Abstract
Leptin receptors (ObRs) in the forebrain and hindbrain have been independently recognized as important mediators of leptin responses. It is unclear how leptin activity in these areas is integrated. We tested whether both forebrain and hindbrain ObRs have to be activated simultaneously to change energy balance and to maintain metabolic homeostasis. Previous studies used acute leptin injections in either the third ventricle (1–5 μg) or the fourth ventricle (3–10 μg); here we used 12-day infusions of low doses of leptin in one or both ventricles (0.1 μg/24 h in third, 0.6 μg/24 h in fourth). Male Sprague Dawley rats were fitted with third and fourth ventricle cannulas, and saline or leptin was infused from Alzet pumps for 6 or 12 days. Rats that received leptin into only the third or the fourth ventricle were not different from controls that received saline in both ventricles. By contrast, rats with low-dose leptin infusions into both the third and fourth ventricle showed a dramatic 60% reduction in food intake that was reversed on day 6, a 20% weight loss that stabilized on day 6, and a 50% decrease in body fat at day 12 despite the correction of food intake. They displayed normal activity and maintained energy expenditure despite weight loss, indicating inappropriately high thermogenesis that coincided with increased signal transducer and activator of transcription 3 (STAT3) phosphorylation in the brainstem. Altogether, these findings show that with low doses of leptin, chronic activation of both hypothalamic and brainstem ObRs is required to reduce body fat.
The adipokine leptin was originally proposed as a negative feedback signal in the regulation of energy balance (1). It is now clear that leptin regulates multiple endocrine systems including reproduction, adrenal function, and bone remodeling (2). Although the primary function of leptin remains undefined, it is well established that administration of exogenous leptin both centrally and peripherally inhibits food intake, increases energy expenditure, and causes a selective loss of body fat in leptin-sufficient rats (3, 4).
Leptin-induced changes in food intake and energy expenditure are mediated primarily by the long isoform of the leptin receptor (ObRb). The phosphorylations of transcription factor signal transducer and activator of transcription-3 (STAT3) (5), phosphoinositide-3-kinase (PI3K) (6), and ERK1/2 (7) are some of the leptin-induced changes in signaling proteins that are important for transcriptional regulation of orexigenic and anorexigenic peptides and immediate early gene markers. Leptin signaling induces a rapid increase in the expression of suppressor of cytokine signaling-3 (SOCS3) which inhibits STAT3 activation (8). Protein-tyrosine phosphatase (PTP1B) also inhibits leptin action by preventing phosphorylation of leptin receptor (ObR) Janus kinase 2 (JAK2) (9).
ObRs in the hypothalamus have been shown to be important in the regulation of energy balance. We and others have demonstrated that acute high-dose leptin injections into the third ventricle decrease body fat, food intake, and body weight, where doses in the range of 1.5 to 5 μg effectively produce a leptin response (10, 11). Recently, the importance of brainstem nuclei in the regulation of energy balance has been recognized. Acute leptin injections in the fourth ventricle decrease body weight and food intake, although doses lower than 0.83 μg seem to be ineffective in producing a decrease in body weight and food intake (12, 13). The role of hypothalamic and brainstem ObRs in energy balance have been investigated independently, yet it is unclear whether leptin signals in the forebrain and hindbrain integrate to maintain metabolic homeostasis. The studies described here tested whether activation of forebrain or hindbrain ObRs with low doses of leptin had an additive effect on energy balance.
Continuous infusions of subthreshold doses of leptin from mini osmotic pumps were used to chronically activate hypothalamic and brainstem ObRs and facilitate a better understanding of an interaction between the two brain areas. Chronic infusions mimic the presence of factors in the circulation and produce leptin responses with 3- to 10-fold lower doses (0.3 μg/d in third ventricle and 0.6 μg/d in fourth ventricle) (14) compared with acute leptin injections (11, 12). Therefore, we tested whether low doses of leptin that did not have a significant effect on food intake or body weight when applied independently to the third or fourth ventricle acted additively or synergistically when given together.
