Abstract
Schistosomiasis, a high volume neglected tropical disease affecting more than 200 million people worldwide, can only be effectively treated by the tetrahydroisoquinoline drug praziquantel (PZQ). Herein, we describe an efficient approach to access PZQ derivatives by the Ugi 4-component reaction followed by the Pictet-Spengler reaction in a two-step, one-pot procedure. 30 Novel PZQ derivatives are described based on the Ugi 4-component reaction and an X-ray structure of a novel derivative revealing different conformation compared with PZQ is discussed. Several analogues comparable in activity to the drug PZQ have been identified based on an in vitro Schistosoma mansoni worm viability assay.
Keywords: multicomponent reaction, Ugi, Pictet-Spengler, isocyanide, schistosomiasis, neglected tropical disease
Schistosomiasis is a neglected tropical disease affecting more than 200 million people with more than 80% of cases occurring in sub-Sahara Africa.1 Schistosomes have a complex life cycle involving the alternate passage of the infection between the definitive host (human) and an invertebrate intermediate host serving as a vector (various species of freshwater snails depending on the schistosome species).2 The annual mortality rate due to schistosomiasis in sub-Saharan Africa is as high as 280,000.3 There has been recent evidence to suggest a high correlation in women with schistosomiasis and increased susceptibility to HIV/Aids. Women infected with schistosomiasis have as high as a 75% likelihood of suffering from Female Genital Schistosomiasis (FGS) caused by egg deposits in the uterus, cervix, vagina, or vulva which lead to granulomas, fibrosis and angiogenesis. Sandy patches occurring in the vagina due to FGS lead to bleeding and corrode the first HIV entrance barrier, the intact vaginal lumen.4 Thus, it was recently proposed that an effective and low-cost method to reduce HIV/AIDS transmission could be the preventative treatment of the endangered population with the antischistosomal drug praziquantel.5 Praziquantel (PZQ, 1), a tetrahydroisoquinoline derivative is the only commercially available treatment against schistosomiasis. Currently, mass treatment of millions of people in sub-Sahara Africa are performed through the Schistosomiasis Control Initiative (SCI) with the long term goal of eradicating this debilitating disease.6 However, the dependency on one major drug to treat schistosomiasis as well as the current large scale drug administration could potentially lead into the development of drug resistance. Praziquantel itself is a rather simple molecule and several different synthetic routes8 have already been reported, from those detailed in the original patent8a to a recent synthesis based on a radical cyclization.8g The original medicinal chemistry program that identified PZQ at Merck/Bayer generated ~400 PZQ analogues. The great majority of these are variants in a single part of the molecule (the exocyclic amide), and the compounds screened are limited in diversity. Having recognized the key structural motive of the Ugi multicomponent reaction in PZQ we recently devised a short and scalable new synthesis.7 Herein, we report our multicomponent reaction approach to the synthesis of highly diverse PZQ derivatives which are otherwise difficult to access.
Results and Disscussion
The Ugi multicomponent reaction has proven to be a very powerful and reliable reaction which involves a one-pot condensation of isocyanide 2, carbonyl component 3, amine 4 and carboxylic acid 5 under mild conditions to produce a substituted acylaminocarbonamide product 6.9 The PZQ skeleton 7 was then formed by a Pictet-Spengler cyclization under acidic conditions (Scheme 1). Since a high degree of structural diversity can also be introduced by this four component reaction, we extended our research to investigate the scope of this one-pot, two-step sequence. A great deal is already known about the reliability of the Ugi reaction10 however, the Pictet-Spengler cyclization has its limitations as highly electron rich (hetero-)aromatic ethylamines and high reaction temperatures are required.11
Scheme 1.
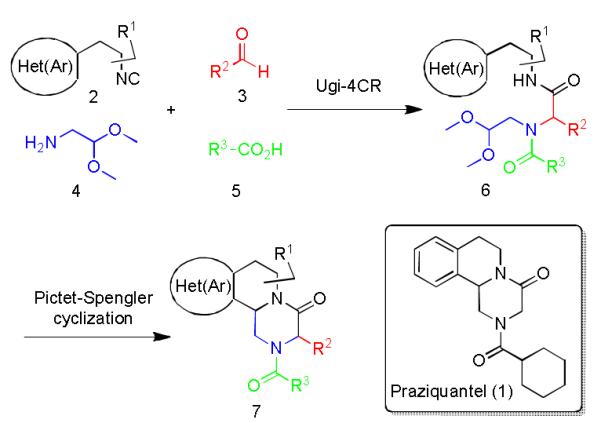
Drug praziquantel (PZQ, 1) and general approach for the multicomponent synthesis of its derivatives 7.
