Abstract
Using a brief-access taste assay, we show in the present report that although phospholipase C β2 knockout (PLCβ2 KO) mice are unresponsive to low- and midrange concentrations of quinine and denatonium, they do significantly avoid licking higher concentrations of these aversive compounds. PLCβ2 KO mice displayed no concentration-dependent licking of the prototypical sweetener sucrose but were similar to wild-type mice in their responses to citric acid and NaCl, notwithstanding some interesting exceptions. Although these findings confirm an essential role for PLCβ2 in taste responsiveness to sucrose and to low- to midrange concentrations of quinine and denatonium in mice as previously reported, they importantly suggest that higher concentrations of the latter two compounds, which are bitter to humans, can engage a PLCβ2-independent taste transduction pathway.
Keywords: licking, mice, phosphoinositide signaling, taste transduction, T1R, T2R
Introduction
There are two general classes of receptors that mediate the transduction of chemical stimuli into intracellular signals in taste receptor cells (see Gilbertson and Boughter, 2003). First, ion channels serve as receptors for salts and acids (see Bigiani et al., 2003). Second, specific families of G-protein–coupled receptors (GPCRs) bind with sugars, artificial sweeteners (and in some cases “sweet” tasting proteins), amino acids, alkaloids, and synthetic “bitter” compounds. Various heteromers of the members from the T1R family (i.e., T1R1, T1R2, and T1R3) of GPCRs are thought to bind with compounds considered sweet or “umami-like” (Hoon et al., 1999; Montmayeur et al., 2001; Nelson et al., 2001, 2002; Li et al., 2002; Zhao et al., 2003; Mueller et al., 2005). The taste-mGluR4, a splice variant of the GPCR found in brain, has also been suggested to serve as a receptor for the prototypical umami compound L-glutamate (Chaudhari and Roper, 1998). The ~30 GPCR members of the T2R family are thought to bind, in some cases with apparent high selectivity, with compounds considered bitter (Adler et al., 2000; Chandrashekar et al., 2000; Bufe et al., 2002; Montmayeur and Matsunami, 2002; Behrens et al., 2004; Mueller et al., 2005).
The intracellular signaling cascade activated by stimulation of taste receptor cells with T1R and T2R ligands appears to involve the β2 subtype of the enzyme phospholipase C (PLC) in rodents (see Perez et al., 2003). Stimulation of taste receptor cells with compounds that are characterized as sweet and bitter leads to an increase in intracellular levels of Ca2+ and inositol 1,4,5-triphosphate (IP3), a second messenger product of the effector PLC (e.g., Hwang et al., 1990; Spielman et al., 1996; Rossler et al., 1998; Yan et al., 2001; Zhao et al., 2002). Moreover, the increase in IP3 levels can be blocked by PLCβ2-specific antibodies (Rossler et al., 1998; Yan et al., 2001). Two Gβγ subunits, Gβ3 and Gγ13, that are known to couple to PLCβ2 are coexpressed with the effector enzyme in a subset of taste receptor cells (Rossler et al., 2000), and Gγ13 has also been shown to colocalize with the type III IP3 receptor (Clapp et al., 2001). Finally, PLCβ2 and the type III IP3 receptor are coexpressed in subsets of taste receptors cells expressing either T1R2 or T2R29 (Asano-Miyoshi et al., 2001).
Zhang et al. (2003) suggested that transduction involving all the known candidate G-protein–coupled taste receptors in mice is completely dependent on PLCβ2 and on the transient receptor potential ion channel M5 (TRPM5). First, consistent with prior findings of others (Asano-Miyoshi et al., 2001; Perez et al., 2002), Zhang et al. (2003) confirmed that both TRPM5 and PLCβ2 are coexpressed in taste receptor cells and completely overlap the expression of either T1Rs or T2Rs. Second, deletion of either the gene encoding for PLCβ2 or the gene encoding TRPM5 in mice eliminated responsiveness to the sugars, artificial sweeteners, amino acids, and bitter ligands tested. This was true as assessed in a brief-access behavioral taste assay as well as from peripheral gustatory nerve recordings. Recently, Mueller et al. (2005) replicated the lack of concentration-dependent avoidance in PLCβ2 null mice tested with a broad panel of putative T2R ligands. Third, in the case of PLCβ2 null mice, gene rescue experiments in cells specifically expressing T2Rs restored responsiveness to the bitter compounds, but T1R ligands remained ineffective stimuli.
