SUMMARY
Developmental dyslexia is a reading disorder, yet deficits also manifest in the magnocellular-dominated dorsal visual system. Uncertainty about whether visual deficits are causal or consequential to reading disability encumbers accurate identification and appropriate treatment of this common learning disability. Using fMRI, we demonstrate in typical readers a relationship between reading ability and activity in area V5/MT during visual motion processing and, as expected, also found lower V5/MT activity for dyslexic children compared to age-matched controls. However, when dyslexics were matched to younger controls on reading ability, no differences emerged, suggesting that weakness in V5/MT may not be causal to dyslexia. To further test for causality, dyslexics underwent a phonological-based reading intervention. Surprisingly, V5/MT activity increased along with intervention-driven reading gains, demonstrating that activity here is mobilized through reading. Our results provide strong evidence that visual magnocellular dysfunction is not causal to dyslexia, but may instead be consequential to impoverished reading.
Keywords: Developmental Dyslexia, Reading Disability, Visual Magnocelluar System, Causality, Reading-Level Design, fMRI, V5/MT, Motion Processing, Intervention
INTRODUCTION
Developmental dyslexia is a common learning disability affecting 8–12% (Rutter et al., 2004) of the population who, as a manifestation of the disorder, struggle to learn to read accurately and fluently (Peterson and Pennington, 2012). The causal mechanisms remain a matter of debate and whilst a linguistically-based theory on weakness in phonological coding (the ability to isolate and manipulate sounds within words) stands as the most widely accepted explanation for dyslexics’ reading problems (Vellutino et al., 2004), other theoretical models remain compelling. Specifically, early psychophysical experiments using sinusoidal gratings demonstrated impaired contrast sensitivity functions in dyslexic individuals under conditions of low spatial and high temporal frequency (Lovegrove et al., 1980), properties known to be subserved by neurons in the magnocellular layers of the lateral geniculate nucleus (LGN - Shapley, 1990). The discovery of size discrepancies in the neurons of the magnocellular layers of the LGN between dyslexics and controls at post-mortem (Livingstone et al., 1991) further fueled the advancement of a transient or magnocellular visual deficit theory of dyslexia (Stein, 2001; Stein and Walsh, 1997). More recently this theory has been bolstered by numerous behavioral and brain imaging studies (Boden and Giaschi, 2007) employing paradigms that rely on the cortical dorsal extensions to the subcortical magnocellular systems (Ungerleider and Mishkin, 1982) including areas V5/MT, MST and parietal cortex. Specifically, individuals with dyslexia of different age groups and language backgrounds show reduced coherent motion detection and speed discrimination compared to controls (Cornelissen et al., 1995; Demb et al., 1997; Hansen et al., 2001; Heim et al., 2010; Meng et al., 2011; Talcott et al., 2000, 2003; Witton et al., 1998), and functional brain imaging studies have revealed reduced or no activation in area V5/MT (Demb et al., 1997; Eden et al., 1996; Heim et al., 2010; but see Vanni et al., 1997).
Understanding the role of the visual magnocellular deficits in dyslexia is critical for the early identification and successful treatment of reading disability. As a precursor to reading problems, magnocellular integrity could serve as an early screening device for children at risk for dyslexia. As a cause of the reading problems, magnocellular function could become integral to treatment. However, the issue of causality is pivotal, as visual magnocellular dysfunction could be an epiphenomenon of the reading disorder rather than its cause. Demonstration of causality has been practiced in studies investigating phonological deficits in dyslexia, and is best achieved via a two-step process (Goswami, 2003): First, a reading level-match design is used, whereby dyslexic children are not only contrasted to chronological age-matched controls, but also younger normal readers matched to the dyslexics on reading level. Deficits manifested in the dyslexics compared to both the age-matched and reading level-matched groups would suggest a causal role in dyslexia (because the dyslexics are impaired given both their developmental and reading level). This can then be tested further by assessing the efficacy of an intervention addressing the same deficit. Such studies (behavioral and more recently brain imaging) have been used to demonstrate not only that there is a causal relationship of phonological awareness on reading (Bradley and Bryant, 1983; Frith and Snowling, 1983; Olson et al., 1989; Snowling, 1980; Hoeft et al., 2006, 2007), but also that there are beneficial effects on reading following phonological training (Alexander and Slinger-Constant, 2004; Eden et al., 2004).
Here we first confirmed the existence of a relationship between reading ability and brain activity in area V5/MT during the perception of visual motion, allowing us to establish agreement with prior studies. Specifically, earlier work reported correlations between reading aptitude and behavioral performance on magnocellular visual tasks (Talcott et al., 2000; Wilmer et al., 2004; Witton et al., 1998) and parallel work has examined the relationship between reading proficiency and brain activity collected during magnocellular tasks (Ben-Shachar et al., 2007; Demb et al., 1997). The latter studies (Ben-Shachar et al., 2007; Demb et al., 1997) employed sinusoidal grating stimuli, whilst the former behavioral studies often employed tasks involving coherent motion random dot kinematogram stimuli. Our first experiment demonstrated consistency with this literature as we found (a) activity in area V5/MT in response to the perception of visual motion in a large group of adults and children with normal reading skills and (b) a correlation between the strength of this V5/MT signal and reading proficiency as measured on standardized tests.
Having verified this relationship for our task, we then used the same task to compare activity in area V5/MT between dyslexic children and their age-matched as well as reading level-matched controls. Between-group differences for both types of comparisons would suggest a causal role for the visual magnocellular deficit, and pave the way for an intervention study that trains the magnocellular visual system, with the goal of improving reading skills. However, our study, while showing differences between dyslexics and controls matched on age, failed to demonstrate differences for the reading level-match comparison, thereby instead suggesting the possibility that altered visual magnocellular function represents an epiphenomenon of dyslexia. That is, magnocellular dysfunction may be a side effect of dyslexia, emerging along with other deficits that are the primary cause of the reading problem (Eden and Zeffiro, 1998; Ramus, 2004). Alternatively, it is possible that magnocellular dysfunction is not actually related to dyslexia per se, but merely reflects magnocellular function in the context of a person’s reading experience. In the case of dyslexia, impoverished visual magnocellular function may simply be the effect of less reading experience. This hypothesis seems reasonable given that visual motion perception improves with age in typically reading children at a time when reading acquisition occurs (Boets et al., 2011), and children exhibit poorer performance on these tasks when compared to adults (Boets et al., 2011; Mitchell and Neville, 2004), suggesting that learning to read may actually “mobilize” the visual magnocellular system. In our third experiment, we tested this specific hypothesis by providing a phonological-based reading intervention (rather than a magnocellular-based intervention), and found that in addition to the expected behavioral gains in phonological awareness and reading, children with dyslexia showed an increase in V5/MT activity following the intervention. Together these results demonstrate that the visual magnocellular dysfunction measured via activity in V5/MT reported in dyslexia by us (Eden et al., 1996) and others (Demb et al., 1997; Heim et al., 2010), as well as the behavioral deficits reported for a range of visual magnocellular tasks (Cornelissen et al., 1995; Hansen et al., 2001; Meng et al., 2011; Talcott et al., 2000, 2003; Witton et al., 1998), is a consequence of reading disability rather than its cause.
RESULTS
Experiment 1: Correlation between Reading Ability and V5/MT Activity
Thirty typically reading individuals participated in the first experiment and included thirteen females and seventeen males with an age span of 7.3 to 31.5 years (mean ± SD: 22.0 ± 6.1). Subjects were selected such that real word reading (Woodcock Johnson (WJ-III) (Woodcock et al., 2001) Word Identification – WID) and pseudoword reading (WJ-III Word Attack – WA) were largely representative of the normal range (WID: range: 94 – 120; mean ± SD: 109 ± 7; WA: range: 93 – 120; mean ± SD: 106 ± 8). Their intelligence also was within or above the normal range, as measured by the Wechsler Abbreviated Scale of Intelligence (WASI (Wechsler, 1999) Full Scale IQ: range: 95 – 137; mean ± SD: 121 ± 9).
