Abstract
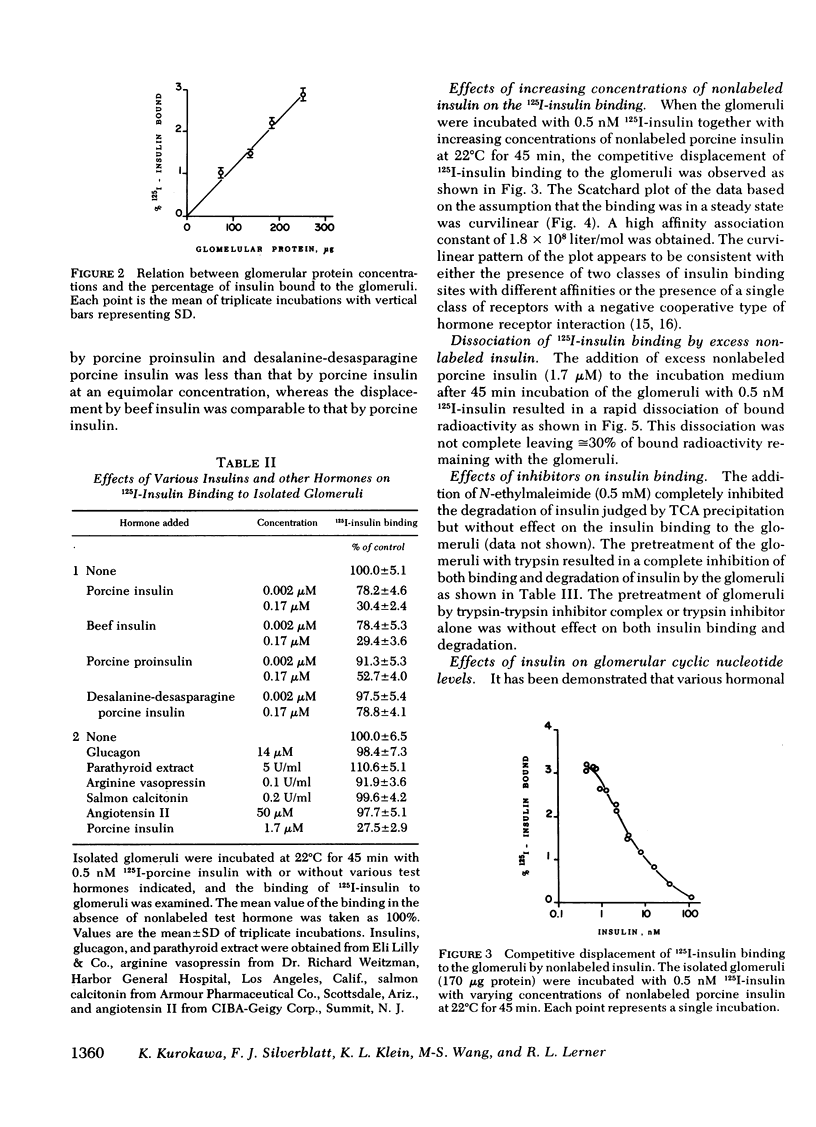
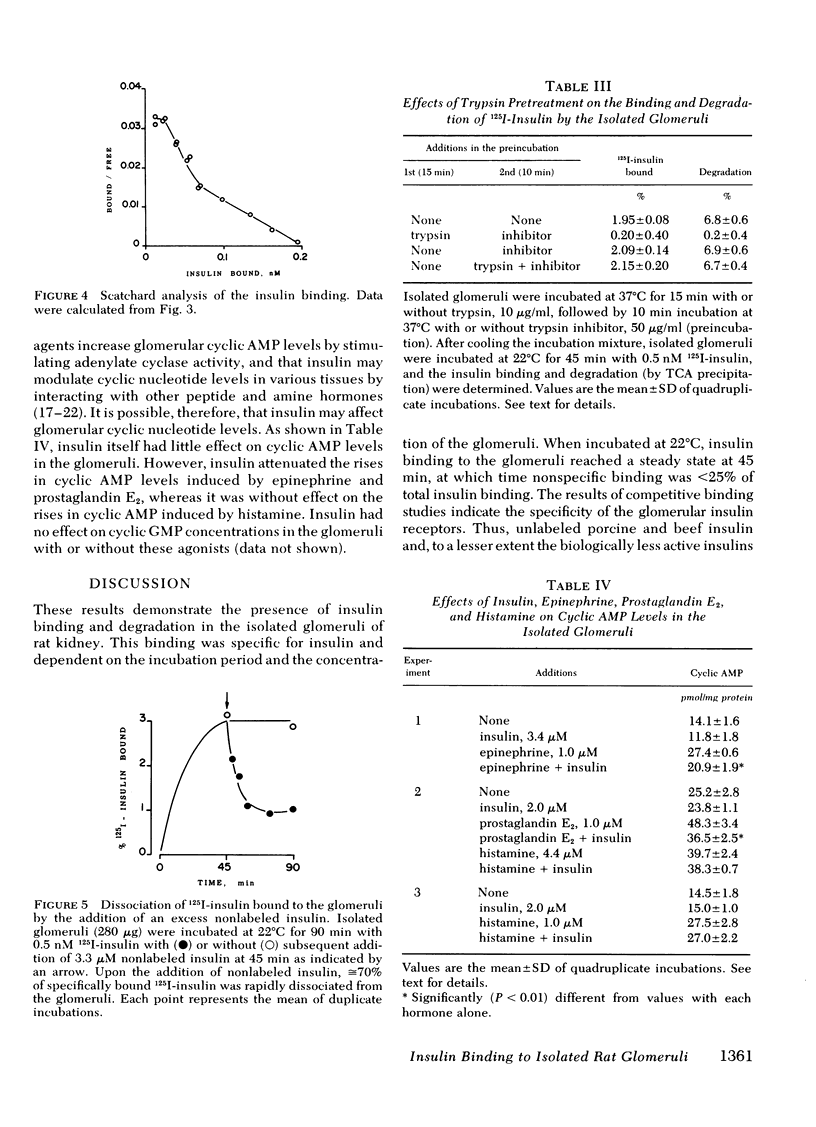
To investigate a possible action of insulin on the glomerulus, the binding 125I-insulin to the isolated glomeruli prepared from rat kidney was examined. When incubated at 22 degrees C, 125I-insulin binding proceeded with time and reached a steady state at 45 min at which time nonspecific binding was less than 25% of total binding. A small fraction of 125I-insulin was degraded during incubation. This binding was specific to insulin in that it was inhibited by unlabeled porcine and beef insulins and to a lesser extent by porcine proinsulin and desalanine-desasparagine insulin, but not by glucagon, parathyroid hormone, vasopressin, calcitonin, and angiotensin II. Increasing concentrations of nonlabeled insulin displaced 125I-insulin binding in a dose-dependent fashion. Scatchard plot of the data was curvilinear consistent with either two classes of receptors with different affinities or a single class of receptors that demonstrate negative cooperativity. The addition of excess nonlabeled insulin to the glomeruli preincubated with 125I-insulin resulted in a rapid dissociation of approximately or equal to 70% of bound 125I-insulin. Insulin decreased the increments in glomerular cyclic AMP levels by epinephrine and by prostaglandin E2, but not those by histamine. These data showed the presence of specific insulin receptors in the glomeruli, and that insulin action may be, at least in part, through modulation of glomerular cyclic AMP concentrations. Such action of insulin may underlie the alteration in glomerular ultrafiltration and the glomerular ultrafiltration and the development of glomerular lesions in diabetes mellitus, a disease in which insulin deficiency or the tissue resistance to insulin exists.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGSTRAND A., BUCHT H. Electron microscopic investigations on the glomerular lesions in diabetes mellitus (diabetic glomerulosclerosis). Lab Invest. 1957 Jul-Aug;6(4):293–300. [PubMed] [Google Scholar]
- Bar R. S., Hoak J. C., Peacock M. L. Insulin receptors in human endothelial cells: identification and characterization. J Clin Endocrinol Metab. 1978 Sep;47(3):699–702. doi: 10.1210/jcem-47-3-699. [DOI] [PubMed] [Google Scholar]
- Baylis C., Deen W. M., Myers B. D., Brenner B. M. Effects of some vasodilator drugs on transcapillary fluid exchange in renal cortex. Am J Physiol. 1976 Apr;230(4):1148–1158. doi: 10.1152/ajplegacy.1976.230.4.1148. [DOI] [PubMed] [Google Scholar]