Despite this compelling support for a critical role for PLCβ2 in the transduction of GPCR taste ligands, there is evidence that additional signaling pathways stimulated by some of the same compounds may be operating in taste receptor cells. Stimulation of taste receptor cells with sucrose or monosodium glutamate leads to increases and decreases, respectively, of intracellular cyclic adenosine monophosphate concentrations (Striem et al., 1991; Bernhardt et al., 1996; Chaudhari et al., 2000). There is also evidence that α-gustducin plays a role in transduction of T1R and T2R ligands through its regulation of phosphodiesterase activity (Wong et al., 1996; Huang et al., 1999; Yan et al., 2001). Moreover, because of their amphipathic properties, some bitter compounds and artificial sweeteners can apparently permeate through the taste receptor cell plasma membrane upon which they could potentially engage a variety of intracellular signaling mechanisms (Peri et al., 2000).
Here we used a brief-access behavioral taste assay in an attempt to confirm and extend the findings that taste is compromised in phospholipase C β2 knockout (PLCβ2 KO) mice. We report that PLCβ2 KO mice do not display appetitive lick responses to sucrose, confirming previous findings (Zhang et al., 2003). However, certain bitter compounds in their higher concentration range remain aversive even to mice lacking PLCβ2.
Materials and methods
Subjects
Breeding pairs of mice that were homozygous null for PLCβ2 were provided by Dr. Dan Wu, and the C57BL/6ByJ wild-type (WT) control breeders were obtained from the Jackson Laboratory (Bar Harbor, ME). The mice were bred at the University of Miami School of Medicine and transferred to the University of Florida for training and testing. At the completion of the behavioral experiment, tail specimens from all the mice were coded and sent to the University of Miami for genotype analysis by polymerase chain reaction. Mice (five males and five females in each group) were housed individually in cages in a colony room where the temperature and lighting were controlled automatically (12:12 h) and were habituated to the laboratory environment for 10 days before testing. During this time, food and purified water were available ad libitum. Testing and training took place during the lights-on phase. At the start of testing, mice were ~10–12 weeks of age. Although rarely necessary, during periods when the animals were placed on a water-restriction schedule, mice that dropped below 80% of their body mass measured under ad libitum drinking conditions received 1 ml of supplemental water 2 h after the end of the testing session. All procedures were approved by the University of Florida Institutional Animal Care and Use Committee.
Taste stimuli
All solutions were prepared daily with purified water (Elix 10; Millipore, Billerica, MA) and reagent grade chemicals and were presented at room temperature. Test stimuli consisted of five concentrations of sucrose (62.5, 125, 250, 500, and 1000 mM; Fisher Scientific, Atlanta, GA) and six concentrations of NaCl (30, 100, 200, 300, 600, and 1000 mM; Fisher Scientific), quinine hydrochloride (0.01, 0.1, 0.3, 1.0, 5.0, and 20.0 mM; ICN Biomedicals, Aurora, OH), denatonium benzoate (0.1, 0.3, 1.0, 5.0, 10.0, and 50.0 mM; ICN Biomedicals), citric acid (0.3, 1, 3, 10, 30, and 100 mM; Fisher Scientific), and purified water.
Procedure
Testing took place in a gustometer (Davis MS-160, DiLog Instruments, Tallahassee, FL) as described by Glendinning et al. (2002). This device allowed mice access to a single tube containing a taste stimulus for a brief period of time (5 s), and then, after a 7.5-s interpresentation interval, the tube was changed, via a motorized table, for the next trial. Presentation order was randomized within blocks. Unconditioned lick responses were recorded for later analysis. The sessions were 30 min in duration during which mice could initiate as many trials as possible.
After being placed on an ~23.5-h restricted water-access schedule, the animals were presented with a stationary tube of water and trained to lick in the gustometer for one session. After training, mice were tested over 5 weeks, one stimulus per week (1 week = 5 consecutive days, Monday–Friday). Two different testing protocols were used based, in part, on the recommendations of Glendinning et al. (2002): one for normally preferred stimuli (i.e., sucrose) and one for normally avoided substances such as quinine, denatonium, citric acid, and NaCl. For sucrose, animals received 2 days of testing with the five stimulus concentrations and purified water while under the water-restriction schedule. The water bottles were then replaced on the home cages, and the mice were tested with sucrose for three consecutive days nondeprived. For quinine, denatonium, NaCl, and citric acid, the mice were tested under the 23.5-h water-restriction schedule for all 5 days. On the first 2 days, the animals received, in 5-s trials, purified water delivered from eight tubes. On the next 3 days, the mice were tested with purified water and six stimulus concentrations of a single compound. A water rinse (10 lick maximum) presentation was interposed between the test trials for all stimuli to help control for potential carryover effects (see St John et al., 1994; Boughter et al., 2002). For half the animals, the order of testing was sucrose, NaCl, quinine, denatonium, and citric acid. For the other half, the quinine and denatonium order was reversed to counterbalance the potential influence of exposure of one bitter taste stimulus on the subsequent responsiveness to the other.