Materials and Methods
Male Sprague Dawley rats (Harlan Laboratories, Inc, Indianapolis, Indiana) were housed individually in hanging wire mesh cages in a room maintained at 20°C to 23°C with lights on for 12 hours per day from 7:00 am. They had free access to chow (Harlan Teklad Rodent Diet 8604) and water unless stated otherwise. Each rat had a Nylabone (Nylabone Products, Neptune, New Jersey) in their cage for enrichment. All animal procedures were approved by the Institutional Animal Care and Use Committee of Georgia Regents University. For each experiment, the rats were allowed to adapt to their environment for 1 week before performing surgeries. Measurement of baseline food intake was initiated 1 week after the rats recovered from placement of ventricular infusion cannulae.
Experiment 1
This study was designed to test the effect of low-dose leptin (recombinant rat leptin; R&D Systems, Minneapolis, Minnesota) when infused into both the third ventricle and fourth ventricle to determine whether both forebrain and hindbrain ObRs need to be activated to produce a significant change in energy balance.
A total of 36 male Sprague Dawley rats, in 3 cohorts of 12 with each treatment group represented in each cohort, were housed and maintained as described above. Each rat was fitted with a third and fourth ventricle infusion cannula (Plastics One Inc, Roanoake, Virginia) and allowed to recover from surgery for 1 week. The coordinates for the third ventricle infusion cannula in relation to the midline at bregma were 2.8 mm posterior, 0 mm lateral, and 9 mm ventrodorsal, and coordinates for the fourth ventricle infusion cannula in relation to the midline at the occipital suture were 2.5 mm anterior, 0 mm lateral, and 6.5 mm ventrodosral. The rats were then housed in individual indirect calorimeter cages (TSE LabMaster Metabolic Research Platform; TSE Systems International, Chesterfield, Missouri). Oxygen consumption, carbon dioxide production, and activity were sampled for 3 minutes from each cage every 39 minutes. Values measured during the last minute of the 3 minutes were used to calculate energy expenditure expressed both as kilocalories per hour per rat and per unit metabolic body weight (kilocalories per hour per weight0.75) and respiratory exchange ratio (RER) as an index of macronutrient oxidation. Total activity was measured by Inframot (TSE Systems). Baseline measures were recorded for 3 days, and the rats were then divided into 4 weight-matched groups. An Alzet mini osmotic pump (model 2002; DURECT Corporation, Cupertino, California) with an infusion rate of 0.25 μl/h was connected to each cannula to deliver either saline or 0.1 μg/day of leptin in the third ventricle or 0.6 μg/day of leptin in the fourth ventricle such that there were 4 treatment groups: saline-saline (SS), saline-leptin (SL), leptin-saline (LS), and leptin-leptin (LL) (first letter indicating infusion in third ventricle and second letter indicating infusion in fourth ventricle). At the same time as the pumps were placed, the rats were fitted with a Thermicron iButton (DS1922L; Embedded Data Systems, Lawrenceburg, Kentucky) placed on top of the intracapsular brown adipose tissue (IBAT). The iButton was set to record temperature every 30 minutes with a precision of 0.06°C starting 24 hours after pump placement and continuing until the end of the study. Daily measures of food intake, water intake, RER, energy expenditure, and activity were recorded for 12 days after the pumps were attached. The doses of leptin infused were chosen based on a dose-response study carried out in our laboratory (14). At the end of the study, the rats were euthanized and the inguinal, epididymal, retroperitoneal, mesenteric, and IBAT depots were weighed. Tissue blocks of hypothalamus and brainstem were collected (15) to determine activation of leptin signaling proteins and ObR expression using Western blot analysis as described previously (16). All primary antibodies were obtained from Cell Signaling Technology, Inc (Danvers, Massachusetts) except anti-PTP1B obtained from Abcam (ab2009; Abcam Plc, Cambridge, Massachusetts) and anti-ObR obtained from Neuromics (GT15059; Neuromics, Edina, Minnesota). Trunk blood was collected to measure serum leptin and insulin by RIA (rat leptin RIA kit and rat insulin RIA kit; Millipore Corporation, Billerica, Massachusetts). Serum glucose was measured using Easy Gluco blood glucose test trips (US Diagnostics Inc, Huntsville, Alabama). Liver lipid and glycogen content were measured as described previously (17). The carcass was analyzed for composition (18).
