Abstract
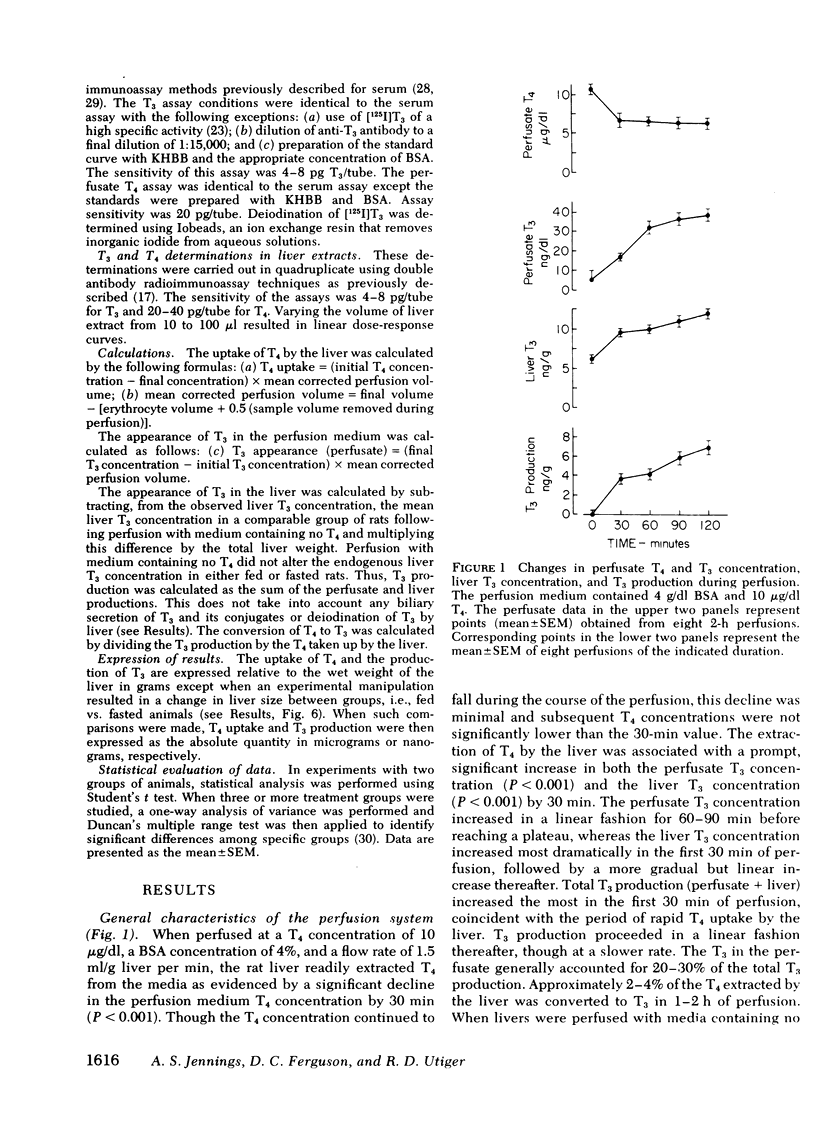
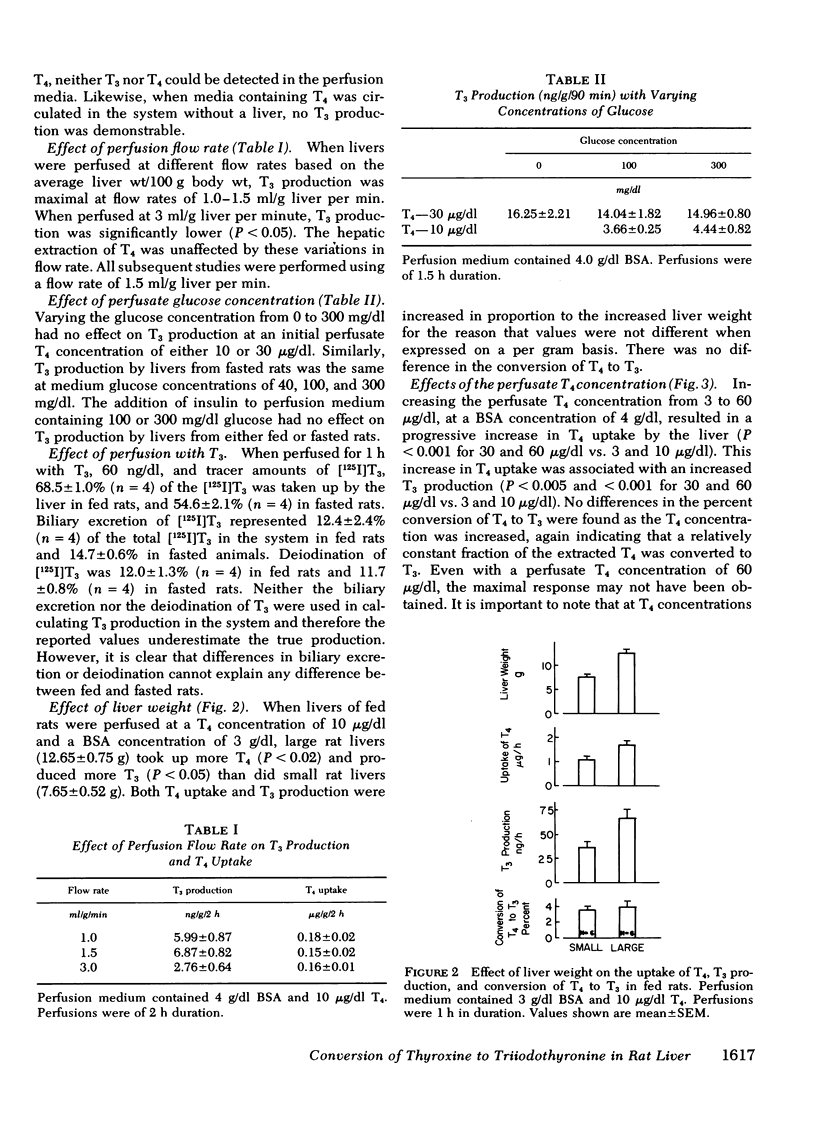
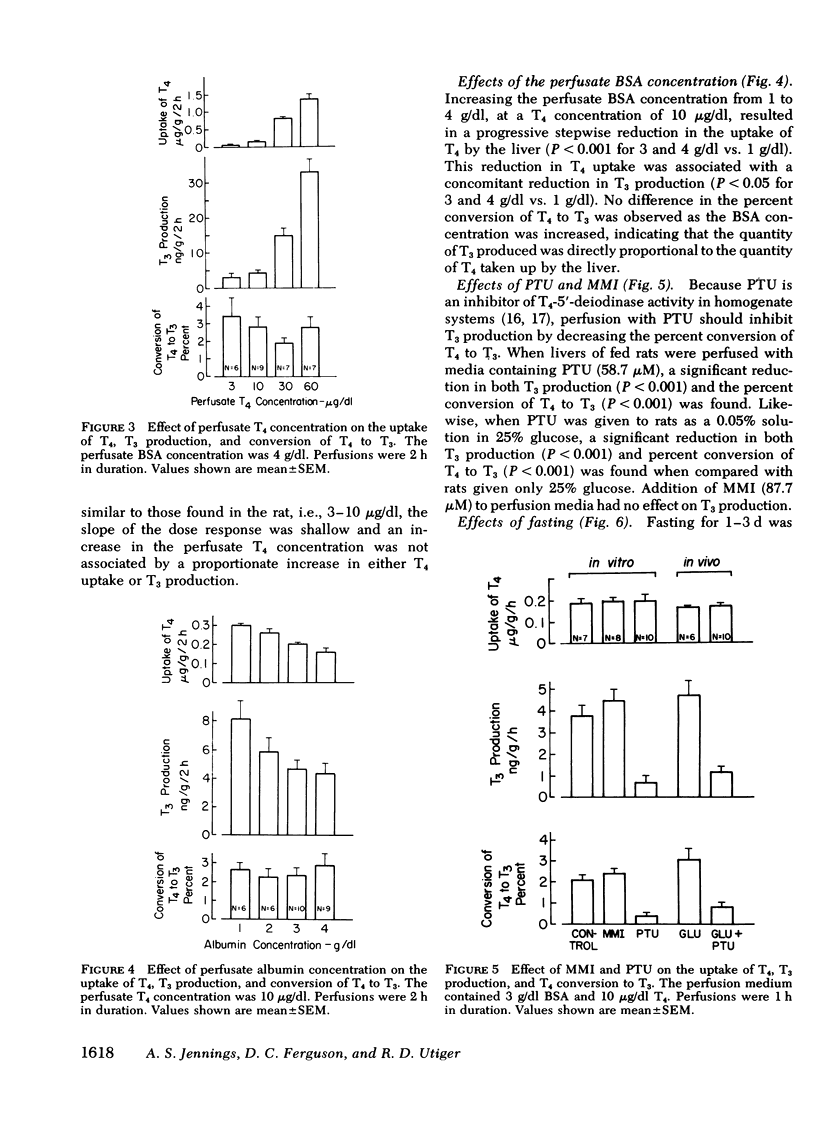
This study was undertaken to determine what factors control the conversion of thyroxine (T4) to triiodothyronine (T3) in rat liver under conditions approximating those found in vivo. Conversion of T4 to T3 was studied in the isolated perfused rat liver, a preparation in which the cellular and structural integrity is maintained and that can perform most of the physiologic functions of the liver. The perfused liver readily extracted T4 from perfusion medium and converted it to T3. Production of T3 by the perfused liver was a function of the size of the liver, the uptake of T4 by the liver, and the presence of T4-5′-deiodinase activity. Production of T3 was increased by increasing the uptake of T4 by liver, which could be accomplished by increasing the liver size, by increasing the perfusate T4 concentration, or by decreasing the perfusate albumin concentration. These changes occurred without altering the conversion of T4 to T3. The liver had a large capacity for extracting T4 and for T4-5′-deiodination to T3, which was not saturated at a T4 concentration of 60 μg/dl. Production of T3 was decreased by inhibiting hepatic T4-5′-deiodinase with propylthiouracil, which decreased T3 production by decreasing the conversion of T4 to T3. Propylthiouracil did not alter hepatic T4 uptake.
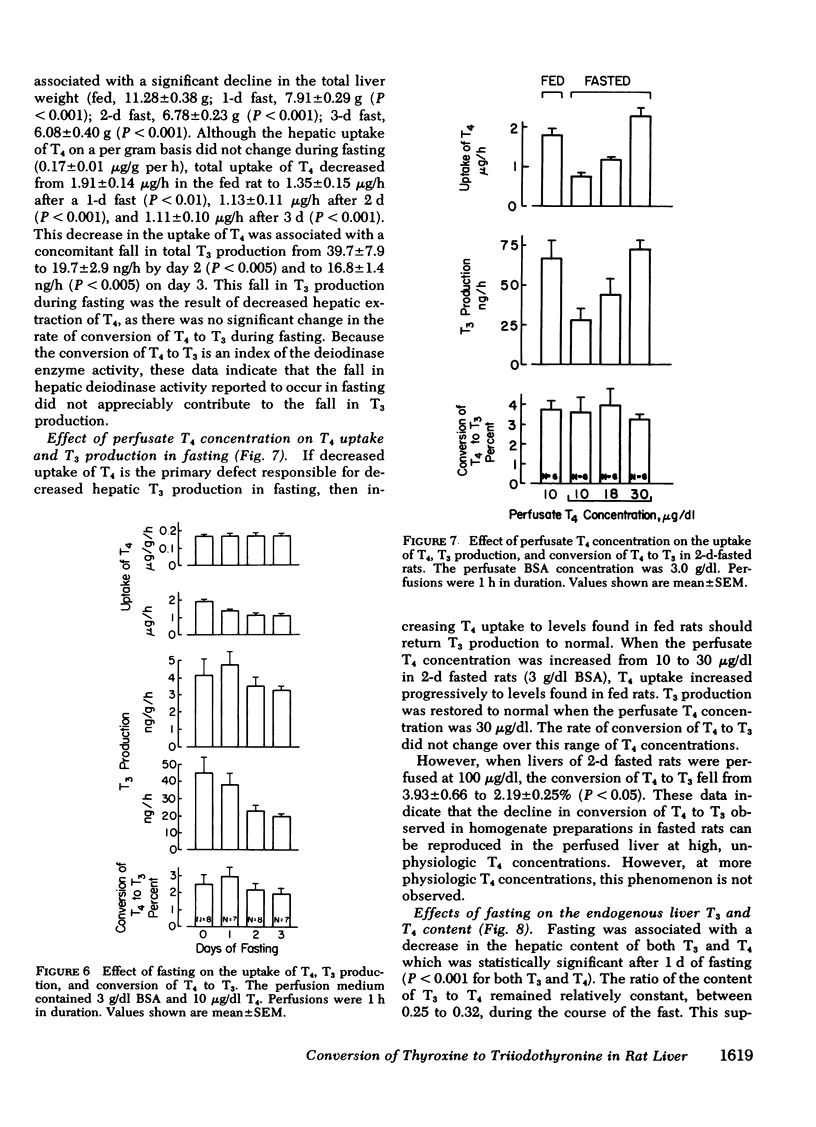
Fasting resulted in a progressive decrease in hepatic T4 uptake to 42% of control levels by the 3rd d of fasting; this was accompanied by a proportionate decrease in T3 production. The rate of conversion of T4 to T3 did not change during fasting. When T4 uptake in 2-d-fasted rat livers was raised to levels found in fed rats by increasing the perfusate T4 concentration from 10 to 30 μg/dl, T3 production returned to normal. Again, no change in the rate of conversion of T4 to T3 was observed.