Data analysis
When the mice were tested in a nondeprived condition (i.e., with sucrose), the average number of licks per trial directed at each concentration, collapsed across test sessions, was divided by an animal’s maximum potential lick rate per trial based on the mean of the interlick interval distribution during training, yielding a Standardized Lick Ratio (see Glendinning et al., 2002). Standardizing lick responses in this fashion controls for individual differences in maximal lick rates. For all other stimuli, the average number of licks per trial directed at each concentration during test days was divided by the average number of water licks per trial, yielding a Tastant/Water Lick Ratio. This ratio controls for individual differences in lick rates and motivational state. Because this ratio incorporates licks to water in its calculation, we did not include these licks in the determination of concentration dependence for the normally avoided stimuli tested (i.e., quinine, denatonium, citric acid, and NaCl).
The ratios were analyzed with standard analyses of variance (ANOVAs). When a genotype × concentration interaction was significant, one-way ANOVAs were conducted within each strain to test for simple effects of concentration. Because latency values tend not to be normally distributed, the latency to initiate the first lick in a trial was analyzed non-parametrically with the Friedman test. The conventional P ≤ 0.05 was applied as the statistical rejection criterion. Only mice that had at least one trial at every concentration were included in the analysis of a given stimulus. In general, all the mice initiated many trials, but the sample sizes analyzed for the nondeprived conditions for the PLCβ2 KO mice tested with sucrose (n = 7) were lower.
Curves were fit to the mean data for each group using a two- or three-parameter logistic function of the form:
where x = log10 concentration, c = log10 concentration at the inflection point, and b = slope. For sucrose, a = the asymptotic Standardized Lick Ratio and d = 0. For quinine, denatonium, and citric acid, a = 1.0 and d = 0. For NaCl, a = 1.0 and d = minimum asymptote of Tastant/Water Lick Ratio. These logistic functions helped quantify the differences in stimulus sensitivity between the WT and KO mice.
Results
Sucrose
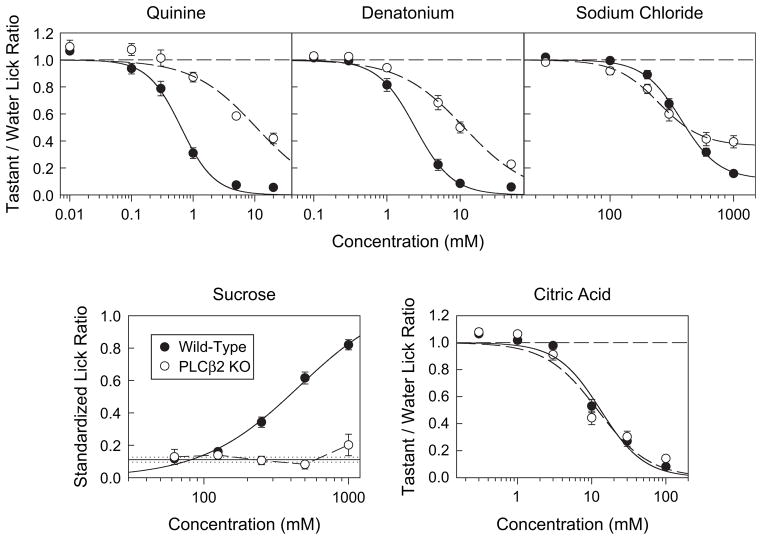
The PLCβ2 KO mice were unresponsive to sucrose up to 1000 mM and significantly differed from the WT mice (Figure 1; Tables 1 and 2), which clearly showed a concentration-dependent increase in licking producing a c value of 456 mM.
Figure 1.
Top panels and bottom right: mean (±SE) Tastant/Water Lick Ratios for WT (black circles, n = 10) and PLCβ2 KO (open circles, n = 10) mice licking quinine hydrochloride, denatonium benzoate, NaCl, and citric acid. Dashed line represents the mean for water. Bottom left panel: mean (±SE) Standardized Lick Ratios for WT (black circles, n = 10) and PLCβ2 KO (open circles, n = 7) mice licking sucrose. Solid and dotted lines represent the mean and SE for water, respectively. Only mice that had at least one trial at every concentration were included in the analysis of a given stimulus.
Table 1.