Experiment 2
In experiment 1, rats infused with leptin in both third and fourth ventricles (LL) showed a decrease in food intake that was reversed by day 6. At the end of the experiment on day 12, however, there was a dramatic decrease in body fat despite the reversal in food intake. The purpose of this experiment was to test whether LL rats were leptin-responsive throughout the 12 days of the experiment or whether the restoration of food intake in these rats was an indication of leptin resistance. The experiment was also designed to determine the differences in leptin response at different times during the course of the experiment.
A total of 60 Male Sprague Dawley rats, in 2 cohorts, were housed and maintained in wire mesh cages throughout the study. Surgical procedures were the same as in experiment 1. After 1 week of recovery from cannula placement, baseline food intakes and body weights were recorded for 5 days. Alzet mini osmotic pumps were attached to infusion cannula to infuse saline or leptin such that there were 8 groups: SS, LL, SL, LS (infusions for 12 days, similar to study 1), and 2 sets of additional groups, SS6 and LL6 (rats killed on day 6 of infusion) and SS-cut and LL-cut (infusions stopped on day 6 by cutting the tubing connecting the pump to the cannula, and rats killed on day 12). Daily body weight and food intake were measured. On day 6, 1 set (SS6 and LL6) of rats was killed and their tissues were collected. Infusion pump connections to the cannulas from a second set of rats (SS-cut and LL-cut) were cut and the animals were maintained through to day 12 of the experiment.
At the end of the study, inguinal, epididymal, retroperitoneal, mesenteric, and IBAT depots were weighed. Trunk blood was collected to measure serum glucose, leptin, and insulin. Liver lipid and glycogen content were measured, and carcass composition was determined. Inguinal and IBAT pads were collected for Western blot analysis of uncoupling protein 1 (UCP1) and mitochondrial cytochrome C oxidase (MTCO1) and real-time PCR of leptin mRNA in inguinal fat. Total RNA from inguinal fat was isolated using Trizol reagent (Invitrogen Life Technologies, Carlsbad, California) according to the manufacturer's directions, except that the homogenized samples were allowed to stand at room temperature for 1 hour in between the addition of chloroform and centrifugation. Leptin mRNA expression was detected by quantitative RT-PCR. After total RNA extraction, 1 μg RNA was treated with 1 μL deoxyribonuclease I (Invitrogen), 1 μL 10× deoxyribonuclease I reaction buffer, and nuclease-free water to 10 μL. After the treatment of RNA with deoxyribonuclease I, RNA was used with the Promega reverse transcription system (Promega Corporation, Madison Wisconsin). A control containing no avian myeloblastosis virus reverse transcriptase was used to ensure that all reverse transcription was complete. After reverse transcription, cDNA was treated with ribonuclease H (Invitrogen). First-strand cDNA PCR was then performed using iQ SYBR Green Supermix (Bio-Rad, Hercules, California). The forward primer used for leptin was 5′-TTGTCACCAGGATCAATGACATTT-3′, and the reverse primer used was 5′-GACAAACTCAGAATGGGGTGAAG-3′. Quantitative RT-PCR was performed using the Bio-Rad iCycler iQ system. Cycling conditions were as follows: initial denaturation at 95°C for 3 minutes and 40 cycles of 95°C for 30 seconds, 58°C for 30 seconds, and 72°C for 30 seconds. Melting-curve analysis was performed immediately after quantitative PCR amplification. Tissue blocks of hypothalamus and brainstem were collected only from SS6 and LL6 rats (15) to determine activation of leptin signaling proteins and ObR expression on day 6 using Western blot analysis (16).
Data analysis
Statistically significant differences between treatment groups were determined using Statistica software version 9.0 (StatSoft, Tulsa, Oklahoma). Differences were considered significant at P < .05. Single end point measures were compared by unpaired t test, 1-way ANOVA, or 2-way ANOVA depending upon experimental design. Daily measures of food intake, body weight, energy expenditure, RER, and activity were compared by repeated-measures ANOVA. Post hoc differences were determined using Duncan's multiple-range test or unpaired t test.
