Abstract
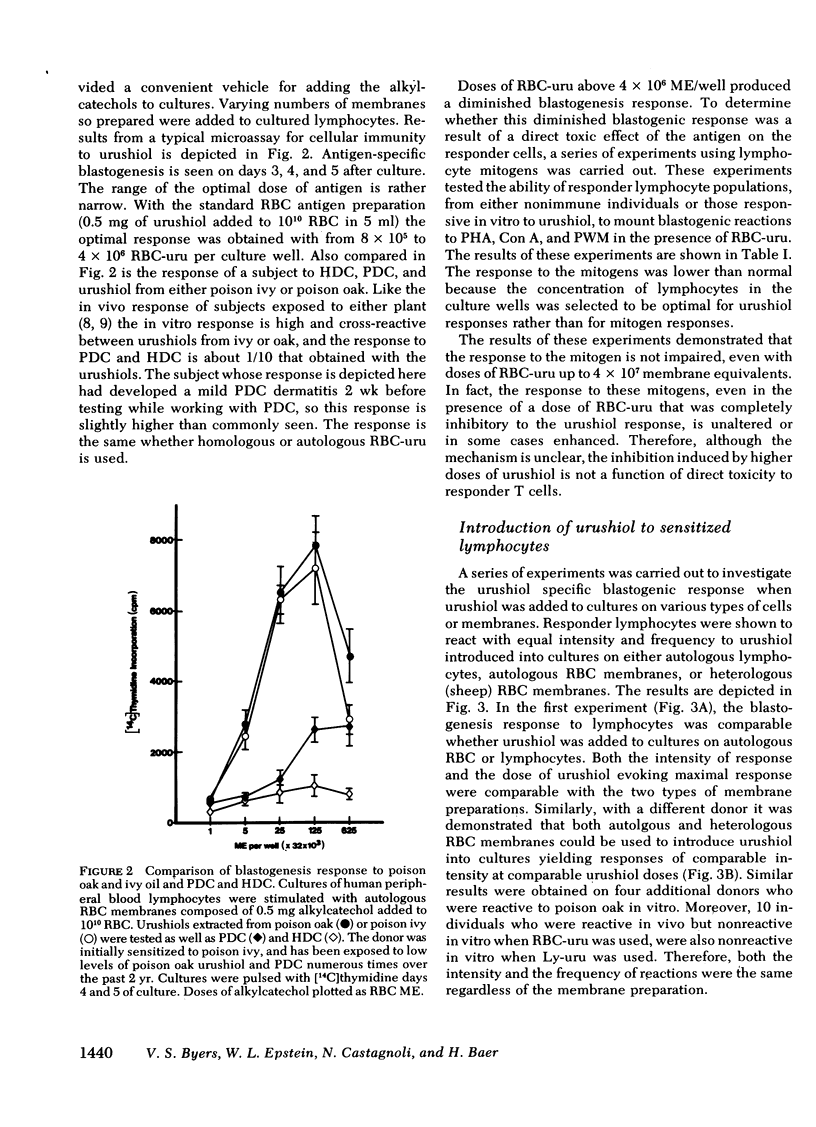
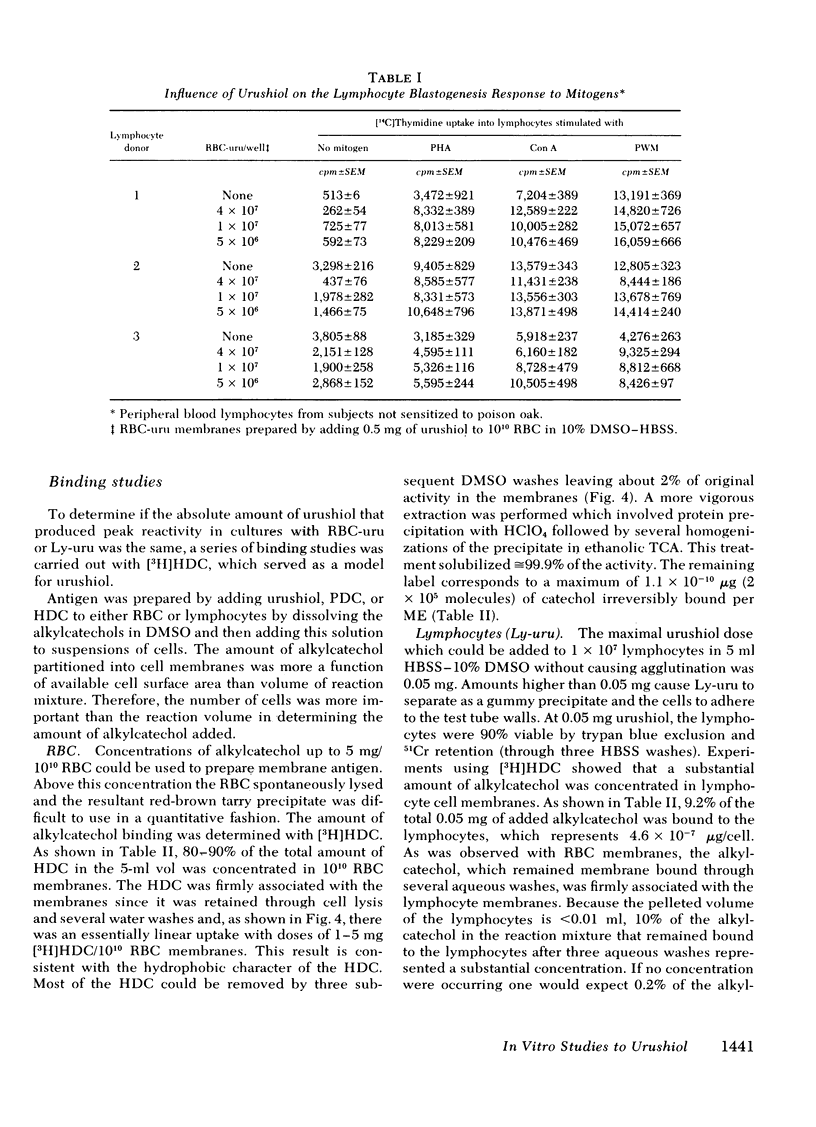
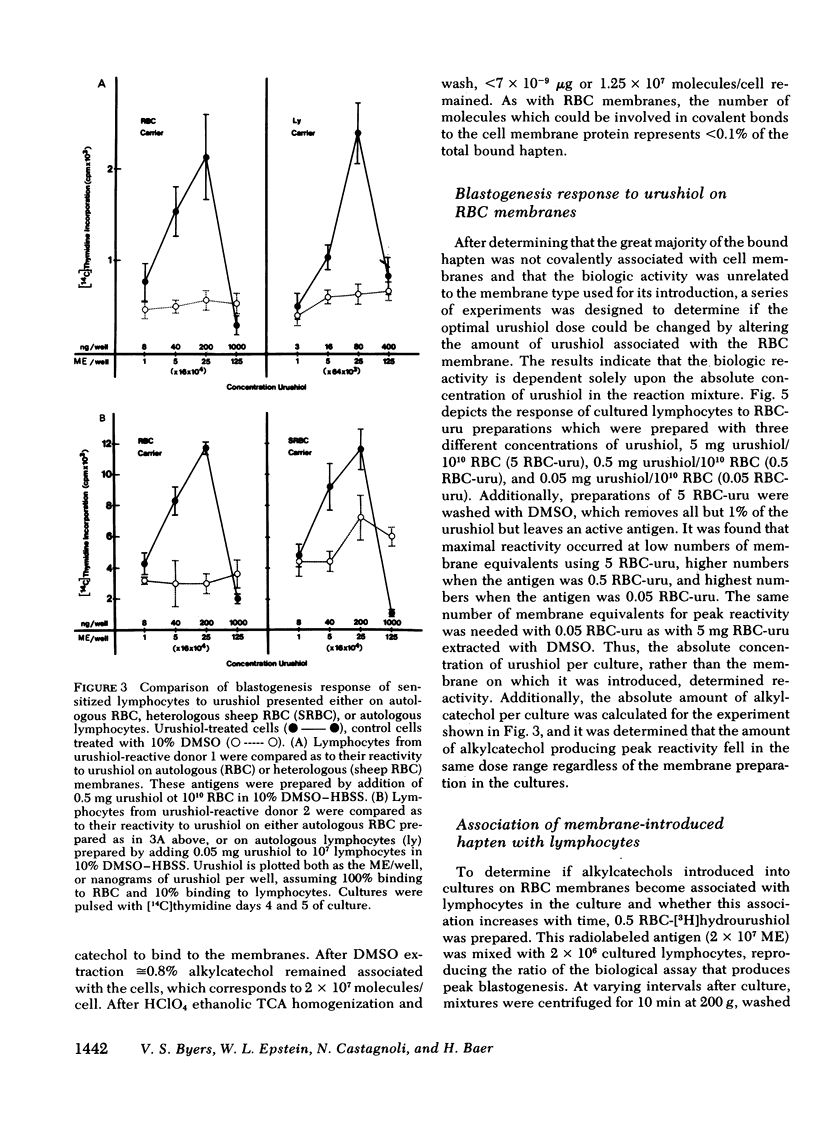
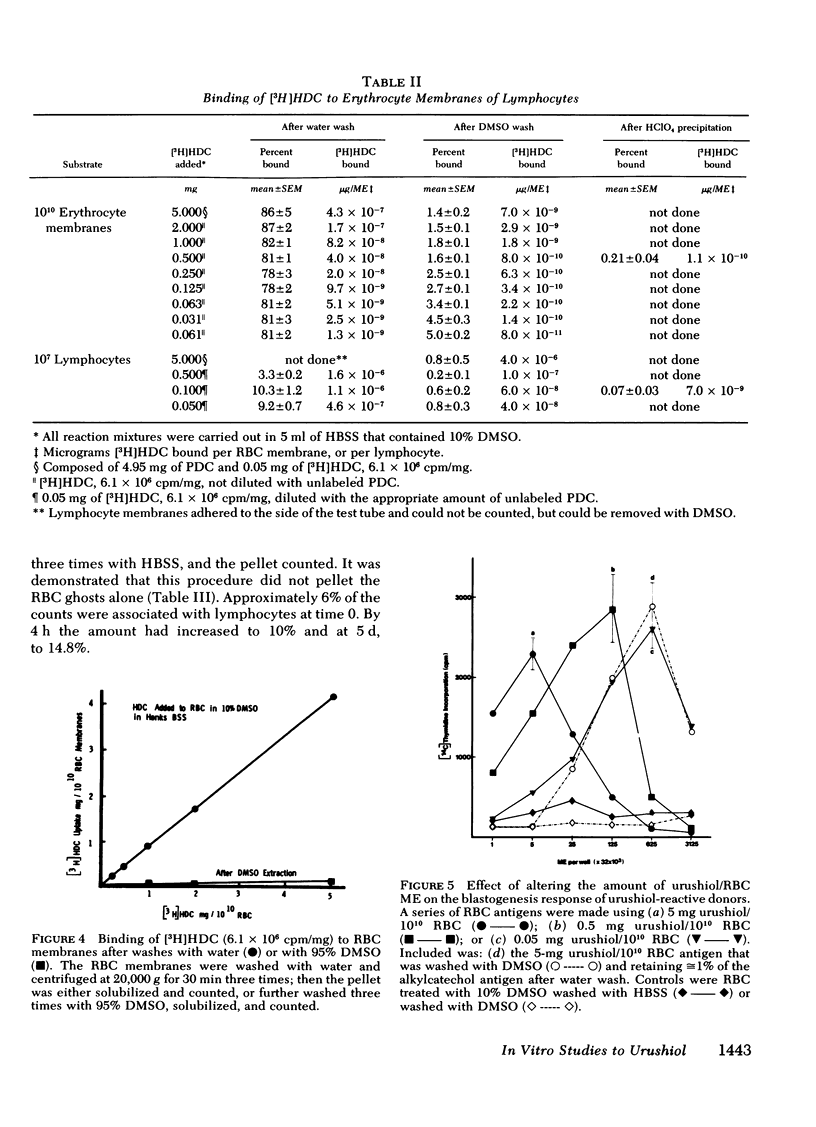
Poison oak, ivy, and sumac dermatitis is a T-cell-mediated reaction against urushiol, the oil found in the leaf of the plants. This hapten is extremely lipophilic and concentrates in cell membranes. A blastogenesis assay employing peripheral blood lymphocytes obtained from humans sensitized to urushiol is described. The reactivity appears 1--3 wk after exposure and persists from 6 wk to 2 mon. The dose-response range is narrow, with inhibition occurring at higher antigen concentrations. Urushiol introduced into the in vitro culture on autologous lymphocytes, erythrocytes and heterologous erythrocytes produces equal results as measured by the optimal urushiol dose, the intensity of reaction, and the frequency of positive reactors. This suggests that the urushiol is passed from introducer to some other presenter cell. Although the blastogenically reactive cell is a T cell, there is also a requirement for an accessory cell, found in the non-T-cell population, for reactivity. Evidence is presented that this cell is a macrophage.