Results from two-way genotype × concentration ANOVAs
| Stimuli | Genotype | Concentration | Interaction |
|---|---|---|---|
| Sucrose | F(1,15) = 71.8, P < 0.001 | F(5,75) = 62.0, P < 0.001 | F(5,75) = 50.3, P < 0.001 |
| NaCl | F(1,18) = 0.03, P = 0.861 | F(5,90) = 297.3, P < 0.001 | F(5,90) = 14.0, P < 0.001 |
| Quinine | F(1,18) = 58.5, P < 0.001 | F(5,90) = 319.5, P < 0.001 | F(5,90) = 27.4, P < 0.001 |
| Denatonium | F(1,18) = 50.4, P < 0.001 | F(5,90) = 503.1, P < 0.001 | F(5,90) = 31.0, P < 0.001 |
| Citric acid | F(1,18) < 0.1, P = 0.988 | F(5,90) = 455.7, P < 0.001 | F(5,90) = 2.5, P < 0.05 |
Boldfaced values signify statistical significance.
Table 2.
Results from one-way ANOVAs conducted within each strain to test for simple effects of concentration
| Stimuli | WT | KO |
|---|---|---|
| Sucrose | F(4,36) = 169.6, P < 0.001 | F(4,24) = 1.3, P = 0.28 |
| NaCl | F(5,45) = 348.6, P < 0.001 | F(5,45) = 72.4, P < 0.001 |
| Quinine | F(5,45) = 306.4, P < 0.001 | F(5,45) = 82.8, P < 0.001 |
| Denatonium | F(5,45) = 290.3, P < 0.001 | F(5,45) = 230.6, P < 0.001 |
| Citric acid | F(5,45) = 218.5, P < 0.001 | F(5,45) = 241.6, P < 0.001 |
Boldfaced values signify statistical significance.
Quinine and denatonium
Although the concentration-response functions of PLCβ2 KO mice were markedly shifted to the right and were significantly different from their WT controls for both quinine and denatonium, these animals, nonetheless, showed clear concentration-dependent decreases (Figure 1; Tables 1 and 2). The c values for the mean curves of the WT mice were 0.638 and 2.43 mM for quinine and denatonium, respectively, and the corresponding c values for the mean PLCβ2 KO curves were 10.59 and 11.35 mM.
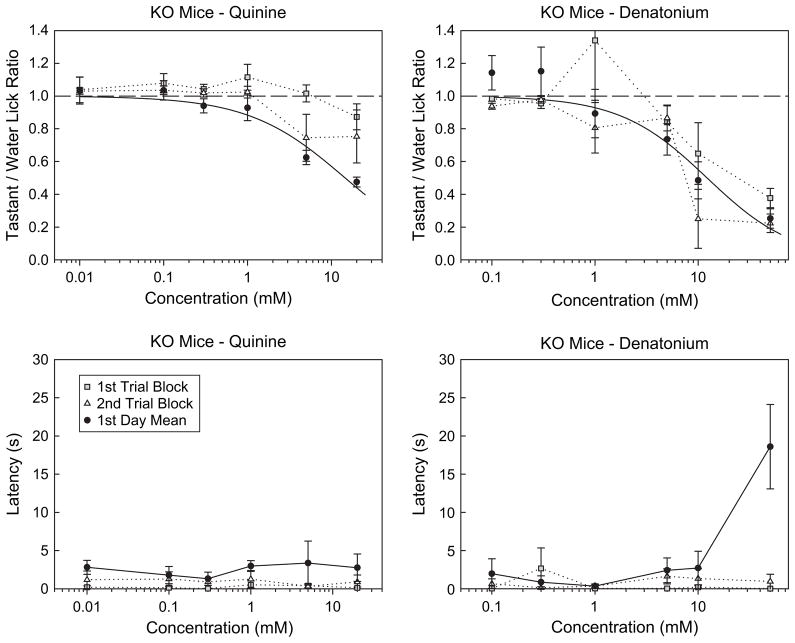
We analyzed the responses of the PLCβ2 KO mice on the first day of testing to assess when the mice actually started to avoid the stimuli. For this test, we only used mice that did not have prior experience with a bitter stimulus, and thus, the sample size was cut in half. Clear avoidance was evident on the first day of testing [quinine: F(5,20) = 21.0, P < 0.001; denatonium: F(5,20) = 18.5, P < 0.001]. In fact, clear avoidance was evident in the first [denatonium: F(5,20) = 3.2, P < 0.05] or at least by the second [quinine: F(5,20) = 3.3, P < 0.05; denatonium: F(5,20) = 13.5, P < 0.001] set of trials (Figure 2). Although the degree of lick suppression observed early in the session was sometimes attenuated relative to what was observed for the entire session, this is likely due to the high initial level of motivation in the water-deprived mice coupled with a weak signal.
Figure 2.