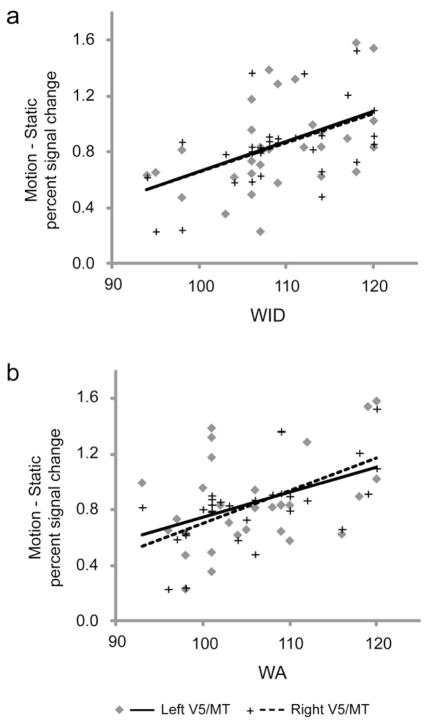
fMRI data were collected during a motion direction detection task (Motion) and a static density detection control task (Static). We identified the V5/MT region of interest bilaterally in each subject individually via the contrast of Motion vs. Static (see Experimental Procedures for details), and correlated average percent signal change within these subject-specific regions for this contrast with standardized measures of real and pseudoword reading. Average Talairach co-ordinates of area V5/MT in these subjects were: Left: −46±5, −71±5, 9±4; Right: 47±5, −67±5, 7±5, similar to those reported in previous studies (Dumoulin et al., 2000; Eden et al., 1996; Mendola et al., 1999; Tootell et al., 1995; Watson et al., 1993). Activity in bilateral V5/MT was significantly correlated with age-referenced, standardized scores for both WID (left V5/MT: r = 0.46; p = 0.009; right V5/MT: r = 0.52; p = 0.003; two-tailed; Figure 1a) and WA (left V5/MT: r = 0.41; p = 0.024; right V5/MT: r = 0.61; p = 0.0003; two-tailed; Figure 1b). Similar correlation analyses with Static vs. a resting baseline (Fixation) condition revealed no relationships with these measures of reading (all p > 0.1), providing further evidence for the specificity of the relationship between motion perception and reading ability independent of age. All subjects performed with high accuracy (ACC) on the in-scanner Motion (ACC mean ± SD (%): 98.8 ± 2.5; Reaction Time (RT) mean ± SD (ms): 1079 ± 351) and Static (ACC mean ± SD (%): 99.7 ± 1.0; RT mean ± SD (ms): 857 ± 203) tasks.
Figure 1. Task-related activity in area V5/MT in response to motion perception in typical readers (Experiment 1).
Positive correlations computed within the bilateral V5/MT regions of interest between motion-specific activity and standardized measures of real word reading (WJ-III Word Identification (A) and pseudoword reading (WJ-III Word Attack (B). Correlation coefficients (r) and p-values (two-tailed) for the correlations are as follows: Word Identification: Left: 0.46/0.009, Right: 0.52/0.003; Word Attack: Left: 0.41/0.024, Right: 0.61/0.0003.
Thus, consistent with previous studies (Ben-Shachar et al., 2007; Demb et al., 1997; Talcott et al., 2000; Wilmer et al., 2004; Witton et al., 1998) our data derived from these specific tasks also demonstrate a relationship between visual magnocellular function and reading.
Experiment 2: Comparisons of V5/MT Activity between Children with Dyslexia and their Age- and Reading-Matched Controls
Fourteen dyslexic and fourteen control children were matched on chronological age, and twelve dyslexic and twelve control children were matched on reading level (Table 1). Between-group differences in behavior between the dyslexics and the controls for both the age-matched and reading level-matched comparisons were assessed via two-sample t-tests (two-tailed). As expected, for the age-matched group comparisons, the dyslexic group (Dysage) had significantly poorer reading skills (t(26) = 11.00; p < 0.001) than their typically reading (Conage) counterparts (as measured by the WJ-III WID) despite being of the same chronological age. Also, as is inherent in the design, the dyslexics in the reading level comparison (Dysread) were significantly older (t(22) = −4.48; p < 0.001) than their reading level-matched controls (Conread). Studies in dyslexia typically match groups on Performance IQ as the Verbal IQ component of the Full-Scale IQ is influenced by reading. All children had normal or above normal Performance IQ scores on the WASI. For the critical comparison, dyslexics versus controls matched for reading level, the groups were matched on Performance IQ (t(22) = 1.38; p = 0.185). This was not the case for the dyslexic and control groups matched on chronological age (t(26) = 4.44; p < 0.001), and while this is less important for the question at hand, we nevertheless examined all behavioral and functional analyses on a subset of these groups matched for Performance IQ (6 individuals per subgroup: controls: mean ± SD = 111 ± 5.0; dyslexics: 106 ± 4.3; two-tailed test: t(10) = 1.93; p = 0.083), to verify that all results reported here were independent of this IQ difference.
Table 1.
Subject Demographic Information and In-Scanner Performance (Experiment 2)
| Conage | Dysage | p-value | Conread | Dysread | p-value | |
|---|---|---|---|---|---|---|
| N | 14 | 14 | - | 12 | 12 | - |
| Sex (female/male) | 5/9 | 5/9 | - | 7/5 | 6/6 | - |
| Age | 9.1 ± 2.2 | 9.9 ± 1.3 | 0.228 | 7.5 ± 0.9 | 10.4 ± 2.1 | < 0.001 |
| PIQ | 120 ± 16 | 98 ±10 | < 0.001 | 107 ± 14 | 101 ± 9.0 | 0.185 |
| WJ-III Word ID* | 121 ± 10 | 77 ± 11 | < 0.001 | 3.4 ± 1.4 | 3.1 ± 2.0 | 0.657 |
| Motion accuracy (%) | 98.9 ± 2.9 | 89.9 ± 18 | 0.093 | 89.8 ± 15 | 89.5 ± 20 | 0.969 |
| Static accuracy (%) | 98.2 ± 4.2 | 96.1 ± 11 | 0.488 | 94.2 ± 15 | 96.3 ± 12 | 0.702 |
| Motion - Static accuracy (%)+ | 0.71 ± 5.5 | −6.15 ± 19 | 0.203 | −4.41 ± 20 | −6.78 ± 20 | 0.771 |
| Motion reaction time (ms) | 1352 ± 352 | 1416 ± 303 | 0.613 | 1488 ± 351 | 1377 ± 290 | 0.410 |
| Static reaction time (ms) | 1097 ± 267 | 1177 ± 263 | 0.432 | 1014 ± 187 | 1134 ± 191 | 0.135 |
| Motion - Static reaction time (ms)+ | 255 ± 299 | 239 ± 356 | 0.770 | 474 ± 334 | 243 ± 336 | 0.106 |
Woodcock-Johnson III Word Identification: Standardized scores are presented for age-matched groups, while grade equivalencies are presented for reading level-matched groups;
Accuracy and reaction time are presented for the difference of Motion - Static consistent with the comparison of these conditions for statistical map generation for the identification of activity in V5/MT. P-values represent two-tailed tests.
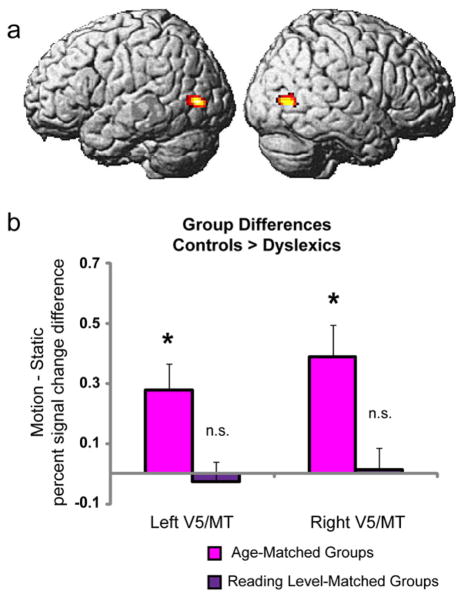
A group map including all subjects from Experiment 1, none of whom were included in Experiments 2 or 3, was used to independently (Kriegeskorte et al., 2010; Poldrack, 2006) define bilateral regions of interest in area V5/MT (one-sample t-test (n = 30) performed at the whole-brain level: family-wise error (FWE) corrected p < 0.05 for the Motion vs. Static contrast). This map yielded only two activation clusters at Talairach co-ordinates −42, −75, −7 (Left V5/MT) and 46, −66, −7 (Right V5/MT; Figure 2a). Percent signal change for this contrast was computed for each subject within these regions, and an ANOVA treating Hemisphere as a within-subject factor and Group as a between-subject factor revealed between-group differences (Controls > Dyslexics) in bilateral V5/MT activity for the age-matched comparison (Figure 2b). Specifically, there was a main effect of Group (F1,26 = 11.8, p = 0.001), and post-hoc t-tests revealed that V5/MT motion-specific activity was greater for the typical readers (Conage group) than for the dyslexics (Dysage group) in both left (t(26) = 2.24; p = 0.034; two-tailed) and right (t(26) = 2.61; p = 0.015; two-tailed) hemispheres. There was no main effect of Hemisphere (F1,26 = 0.68, p = 0.414) and no interaction of Group × Hemisphere (F1,26= 0.33, p= 0.567). This same result was observed when the subset of subjects matched on Performance IQ was analyzed (left V5/MT: t(10) = 2.40; p = 0.038; right V5/MT: t(10) = 2.83; p = 0.018; two-tailed). Having replicated findings of V5/MT hypoactivity in dyslexia as previously reported in adults (Demb et al., 1997; Eden et al., 1996) and children matched on age (Heim et al., 2010), the critical novel comparison involved the groups matched for reading level. Here the ANOVA did not reveal a significant effect of Group (F1,22 = 0.01, p= 0.938). We also did not observe a significant effect of Hemisphere (F1,22 = 0.02, p= 0.895), or interaction of Hemisphere × Group (F1,22 = 0.07, p= 0.787). Simple t-tests did not reveal significant differences between the Conread group and the Dysread group in V5/MT activity in either hemisphere (left: t(22) = −0.26; p = 0.799; right: t(22) = 0.13; p = 0.895; two-tailed). Evidence of a between-group difference would have lent support to the theory of a causal role for magnocellular deficits in dyslexia.