Since a large number of aldehydes (R2CHO) and carboxylic acids (R3CO2H) are commercially available, we started our investigations with the synthesis of several suitable (hetero)phenylethylamine derived isocyanides (R1NC). Eight isocyanides 2(a-h) were prepared either by a classical one-step “Hoffmann procedure” or by an “Ugi 2-step method”.12 By mixing isocyanide, aminoacetaldehyde-dimethylacetale 4, formaldehyde and cyclohexanecarboxylic acid (in a ratio of 1:1:1.2:1) in anhydrous methanol under ambient temperature, the Ugi condensation product formed in very good to quantitative yield, which was dried in vacuum and used directly in the next step transformation. Depending on the nature of the (hetero-)arylethylamine the yield of the Pictet-Spengler cyclization varied greatly.
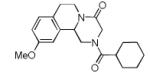
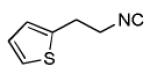
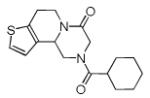
We utilized a modified cyclisation condition (CH3SO3H, MgSO4, 1,2-dichloroethane, 80 °C) as published in an earlier PZQ synthesis to carry out the Pictet-Spengler cyclization.13 Ugi products based on electron rich or electron neutral (hetero)phenylethylamine derived isocyanides always reacted better and gave final PZQ derivatives in good to excellent yields (Table 1, entries 1-3). The phenylalanine derived methyl ester isocyanide reacted rather sluggish in the Pictet-Spengler step, and only small amount of cyclized product was identified (Table 1, entry 4). In the case of tryptophan derived isocyanide, two diastereomers were isolated in a 2.4:1 ratio (trans/cis) in good overall yield (Table 1, entry 5).14 The relative stereochemistry was assigned on the basis of 2D NOESY experiments (see the Supporting Information). The 4-fluoro phenylethylamine derived isocyanide was unreactive in the Pictet-Spengler step (Table 1, entry 6) due to the electron-deficient nature of the aromatic ring. Attempts to prepare a five or seven-membered ring homologue of PZQ failed (Table 1, entry 7-8). This is consistent with the literature that no cyclization of N-acyiminium ion yielding 5-membered product or 7-membered product has been reported.15
Table 1.
Varations of isocyanides and praziquantel derivatives 8(a-e)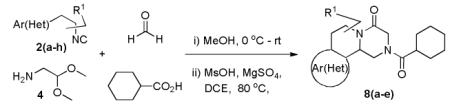
| Entry | R1NC 2(a-h) |
Product |
8 (a-e) |
Yield (%) |
|---|---|---|---|---|
| 1 |
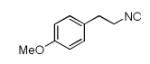
|
|
8a | 46c |
| 2 |
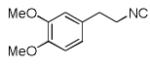
|
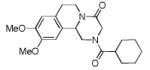
|
8b | 48c |
| 3 |
|
|
8c | 34c |
| 4 |
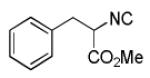
|
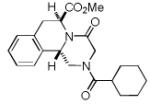
|
8d a | 16c |
| 5 |
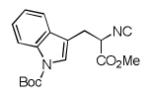
|
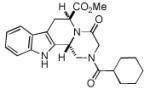
|
8e b | 45c |
| 6 |
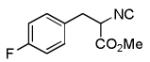
|
- | - | 76d |
| 7 |
|
- | - | 73d |
| 8 |
|
- | - | 58d |
Only trans-8d was identified.
Two diastereomers were isolated in a ratio of 2.4:1 (trans/cis).
Overall isolated yield for two steps.
Isolated yield for the Ugi reaction.