Top panels: mean (±SE) Tastant/Water Lick Ratios for PLCβ2 KO (n = 5) mice licking quinine hydrochloride and denatonium benzoate on the first (gray squares) and second (open triangles) set of trials on the first day of testing and for all trials on the first day of testing (black circles). The upper error bar for the mean at 1 mM denatonium on the first set of trials is not shown. Bottom panels: median (±median absolute deviation) latency for PLCβ2 KO (n = 5) mice to first initiate a trial when licking quinine hydrochloride and denatonium benzoate on the first (gray squares) and second (open triangles) set of trials on the first day of testing and for all trials on the first day of testing (black circles). Only KO mice that did not have prior experience with a bitter stimulus were included in the analysis, and thus, the sample size was cut in half.
We also analyzed the latency of the PLCβ2 KO mice to approach the spout upon presentation of the stimulus tube on the first day of testing to assess whether a nonoral cue (e.g., olfaction) could have contributed to responsiveness (Figure 2). For this test, we also only used mice that did not have prior experience with a bitter stimulus, and thus, the sample size was cut in half. There was a significant concentration-dependent increase in the latency to lick denatonium, but no increase was observed when quinine was the stimulus for PLCβ2 KO mice on the first day of testing (quinine: P = 0.830; denatonium: P < 0.05, Friedman test). Furthermore, if the highest concentration of denatonium (50 mM) was removed from the analysis, no concentration-dependent change in latency was statistically evident (P = 0.570). During the first two blocks of trials, no concentration-dependent change in the latency to first lick quinine or denatonium was observed (quinine: both P values >0.788; denatonium: both P values >0.708). These results indicate that the lick avoidance displayed by the PLCβ2 KO mice during the initial exposure on the first day of testing was not likely the result of some unconditioned aversive odor associated with the stimulus. Moreover, except for the highest concentration of denatonium, there was no strong evidence from the latency data on the first day that nonoral cues guided the responses of the PLCβ2 KO mice.
Citric acid and NaCl
The citric acid concentration-response functions representing WT mice (c value = 13.2 mM) and PLCβ2 KO mice (c value = 12.0 mM) were clearly very similar, but the two groups did significantly differ at the highest concentration; the PLCβ2 KO mice displayed slightly less avoidance (P < 0.05, t-test; Figure 1). The results for NaCl were somewhat more complex. Both WT and PLCβ2 KO mice showed clear concentration-dependent lick avoidance, but there was a significant genotype × concentration interaction for NaCl responses (Figure 1; Tables 1 and 2). Independent t-tests indicated that the PLCβ2 KO animals showed greater lick avoidance at the 100 and 200 mM NaCl concentrations (both P values <0.05) relative to the WT controls but showed less lick avoidance at 1000 mM (P < 0.001). Although the differences between the genotypes at the two lower concentrations were significant, they were unremarkable, but the difference between the responsiveness of WT and PLCβ2 KO mice at 1.0 M NaCl was very distinct. In fact, there was no overlap in the relative distributions of the taste/water lick ratio scores for the two genotypes at this concentration. Because the PLCβ2 KO mice displayed a minimum asymptote, at least within this concentration range, the d parameter was added to the logistic function to calculate the fit. When this was done, the c value (248 mM) for the PLCβ2 KO mice was actually slightly lower than that for the WT mice (375 mM), indicating that the former genotype was just as sensitive to NaCl as, or more so than, the latter group. However, at high concentrations, the responsivenss of PLCβ2 KO mice to NaCl appeared to be compromised.
Discussion
Mice that had the gene encoding for the effector enzyme PLCβ2 deleted were unresponsive to all concentrations of sucrose tested here as assessed in a brief-access behavioral taste assay, replicating the findings of Zhang et al. (2003). Because the brief-access taste test relies on the hedonic characteristics of the taste stimuli to drive responsiveness, we cannot conclusively rule out the possibility that these KO mice could still detect sucrose. In other words, it is possible that the gene deletion merely altered the affective properties of the stimulus such that it was no longer preferred or avoided even though the compound may have generated a detectable taste and that this change was obscure to the measure. Moreover, only one prototypical sweetener was tested here. Zhang et al. (2003), however, tested other sweet-tasting compounds in addition to sucrose and found that the PLCβ2 KO mice were similarly unresponsive in the brief-access test. Furthermore, the chorda tympani and glossopharyngeal nerves, which transmit taste signals to the brain from the anterior and posterior tongue, respectively, display no activity in response to sweeteners applied to their receptor fields in mice lacking PLCβ2 (Zhang et al., 2003). Thus, with the above considerations in mind, our data are in complete agreement with the hypothesis that PLCβ2 is a critical intermediary enzyme in the taste transduction of sucrose.