Figure 2. Task related activity in area V5/MT in response to motion perception in typical and dyslexic children (Experiment 2).
(A) Regions of interest in bilateral V5/MT were independently generated via a group map for motion versus static in Experiment 1, using whole-brain random effects analysis and a one-sample t-test; family-wise error corrected threshold of p < 0.05. (B) Between-group differences (Controls > Dyslexics) for percent signal change for Motion vs. Static within area V5/MT (as defined and shown in (A)): Age-matched (left bars) and reading level-matched (right bars) comparisons of controls greater than dyslexics. Greater activity (two-tailed tests; Left: *p = 0.034; Right: *p = 0.015) was observed in the controls compared to the dyslexics matched on age in bilateral V5/MT. There were no differences between controls and dyslexics when matched on reading level. Percent signal change values (Motion vs. Static) for the individual groups were as follows: (mean ± SEM: Left V5/MT: Conage: 0.537 ± 0.076; Dysage: 0.259 ± 0.098; Conread: 0.370 ± 0.069; Dysread: 0.396 ± 0.072; Right V5/MT: Conage: 0.674 ± 0.100; Dysage: 0.283 ± 0.111; Conread: 0.400 ± 0.063; Dysread: 0.385 ± 0.086). Error bars represent the standard error of the difference between sample means.
As shown in Table 1, accuracy (ACC) and reaction time (RT) did not differ between the groups (two-tailed tests) on task performance inside the scanner for either Motion or Static conditions, nor when the difference between conditions for the contrast of interest (Motion–Static) was considered. These data confirm that in-scanner task performance was equally easy for all groups. The task was deliberately designed not to be challenging, allowing fMRI data to be interpreted without concerns for between-group performance differences (Price and Friston, 2002; Price et al., 2006). As such, it does not contain the full range of performances typically elicited by psychophysical magnocellular tasks that in prior studies have been used to demonstrate a correlation with V5/MT activity (Demb et al., 1998; Koldewyn et al., 2011).
Together, whilst we demonstrated once again that dyslexics differ in visual magnocellular function, our reading level-match experiment does not support the notion that this deficit is causal to the reading disability.
Experiment 3: Changes in Neural Activity in V5/MT following Reading Intervention
To test whether reading improvements in dyslexic children leads to greater activity in area V5/MT, we compared brain activity during visual motion perception in twenty-two children with dyslexia (age: 9.6 ± 1.4) prior to and following an eight week intervention involving tutoring of phonological and orthographic constructs (Bell, 1997). The efficacy of the reading intervention was tested by comparing reading gains made during this intervention period with any gains that occurred during a control period. That is, in addition to the reading intervention each child also participated in either (a) an active control period, during which a math intervention was provided by the same tutors with the same intensity as the reading intervention or (b) a no intervention developmental control period. For the purpose of the present study, we collapsed across these two types of control periods (see Experimental Procedures for details). All subjects were seen at three time points. During the intervening two periods of eight weeks, either the active reading intervention or control period took place, with the order being randomized across subjects.
As expected, the reading intervention delivered by tutors working with small groups of children lead to significant improvements in phonological awareness and single real word reading skills. No such gains were observed during the control period. Specifically, one-way repeated measures ANOVA (n = 22) on the within-group behavioral data from all three time-points (i.e. prior to the first eight week period, following the first eight week period and following the second eight week period) showed that children improved in reading of real words (WID: (F2,19 = 12.8, p< 0.0001)), reading of pseudowords (WA: (F2,19 = 7.77, p= 0.001)) and phonological awareness (Lindamood Auditory Conceptualization – LAC3 (Lindamood and Lindamood, 2004): (F2,19 = 2.46, p= 0.098)). Importantly, post hoc t-tests (two-tailed) revealed these gains to follow the reading intervention period (Standard Scores: WID (mean ± SD): Pre- = 79 ± 7; Post- = 87 ± 9; t(21) = 6.07; p < 0.0001; WA (mean ± SD): Pre- = 93 ± 7; Post- = 97 ± 9; t(21) = 4.56; p = 0.0002); LAC (mean ± SD): Pre- = 99 ± 8; Post- = 103 ± 11; t(21) = 2.44; p = 0.024; but not the control period (WID: Pre- = 85 ± 9; Post- = 85 ± 12; t(21) = 0.21; p = 0.833; WA: Pre- = 97 ± 8; Post- = 97 ± 9; t(21) = 0.38; p = 0.701; LAC: Pre- = 103 ± 11; Post- = 102 ± 9; t(21) = −0.84; p = 0.409; – Table 2). This demonstrated that these gains were specific to the reading intervention itself, rather than being attributed to development, or a Hawthorn effect due to the tutoring (i.e. there were no gains in reading skills following the intervention focusing on math, or due to development).
Table 2.
Reading and In-Scanner Performance changes following Intervention (Experiment 3)
| Reading Intervention Period | Control Period | |||||
|---|---|---|---|---|---|---|
| Pre- | Post- | p-value | Pre- | Post- | p-value | |
| LAC-3 (phonemic awareness) | 99 ± 8 | 103 ± 11 | 0.024 | 103 ± 11 | 102 ± 9 | 0.409 |
| WJ-III WID (real words) | 79 ± 7 | 87 ± 9 | < 0.001 | 85 ± 9 | 85 ± 12 | 0.833 |
| WJ-III WA (pseudo words) | 93 ± 7 | 97 ± 9 | < 0.001 | 97 ± 8 | 97 ± 9 | 0.701 |
| Motion accuracy (%) | 82.1 ± 27 | 93.8 ± 9.8 | 0.046 | 88.3 ± 21 | 91.2 ± 16 | 0.549 |
| Static accuracy (%) | 96.2 ± 8.6 | 99.3 ± 1.6 | 0.117 | 98.4 ± 3.5 | 99.0 ± 2.0 | 0.427 |
| Motion - Static accuracy (%)+ | −14.1 ± 29 | −5.47 ± 10 | 0.154 | −10.1 ± 20 | −7.76 ± 16 | 0.657 |
| Motion reaction time (ms) | 1216 ± 317 | 1205 ± 301 | 0.857 | 1222 ± 294 | 1124 ± 250 | 0.032 |
| Static reaction time (ms) | 1006 ± 214 | 956 ± 162 | 0.163 | 972 ± 144 | 876 ± 143 | 0.001 |
| Motion - Static reaction time (ms)+ | 210 ± 290 | 249 ± 216 | 0.487 | 250 ± 227 | 248 ± 263 | 0.957 |
LAC: Lindamood-Bell Auditory Conceptualization test; WJ-III: Woodcock Johnson III tests of achievement.
Accuracy and reaction time are presented for the difference of Motion - Static consistent with the comparison of these conditions for statistical map generation for the identification of activity in V5/MT. F values yielded from repeated measures ANOVA. P-values represent two-tailed tests.