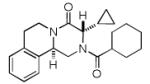
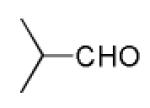
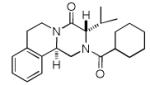
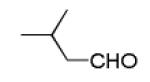
Next, we investigated different aldehydes in order to produce structurally diversified PZQ derivatives. All aldehydes including acyclic, cyclic aldehyde as well as aryl aldehydes reacted very well in the Ugi-4CR reaction and the subsequent Pictet-Spengler in most cases led to the final PZQ derivatives 9(a-g) in acceptable overall yields (Table 2, entries 1-7).
Table 2.
Variations of aldehydes and praziquantel derivatives 9(a-g)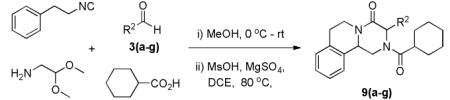
| Entry | R2CHO 3(a-g) |
Producta |
9 (a-g) |
Yield (%)b |
|---|---|---|---|---|
| 1 |
|
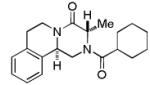
|
9a | 21 |
| 2 |
|
|
9b | 29 |
| 3 |
|
|
9c | 33 |
| 4 |
|
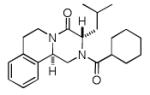
|
9d | 34 |
| 5 |
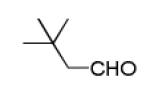
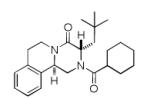
|
|
9e | 23 |
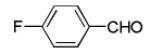
| 6 |
|
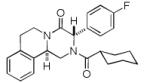
|
9f | 16 |
| 7 |
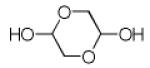
|
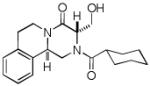
|
9g | 17 |
Relative stereochemistry as drawn.
Overall isolated yield for two steps.
It should be noted that the Pictet-Spengler cyclization occurs with remarkably high diastereoselectivity and only one diastereomer was formed according to NMR experiments. The relative stereochemistry was assigned on the basis of 2D NOESY experiments (see the Supporting Information). In addition the structure and relative stereochemistry of compound 9e was unequivocally determined by X-ray diffraction analysis (see the Supporting Information). In almost all of the cases, only trans-fused product was formed (Table 2, entries 1-6). While the relative configuration of the final product 9g from glycolaldehyde dimer is opposite to the others (Table 2, entry 7). This could be interpreted by an intramolecular H-bond binding between the hydroxyl group and the carbonyl group through a six-membered ring in the Pictet-Spengler cyclization precursor, which favors the formation of the cis-fused cycloproduct 9g (see the Supporting Information).
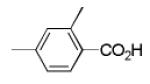
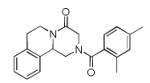
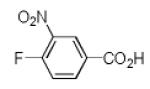
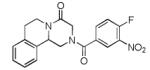
Finally, the scope and limitations of the carboxylic acid component (R3CO2H) was also investigated. Aromatic and aliphatic acids as well as functionalized acids reacted nicely in the Ugi-4CR (Table 3, entries 1-7). We also prepared conjugates of known antibiotic of the quinolinone type, such as 4-methoxyquinoline antibiotics (Table 3, entry 4) and nalidixic acid (Table 3, entry 5), a gyrase inhibitors.16 The substrate scope in the carboxylic acid part is amazingly broad with the possibility of generating more complicated PZQ derivatives with unprecedented structural complexity.
Table 3.
Variations of carboxylic acids and praziquantel derivatives 10(a-h)
| Entry | R3CO2H 5(a-h) |
Product |
10 (a-h) |
Yield (%)a |
|---|---|---|---|---|
| 1 |
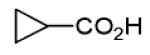
|
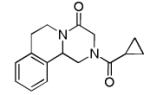
|
10a | 58 |
| 2 |
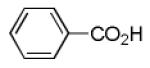
|
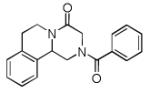
|
10b | 42 |
| 3 |
|
|
10c | 44 |
| 4 |
|
|
10d | 25 |
| 5 |
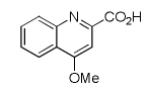
|
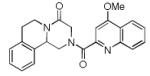
|
10e | 29 |
| 6 |
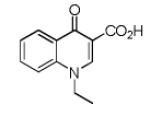
|
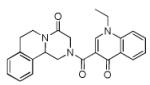
|
10f | 10 |
| 7 |
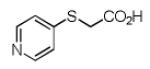
|
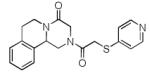
|
10g | 19 |
| 8 |
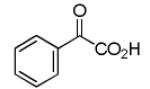
|
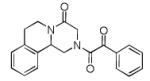
|
10h | 25 |
Overall isolated yield for two steps.