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L., Wood P. J., Mayhew B. Control of the immune response. I. Depression of DNA synthesis by immune lymph node cells. Immunology. 1975 Dec;29(6):1057–1065. [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L., Zembala M., Perera M. A., Mayhew B., Thomas W. R. Production of immunity and unresponsiveness in the mouse by feeding contact sensitizing agents and the role of suppressor cells in the peyer's patches, mesenteric lymph nodes and other lymphoid tissues. Cell Immunol. 1977 Sep;33(1):145–155. doi: 10.1016/0008-8749(77)90142-3. [DOI] [PubMed] [Google Scholar]
- Byers V. S., Castagnoli N., Jr, Epstein W. L. In vitro studies of poison oak immunity. II. Effect of urushiol analogues on the human in vitro response. J Clin Invest. 1979 Nov;64(5):1449–1456. doi: 10.1172/JCI109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers V. S., Levin A. S., Johnston J. O., Hackett A. J. Quantitative immunofluorescence studies of the tumor antigen-bearing cell in giant cell tumor of bone and osteogenic sarcoma. Cancer Res. 1975 Sep;35(9):2520–2531. [PubMed] [Google Scholar]
- Corbett M. D., Billets S. Characterization of poison oak urushiol. J Pharm Sci. 1975 Oct;64(10):1715–1718. doi: 10.1002/jps.2600641032. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Forman J., Vitetta E. S., Hart D. A., Klein J. Relationship between trinitrophenyl and H-2 antigens on trinitrophenyl-modified spleen cells. I. H-2 antigens on cells treated with trinitrobenzene sulfonic acid are derivatized. J Immunol. 1977 Mar;118(3):797–802. [PubMed] [Google Scholar]
- Johnson R. A., Haer H., Kirkpatrick C. H., Dawson C. R., Khurana R. G. Comparison of the contact allergenicity of the four pentadecylcatechols derived from poison ivy urushiol in human subjects. J Allergy Clin Immunol. 1972 Jan;49(1):27–35. doi: 10.1016/0091-6749(72)90120-0. [DOI] [PubMed] [Google Scholar]
- KLIGMAN A. M. Poison ivy (Rhus) dermatitis; an experimental study. AMA Arch Derm. 1958 Feb;77(2):149–180. doi: 10.1001/archderm.1958.01560020001001. [DOI] [PubMed] [Google Scholar]
- Li C. Y., Lam K. W., Yam L. T. Esterases in human leukocytes. J Histochem Cytochem. 1973 Jan;21(1):1–12. doi: 10.1177/21.1.1. [DOI] [PubMed] [Google Scholar]
- Littman B. H., David J. R., Rocklin R. E. Migration inhibitory factor (MIF) and proliferative responses by human lymphocyte subpopulations separated by sheep erythrocyte rosette formation. Cell Immunol. 1976 Jun 15;24(2):241–249. doi: 10.1016/0008-8749(76)90209-4. [DOI] [PubMed] [Google Scholar]
- Miller A. E., Levis W. R. Lymphocyte transformation during dinitrochlorobenzene contact sensitization. An in vitro and in vivo evaluation of the primary immune response in man. J Clin Invest. 1973 Aug;52(8):1925–1930. doi: 10.1172/JCI107376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. D., Sy M. S., Claman H. N. Suppressor T cell mechanisms in contact sensitivity. I. Efferent blockade by syninduced suppressor T cells. J Immunol. 1978 Jul;121(1):265–273. [PubMed] [Google Scholar]
- Rosenberg S. A., Lipsky P. E. Monocyte dependence of pokeweed mitogen-induced differentiation of immunoglobulin-secreting cells from human peripheral blood mononuclear cells. J Immunol. 1979 Mar;122(3):926–931. [PubMed] [Google Scholar]
- Vitetta E. S., Hart D. A., Forman J. Relationship between trinitrophenol and H-2 antigens on trinitrophenyl-modified spleen cells. III. Quantitative aspects of trinitrophenol binding on cells treated with trinitrobenzene sulfonic acid. J Immunol. 1978 Sep;121(3):997–1001. [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]