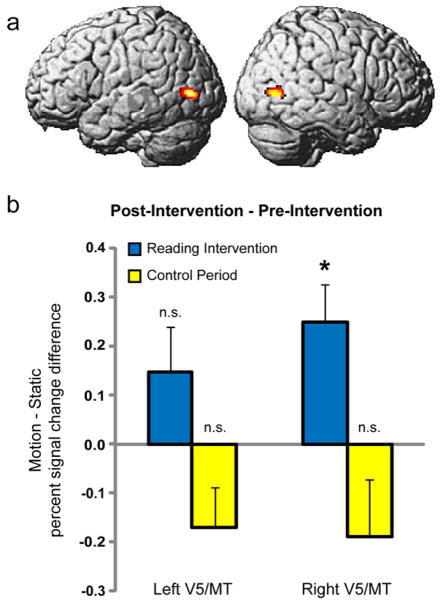
As in Experiment 2, percent signal change values for Motion greater than Static were computed for each subject within the independently defined ROIs from Experiment 1 in bilateral V5/MT (Figure 3a) at all three time-points. Values were entered into a repeated measures ANOVA for left and right hemispheres separately. A significant main effect of Time-Point was observed in the right hemisphere (F2,19 = 3.27, p= 0.048), but not the left hemisphere (F2,19 = 0.06, p= 0.941). Post-hoc t-tests (two-tailed; n = 22) revealed that average percent signal change increased significantly in right hemisphere area V5/MT following the reading intervention period (t(21) = 2.82; p = 0.010; Figure 3), with the increase in left hemisphere not being significant (t(21) = 1.61; p = 0.123). Importantly, the increase in the activity of right area V5/MT underlying motion processing was specific to the reading intervention, as no such intervention-induced increase was observed during the control period. In fact, a non-significant decrease in activity was observed following the control period in the left (t(21) = −2.06, p= 0.051) and right hemispheres (t(21) = −0.06; p = 0.118), and change in activity was greater for the reading intervention than the control period in both hemispheres (left: (t(21) = 2.07; p = 0.054); right: (t(21) = 2.71; p = 0.013); two-tailed)).
Figure 3. Task-related activity in area V5/MT in response to motion perception in dyslexic children prior to and following phonologically-based reading intervention (Experiment 3).
(A) Regions of interest in bilateral V5/MT generated from Experiment 1 (also used in Experiment 2). (B). Percent signal change difference for Motion vs. Static following the reading intervention within area V5/MT as defined in (A). Positive values indicate increases in activity following the reading intervention. A significant increase (*p = 0.010; two-tailed test) was observed in right V5/MT following the reading intervention, but not the control period, demonstrating that this increase in activity was specific to the reading intervention. Increased activity in left V5/MT following the reading intervention period did not achieve significance. Percent signal change values (Motion vs. Static) were as follows: (mean ± SEM: Left V5/MT: Pre-Reading Intervention: 0.344 ± 0.052; Post-Reading Intervention: 0.493 ± 0.072; Pre-Control Period: 0.474 ± 0.064; Post-Control Period: 0.304 ± 0.053; Right V5/MT: Pre-Reading Intervention: 0.255 ± 0.089; Post-Reading Intervention: 0.504 ± 0.069; Pre-Control Period: 0.490 ± 0.072; Post-Control Period: 0.302 ± 0.087). Error bars represent the standard error of the difference between sample means.
As shown in Table 2, there was a significant improvement for in-scanner task performance measured by greater accuracy for the Motion task following the reading intervention period, but not the Control period. During the Control period, subject reaction times decreased for both Motion and Static conditions. However, none of these changes were significant when considering accuracy and reaction time for Motion-Static.
DISCUSSION
Understanding the causal factors underlying developmental dyslexia is critical for early identification and successful treatment of the disorder. Investigations into the visual magnocellular deficit in dyslexia have involved behavioral as well as brain anatomical studies spanning three decades. It has been argued that such magnocellular dysfunction is the cause of reading problems (Stein, 2001). However, significant controversy remains surrounding the visual magnocellular deficit and its role in mediating reading difficulties (Danelli et al., 2012; Hulme, 1988; Vellutino et al., 2004), especially in light of well documented language-based deficits in phonological coding that are thought to be directly responsible for dyslexics’ reading impairments. An alternative position to the causal hypothesis of magnocellular deficits is that visual symptoms are an epiphenomenon of dyslexia. For example, it has been proposed that alteration of multiple neighboring brain regions within occipito-temporal and temporo-parietal cortex, some of which are responsible for reading whilst others are involved in visual motion processing, could also account for the co-occurrence of both phonological and visual deficits reported in dyslexia (Eden and Zeffiro, 1998). Another theory is that the anatomical alterations in perisylvian cortex that eventually give rise to reading problems also disturb the typical course of prenatal brain development, resulting in additional microstructural anomalies in the brain, which in turn cause other problems, including visual deficits (Ramus, 2004). Both of these models are consistent with the observed differences in behavior and brain function in dyslexia associated with magnocellular function. Importantly, both models view the visual symptoms as a side effect, recognizing that it is the phonological deficits (and not the visual deficits) that are driving the reading problems. Which of these models is correct, and whether there is a causal role of visual magnocellular deficits in dyslexia has to be determined in order to ensure accurate diagnosis of dyslexia, and to develop and apply appropriate and effective interventions.
Our study was designed to address this issue directly. First, we demonstrated in a group of children and adults, a correlation between signal change in area V5/MT and reading ability. Our finding is consistent with other studies showing correlations between reading and behavioral measures of visual magnocellular function (Talcott et al., 2000; Wilmer et al., 2004; Witton et al., 1998), which have often been used to invoke the argument that the relationship is causal. However, demonstration of a correlation between V5/MT activity and reading in this and other studies does not allow us to infer the directionality of this relationship. To test for causality, we compared magnocellular activity in area V5/MT between dyslexic children and younger controls matched for reading ability and found that dyslexics and controls matched on reading level did not differ in their activity (whilst those matched on age did). These results confirm differences between dyslexics and controls in visual magnocellular function, but they do not support a causal role for these magnocellular deficits in reading disability. Differences in brain function have been reported for children with dyslexia compared to younger controls on a task requiring phonological manipulation of visually presented words (Hoeft et al., 2006, 2007). As such it is possible to demonstrate causal brain differences in dyslexia using fMRI. However, the fact that the study by Hoeft and colleagues involved phonological manipulation, once again speaks to the more likely causal brain basis of dyslexia involving language.
Having established that the visual magnocellular deficit is likely to be an epiphenomenon of dyslexia, we then provided the dyslexic children with a phonological-based reading intervention which resulted in better reading ability, and somewhat surprisingly, also in greater activity in right area V5/MT during visual motion perception. This final result is important in that it reveals information about the mechanism by which phonological and visual deficits may come to co-exist in dyslexia. Specifically, they do not support the above-mentioned models (Eden and Zeffiro, 1998; Ramus, 2004) that have argued that dyslexia is best described as a condition that gives rise to sensory deficits in addition to the language-based problem. Instead our results demonstrate that the acquisition of reading has a positive influence on magnocellular visual system function, as demonstrated by the increase in right V5/MT activity following reading gains in the dyslexics. Since dyslexia impedes reading acquisition, it is most likely that the differences in magnocellular function reported here and elsewhere between dyslexics and their typically reading peers, may be attributed to their lower reading level and less reading experience. In other words, the magnocellular visual deficit is a consequence and not the cause of impoverished reading.
Several ideas have been put forward to explain the mechanism by which weaknesses in the magnocellular visual system might affect reading (Boden and Giaschi, 2007; Stein, 2001). It has been argued that the magnocellular system is involved in direction of visual attention, visual search and eye movements, and that these problems directly impact a person’s ability to read accurately (Eden et al., 1994). However, since our results do not support a causal relationship, it becomes necessary to look at the other side of the same coin and consider how subdued magnocellular function in dyslexia might be a result of lower reading ability. For example, extensive eye movements associated with reading experience may serve to train processes linked to the dorsal magnocellular system such as oculomotor control, visual attention and spatial position encoding (Boets et al., 2011). From this viewpoint, one can agree on a relationship between reading and magnocellular function, even if the precise mechanisms are not well understood. However, the directionality is likely that learning to read is followed by changes in the magnocellular system and not vice versa. Further, this theory would hold that reading acquisition exerts an influence on the size of neurons in the magnocellular layers of the LGN, or the amount of activity in area V5/MT, with the degree of influence modulated by the amount of reading experience. This model provides a parsimonious account of the findings reported to date.