- Beisswenger P. J. Glomerular basement membrane: biosynthesis and chemical composition in the streptozotocin diabetic rat. J Clin Invest. 1976 Oct;58(4):844–852. doi: 10.1172/JCI108537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. P., Khalifa A. Effect of diabetes and insulin on rat renal glomerular protocollagen hydroxylase activities. Biochim Biophys Acta. 1977 Jan 24;496(1):88–94. doi: 10.1016/0304-4165(77)90117-9. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Membrane receptors. Annu Rev Biochem. 1974;43(0):169–214. doi: 10.1146/annurev.bi.43.070174.001125. [DOI] [PubMed] [Google Scholar]
- De Meyts P., Roth J. Cooperativity in ligand binding: a new graphic analysis. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1118–1126. doi: 10.1016/0006-291x(75)90473-8. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Cooke C. R., Andres R., Faloona G. R., Davis P. J. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest. 1975 Apr;55(4):845–855. doi: 10.1172/JCI107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Goldberg M., Agus Z. S. The effects of glucose and insulin on renal electrolyte transport. J Clin Invest. 1976 Jul;58(1):83–90. doi: 10.1172/JCI108463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzel J., Schwartz M. Abnormally increased glomerular filtration rate in short-term insulin-treated diabetic subjects. Diabetes. 1967 Apr;16(4):264–267. doi: 10.2337/diab.16.4.264. [DOI] [PubMed] [Google Scholar]
- Dousa T. P., Barnes L. D., Ong S. H., Steiner A. L. Immunohistochemical localization of 3':5'-cyclic AMP and 3':5'-cyclic GMP in rat renal cortex: effect of parathyroid hormone. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3569–3573. doi: 10.1073/pnas.74.8.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR M. G., HOPPER J., Jr, MOON H. D. Diabetic glomerulosclerosis: electron and light microscopic studies. Am J Pathol. 1959 Jul-Aug;35(4):721–753. [PMC free article] [PubMed] [Google Scholar]
- Freychet P., Kahn R., Roth J., Neville D. M., Jr Insulin interactions with liver plasma membranes. Independence of binding of the hormone and its degradation. J Biol Chem. 1972 Jun 25;247(12):3953–3961. [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Insulin receptors in the liver: specific binding of ( 125 I)insulin to the plasma membrane and its relation to insulin bioactivity. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1833–1837. doi: 10.1073/pnas.68.8.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Jones A. L., Hradek G. T., Wong K. Y., Mooney J. S. Entry of insulin into human cultured lymphocytes: electron microscope autoradiographic analysis. Science. 1978 Nov 17;202(4369):760–763. doi: 10.1126/science.715440. [DOI] [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Freychet P., LeCam A., Orci L. Intracellular translocation of iodine-125-labeled insulin: direct demonstration in isolated hepatocytes. Science. 1978 May 19;200(4343):782–785. doi: 10.1126/science.644321. [DOI] [PubMed] [Google Scholar]
- Ichikawa I., Brenner B. M. Evidence for glomerular actions of ADH and dibutyryl cyclic AMP in the rat. Am J Physiol. 1977 Aug;233(2):F102–F117. doi: 10.1152/ajprenal.1977.233.2.F102. [DOI] [PubMed] [Google Scholar]
- Ichikawa I., Humes H. D., Dousa T. P., Brenner B. M. Influence of parathyroid hormone on glomerular ultrafiltration in the rat. Am J Physiol. 1978 May;234(5):F393–F401. doi: 10.1152/ajprenal.1978.234.5.F393. [DOI] [PubMed] [Google Scholar]
- KURTZ S. M., FELDMAN J. D. Experimental studies on the formation of the glomerular basement membrane. J Ultrastruct Res. 1962 Feb;6:19–27. doi: 10.1016/s0022-5320(62)90058-8. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Freychet P., Roth J., Neville D. M., Jr Quantitative aspects of the insulin-receptor interaction in liver plasma membranes. J Biol Chem. 1974 Apr 10;249(7):2249–2257. [PubMed] [Google Scholar]
- Khalifa A., Cohen M. P. Glomerular protocollagen lysyl-hydroxylase activity in streptozotocin diabetes. Biochim Biophys Acta. 1975 Mar 28;386(1):332–339. doi: 10.1016/0005-2795(75)90275-5. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. Glomerular filtration rate and renal plasma flow in normal and diabetic man during elevation of blood sugar levels. Scand J Clin Lab Invest. 1971 Oct;28(2):177–182. doi: 10.3109/00365517109086898. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. Kidney function and glomerular permeability to macromolecules in early juvenile diabetes. Scand J Clin Lab Invest. 1971 Sep;28(1):79–90. doi: 10.3109/00365517109090666. [DOI] [PubMed] [Google Scholar]
- Norgaard J. O. A new method for the isolation of ultrastructurally preserved glomeruli. Kidney Int. 1976 Mar;9(3):278–285. doi: 10.1038/ki.1976.30. [DOI] [PubMed] [Google Scholar]