These results indicate that the decreased hepatic T3 production during fasting primarily results from decreased hepatic uptake of T4, rather than from changes in T4-5′-deiodinase activity. Thus, these studies have delineated a new mechanism that functions independently of enzyme quantity or activity whereby production of T3 from T4 is regulated.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBRIGHT E. C., LARSON F. C., TUST R. H. In vitro conversion of thyroxin to triiodothyronine by kidney slices. Proc Soc Exp Biol Med. 1954 May;86(1):137–140. doi: 10.3181/00379727-86-21031. [DOI] [PubMed] [Google Scholar]
- BECKER D. V., PRUDDEN J. F. The metabolism of I 131-labeled thyroxine, triiodothyronine and diiodotyrosine by an isolated perfused rabbit liver. Endocrinology. 1959 Jan;64(1):136–148. doi: 10.1210/endo-64-1-136. [DOI] [PubMed] [Google Scholar]
- Balsam A., Ingbar S. H. Observations on the factors that control the generation of triiodothyronine from thyroxine in rat liver and the nature of the defect induced by fasting. J Clin Invest. 1979 Jun;63(6):1145–1156. doi: 10.1172/JCI109408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsam A., Ingbar S. H. The influence of fasting, diabetes, and several pharmacological agents on the pathways of thyroxine metabolism in rat liver. J Clin Invest. 1978 Aug;62(2):415–424. doi: 10.1172/JCI109143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsam A., Sexton F., Ingbar S. H. The effect of thyroidectomy, hypophysectomy, and hormone replacement on the formation of triiodothyronine from thyroxine in rat liver and kidney. Endocrinology. 1978 Nov;103(5):1759–1767. doi: 10.1210/endo-103-5-1759. [DOI] [PubMed] [Google Scholar]
- Braverman L. E., Ingbar S. H., Sterling K. Conversion of thyroxine (T4) to triiodothyronine (T3) in athyreotic human subjects. J Clin Invest. 1970 May;49(5):855–864. doi: 10.1172/JCI106304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman L. E., Vagenakis A., Downs P., Foster A. E., Sterling K., Ingbar S. H. Effects of replacement doses of sodium L-thyroxine on the peripheral metabolism of thyroxine and triiodothyronine in man. J Clin Invest. 1973 May;52(5):1010–1017. doi: 10.1172/JCI107265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman K. D., Lukes Y., Wright F. D., Wartofsky L. Reduction in hepatic triiodothyronine binding capacity induced by fasting. Endocrinology. 1977 Oct;101(4):1331–1334. doi: 10.1210/endo-101-4-1331. [DOI] [PubMed] [Google Scholar]