Our findings that the visual motion perception system is affected by learning to read and not the other way around is consistent with the observation that lesions to area V5/MT do not impair the normal reading process. A patient with severe impaired motion perception (akinatopsia) following thrombosis of the superior sagittal sinus, that resulted in damage encompassing area V5/MT, maintained normal reading ability (Zihl et al., 1983). A recent study in normal controls revealed that inhibition of area V5/MT through transcranial magnetic stimulation does not disrupt word reading under conditions of normal orthography, but only when the words are in motion (Rauschecker et al., 2011).
It is important to keep in mind that reading is a uniquely human skill that is explicitly taught over several years of formal schooling. During this time, significant functional changes occur as a direct consequence of learning to read, as has been shown with fMRI (Gaillard et al., 2003; Schlaggar, 2002; Turkeltaub et al., 2003). However, reading does not have a sufficiently long evolutionary history which would reserve dedicated neural populations specifically to this skill. Therefore reading makes use of brain areas that were most likely dedicated to other functions, an idea that has been captured in the “neuronal recycling hypothesis” (Dehaene et al., 2010). As such, the process of learning to read most likely results in diminishing of some skills while at the same time promoting others. The consequential outcomes of reading acquisition has been elegantly revealed in studies contrasting literates with illiterates, demonstrating that the profound anatomical and physiological effects learning to read has on the brain exist within and well beyond brain regions directly associated with reading (Carreiras et al., 2009). Relevant to the present study, positive consequences have been shown to be exerted by reading acquisition on visual performance on a contour integration task, where literates outperform illiterates (Szwed et al., 2012). Based on our observations in dyslexia, we would predict that motion perception and activity in area V5/MT would also be weaker in illiterates than in literates, a hypothesis that needs to be tested in future work.
Other observed experience-dependent changes in the visual system in normally reading individuals are relevant to our findings. For example, increase in gray matter volume in areas V2/V3 follows color category training (Kwok et al., 2011), and in area V5/MT after intensive practice and improvement in juggling (Draganski et al., 2004). At the level of brain function, glucose metabolism increases in area V5/MT following speech learning in deaf individuals who were recipients of cochlear implantations (Kang et al., 2004). It has been suggested that the dorsal visual stream, which houses area V5/MT, is more malleable to change than the ventral visual stream because its developmental trajectory is relatively longer. Specifically, electrophysiological studies by Neville and colleagues contrasting children and adults found greater between-group differences for amplitude and latency of responses to dorsal stream processes, indicating slower development here relative to the ventral stream (Mitchell and Neville, 2004). While this observation of differential development and susceptibility has been used to explain why dyslexia involves dorsal stream dysfunction, our study suggests that maturation alone may not be the only element driving normal dorsal stream development, but that learning to read has an important catalytic role in this process. This is consistent with the idea that reading acquisition “mobilizes” dorsal stream functions, as suggested by Boets and colleagues, who observed improved performance in coherent motion detection from kindergarten to first grade in typically reading children (i.e. following the onset of formal reading instruction), with adults performing even better than both groups of children (Boets et al., 2011). Critically, our results caution against the use of magnocellular dorsal integrity as a biological marker for early-detection of dyslexia, or for other conditions which manifest in reduced reading proficiency. Likewise, weaknesses in visual motion perception in other disorders such as autism and William’s syndrome (Atkinson et al., 1997; Milne et al., 2002), that to date have been ascribed to dorsal stream malleability, may have to be revisited in the context of the current findings which suggest that lower magnocellular function might be due to less reading experience in these populations. At the same time, our observations are specific to visual motion processing and area V5/MT, and therefore do not speak to other dorsal stream mechanisms that have been implicated as being predictive of, and causal to reading disability, such as visual-spatial attention (Franceschini et al., 2012).
The precise mechanisms by which advances in reading might mobilize visual dorsal stream function cannot be elucidated from our study. The most likely scenario is the one already described above, that changes in the visual magnocellular system are due to the mechanical aspects of the reading process. Interestingly, a recent study demonstrated considerable overlap of activity in visual extrastriate regions during single-pseudoword reading and visual motion processing in typical readers (Danelli et al., 2012). These results raise the possibility of involvement of these areas in the aberrant interactions between reading and magnocellular systems in dyslexia. However, brain imaging studies on reading primarily focus on decoding of single words rather than more ecologically valid sentences or passages, thereby avoiding the very mechanisms that are important to the understanding of the role of visual magnocellular systems in reading. Other technologies have been employed to study the role of eye movements in word processing, and could be expanded to dyslexia (Temereanca et al., 2012). To examine the possibility that there might be a direct link between neural systems underlying the linguistic aspects of reading and area V5/MT at the cortical level, we examined whether resting-state connectivity between right V5/MT and left hemisphere reading areas (i.e. the inferior frontal gyrus, the posterior superior temporal gyrus, the inferior parietal lobule, and the visual word form area) increased following the reading intervention period. Our results however did not show an increase in connectivity between right V5/MT and any of these language regions.
In sum, our results demonstrate that the reading problems experienced by children with dyslexia are not a consequence of visual magnocellular dysfunction. While visual magnocellular weakness does manifest in dyslexia, it is not the cause of the reading problem. Secondly, the weaknesses in the magnocellular visual system, indexed in this study by the amount of activity in area V5/MT during the perception of visual motion, do not represent a symptom of dyslexia. They are not, as previous models assumed, part of a common etiology with different behavioral manifestations and thereby an integral part of the pathophysiology of dyslexia. Rather, they are a secondary consequence of reading experience itself. We suggest that phonological deficits, by restricting the amount and quality of reading in dyslexics, limit the opportunity for reading to induce changes in the visual magnocellular system (by mechanisms which remain to be determined). As such, reading itself can be thought of as an environmental influence that bears on functional and anatomical aspects of the brain, and in the case of reading disability, these changes are not invoked to the same degree as they are in typical readers. In the context of the observed differences at the level of the LGN, larger neurons in the controls relative to the dyslexic at post mortem could be due to extensive versus limited experience with reading over a lifetime. The same explanation holds to account for the differences between dyslexics and age-matched controls in behavioral studies of magnocellular function and brain imaging studies of V5/MT. Together our results represent not only an important advancement in understanding the etiology of developmental dyslexia, but also offer a reinterpretation of the existing data on visual magnocellular dysfunction in dyslexia. They also contribute to an important growing body of work that explains how experience, in this case for reading, alters the functional organization of the brain.
EXPERIMENTAL PROCEDURES
Participants
Subjects participating in all three experiments were native English speakers with no history of neurological or psychiatric disorder, and all had normal or corrected-to-normal visual acuity. Written informed consent was obtained from the subjects themselves or from the subjects’ parents (in the case of pediatric participants), and all procedures were approved by the Georgetown University Institutional Review Board. All subjects completed a battery of behavioral tests to evaluate intelligence and proficiency on reading and reading related skills, including the Wechsler Abbreviated Scale of Intelligence Verbal and Performance tests (IQ), Woodcock Johnson (WJ-III) Word Identification (WID – single real word reading), and Woodcock Johnson Word Attack (WA – single pseudoword reading). Subjects in Experiment 3 also completed the Lindamood-Bell test of Auditory Conceptualization (LAC3 – phonemic awareness). Inclusion criteria for all subjects was a Full scale IQ standard score equal and greater than 80. Inclusion criteria for the typically reading adults (Experiment 1) and children (Experiments 1, and 2) was a WJ-III WID and WA standard score > 92. Inclusion criteria for the dyslexic children (Experiments 2 and 3) was a WJ-III WID or/and WA standard score ≤ 93, and a documented diagnosis of dyslexia.
For Experiments 2 and 3, Attention Deficit Disorder (ADHD) was not considered exclusionary for this study. ADHD symptoms were assessed via the short form of the Conner’s Parent Rating Scale (Conners, 1990). The parents of eighteen dyslexic subjects returned the Connors Parent Rating Scale. Assuming a normal T-score range of 40–60 (plus or minus one standard deviation around the mean), two of these had elevated ADHD Index scores. Of the 23 typically reading participants who served as controls for the dyslexics, 18 Connors Parent Rating Scale (Short Form – Conners, 1990) were returned by the parents, and three subjects had elevated ADHD Index T-scores.