Currently, the molecular target(s), the detailed mode-of-action, and the mechanism of possible resistance to PZQ remains unknown despite many reported mechanistic studies.17 The synthesis of radio- or fluorescent-labeled PZQ derivatives, coupled with modern proteomics might be helpful to perform affinity and target fishing studies. With this general access to multiple functionalized PZQ derivatives, there are many different choices for site-specific labeling (Table 4). For example, as for derivative 10d, the dansyl fluorescent label was introduced via an aromatic nucleophilic amine substitution (11g). Derivatives 11(a-g), on the other hand showed increased water solubility and might show different pharmacodynamic properties as opposed to the highly hydrophobic PZQ derivatives.
Table 4.
Late-stage modification of praziquantel derivative 8d and 10d
Condition A, Amine, 20% TBD, 60 °C.
Condition B: Amine, K2CO3, DMF, rt.
Isolated yield. TBD = 1,5,7-triazabicyclo[4.4.0]dec-5-ene.
With these representative PZQ derivatives in hand, the antischistosomal activities were assessed by performing schistosome worm viability assays as a primary screen. Schistosoma mansoni cercariae shed from Biomphalariae alexandrina snails were used to infect hamsters to obtain adult schistosomes as previously described.18 The PZQ derivatives and the analytical standard PZQ were prepared in stock solutions of 5 mM and were then serially diluted using complete media and were added to Petri dishes containing the freshly harvested schistosomes. On the day 5, the number of living/dead worms was recorded and compared to parallel values in negative control petri dishes containing pure medium alone or medium with DMSO and positive control media containing comparable concentrations from the mother PZQ. The percentages of Schistosoma mansoni worm death in vitro under the influences of different PZQ derivatives in different concentrations versus untreated and DMSO negative controls and positive controls treated with the mother drug PZQ were determined. Different EC50’s (the concentration at which 50% of worms was recorded to be dead) and the significance between different EC50 values was calculated using computerized program “Pharm/PCS” Version 4.2 (Pharmacologic calculation system) by a plot of the percent of worm mortality (versus living worms) against the concentration of the drug.
Selected EC50 values were shown in Table 5. Untreated and DMSO-treated controls had no observed mortality. The mother drug PZQ was the most effective compound studied, with 100% of the worms dying with concentrations from 0.8, 1.5, 3.0, 6.0, 12.5, 25, 50, 75 and 100 μM. The 50% effective concentrations (EC50) of promising PZQ derivatives ranged from 0.37 to 9.9 μM. Not surprisingly, the herein synthesized PZQ revealed an EC50 value of 0.37 μM which is not significantly different from that of the analytical standard PZQ (0.18 μM). The EC50 for compounds 8c, 9a, 10b, 8a and 10d were 0.9, 1.3, 3.9, 9.7 and 9.9 μM respectively. PZQ derivatives 8d, 8e, 9d, 9e, 9f, 10c, 10f, 11b and 10e showed weak schistosoma mansoni worm death (EC50 from 30 μM −100 μM) respectively. Compounds 8b, 9c, 10g, 10h and 11a-g did not reveal any antischistosomal activity.
Table 5.
50% effective concentration of schistosoma mansoni worm killing for different PZQ derivatives
| Compound | EC50 [μM]a | Compound | EC50 [μM]a |
|---|---|---|---|
| S.mansoni PZQ | 0.18 | 10b | 3.9* # |
| PZQ (synthetic) | 0.37c | 10c | 46.8* # |
| 8a | 9.7* # | 10d | 9.9* # |
| 8b | NE | 10e | >100* # |
| 8c | 0.9* # | 10f | >100* # |
| 8d | >100* # | 10g | NE |
| 8e | >100* # | 10h | NE |
| 9a | 1.3* # | 11a | NE |
| 9b | >100* # | 11b | >100* # |
| 9c | NE | 11c | NE |
| 9d | 43.7* # | 11d | NE |
| 9e | >100* # | 11e | NE |
| 9f | >100* # | 11f | NE |
| 10a | NE | 11g | NE |
NE = No effect.
