Abstract
The serum and urine polyamines putrescine, spermidine, and spermine were measured in 112 normal subjects from 0 to 70 yr of age, and in three groups of short children from 7 to 20 yr: 21 growth hormone (GH) deficient patients, 20 normal variant short stature children, and 9 girls with 45, X Turner's syndrome. Urine polyamines were expressed as micromoles per gram of creatinine or per kilogram body weight, and serum polyamines were expressed as nanomoles per milliliter.
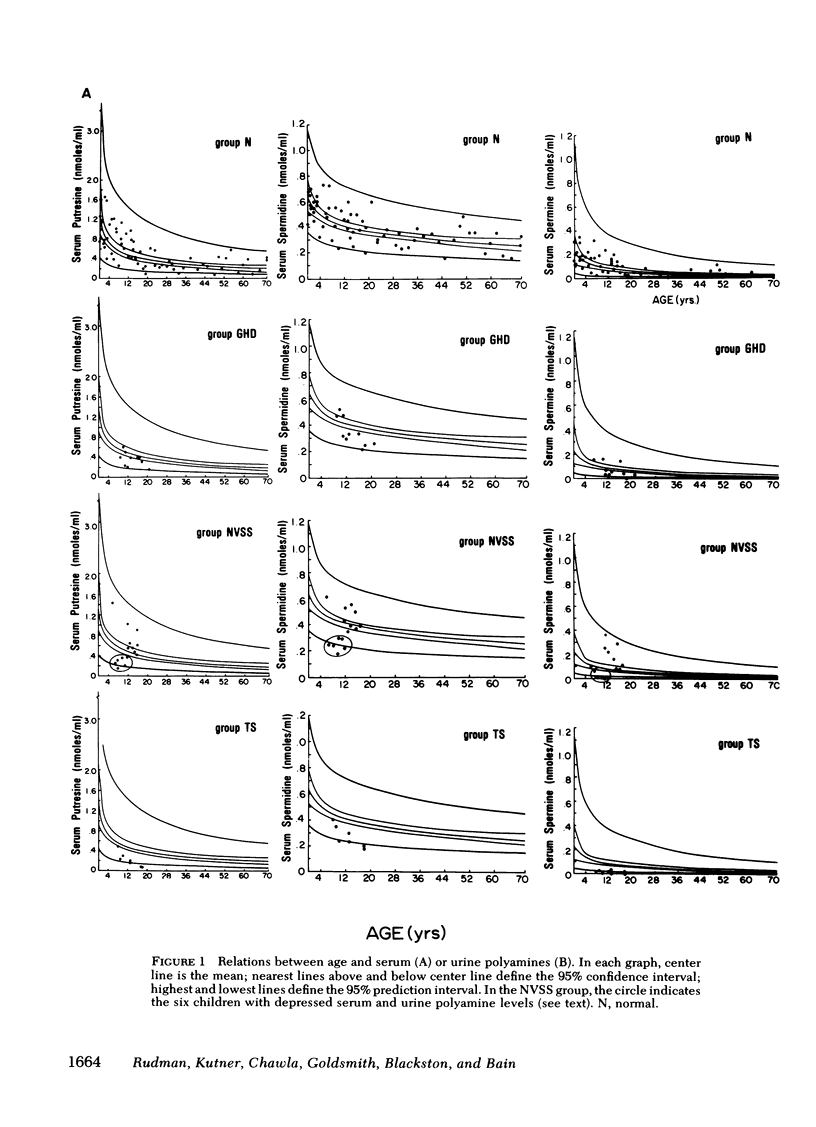
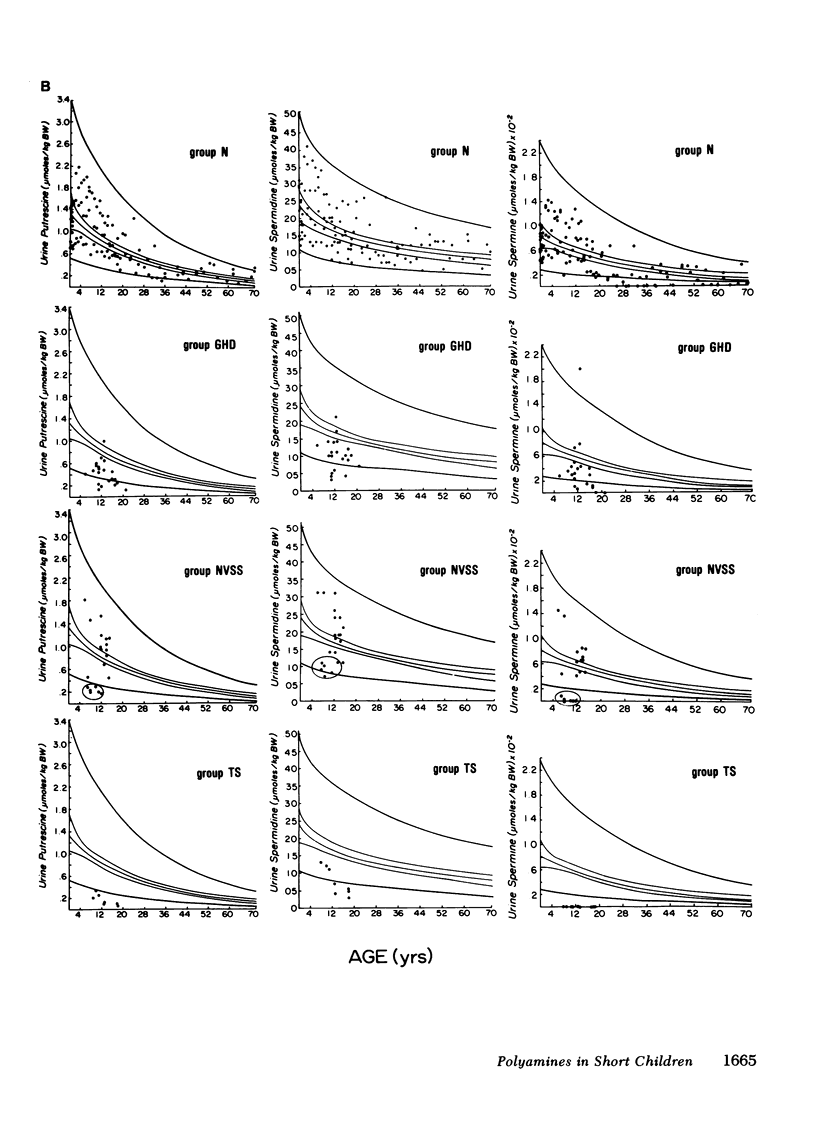