Experiment 1
Thirty typically reading individuals (13 females; ages 7.3 – 31.5 yrs; mean ± SD: 21.9 ± 6.1) were included in this analysis. All subjects were within or above the normal range for intelligence (WASI Full Scale IQ: range: 95 – 137; mean ± SD: 121 ± 9), and within the normal range for real word reading (WJ-III WID: range: 94 – 120; mean ± SD: 109 ± 7;) and pseudoword reading (WJ-III WA: range: 93 – 120; mean ± SD: 106 ± 8).
Experiment 2
The dyslexic group entered into the age-matched comparison with controls (Dysage group) consisted of fourteen individuals (5 females; ages 7.4 – 11.9 yrs; mean ± SD: 9.9 ± 1.3). All subjects in this group were within the normal or above normal range for intelligence (WASI Full Scale IQ: range: 80 – 123; mean ± SD: 104 ± 10). Average reading level was low for this group for both real word and pseudoword reading (WJ-III WID: range: 49 – 91; mean ± SD: 77 ± 11; WJ-III WA: range: 47 – 98; mean ± SD: 88 ± 13). The Conage group consisted of fourteen typically reading individuals matched to the Dysage group on average age (5 females; ages 7.1 – 13.4 yrs; mean ± SD: 9.1 ± 2.2). These control subjects were within or above the normal range for intelligence (WASI Full Scale IQ: range: 106 – 149; mean ± SD: 122 ± 14), real word reading (WJ-III WID: range: 98 – 140; mean ± SD: 121 ± 10) and pseudoword reading (WJ-III WA: range: 100 – 140; mean ± SD: 119 ± 12). For the reading level-match comparison, the Dysread group consisted of twelve individuals with dyslexia (6 females; ages 9.1 – 15.8 yrs; mean ± SD: 10.4 ± 2.1). Ten of these individuals were also included in the Dysage group. All individuals had normal or above normal intelligence (WASI Full Scale IQ: range: 88 – 123; mean ± SD: 106 ± 8), but low real word (WJ-III WID: range: 71 – 96; mean ± SD: 83 ± 9) and pseudoword reading ability (WJ-III WA: range: 83 – 109; mean ± SD: 94 ± 7). The Conread group consisted of twelve typically reading individuals, three of whom were also included in the Conage group. Average age for this group was, by design, lower than for the Dysread group (5 females; ages 6.7 – 9.8 yrs; mean ± SD: 7.5 ± 0.9). Subjects in this control group had normal or above normal intelligence (WASI Full Scale IQ: range: 95 – 135; mean ± SD: 117 ± 14), and normal age-equivalent real word (WJ-III WID: range: 98 – 132; mean ± SD: 118 ± 10) and pseudoword reading ability (WJ-III WA: range: 100 – 130; mean ± SD: 117 ± 10). Performance IQ for all four groups is presented in Table 1. For the age-matched comparison, a second analysis was performed in a subset of individuals matched on Performance IQ (n = 6) to ensure that any observed differences were independent of the IQ difference.
Experiment 3
Twenty-two children with dyslexia (9 females; ages 7.4 – 12.0) participated in three scanning sessions, the first prior to the beginning of any intervention, and the second and third following two eight week periods. All subjects were within or above the normal range for intelligence (WASI Full Scale IQ: range: 98 – 124; mean ± SD: 109 ± 8), low real word reading WJ-III WID: range: 62 – 93; mean ± SD: 79 ± 7.7), pseudoword reading (WJ-III WA: range: 77 – 109; mean ± SD: 93 ± 6.3) and phonemic awareness scores prior to the intervention (LAC-3: range: 87 – 115; mean ± SD: 100 ± 7.5). Based on random assignment, some subjects underwent reading intervention during the first eight week period, followed by the math intervention during the second eight week period (n = 8); a second group received a math intervention first, followed by the reading intervention (n = 6); the third group received the reading intervention followed by no intervention (n = 8). For the analysis, the periods of no intervention and math intervention were combined into a control period to provide a control comparison for the periods during which the same children received the reading intervention.
fMRI Task
We used an fMRI task involving coherent motion detection (Motion) to examine activity in area V5/MT. During this task, subjects maintained central fixation while viewing a set of low contrast dots moving in various directions on a black background, with 40% coherence in the horizontal direction. Task difficulty was set at a level to ensure good performance by all subjects in all three experiments, thereby avoiding performance differences between dyslexic and controls (Experiment 2) that can obscure the interpretation of the between-group differences of fMRI data (Price and Friston, 2002; Price et al., 2006). Via button press, subjects were asked to indicate the direction of motion. A control condition involved presentation of static dots (Static), during which subjects performed a density judgment on the left and right visual field, while maintaining central fixation. Density contrast between hemifields varied from 35% to 65%. Stimuli were presented using a block design paradigm. Motion and Static blocks were separated by intervening passive Fixation periods that lasted 18s each, and during which a crosshair was presented in the center of the screen. Motion and Static blocks lasted for 42s each, and consisted of trials during which the stimulus was presented for 1.2s followed by a crosshair for 3s. Ten such trials were presented in each block and a single run consisted of two blocks each of the Motion and Static stimuli. Pediatric participants underwent a training session in a mock scanner prior to the experiment to familiarize them with the task and the MRI environment.
fMRI Acquisition
Data were acquired using a 3T Siemens Trio scanner located in the Center for Functional and Molecular Imaging at the Georgetown University Medical Center, Washington, DC. For each run, eighty-nine functional images consisting of 50 contiguous whole-brain axial slices were acquired using a blipped echo-planar imaging (EPI) sequence and the following parameters: TR = 3s, TE = 30ms, Flip Angle = 90°, FOV = 192mm, Slice Thickness = 2.8mm (0.2mm inter-slice gap), In-plane resolution = 64 × 64, voxel size = 3mm isotropic.
Statistical Analysis
SPM8 was used in analysis of functional MRI datasets. The first five scans of each run were discarded to account for T1 saturation effects. Resulting datasets were realigned to the mean of the remaining images, normalized to the Montreal Neurological Institute (MNI) EPI template, re-sampled to an isotropic voxel size of 2mm3, and smoothed with a Gaussian kernel of 8mm full width at half maximum (FWHM). Statistical analysis was performed based on the general linear model. Functional datasets were high pass filtered with a cut-off of 128s to account for signal drift, and corrected for auto-correlations using an AR(1) model. Stimulus onsets were modeled using the SPM canonical hemodynamic response function, and within-subject parametric maps were created for the motion specific contrast (Motion > Static). Area V5/MT was functionally identified via its responsivity to the visual motion stimulus. In Experiment 1, V5/MT was identified individually in each subject via the contrast of Motion vs. Static. For this single-subject analysis, we searched for clusters within Talairach co-ordinates bounded by previously defined anatomical volumes: x = lateral to ±35; y = posterior to −60; z = −9 to +13 (Dumoulin et al., 2000; Tootell et al., 1995; Watson et al., 1993); To avoid circularity, this identification of V5/MT was performed using half the data acquired, while the other half was utilized in percent signal change calculation. Allocation of task blocks for this split between the two halves of the run was randomized across subjects. Data from Experiment 1 was also used to determine the ROI used in Experiments 2 and 3, however, this time using a different analysis, since Experiment 1 involved a different group of subjects than those participating in Experiments 2 and 3. An independent ROI was identified via a second level random effects whole-brain analysis (no anatomical boundaries or masks were used here) performed using a one-sample t-test to combine activation for the motion specific contrast over all the subjects in Experiment 1. Clusters surviving a family-wise error corrected threshold of p < 0.05 were observed within bilateral V5/MT (but nowhere else in the brain), extracted using Marsbar, and utilized as ROIs. The percent signal change within these bilateral regions for the Motion vs. Static contrast was extracted for each subject and utilized in further analysis for Experiments 2 and 3. For all three experiments, analysis were repeated and similar results were observed using a voxel-wise approach and small-volume correction within the afore-mentioned clusters.
Intervention
The reading intervention “Seeing Stars” (Bell, 1997) was administered by trained employees of Lindamood-Bell Learning Processes to small groups of students at their school for three hours a day, five days a week over an eight week period. This program addresses visualization of letters, syllables, multisyllables, and words as well as motor/tactile and articulatory aspects of word presentation, thereby promoting visual imagery of orthographic presentations as well as phonological awareness. During the Control Period, some children received a math intervention (whilst the remaining children served as a developmental control). The math intervention “On Cloud Nine” (Tuley and Bell, 1997) was employed for this study as it was created by the same company that devised the reading intervention, and delivered by the same tutors in similar student/tutor ratios. The math intervention emphasizes the use of visualization and articulatory strategies to solve mathematical processes such as counting, addition, subtraction and fractions. The use of the math intervention was to control for a placebo effect that might be driving the reading intervention, rather than for the purpose of addressing any math deficits.