Significant difference from the mother brand of PZQ at P<0.05.
Significant difference from the synthetic PZQ at P<0.05.
comparable sensitivity to mother PZQ with no statistically significant difference.
We aligned the solid state structure of compound 9e with PZQ based on the common phenyl group (Figure 1). Interestingly, it can be seen that the bulky neopentyl group in 9e induces a dramatic conformational change of the diketopiperazine (DKP) moiety and especially the cyclohexylcarbonyl moiety. The DKP in PZQ is almost planar whereas the DKP ring in 9e adopts a boat-like conformation. Additionally, the cyclohexylcarbonyl moiety in PZQ exits the DKP ring in-plane and is almost coplanar with the DKP, the cyclohexylcarbonyl moiety in 9e, however, is turned by 180° and resides on top of the phenyl ring. The dramatically decreased activities of 9(a-f), where various R2 groups (mainly hydrophobic alkyl substituentes) were introduced, together with the conformation change, may indicate that this part of the structure may not be a good choice for variation in the development of new PZQ alternatives. Modification of derivatives 8d and 10d also resulted in a total loss of activities. Variation of aromatic ring (R1) and cyclohexyl ring part (R3) led to slightly decreased activity. Analogue 8c which bears a smaller heteroaromatic ring displayed less activity was still comparable to mother PZQ. The results from 10b and 10d are also interesting, where the nature of the substituent’s on the aromatic ring play an important role in their activity.
Figure 1.
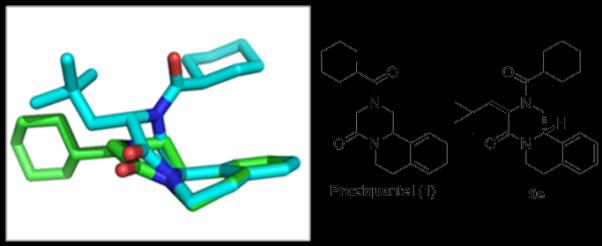
X-ray structure of praziquantel 1 (green sticks, CCDC 286150) aligned with derivative 9e (cyan sticks, CCDC 768130) based on the phenyl rings.
Conclusions
We have developed a convergent and versatile synthetic method that allows easily accessible and highly diversified PZQ derivatives to be synthesized for extensive SAR studies. This approach comprises a versatile usage of MCR chemistry, an Ugi-4CR as the key step, followed by a Pictet-Spengler ring closure, which was carried out in a sequential one-pot, two-step procedure. This approach allows access to a different chemical space compared to previous PZQ analogues, particularly derivatives with substituent R1 and R2, which are otherwise very difficult to access. Several compounds have been identified, however slightly less active than the mother drug PZQ. Noteworthy, the herein and recently described Ugi-Pictet-Spengler approach towards PZQ is the shortest and scalable approach towards PZQ.7,13 Future bioactivity guided optimization of PZQ derivatives to overcome the known limitations of the PZQ, including inefficacy against immature parasites, quick first pass metabolism and parasites with diminished responsiveness is in progress.
Supplementary Material
Acknowledgments
This research has been partially supported by NIH grant 1R21GM087617-01, 1R01GM097082-01 and 1P41GM094055-01 (to AD) and the University of Pittsburgh.