Results
Experiment 1: Low doses of leptin produce a dramatic leptin response when infused centrally into both the third and fourth ventricles for 12 days
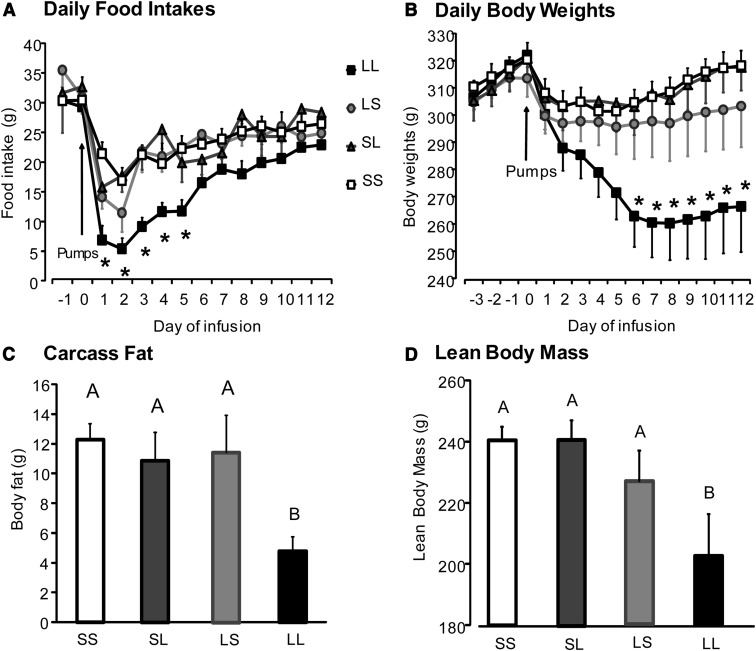
Rats receiving low-dose leptin infusions into either the third ventricle (LS) or the fourth ventricle (SL) showed no differences in body weight during the experiment or in body fat or lean body mass when compared with saline-infused controls (SS) at the end of the 12 days of infusion (Figure 1, B–D). Rats receiving low-dose leptin infusions into both the third and fourth ventricles (LL rats) showed a significant reduction in energy intake for the first 5 days of leptin infusion compared with control SS rats (P < .03) after which their intake returned to the same as that of control rats through the rest of the experiment. The energy intake of LS and SL rats was no different from that of controls (SS) on any of the 11 days of the experiment (Figure 1A). LL rats showed a significant loss in body weight by day 6 (P < .04) as compared with rats with single ventricle leptin infusion in either third or fourth ventricles (LS and SL) or with controls (SS), and this weight loss was maintained through the rest of the experiment (Figure 1B). The decrease in body weight of LL rats after 12 days (P < .02) was reflected in a dramatic 50% reduction in body fat (P < .008) and a 16% reduction in lean body mass (P < .009) compared with SS controls (Figure 1, C and D). Leptin reduced the size of all fat depots weighed in LL rats except the mesenteric fat (Table 1) and IBAT depots (data not shown). Leptin reduced the size of the epididymal and retroperitoneal fat depot by a larger percentage (80%) than inguinal fat (66%). The energy stores of the liver were depleted with liver lipid significantly lower in LL rats than in LS, SL, and control rats.
Figure 1.
Food intake, body weight, body fat, and lean body mass of rats from experiment 1 infused with saline (SS), low-dose leptin in either third or fourth ventricle (LS or SL), or low-dose leptin in both ventricles (LL) for 12 days. Data are means ± SEM for 9 rats. *, Significant difference between rats infused in both ventricles (LL) and all other groups (SS, LS, and SL) at P < .05. Data for body fat or lean body mass that do not share a common superscript are significantly different at P < .05.
Table 1.