HIGHLIGHTS.
Activity in V5/MT for visual motion processing is correlated with reading ability; Dyslexics have reduced V5/MT activity when compared to age-matched controls; V5/MT activity is similar for dyslexics and reading-matched controls; Intervention induces reading gains and increased V5/MT activity in dyslexia.
Acknowledgments
We would like to thank Corinna Moore, Emily Curran, Iain DeWitt, Megan Luetje, Alison Merikangas, Ashley Wall-Piche, Robert Twomey and Jenni Rosenberg, for their assistance in fMRI data collection and Emma Cole, Martha Miranda, Gina Smith, and Kelly Crain for collection of behavioral psychometric measures. We thank our subjects for their participation, and the students, parents and staff at the Jemicy School for their involvement. We thank Peter Turkeltaub and three anonymous reviewers for providing constructive comments on the manuscript. This work was supported by the National Institute of Child Health and Human Development (P50 HD40095 and R01 HD056107).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander AW, Slinger-Constant A-M. Current status of treatments for dyslexia: critical review. J Child Neurol. 2004;19:744–758. doi: 10.1177/08830738040190100401. [DOI] [PubMed] [Google Scholar]
- Atkinson J, King J, Braddick O, Nokes L, Anker S, Braddick F. A specific deficit of dorsal stream function in Williams’ syndrome. Neuroreport. 1997;8:1919–1922. doi: 10.1097/00001756-199705260-00025. [DOI] [PubMed] [Google Scholar]
- Bell N. Seeing Stars. San Luis Obispo, CA: Gander Publishing; 1997. [Google Scholar]
- Ben-Shachar M, Dougherty RF, Deutsch GK, Wandell BA. Contrast responsivity in MT+ correlates with phonological awareness and reading measures in children. NeuroImage. 2007;37:1396–1406. doi: 10.1016/j.neuroimage.2007.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden C, Giaschi D. M-stream deficits and reading-related visual processes in developmental dyslexia. Psychol Bull. 2007;133:346–366. doi: 10.1037/0033-2909.133.2.346. [DOI] [PubMed] [Google Scholar]
- Boets B, Vandermosten M, Cornelissen P, Wouters J, Ghesquière P. Coherent Motion Sensitivity and Reading Development in the Transition From Prereading to Reading Stage. Child Development. 2011;82:854–869. doi: 10.1111/j.1467-8624.2010.01527.x. [DOI] [PubMed] [Google Scholar]
- Bradley L, Bryant PE. Categorizing sounds and learning to read—a causal connection. Nature. 1983;301:419–421. [Google Scholar]
- Carreiras M, Seghier ML, Baquero S, Estévez A, Lozano A, Devlin JT, Price CJ. An anatomical signature for literacy. Nature. 2009;461:983–986. doi: 10.1038/nature08461. [DOI] [PubMed] [Google Scholar]
- Cornelissen P, Richardson A, Mason A, Fowler S, Stein J. Contrast sensitivity and coherent motion detection measured at photopic luminance levels in dyslexics and controls. Vision Res. 1995;35:1483–1494. doi: 10.1016/0042-6989(95)98728-r. [DOI] [PubMed] [Google Scholar]
- Danelli L, Berlingeri M, Bottini G, Ferri F, Vacchi L, Sberna M, Paulesu E. Neural intersections of the phonological, visual magnocellular and motor/cerebellar systems in normal readers: Implications for imaging studies on dyslexia. Human Brain Mapping 2012. 2012 Jun 27; doi: 10.1002/hbm.22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Pegado F, Braga LW, Ventura P, Filho GN, Jobert A, Dehaene-Lambertz G, Kolinsky R, Morais J, Cohen L. How Learning to Read Changes the Cortical Networks for Vision and Language. Science. 2010;330:1359–1364. doi: 10.1126/science.1194140. [DOI] [PubMed] [Google Scholar]
- Demb JB, Boynton GM, Heeger DJ. Brain activity in visual cortex predicts individual differences in reading performance. Proc Natl Acad Sci USA. 1997;94:13363–13366. doi: 10.1073/pnas.94.24.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Boynton GM, Heeger DJ. Functional magnetic resonance imaging of early visual pathways in dyslexia. J Neurosci. 1998;18:6939–6951. doi: 10.1523/JNEUROSCI.18-17-06939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Dumoulin SO, Bittar RG, Kabani NJ, Baker CL, Jr, Le Goualher G, Bruce Pike G, Evans AC. A new anatomical landmark for reliable identification of human area V5/MT: a quantitative analysis of sulcal patterning. Cereb Cortex. 2000;10:454–463. doi: 10.1093/cercor/10.5.454. [DOI] [PubMed] [Google Scholar]
- Eden GF, Zeffiro TA. Neural systems affected in developmental dyslexia revealed by functional neuroimaging. Neuron. 1998;21:279–282. doi: 10.1016/s0896-6273(00)80537-1. [DOI] [PubMed] [Google Scholar]
- Eden GF, Stein JF, Wood HM, Wood FB. Differences in eye movements and reading problems in dyslexic and normal children. Vision Res. 1994;34:1345–1358. doi: 10.1016/0042-6989(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Eden GF, VanMeter JW, Rumsey JM, Maisog JM, Woods RP, Zeffiro TA. Abnormal processing of visual motion in dyslexia revealed by functional brain imaging. Nature. 1996;382:66–69. doi: 10.1038/382066a0. [DOI] [PubMed] [Google Scholar]
- Eden GF, Jones KM, Cappell K, Gareau L, Wood FB, Zeffiro TA, Dietz NAE, Agnew JA, Flowers DL. Neural changes following remediation in adult developmental dyslexia. Neuron. 2004;44:411–422. doi: 10.1016/j.neuron.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Franceschini S, Gori S, Ruffino M, Pedrolli K, Facoetti A. A causal link between visual spatial attention and reading acquisition. Curr Biol. 2012;22:814–819. doi: 10.1016/j.cub.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Frith U, Snowling MJ. Reading for meaning and reading for sound in autistic and dyslexic children. British Journal of Developmental Psychology. 1983;1:329–342. [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, Braniecki SH, McKinney CM, Hunter K, Xu B, et al. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Human Brain Mapping. 2003;18:176–185. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami U. Why theories about developmental dyslexia require developmental designs. Trends Cogn Sci (Regul Ed) 2003;7:534–540. doi: 10.1016/j.tics.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Hansen PC, Stein JF, Orde SR, Winter JL, Talcott JB. Are dyslexics’ visual deficits limited to measures of dorsal stream function? Neuroreport. 2001;12:1527–1530. doi: 10.1097/00001756-200105250-00045. [DOI] [PubMed] [Google Scholar]
- Heim S, Grande M, Pape-Neumann J, Van Ermingen M, Meffert E, Grabowska A, Huber W, Amunts K. Interaction of phonological awareness and “magnocellular” processing during normal and dyslexic reading: behavioural and fMRI investigations. Dyslexia. 2010;16:258–282. doi: 10.1002/dys.409. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, McMillon G, Taylor-Hill H, Martindale JL, Meyler A, Keller TA, Siok WT, Deutsch GK, Just MA, et al. Neural basis of dyslexia: a comparison between dyslexic and nondyslexic children equated for reading ability. J Neurosci. 2006;26:10700–10708. doi: 10.1523/JNEUROSCI.4931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, McMillon G, Kolchugina G, Black JM, Faizi A, et al. Functional and morphometric brain dissociation between dyslexia and reading ability. Proc Natl Acad Sci USA. 2007;104:4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme C. The implausibility of low-level visual deficits as a cause of children’s reading difficulties. Cognitive Neuropsychology. 1988;5:369–374. [Google Scholar]
- Kang E, Lee DS, Kang H, Lee JS, Oh SH, Lee MC, Kim CS. Neural changes associated with speech learning in deaf children following cochlear implantation. Neuroimage. 2004;22:1173–1181. doi: 10.1016/j.neuroimage.2004.02.036. [DOI] [PubMed] [Google Scholar]