In normals, the three polyamines were highest in urine and serum at birth. The mean levels declined progressively with age, the rate of change decreasing with age. The mean for the normal subjects, and its 95% confidence and prediction intervals, were estimated from birth to age 70 for each serum and urine polyamine.
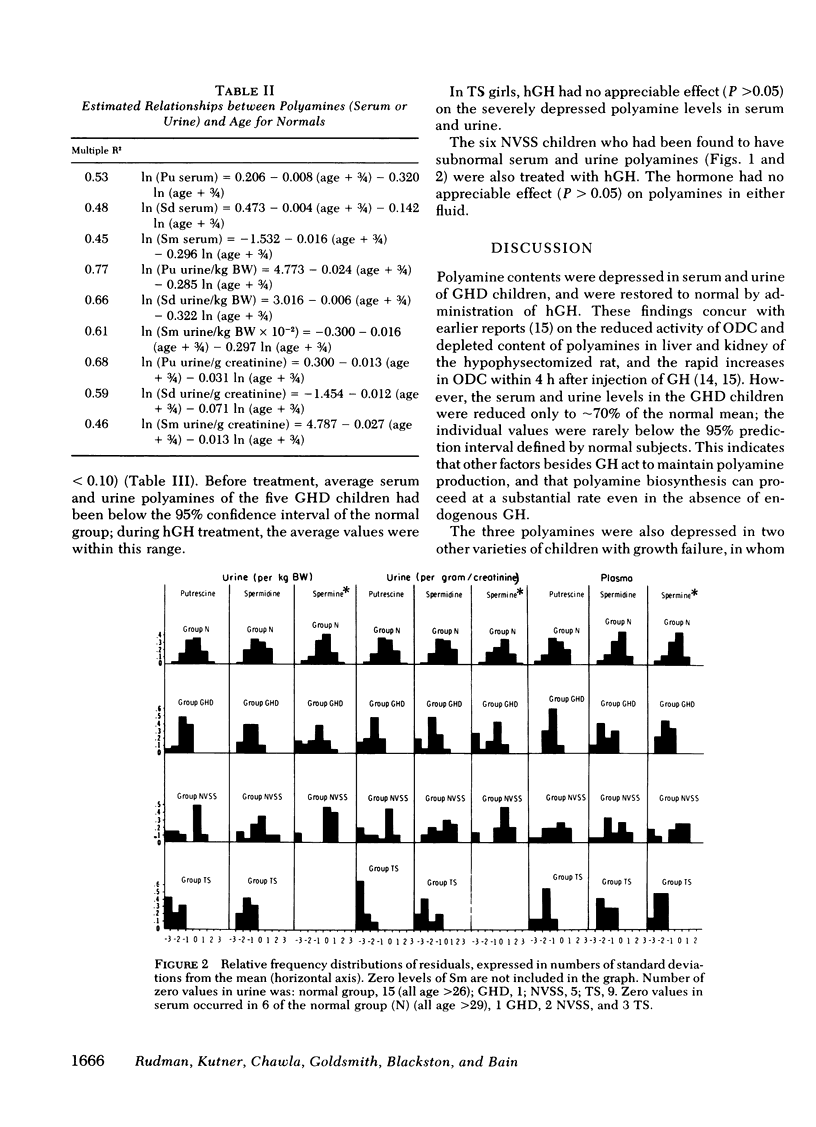
In GH-deficient children, serum and urine values were significantly lower (P < 0.05) than the age-specific normal values (with the exception of serum spermidine and spermine), averaging 25-55% below normal. This abnormality was corrected during 1 wk of treatment with human GH.