- Olefsky J., Johnson J., Liu F., Edwards P., Baur S. Comparison of 125-I-insulin binding and degradation to isolated rat hepatocytes and liver membranes. Diabetes. 1975 Sep;24(9):801–810. doi: 10.2337/diab.24.9.801. [DOI] [PubMed] [Google Scholar]
- Olefsky J., Reaven G. M. The human lymphocyte: a model for the study of insulin-receptor interaction. J Clin Endocrinol Metab. 1974 Apr;38(4):554–560. doi: 10.1210/jcem-38-4-554. [DOI] [PubMed] [Google Scholar]
- Roth J. Peptide hormone binding to receptors: a review of direct studies in vitro. Metabolism. 1973 Aug;22(8):1059–1073. doi: 10.1016/0026-0495(73)90225-4. [DOI] [PubMed] [Google Scholar]
- STALDER G., SCHMID R. Severe functional disorders of glomerular capillaries and renal hemodynamics in treated diabetes mellitus during childhood. Ann Paediatr. 1959 Sep;193:129–138. [PubMed] [Google Scholar]
- Schlondorff D., Yoo P., Alpert B. E. Stimulation of adenylate cyclase in isolated rat glomeruli by prostaglandins. Am J Physiol. 1978 Nov;235(5):F458–F464. doi: 10.1152/ajprenal.1978.235.5.F458. [DOI] [PubMed] [Google Scholar]
- Spiro R. G., Spiro M. J. Effect of diabetes on the biosynthesis of the renal glomerular basement membrane. Studies on the glucosyltransferase. Diabetes. 1971 Oct;20(10):641–648. doi: 10.2337/diab.20.10.641. [DOI] [PubMed] [Google Scholar]
- Sraer J. D., Sraer J., Ardaillou R., Mimoune O. Evidence for renal glomerular receptors for angiotensin II. Kidney Int. 1974 Oct;6(4):241–246. doi: 10.1038/ki.1974.105. [DOI] [PubMed] [Google Scholar]
- Sraer J., Sraer J. D., Chansel D., Jueppner H., Hesch R. D., Ardaillou R. Evidence for glomerular receptors for parathyroid hormone. Am J Physiol. 1978 Aug;235(2):F96–103. doi: 10.1152/ajprenal.1978.235.2.F96. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Pagliara A. S., Chase L. R., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. II. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in mammalian tissues and body fluids. J Biol Chem. 1972 Feb 25;247(4):1114–1120. [PubMed] [Google Scholar]
- Terris S., Steiner D. F. Binding and degradation of 125I-insulin by rat hepatocytes. J Biol Chem. 1975 Nov 10;250(21):8389–8398. [PubMed] [Google Scholar]
- Torres V. E., Northrup T. E., Edwards R. M., Shah S. V., Dousa T. P. Modulation of cyclic nucleotides in islated rat glomeruli: role of histamine, carbamylcholine, parathyroid hormone, and angiotensin-II. J Clin Invest. 1978 Dec;62(6):1334–1343. doi: 10.1172/JCI109254. [DOI] [PMC free article] [PubMed] [Google Scholar]