- Cavalieri R. R. Impaired peripheral conversion of thyroxine to triiodothyronine,. Annu Rev Med. 1977;28:57–65. doi: 10.1146/annurev.me.28.020177.000421. [DOI] [PubMed] [Google Scholar]
- Cavalieri R. R., Searle G. L. The kinetics of distribution between plasma and liver of 131-I-labeled L-thyroxine in man: observations of subjects with normal and decreased serum thyroxine-binding globulin. J Clin Invest. 1966 Jun;45(6):939–949. doi: 10.1172/JCI105409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I. J. A radioimmunoassay for measurement of 3,3',5'-triiodothyronine (reverse T3). J Clin Invest. 1974 Sep;54(3):583–592. doi: 10.1172/JCI107795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I. J. A radioimmunoassay for measurement of thyroxine in unextracted serum. J Clin Endocrinol Metab. 1972 Jun;34(6):938–947. doi: 10.1210/jcem-34-6-938. [DOI] [PubMed] [Google Scholar]
- Chopra I. J. A study of extrathyroidal conversion of thyroxine (T4) to 3,3',5-triiodothyronine (T3) in vitro. Endocrinology. 1977 Aug;101(2):453–463. doi: 10.1210/endo-101-2-453. [DOI] [PubMed] [Google Scholar]
- Chopra I. J. An assessment of daily production and significance of thyroidal secretion of 3, 3', 5'-triiodothyronine (reverse T3) in man. J Clin Invest. 1976 Jul;58(1):32–40. doi: 10.1172/JCI108456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I. J. Sulfhydryl groups and the monodeiodination of thyroxine to triiodothyronine. Science. 1978 Feb 24;199(4331):904–906. doi: 10.1126/science.622575. [DOI] [PubMed] [Google Scholar]
- DOWLING J. T., FREINKEL N., INGBAR S. H. The influence of extracellular thyroxine-binding protein upon the accumulation of thyroxine by tissue slices. J Clin Invest. 1957 Jan;36(1 Pt 1):25–37. doi: 10.1172/JCI103406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P. J., Handwerger B. S., Glaser F. Physical properties of a dog liver and kidney cytosol protein that binds thyroid hormone. J Biol Chem. 1974 Oct 10;249(19):6208–6217. [PubMed] [Google Scholar]