In Turner's syndrome, serum and urine values were significantly reduced (P < 0.05), averaging 35-80% below age-specific normals. GH treatment had no corrective effect.
In 6 of 20 normal variant short stature children, polyamine levels were significantly (P < 0.01) subnormal, averaging 50-80% below age-specific normals in both serum and urine. Treatment with GH had no corrective effect.
These data show that levels of polyamines in serum and urine are correlated with linear growth primarily during the first decade of life. Subnormal polyamine levels are generally associated with growth retardation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barros C., Giudice G. Effect of polyamines on ribosomal RNA synthesis during sea urchin development. Exp Cell Res. 1968 Jun;50(3):671–674. doi: 10.1016/0014-4827(68)90434-5. [DOI] [PubMed] [Google Scholar]
- Desser H., Höcker P., Weiser M., Böhnel J. The content of unbound polyamines in blood plasma and leukocytes of patients with polycythemia vera. Clin Chim Acta. 1975 Sep 16;63(3):243–247. doi: 10.1016/0009-8981(75)90044-3. [DOI] [PubMed] [Google Scholar]
- Dreyfuss F., Chayen R., Dreyfuss G., Dvir R., Ratan J. Polyamine excretion in the urine of cancer patients. Isr J Med Sci. 1975 Aug;11(8):785–795. [PubMed] [Google Scholar]
- Durie B. G., Salmon S. E., Russell D. H. Polyamines as markers of response and disease activity in cancer chemotherapy. Cancer Res. 1977 Jan;37(1):214–221. [PubMed] [Google Scholar]
- Fair W. R., Wehner N., Brorsson U. Urinary polyamine levels in the diagnosis of carcinoma of the prostate. J Urol. 1975 Jul;114(1):88–92. doi: 10.1016/s0022-5347(17)66951-9. [DOI] [PubMed] [Google Scholar]
- Fleisher J. H., Russell D. H. Estimation of urinary diamines and polyamines by thin-layer chromatography. J Chromatogr. 1975 Jul 16;110(2):335–340. doi: 10.1016/0021-9673(75)85014-x. [DOI] [PubMed] [Google Scholar]
- Fujita K., Nagatsu T., Maruta K., Ito M., Senba H. Urinary putrescine, spermidine, and spermine in human blood and solid cancers and in an experimental gastric tumor of rats. Cancer Res. 1976 Apr;36(4):1320–1324. [PubMed] [Google Scholar]
- Ham R. G. Putrescine and related amines as growth factors for a mammalian cell line. Biochem Biophys Res Commun. 1964;14:34–38. doi: 10.1016/0006-291x(63)90206-7. [DOI] [PubMed] [Google Scholar]
- Henningsson S., Rosengren E. The effect of nandrolone, an anabolic steroid on putrescine metabolism in the mouse. Br J Pharmacol. 1976 Nov;58(3):401–406. doi: 10.1111/j.1476-5381.1976.tb07717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyo J. L. Changes in polyamine content of rat liver following hypophysectomy and treatment with growth hormone. Biochem Biophys Res Commun. 1966 Apr 19;23(2):150–155. doi: 10.1016/0006-291x(66)90520-1. [DOI] [PubMed] [Google Scholar]
- Levy C. C., Mitch W. E., Schmukler M. Effect of polyamines on a ribonuclease which hydrolyzes ribonucleic acid at uridylic acid residues. J Biol Chem. 1973 Aug 25;248(16):5712–5719. [PubMed] [Google Scholar]
- Lipton A., Sheehan L. M., Kessler G. F., Jr Urinary polyamine levels in human cancer. Cancer. 1975 Feb;35(2):464–468. doi: 10.1002/1097-0142(197502)35:2<464::aid-cncr2820350225>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Marton L. J., Heby O., Levin V. A., Lubich W. P., Crafts D. C., Wilson C. B. The relationship of polyamines in cerebrospinal fluid to the presence of central nervous system tumors. Cancer Res. 1976 Mar;36(3):973–977. [PubMed] [Google Scholar]