Body Weights, Body Composition, Energy Stores, Serum Hormone Levels, and IBAT Temperatures of Rats From Experiment 1
| SS | LS | SL | LL | |
|---|---|---|---|---|
| Start weight, g | 320 ± 6 | 314 ± 7 | 321 ± 7 | 323 ± 4 |
| End weight, g | 319 ± 5b | 303 ± 15b | 318 ± 9b | 267 ± 17c |
| Liver weight, g | 11.0 ± 0.5 | 9.9 ± 0.9 | 10.7 ± 0.9 | 9.2 ± 1.0 |
| Liver lipid, mg | 429 ± 21b | 359 ± 39b | 414 ± 37b | 322 ± 40c |
| Liver glycogen, mg | 138 ± 20 | 83 ± 16 | 114 ± 33 | 104 ± 29 |
| Fat depots | ||||
| Inguinal, g | 3.02 ± 0.38b | 1.87 ± 0.48b | 2.51 ± 0.43b | 1.02 ± 0.13c |
| Epididymal, g | 2.05 ± 0.23b | 1.84 ± 0.45b | 1.71 ± 0.34b | 0.45 ± 0.11c |
| Retroperitoneal, g | 0.62 ± 0.12b | 0.63 ± 0.21b | 0.59 ± 0.17b | 0.09 ± 0.03c |
| Mesenteric, g | 0.80 ± 0.18 | 0.63 ± 0.18 | 0.65 ± 0.17 | 0.38 ± 0.14 |
| Serum measures | ||||
| Leptin, ng/mL | 1.28 ± 0.13 | 1.40 ± 0.24 | 1.36 ± 0.11 | 0.95 ± 0.11 |
| Insulin, ng/mL | 2.55 ± 0.56 | 2.29 ± 0.60 | 1.91 ± 0.55 | 1.52 ± 0.67 |
| Range | 0.27–5.09 | 0.29–4.67 | 0.21–7.14 | 0.43–4.08 |
| Glucose, mg/dL | 117 ± 9 | 125 ± 11 | 122 ± 16 | 116 ± 18 |
| Range | 72–152 | 84–156 | 71–191 | 84–160 |
| IBAT temperature, °C | ||||
| Daytime temperature | 36.55 ± 0.08b | 36.51 ± 0.06b,c | 36.65 ± 0.07b,c | 36.82 ± 0.12c |
| Nighttime temperature | 37.44 ± 0.01 | 37.41 ± 0.04 | 37.59 ± 0.04 | 37.70 ± 0.11 |
Data are means ± SEM for groups of 8 rats. All measures were made after 12 days of infusion.
Values for a specific parameter that do not share a common superscript are significantly different at P < .05.
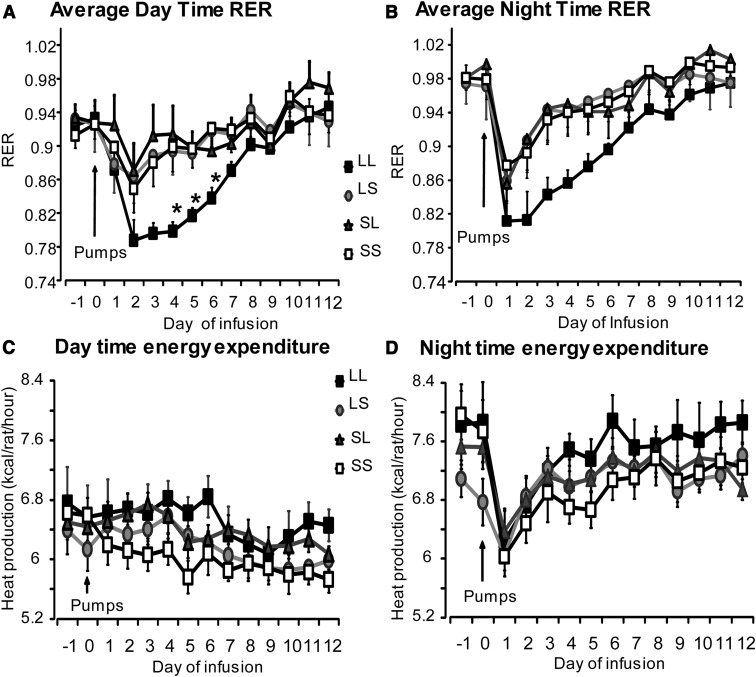
RER, an indicator of the type of fuel metabolized for energy utilization, closely tracked food intake. LL rats had a significantly lower daytime RER (0.75–0.87) compared with LS, SL, and SS rats on days 3, 4, and 5 (Figure 2A) (P < .02), suggesting oxidation of endogenous fat and protein for energy metabolism when their food intake was low. The daytime RER in LL rats was no different from the other treatment groups from day 6 onward (RER, 0.9–1.05), suggesting that the rats were using primarily carbohydrate,which paralleled the restoration of their consumption of the high-carbohydrate chow diet from days 6 through 11. Night-time RER tended to be lower in LL rats compared with control SS rats on days 2, 3, and 4, but these differences were not statistically significant (P < .06) (Figure 2B).
Figure 2.
RER and energy expenditure of rats from experiment 1 infused with PBS (SS), low-dose leptin in either third or fourth ventricle (LS or SL), or low-dose leptin in both ventricles (LL) for 12 days. Data are means ± SEM for 9 rats. *, Significant difference between LL rats and all other groups at P < .05.