- Koldewyn K, Whitney D, Rivera SM. Neural correlates of coherent and biological motion perception in autism. Developmental Science. 2011;14:1075–1088. doi: 10.1111/j.1467-7687.2011.01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Lindquist MA, Nichols TE, Poldrack RA, Vul E. Everything you never wanted to know about circular analysis, but were afraid to ask. J Cereb Blood Flow Metab. 2010;30:1551–1557. doi: 10.1038/jcbfm.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok V, Niu Z, Kay P, Zhou K, Mo L, Jin Z, So KF, Tan LH. Learning new color names produces rapid increase in gray matter in the intact adult human cortex. Proceedings of the National Academy of Sciences. 2011;108:6686–6688. doi: 10.1073/pnas.1103217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindamood PC, Lindamood P. Lindamood-bell auditory conceptualization test. Austin, TX: Pro-Ed Incorporated; 2004. [Google Scholar]
- Livingstone MS, Rosen GD, Drislane FW, Galaburda AM. Physiological and anatomical evidence for a magnocellular defect in developmental dyslexia. Proc Natl Acad Sci USA. 1991;88:7943–7947. doi: 10.1073/pnas.88.18.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovegrove WJ, Bowling A, Badcock D, Blackwood M. Specific reading disability: differences in contrast sensitivity as a function of spatial frequency. Science. 1980;210:439–440. doi: 10.1126/science.7433985. [DOI] [PubMed] [Google Scholar]
- Mendola JD, Dale AM, Fischl B, Liu AK, Tootell RB. The representation of illusory and real contours in human cortical visual areas revealed by functional magnetic resonance imaging. J Neurosci. 1999;19:8560–8572. doi: 10.1523/JNEUROSCI.19-19-08560.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Cheng-Lai A, Zeng B, Stein JF, Zhou X. Dynamic visual perception and reading development in Chinese school children. Annals of Dyslexia. 2011;61:161–176. doi: 10.1007/s11881-010-0049-2. [DOI] [PubMed] [Google Scholar]
- Milne E, Swettenham J, Hansen P, Campbell R, Jeffries H, Plaisted K. High motion coherence thresholds in children with autism. J Child Psychol Psychiatry. 2002;43:255–263. doi: 10.1111/1469-7610.00018. [DOI] [PubMed] [Google Scholar]
- Mitchell TV, Neville HJ. Asynchronies in the development of electrophysiological responses to motion and color. J Cogn Neurosci. 2004;16:1363–1374. doi: 10.1162/0898929042304750. [DOI] [PubMed] [Google Scholar]
- Olson R, Wise B, Conners F, Rack J, Fulker D. Specific deficits in component reading and language skills: genetic and environmental influences. J Learn Disabil. 1989;22:339–348. doi: 10.1177/002221948902200604. [DOI] [PubMed] [Google Scholar]
- Peterson RL, Pennington BF. Developmental dyslexia. Lancet. 2012;379:1997–2007. doi: 10.1016/S0140-6736(12)60198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Region of interest analysis for fMRI. Social Cognitive and Affective Neuroscience. 2006;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Functional imaging studies of neuropsychological patients: applications and limitations. Neurocase. 2002;8:345–354. doi: 10.1076/neur.8.4.345.16186. [DOI] [PubMed] [Google Scholar]
- Price CJ, Crinion J, Friston KJ. Design and analysis of fMRI studies with neurologically impaired patients. J Magn Reson Imaging. 2006;23:816–826. doi: 10.1002/jmri.20580. [DOI] [PubMed] [Google Scholar]
- Ramus F. Neurobiology of dyslexia: a reinterpretation of the data. Trends Neurosci. 2004;27:720–726. doi: 10.1016/j.tins.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Rauschecker AM, Bowen RF, Perry LM, Kevan AM, Dougherty RF, Wandell BA. Visual Feature-Tolerance in the Reading Network. Neuron. 2011;71:941–953. doi: 10.1016/j.neuron.2011.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Caspi A, Fergusson D, Horwood LJ, Goodman R, Maughan B, Moffitt TE, Meltzer H, Carroll J. Sex differences in developmental reading disability: new findings from 4 epidemiological studies. JAMA. 2004;291:2007–2012. doi: 10.1001/jama.291.16.2007. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL. Functional Neuroanatomical Differences Between Adults and School-Age Children in the Processing of Single Words. Science. 2002;296:1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Shapley R. Visual sensitivity and parallel retinocortical channels. Annu Rev Psychol. 1990;41:635–658. doi: 10.1146/annurev.ps.41.020190.003223. [DOI] [PubMed] [Google Scholar]
- Snowling MJ. The development of grapheme-phoneme correspondence in normal and dyslexic readers. J Exp Child Psychol. 1980;29:294–305. doi: 10.1016/0022-0965(80)90021-1. [DOI] [PubMed] [Google Scholar]
- Stein J. The magnocellular theory of developmental dyslexia. Dyslexia. 2001;7:12–36. doi: 10.1002/dys.186. [DOI] [PubMed] [Google Scholar]
- Stein J, Walsh V. To see but not to read; the magnocellular theory of dyslexia. Trends Neurosci. 1997;20:147–152. doi: 10.1016/s0166-2236(96)01005-3. [DOI] [PubMed] [Google Scholar]
- Szwed M, Ventura P, Querido L, Cohen L, Dehaene S. Reading acquisition enhances an early visual process of contour integration. Developmental Science. 2012;15:139–149. doi: 10.1111/j.1467-7687.2011.01102.x. [DOI] [PubMed] [Google Scholar]
- Talcott JB, Witton C, McLean MF, Hansen PC, Rees A, Green GG, Stein JF. Dynamic sensory sensitivity and children’s word decoding skills. Proc Natl Acad Sci USA. 2000;97:2952–2957. doi: 10.1073/pnas.040546597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talcott JB, Gram A, Van Ingelghem M, Witton C, Stein JF, Toennessen FE. Impaired sensitivity to dynamic stimuli in poor readers of a regular orthography. Brain and Language. 2003;87:259–266. doi: 10.1016/s0093-934x(03)00105-6. [DOI] [PubMed] [Google Scholar]
- Temereanca S, Hamalainen MS, Kuperberg GR, Stufflebeam SM, Halgren E, Brown EN. Eye Movements Modulate the Spatiotemporal Dynamics of Word Processing. Journal of Neuroscience. 2012;32:4482–4494. doi: 10.1523/JNEUROSCI.5571-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RB, Reppas JB, Kwong KK, Malach R, Born RT, Brady TJ, Rosen BR, Belliveau JW. Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J Neurosci. 1995;15:3215–3230. doi: 10.1523/JNEUROSCI.15-04-03215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuley K, Bell N. On cloud nine : visualizing and verbalizing for math. San Luis Obispo, CA: Gander Pub; 1997. [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat Neurosci. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Mansfield RJW, Goodale MA, editors. The Analysis of Visual Behavior. Cambridge, MA: The MIT Press; 1982. pp. 549–586. [Google Scholar]
- Vanni S, Uusitalo MA, Kiesilä P, Hari R. Visual motion activates V5 in dyslexics. Neuroreport. 1997;8:1939–1942. doi: 10.1097/00001756-199705260-00029. [DOI] [PubMed] [Google Scholar]
- Vellutino FR, Fletcher JM, Snowling MJ, Scanlon DM. Specific reading disability (dyslexia): what have we learned in the past four decades? J Child Psychol Psychiatry. 2004;45:2–40. doi: 10.1046/j.0021-9630.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- Watson JD, Myers R, Frackowiak RS, Hajnal JV, Woods RP, Mazziotta JC, Shipp S, Zeki S. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb Cortex. 1993;3:79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence (WASI) San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Wilmer JB, Richardson AJ, Chen Y, Stein JF. Two visual motion processing deficits in developmental dyslexia associated with different reading skills deficits. J Cogn Neurosci. 2004;16:528– 540. doi: 10.1162/089892904323057272. [DOI] [PubMed] [Google Scholar]
- Witton C, Talcott JB, Hansen PC, Richardson AJ, Griffiths TD, Rees A, Stein JF, Green GG. Sensitivity to dynamic auditory and visual stimuli predicts nonword reading ability in both dyslexic and normal readers. Curr Biol. 1998;8:791–797. doi: 10.1016/s0960-9822(98)70320-3. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III tests of achievement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- Zihl J, Von Cramon D, Mai N. Selective Disturbance of Movement Vision after Bilateral Brain Damage. Brain. 1983;106:313–340. doi: 10.1093/brain/106.2.313. [DOI] [PubMed] [Google Scholar]