- Eisenstein Z., Hagg S., Vagenakis A. G., Fang S. L., Ransil B., Burger A., Balsam A., Braverman L. E., Ingbar S. H. Effect of starvation on the production and peripheral metabolism of 3,3',5'-triiodothyronine in euthyroid obese subjects. J Clin Endocrinol Metab. 1978 Oct;47(4):889–893. doi: 10.1210/jcem-47-4-889. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Park C. R. Control of gluconeogenesis in liver. I. General features of gluconeogenesis in the perfused livers of rats. J Biol Chem. 1967 Jun 10;242(11):2622–2636. [PubMed] [Google Scholar]
- Exton J. H. The perfused rat liver. Methods Enzymol. 1975;39:25–36. doi: 10.1016/s0076-6879(75)39006-x. [DOI] [PubMed] [Google Scholar]
- FLOCK E. V., BOLLMAN J. L. The metabolism of thyroxine and triiodothyronine in the eviscerated rat. J Biol Chem. 1955 Jun;214(2):709–721. [PubMed] [Google Scholar]
- Flock E. V., Owen C. A., Jr Metabolism of thyroid hormones and some derivatives in isolated, perfused rat liver. Am J Physiol. 1965 Nov;209(5):1039–1045. doi: 10.1152/ajplegacy.1965.209.5.1039. [DOI] [PubMed] [Google Scholar]
- GRINBERG R., VOLPERT E. M., WERNER S. C. In vivo deiodination of labeled L-thyroxine to L-3,5,3'-triiodothyronine in mouse and human pituitaries. J Clin Endocrinol Metab. 1963 Feb;23:140–142. doi: 10.1210/jcem-23-2-140. [DOI] [PubMed] [Google Scholar]
- Gorman C. A., Flock E. V., Owen C. A., Jr, Paris J. Factors affecting exchange of thyroid hormones between liver and blood. Endocrinology. 1966 Aug;79(2):391–405. doi: 10.1210/endo-79-2-391. [DOI] [PubMed] [Google Scholar]
- Green W. L. Metabolism of thyroid hormones by rat thyroid tissue in vitro. Endocrinology. 1978 Sep;103(3):826–837. doi: 10.1210/endo-103-3-826. [DOI] [PubMed] [Google Scholar]
- HOGNESS J. R., BERG M., VANARSDEL P. P., Jr, WILLIAMS R. H. Tissue conversion of thyroxine to triiodothyronine. Proc Soc Exp Biol Med. 1955 Oct;90(1):93–97. doi: 10.3181/00379727-90-21949. [DOI] [PubMed] [Google Scholar]