References and notes
- 1.Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, Savioli L. Control of neglected tropical diseases. N Engl J Med. 2007;357:1018–1027. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- 2.Strickland GT, Ramirez BL. Schistosomiasis. In: Strickland GT, editor. Hunter’s tropical medicine and emerging infectious diseases. 8th ed W. B. Saunders; Philadelphia: 2000. pp. 804–832. [Google Scholar]
- 3.Van der Werf MJ, DeVlas SJ, Brooker S, Looman CW, Nagelkerke NJ, Habbema JD, Engels D. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Tropica. 2003;86:125–139. doi: 10.1016/s0001-706x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 4 a).Feldmeier H, Krantz I, Poggensee G. Female genital schistosomiasis as a risk-factor for the transmission of HIV. Int J STD AIDS. 1994;5:368–372. doi: 10.1177/095646249400500517. [DOI] [PubMed] [Google Scholar]; b) Poggensee G, Feldmeier H. Female genital schistosomiasis: facts and hypotheses. Acta Trop. 2001;79:193–210. doi: 10.1016/s0001-706x(01)00086-9. [DOI] [PubMed] [Google Scholar]; c) Kjetland EF, Ndhlovu PD, Gomo E, Mduluza T, Midzi N, Gwanzura L, Mason PR, Sandvik L, Friis H, Gundersen SG. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS. 2006;20:593–600. doi: 10.1097/01.aids.0000210614.45212.0a. [DOI] [PubMed] [Google Scholar]
- 5.Hotez PJ, Fenwick A, Kjetland EF. Africa’s 32 cents solution for HIV/AIDS. PLoS Negl Trop Dis. 2009;3:e430. doi: 10.1371/journal.pntd.0000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotez P, Raff S, Fenwick A, Richards F, Molyneux DH. Recent progress in integrated neglected tropical disease control. Trends in Parasitology. 2007;12:511–514. doi: 10.1016/j.pt.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Dömling A, Khoury K. Praziquantel and Schistosomiasis. Chem Med Chem. 2010;5:1420–1434. doi: 10.1002/cmdc.201000202. [DOI] [PubMed] [Google Scholar]
- 8 a).Seubert J, Pohlke R, Loebich F. Synthesis and properties of praziquantel, a novel broad spectrum anthelmintic with excellent activity against Schistosomes and Cestodes. Experientia. 1977;33:1036–1037. doi: 10.1007/BF01945954. [DOI] [PubMed] [Google Scholar]; b) Frehel D, Maffrand JP. New syntheses of Praziquantel-2-(cyclohexylcarbonyl)-1,2,3,6,7,11b-hexahydro-4H-pyrazino (2,1-a)isoquinolin-4-one. Heterocycles. 1983;20:1731–1735. [Google Scholar]; c) Berkowitz WF, John TV. An internal imino-Diels-Alder route to a tetrahydroisoquinoline. J Org Chem. 1984;49:5269–5271. [Google Scholar]; d) Yuste F, Pallás Y, Barrios H, Ortiz B, Sanchez Obregon R. A short synthesis of praziquantel. J Heterocyclic Chem. 1986;23:189–190. [Google Scholar]; e) Kim JH, Lee YS, Park H, Kim CS. Synthesis of praziquantel via N-acyliminium ion cyclization of amido acetals through seceral synthetic routes. Heterocycles. 1998;48:2279–2285. [Google Scholar]; f) Kim JK, Lee YS, Park H, Kim CS. Formation of pyrazinoisoquinoline ring system by the tandem amidoalkylation and N-acyliminium ion cyclization: An efficient synthesis of Praziquantel. Tetrahedron. 1998;54:7395–7400. [Google Scholar]; g) Todd MH, Ndubaku C, Bartlett PA. Amino Acid-Derived Heterocycles: Lewis Acid-Catalyzed and Radical Cyclizations from Peptide Acetals. J Org Chem. 2002;67:3985–3988. doi: 10.1021/jo010990m. [DOI] [PubMed] [Google Scholar]
- 9 a).Ugi I, Dömling A. Multicomponent reactions with isocyanides. Angew Chem Int Ed. 2000;39:3168–3210. doi: 10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]; b) Dömling A. Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry. Chem Rev. 2006;106:17–89. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]; c) Liu H-X, Dömling A. One-Pot Synthesis of Highly Functionalized Seleno Amino Acid Derivatives. Chem Biol Drug Des. 2009;74:302–308. doi: 10.1111/j.1747-0285.2009.00854.x. [DOI] [PubMed] [Google Scholar]
