selective inhibitions of the medullary portion. J Clin Invest. 1979 Aug;64(2):495–502. doi: 10.1172/JCI109487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes J. B., Kokko J. P. Inhibition of sodium transport by prostaglandin E2 across the isolated, perfused rabbit collecting tubule. J Clin Invest. 1977 Jun;59(6):1099–1104. doi: 10.1172/JCI108733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandhoy J. W., Ott C. E., Schneider E. G., Willis L. R., Beck N. P., Davis B. B., Knox F. G. Effects of prostaglandins E1 and E2 on renal sodium reabsorption and Starling forces. Am J Physiol. 1974 May;226(5):1015–1021. doi: 10.1152/ajplegacy.1974.226.5.1015. [DOI] [PubMed] [Google Scholar]
- Susic D., Sparks J. C. Effects of aspirin on renal sodium excretion, blood pressure, and plasma and extracellular fluid volume in salt-loaded rats. Prostaglandins. 1975 Nov;10(5):825–831. doi: 10.1016/0090-6980(75)90011-8. [DOI] [PubMed] [Google Scholar]
- Wright F. S. Increasing magnitude of electrical potential along the renal distal tubule. Am J Physiol. 1971 Mar;220(3):624–638. doi: 10.1152/ajplegacy.1971.220.3.624. [DOI] [PubMed] [Google Scholar]