- Harris A. R., Fang S. L., Hinerfeld L., Braverman L. E., Vagenakis A. G. The role of sulfhydryl groups on the impaired hepatic 3',3,5-triiodothyronine generation from thyroxine in the hypothyroid, starved, fetal, and neonatal rodent. J Clin Invest. 1979 Mar;63(3):516–524. doi: 10.1172/JCI109330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. R., Fang S. L., Prosky J., Braverman L. E., Vagenakis A. G. Decreased outer ring monodeiodination of thyroxine and reverse triiodothyronine in the fetal and neonatal rat. Endocrinology. 1978 Dec;103(6):2216–2222. doi: 10.1210/endo-103-6-2216. [DOI] [PubMed] [Google Scholar]
- Harris A. R., Fang S. L., Vagenakis A. G., Braverman L. E. Effect of starvation, nutriment replacement, and hypothyroidism on in vitro hepatic T4 to T3 conversion in the rat. Metabolism. 1978 Nov;27(11):1680–1690. doi: 10.1016/0026-0495(78)90290-1. [DOI] [PubMed] [Google Scholar]
- Hillier A. P. The mechanism of thyroxine transfer from plasma to tissue binding sites. J Physiol. 1971 Sep;217(3):635–639. doi: 10.1113/jphysiol.1971.sp009590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. M. Subcellular alterations causing reduced hepatic thyroxine-5'-monodeiodinase activity in fasted fats. Endocrinology. 1979 Jan;104(1):58–64. doi: 10.1210/endo-104-1-58. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Utiger R. D. Iodothyronine metabolism in liver and kidney homogenates from hyperthyroid and hypothyroid rats. Endocrinology. 1978 Jul;103(1):156–161. doi: 10.1210/endo-103-1-156. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Utiger R. D. Iodothyronine metabolism in rat liver homogenates. J Clin Invest. 1978 Feb;61(2):459–471. doi: 10.1172/JCI108957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochupillai N., Yalow R. S. Preparation, purification, and stability of high specific activity 125I-labeled thyronines. Endocrinology. 1978 Jan;102(1):128–135. doi: 10.1210/endo-102-1-128. [DOI] [PubMed] [Google Scholar]