The results from the present work also confirm the important role of PLCβ2 in behavioral responsiveness to the prototypical bitter ligands, quinine and denatonium, but in a more qualified manner. The PLCβ2 KO mice displayed no avoidance of low- and midrange concentrations of quinine and denatonium relative to WT controls. They did, however, avoid higher stimulus concentrations. Indeed, it appeared as though the PLCβ2 KO mice had a concentration-avoidance function that was shifted to the right by about 1.22 log10 units for quinine and 0.669 log10 units for denatonium. In this respect, our results differ from those of Zhang et al. (2003) and Mueller et al. (2005), who found that PLCβ2 KO mice displayed no avoidance to any concentration of quinine or denatonium tested.
In the present study, there were only very minor differences between PLCβ2 KO and WT mice in their lick responses to citric acid. Although the NaCl concentration-response functions for the two genotypes were quite similar, the PLCβ2 KO mice distinctly licked 1.0 M NaCl more than the WT controls without any overlap in the respective distributions of the Tastant/Water Lick Ratios. The basis of this difference remains unclear and is worthy of further experimental scrutiny. Interestingly, a study that assessed the taste responsiveness of α-gustducin KO mice also found attenuated lick responsiveness to high concentrations of NaCl in Gαgust −/ − mice (Glendinning et al., 2005). These mice showed reduced behavioral responsiveness to 0.6 and 1.0 M NaCl when compared with Gαgust +/− controls. These parallel findings, in independent experiments, although unexplained, increase our confidence that our results are genuine. Nevertheless, the important point is that, on the whole, the responses between the PLCβ2 KO and WT mice to the ionic stimuli were much more similar than they were different.
At issue is whether the necessity of PLCβ2 for the taste transduction of compounds such as quinine and denatonium is absolute as implied by the findings of Zhang et al. (2003) and Mueller et al. (2005) or partial as suggested here. With sweet-tasting ligands, the necessity of the enzyme appears to be complete, notwithstanding the limitations of the brief-access procedure, at least for the concentrations and compounds tested across the two studies. The disparity between the data sets regarding bitter-tasting ligands, however, remains to be fully understood. We confirmed the genetic status of the PLCβ2 KO and WT mice by conducting “blind” genotyping. The PLCβ2 KO mice in the three studies [i.e., the present findings and those of Zhang et al. (2003) and Mueller etal.(2005)]were derived from an identical strain and obtained from an identical source. However, in the present study, the KO animals originally derived from the 129S vs train had been back-crossed for seven generations with C57BL/6 mice, where as in the studies of Zhang et al. (2003) and Mueller et al. (2005), the PLCβ2 KO mice were back crossed with SWR mice prior to testing. Perhaps, this difference in genetic background is significant.
We cannot entirely dismiss the possibility that olfaction somehow played a role in the behavior of the PLCβ2 KO mice, but several observations militate against this possibility. Although there were some concentration-dependent effects on latency to approach the drinking spout for PLCβ2 KO mice when denatonium was the stimulus, these effects were not evident on the first two sets of trials but the avoidance was, suggesting that the responsiveness was due to the taste, or at least oral sensory, properties of the compounds and not due to some unconditional aversive olfactory characteristic. For quinine, the PLCβ2 KO mice never displayed any concentration dependence in latency to respond, even though they clearly avoided the stimulus by the second set of trials.
We employed a similar behavioral assay used by Zhang et al. (2003) and Mueller et al. (2005), namely, the brief-access taste test (see Boughter et al., 2002; Glendinning et al., 2002). It is true that our maximum concentrations of denatonium and quinine were higher than those used in the prior report, but the PLCβ2 KO mice displayed clear avoidance responses to these stimuli at concentrations that overlapped the three studies. It seems possible, nonetheless, that because the concentration threshold for activation of the PLCβ2-independent pathway is apparently high, it may have been missed in the prior study, especially if it is subject to any kind of variability. Moreover, the incorporation of water rinses into the test used here may have helped increase the sensitivity of the procedure. Parenthetically, it is important to note that none of the mice, PLCβ2 KO or WT alike, showed any obvious deleterious effects of the brief-access testing to the ranges of concentrations we used.
There are several potential mechanisms that could mediate PLCβ2-independent taste transduction of quinine and denatonium, including blockade of K+ channels (e.g., Cummings and Kinnamon, 1992), stimulation of phosphodiesterase activity (e.g., Wong et al., 1996; Huang et al., 1999; Yan et al., 2001), or the possible intracellular effects of some ligands such as quinine that can permeate the taste receptor cell membrane (e.g., Chen and Herness, 1997; Peri et al., 2000). Whether these or other transduction mechanisms, to be yet identified, are responsible for the residual behavioral responsiveness of PLCβ2 KO mice to high concentrations of the bitter-tasting ligands tested here remains to be elucidated.