- 10 a).Ugi I, Steinbrückner C. Über ein neues Kondensations-Prinzip. Angew Chem. 1960;72:267–268. [Google Scholar]; b) Marcaccini S, Torroba T. The use of the Ugi four-component condensation. Nat Protocols. 2007;2:632–639. doi: 10.1038/nprot.2007.71. [DOI] [PubMed] [Google Scholar]
- 11 a).Pictet A, Spengler T. Über die Bildung von Isochinolin-derivaten durch Einwirkung von Methylal auf Phenyl-äthylamin, Phenylalanin und Tyrosin. Berichte der deutschen chemischen Gesellschaft. 1911;44:2030–2036. [Google Scholar]; b) Cox ED, Cook JM. The Pictet-Spengler condensation: a new direction for an old reaction. Chem Rev. 1995;95:1797–1842. [Google Scholar]
- 12 a).Ugi I, Meyr IR. Neue Darstellungsmethode fur Isonitrile. Angew Chem. 1958;70:702–703. [Google Scholar]; b) Hofmann AW. Über eine neue Reihe von Homologen der Cyanwasserstoffsäure. Justus Liebigs Ann Chem. 1867;144:114–120. [Google Scholar]; c) Weber WP, Gokel GW, Ugi I. Phase Transfer Catalysis in the Hofmann Carbylamine Reaction. Angew Chem Int Ed. 1972;11:530–531. [Google Scholar]; d) Grolla AA, Podesta V, Chini MG, DiMicco S, Vallario A, Genazzarni AA, Canonico PL, Bifulco G, Tron GC, Sorba G, Pirali T. Synthesis, biological evaluation, and molecular docking of Ugi products containing a zinc-chelating moiety as novel inhibitors of histone deacetylases. J Med Chem. 2009;52:2776–2785. doi: 10.1021/jm801529c. [DOI] [PubMed] [Google Scholar]
- 13.Cao H-P, Liu H-X, Dömling A. Efficient Multicomponent Reaction Synthesis of the Schistosomiasis Drug Praziquantel. Chem Eur J. 2010;16:12296–12298. doi: 10.1002/chem.201002046. [DOI] [PubMed] [Google Scholar]
- 14.Liu H-X, Dömling A. Efficient and Diverse Synthesis of Indole Derivatives. J Org Chem. 2009;74:6895–6898. doi: 10.1021/jo900986z. [DOI] [PubMed] [Google Scholar]
- 15.Maryanoff BE, Zhang H-C, Cohen JH, Turchi IJ, Maryanoff CA. Cyclizations of N-Acyliminium Ions. Chem Rev. 2004;104:1431–1628. doi: 10.1021/cr0306182. [DOI] [PubMed] [Google Scholar]
- 16.Page WJ, Patrick J. The DNA gyrase inhibitors, nalidixic acid and oxolinic acid, prevent iron-mediated repression of catechol siderophore synthesis inAzotobacter vinelandii. Biol Metals. 1988;1:57–61. doi: 10.1007/BF01128018. [DOI] [PubMed] [Google Scholar]
- 17 a).Greenberg RM. Are Ca2+ channels targets of Praziquantel action? Int J Parasitol. 2005;35:1–9. doi: 10.1016/j.ijpara.2004.09.004. [DOI] [PubMed] [Google Scholar]; b) Doenhoff MJ, Cioli D, Utzinger J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr Opin Infect Dis. 2008;21:659–667. doi: 10.1097/QCO.0b013e328318978f. [DOI] [PubMed] [Google Scholar]
- 18 a).Duvall RH, DeWitt WB. An improved perfusion technique for recovering adult schistosomes from laboratory animals. Am J Trop Med Hyg. 1967;16:483–486. doi: 10.4269/ajtmh.1967.16.483. [DOI] [PubMed] [Google Scholar]; b) Liang YS, Bruce JI, Boy DA. Laboratory cultivation of schistosome vector snails and maintenance of schistosome life cycle. Proceeding of the first Sino-American symposium. 1987;1:34–48. [Google Scholar]; c) Botros S, Pica-Mottoccia L, William S, ElLa-kkani N, Cioli D. Effect of praziquantel on the immature stages of Schistosoma haematobium. Int J Parasitol. 2005;35:1453–1457. doi: 10.1016/j.ijpara.2005.05.002. [DOI] [PubMed] [Google Scholar]; d) Wang L-D, Utzinger J, Zhou X-N. Schistosomiasis control: experiences and lessons from China. Lancet. 2008;372:1793–1795. doi: 10.1016/S0140-6736(08)61358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.