- Lichter M., Fleischner G., Kirsch R., Levi A. J., Kamisaka K., Arias I. M. Ligandin and Z protein in binding of thyroid hormones by the liver. Am J Physiol. 1976 Apr;230(4):1113–1120. doi: 10.1152/ajplegacy.1976.230.4.1113. [DOI] [PubMed] [Google Scholar]
- Lieblich J., Utiger R. D. Triiodothyronine radioimmunoassay. J Clin Invest. 1972 Jan;51(1):157–166. doi: 10.1172/JCI106786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLAGAN N. F., SPROTT W. E. The in-vitro deiodination of thyroxine and triiodothyronine. Lancet. 1954 Aug 21;267(6834):368–369. [PubMed] [Google Scholar]
- Oppenheimer J. H., Bernstein G., Surks M. I. Increased thyroxine turnover and thyroidal function after stimulation of hepatocellular binding of thyroxine by phenobarbital. J Clin Invest. 1968 Jun;47(6):1399–1406. doi: 10.1172/JCI105831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Koerner D., Surks M. I. Limited binding capacity sites for L-triiodothyronine in rat liver nuclei. Nuclear-cytoplasmic interrelation, binding constants, and cross-reactivity with L-thyroxine. J Clin Invest. 1974 Mar;53(3):768–777. doi: 10.1172/JCI107615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITT-RIVERS R., STANBURY J. B., RAPP B. Conversion of thyroxine to 3-5-3'-triiodothyronine in vivo. J Clin Endocrinol Metab. 1955 May;15(5):616–620. doi: 10.1210/jcem-15-5-616. [DOI] [PubMed] [Google Scholar]
- Pittman C. S., Chambers J. B., Jr, Read V. H. The extrathyroidal conversion rate of thyroxine to triiodothyronine in normal man. J Clin Invest. 1971 Jun;50(6):1187–1196. doi: 10.1172/JCI106596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliam N. B., Goldfine I. D. High affinity thyroid hormone binding sites on purified rat liver plasma membranes. Biochem Biophys Res Commun. 1977 Nov 7;79(1):166–172. doi: 10.1016/0006-291x(77)90075-4. [DOI] [PubMed] [Google Scholar]
- RALL J. E., RAWSON R. W., TATA J. R. Metabolism of L-thyroxine and L-3:5:3'-triiodothyronine by brain tissue preparations. Endocrinology. 1957 Jan;60(1):83–98. doi: 10.1210/endo-60-1-83. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Tsai J. S. Thyroid hormone action. Demonstration of similar receptors in isolated nuclei of rat liver and cultured GH1 cells. J Clin Invest. 