Indeed, it is not uncommon to find responsiveness to high concentrations of bitter-tasting compounds in gene deletion preparations that appear to be ageusic to low- and midrange concentrations of the same stimuli. For example, α-gustducin KO mice do not display lick avoidance to a variety of alkaloid compounds at low- and midrange concentrations in a brief-access test but do significantly suppress their lick responses to high concentrations (Glendinning et al., 2005). The same appears to be true for TRPM5 KO mice at least with respect to denatonium (Damak et al., 2005). Although these KO experiments provide convincing support for the “necessity” of these various transduction components, they do not necessarily demonstrate “sufficiency.” For sufficiency to be established, it would be necessary to demonstrate that a proposed transduction pathway could maintain performance and/or responsiveness in the absence of all other putative alternative mechanisms (see Spector, 2000, for a review of these concepts). Thus, it remains possible that once the PLCβ2-independent transduction pathway is identified, its elimination could potentially lead to severely disrupted responsiveness to lower concentrations of bitter-tasting compounds.
Acknowledgments
We would like to thank Angela Newth for her technical assistance and Dr Dianqing Wu for generously providing the breeding pair that generated the animals used in this experiment. We would also like to thank Dr G. Dvoriantchikov and Ms E. Pereira for conducting the genotyping. This study was supported by grants R01-DC04574 (A.C.S.) and R01-DC00374 (S.D.R.).
References
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Asano-Miyoshi M, Abe K, Emori Y. IP(3) receptor type 3 and PLCbeta2 are co-expressed with taste receptors T1R and T2R in rat taste bud cells. Chem Senses. 2001;26:259–265. doi: 10.1093/chemse/26.3.259. [DOI] [PubMed] [Google Scholar]
- Behrens M, Brockhoff A, Kuhn C, Bufe B, Winnig M, Meyerhof W. The human taste receptor hTAS2R14 responds to a variety of different bitter compounds. Biochem Biophys Res Commun. 2004;319:479–485. doi: 10.1016/j.bbrc.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Bernhardt SJ, Naim M, Zehavi U, Lindemann B. Changes in IP3 and cytosolic Ca2+ in response to sugars and non-sugar sweeteners in transduction of sweet taste in the rat. J Physiol. 1996;490(Pt 2):325–336. doi: 10.1113/jphysiol.1996.sp021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigiani A, Ghiaroni V, Fieni F. Channels as taste receptors in vertebrates. Prog Biophys Mol Biol. 2003;83:193–225. doi: 10.1016/s0079-6107(03)00058-0. [DOI] [PubMed] [Google Scholar]
- Boughter JD, Jr, St John SJ, Noel DT, Ndubuizu O, Smith DV. A brief-access test for bitter taste in mice. Chem Senses. 2002;27:133–142. doi: 10.1093/chemse/27.2.133. [DOI] [PubMed] [Google Scholar]
- Bufe B, Hofmann T, Krautwurst D, Raguse JD, Meyerhof W. The human TAS2R16 receptor mediates bitter taste in response to beta-glucopyranosides. Nat Genet. 2002;32:397–401. doi: 10.1038/ng1014. [DOI] [PubMed] [Google Scholar]
- Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Chaudhari N, Landin AM, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nat Neurosci. 2000;3:113–119. doi: 10.1038/72053. [DOI] [PubMed] [Google Scholar]
- Chaudhari N, Roper SD. Molecular and physiological evidence for glutamate (umami) taste transduction via a G protein-coupled receptor. Ann N Y Acad Sci. 1998;855:398–406. doi: 10.1111/j.1749-6632.1998.tb10598.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Herness MS. Electrophysiological actions of quinine on voltage-dependent currents in dissociated rat taste cells. Pflugers Arch. 1997;434:215–226. doi: 10.1007/s004240050388. [DOI] [PubMed] [Google Scholar]
- Clapp TR, Stone LM, Margolskee RF, Kinnamon SC. Immunocytochemical evidence for co-expression of type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci. 2001;2:6. doi: 10.1186/1471-2202-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings TA, Kinnamon SC. Apical K+ channels in Nectu-rus taste cells. Modulation by intracellular factors and taste stimuli. J Gen Physiol. 1992;99:591–613. doi: 10.1085/jgp.99.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damak S, Rong M, Kokrashvilli Z, Yasumatsu K, Glendinning JI, Ninomiya Y, Margolskee RF. Residual responses to bitter, sweet and umami compounds in TRPM5 knockout mice [abstract] Chem Senses. 2005;30:A54. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Boughter JD., Jr Taste transduction: appetizing times in gustation. Neuroreport. 2003;14:905–911. doi: 10.1097/01.wnr.0000074346.81633.76. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Bloom LD, Onishi M, Zheng KH, Damak S, Margolskee RF, Spector AC. Contribution of alpha-gustducin to taste-guided licking responses of mice. Chem Senses. 2005;30:299–316. doi: 10.1093/chemse/bji025. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Gresack J, Spector AC. A high-throughput screening procedure for identifying mice with aberrant taste and oromotor function. Chem Senses. 2002;27:461–474. doi: 10.1093/chemse/27.5.461. [DOI] [PubMed] [Google Scholar]
- Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, Zuker CS. Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell. 1999;96:541–551. doi: 10.1016/s0092-8674(00)80658-3. [DOI] [PubMed] [Google Scholar]
- Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF. Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci. 1999;2:1055–1062. doi: 10.1038/15981. [DOI] [PubMed] [Google Scholar]
- Hwang PM, Verma A, Bredt DS, Snyder SH. Localization of phosphatidylinositol signaling components in rat taste cells: role in bitter taste transduction. Proc Natl Acad Sci USA. 1990;87:7395–7399. doi: 10.1073/pnas.87.19.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci. 2001;4:492–498. doi: 10.1038/87440. [DOI] [PubMed] [Google Scholar]
- Montmayeur JP, Matsunami H. Receptors for bitter and sweet taste. Curr Opin Neurobiol. 2002;12:366–371. doi: 10.1016/s0959-4388(02)00345-8. [DOI] [PubMed] [Google Scholar]
- Mueller KL, Hoon MA, Erlenbach I, Chandrashekar J, Zuker CS, Ryba NJ. The receptors and coding logic for bitter taste. Nature. 2005;434:225–229. doi: 10.1038/nature03352. [DOI] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- Perez CA, Margolskee RF, Kinnamon SC, Ogura T. Making sense with TRP channels: store-operated calcium entry and the ion channel Trpm5 in taste receptor cells. Cell Calcium. 2003;33:541–549. doi: 10.1016/s0143-4160(03)00059-9. [DOI] [PubMed] [Google Scholar]
- Peri I, Mamrud-Brains H, Rodin S, Krizhanovsky V, Shai Y, Nir S, Naim M. Rapid entry of bitter and sweet tastants into liposomes and taste cells: implications for signal transduction. Am J Physiol Cell Physiol. 2000;278:C17–C25. doi: 10.1152/ajpcell.2000.278.1.C17. [DOI] [PubMed] [Google Scholar]
- Rossler P, Boekhoff I, Tareilus E, Beck S, Breer H, Freitag J. G protein betagamma complexes in circumvallate taste cells involved in bitter transduction. Chem Senses. 2000;25:413–421. doi: 10.1093/chemse/25.4.413. [DOI] [PubMed] [Google Scholar]
- Rossler P, Kroner C, Freitag J, Noe J, Breer H. Identification of a phospholipase C beta subtype in rat taste cells. Eur J Cell Biol. 1998;77:253–261. doi: 10.1016/s0171-9335(98)80114-3. [DOI] [PubMed] [Google Scholar]
- Spector AC. Linking gustatory neurobiology to behavior in vertebrates. Neurosci Biobehav Rev. 2000;24:391–416. doi: 10.1016/s0149-7634(00)00013-0. [DOI] [PubMed] [Google Scholar]
- Spielman AI, Nagai H, Sunavala G, Dasso M, Breer H, Boekhoff I, Huque T, Whitney G, Brand JG. Rapid kinetics of second messenger production in bitter taste. Am J Physiol. 1996;270:C926–C931. doi: 10.1152/ajpcell.1996.270.3.C926. [DOI] [PubMed] [Google Scholar]
- St John SJ, Garcea M, Spector AC. Combined, but not single, gustatory nerve transection substantially alters taste-guided licking behavior to quinine in rats. Behav Neurosci. 1994;108:131–140. doi: 10.1037//0735-7044.108.1.131. [DOI] [PubMed] [Google Scholar]
- Striem BJ, Naim M, Lindemann B. Generation of cyclic AMP in taste buds of the rat circumvallate papilla in response to sucrose. Cell Physiol Biochem. 1991;1:46–54. [Google Scholar]
- Wong GT, Gannon KS, Margolskee RF. Transduction of bitter and sweet taste by gustducin. Nature. 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]
- Yan W, Sunavala G, Rosenzweig S, Dasso M, Brand JG, Spielman AI. Bitter taste transduced by PLC-beta(2)-dependent rise in IP(3) and alpha-gustducin-dependent fall in cyclic nucleotides. Am J Physiol Cell Physiol. 2001;280:C742–C751. doi: 10.1152/ajpcell.2001.280.4.C742. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Zhao FL, Lu SG, Herness S. Dual actions of caffeine on voltage-dependent currents and intracellular calcium in taste receptor cells. Am J Physiol Regul Integr Comp Physiol. 2002;283:R115–R129. doi: 10.1152/ajpregu.00410.2001. [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]