- Marton L. J., Russell D. H., Levy C. C. Measurement of putrescine, spermidine, and spermine in physiological fluids by use of an amino acid analyzer. Clin Chem. 1973 Aug;19(8):923–926. [PubMed] [Google Scholar]
- McAnulty P. A., Williams J. P. Polyamine-synthesizing enzymes during recovery from nutritionally induced growth restriction. Biochem Soc Trans. 1975;3(4):521–524. doi: 10.1042/bst0030521. [DOI] [PubMed] [Google Scholar]
- Nicholson W. E., Levine J. H., Orth D. N. Hormonal regulation of renal ornithine decarboxylase activity in the rat. Endocrinology. 1976 Jan;98(1):123–128. doi: 10.1210/endo-98-1-123. [DOI] [PubMed] [Google Scholar]
- Nishioka K., Romsdahl M. M. Elevation of putrescine and spermidine in sera of patients with solid tumors. Clin Chim Acta. 1974 Dec 2;57(2):155–161. doi: 10.1016/0009-8981(74)90424-0. [DOI] [PubMed] [Google Scholar]
- Penny R., Blizzard R. M., Davis W. T. Sequential arginine and insulin tolerance tests on the same day. J Clin Endocrinol Metab. 1969 Nov;29(11):1499–1501. doi: 10.1210/jcem-29-11-1499. [DOI] [PubMed] [Google Scholar]
- Priest J. H., Blackston R. D., Au K. S., Ray S. L. Differences in human X isochromosomes. J Med Genet. 1975 Dec;12(4):378–389. doi: 10.1136/jmg.12.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina A., Cohen S. S. Polyamines and RNA synthesis in a polyauxotrophic strain of E. coli. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1587–1593. doi: 10.1073/pnas.55.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina A., Jänne J. Polyamines and the accumulation of RNA in mammalian systems. Fed Proc. 1970 Jul-Aug;29(4):1568–1574. [PubMed] [Google Scholar]
- Rozovski S. J., Rosso P., Winick M. Effect of malnutrition and rehabilitation on the metabolism of polyamines in rat liver. J Nutr. 1978 Oct;108(10):1680–1690. doi: 10.1093/jn/108.10.1680. [DOI] [PubMed] [Google Scholar]
- Rudman D., Chyatte S. B., Patterson J. H., Gerron G. G., O'Beirne I., Barlow J., Ahmann P., Jordan A., Mosteller R. C. Observations on the responsiveness of human subjects to human growth hormone. Effects of endogenous growth hormone deficiency and myotinic dystrophy. J Clin Invest. 1971 Sep;50(9):1941–1949. doi: 10.1172/JCI106686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudman D., Kutner M. H., Blackston R. D., Jansen R. D., Patterson J. H. Normal variant short stature: subclassification based on responses to exogenous human growth hormone. J Clin Endocrinol Metab. 1979 Jul;49(1):92–99. doi: 10.1210/jcem-49-1-92. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Snyder S. H. Amine synthesis in regenerating rat liver: effect of hypophysectomy and growth hormone on ornithine decarboxylase. Endocrinology. 1969 Feb;84(2):223–228. doi: 10.1210/endo-84-2-223. [DOI] [PubMed] [Google Scholar]
- Sanford E. J., Drago J. R., Rohner T. J., Kessler G. F., Sheehan L., Lipton A. Preliminary evaluation of urinary polyamines in the diagnosis of genitourinary tract malignancy. J Urol. 1975 Feb;113(2):218–221. doi: 10.1016/s0022-5347(17)59448-3. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Kellogg P. D. The effect of isolation conditions on the polyamine content of Escherichia coli ribosomes. J Biol Chem. 1967 Mar 10;242(5):1044–1052. [PubMed] [Google Scholar]