1974 Feb;53(2):656–659. doi: 10.1172/JCI107601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel M., Utiger R. D. Thyroidal and peripheral production of thyroid hormones. Review of recent findings and their clinical implications. Ann Intern Med. 1977 Dec;87(6):760–768. doi: 10.7326/0003-4819-87-6-760. [DOI] [PubMed] [Google Scholar]
- Schussler G. C., Orlando J. Fasting decreases triiodothyronine receptor capacity. Science. 1978 Feb 10;199(4329):686–688. doi: 10.1126/science.204004. [DOI] [PubMed] [Google Scholar]
- Schwartz H. L., Bernstein G., Oppenheimer J. H. Effect of phenobarbital administration on the subcellular distribution of 125-I-thyroxine in rat liver: mportance of microsomal binding. Endocrinology. 1969 Feb;84(2):270–276. doi: 10.1210/endo-84-2-270. [DOI] [PubMed] [Google Scholar]
- Stein L. B., Mishkin S., Fleischner G., Gatmaitan Z., Arias I. M. Effect of fasting on hepatic ligandin, Z protein, and organic anion transfer from plasma in rats. Am J Physiol. 1976 Nov;231(5 Pt 1):1371–1376. doi: 10.1152/ajplegacy.1976.231.5.1371. [DOI] [PubMed] [Google Scholar]
- Sterling K., Milch P. O., Brenner M. A., Lazarus J. H. Thyroid hormone action: the mitochondrial pathway. Science. 1977 Sep 2;197(4307):996–999. doi: 10.1126/science.196334. [DOI] [PubMed] [Google Scholar]
- Sterling K., Milch P. O. Thyroid hormone binding by a component of mitochondrial membrane. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3225–3229. doi: 10.1073/pnas.72.8.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surks M. I., Schadlow A. R., Stock J. M., Oppenheimer J. H. Determination of iodothyronine absorption and conversion of L-thyroxine (T 4 ) to L-triiodothyronine (T 3 ) using turnover rate techniques. J Clin Invest. 1973 Apr;52(4):805–811. doi: 10.1172/JCI107244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeber K. A. L-triiodothyronine and L-reverse-triiodothyronine generation in the human polymorphonuclear leukocyte. J Clin Invest. 1978 Sep;62(3):577–584. doi: 10.1172/JCI109163. [DOI] [PMC free article] [PubMed] [Google Scholar]