Energy expenditure of all treatment groups was higher during the dark period than the light period throughout the experiment. There was no effect of leptin treatment on either day time or night time energy expenditure across the different groups (Figure 2, C and D). This was true whether expenditure was expressed per rat or per metabolic body weight (data not shown). Activity of all treatment groups increased during the dark compared with the light period of the day. There were no differences in activity across treatment groups during the light or dark period on any of the days (data not shown). IBAT temperatures, measured by iButtons, were averaged over 3 hours (11:51 am to 2:51 pm) when temperatures were at the lowest for the day and 3 hours (7.51 pm to 10.51 pm) when temperatures were at their highest on each day of the experiment. The averages for each rat were then averaged for each group. Daytime IBAT temperature of rats that received leptin infusions in both the third and fourth ventricle (LL) was higher than that of control SS rats (P < .056) (Table 1), but there were no differences in nighttime temperature. Serum leptin, insulin, and glucose levels measured at the end of the study were not different across treatment groups, although insulin levels of LL rats tended to be lower than in the other groups (P < .10) (Table 1).
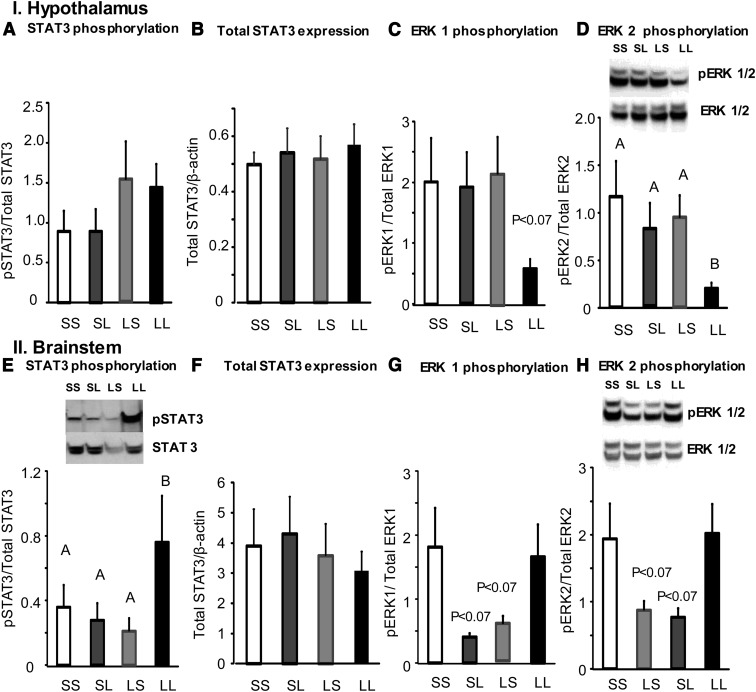
Measurement of activation of leptin signaling proteins in hypothalamic tissue after 12 days of infusion did not show any changes in total STAT3 expression or phosphorylation of STAT3, ERK1 (Figure 3, A–C), or PI3K. There were no differences in SOCS3 or PTP1B expression between groups (data not shown). Surprisingly, infusion of low-dose leptin in both third and fourth ventricles (LL) caused a significant decrease in phosphorylation of ERK2 in hypothalamic tissue (Figure 3D). Measurement of leptin signaling proteins in the brainstem revealed that low-dose leptin infusion in both third and fourth ventricle caused a significant increase in phosphorylation of STAT3 (Figure 3E). Phosphorylation of ERK1/2 tended to be lower in LS and SL groups (P < .07) when compared with SS and LL groups; however, the difference was not statistically significant (Figure 3, G and H). There were no differences in total STAT3 expression (Figure 3F), phosphorylation of PI3K, or expression of SOCS3 and PTP1B in the brainstem across the different treatment groups (data not shown). There were no changes in expression of hypothalamic ObRb or short-form ObR between groups (data not shown). There was not enough protein to measure ObR expression in the brainstem.
Figure 3.
Western blot analysis of total STAT3 expression, phosphorylation (p) of leptin signaling proteins STAT3 and ERK1 and -2 in hypothalamic and brainstem tissue of saline-infused (SS), third or fourth ventricle leptin-infused (LS or SL) or double ventricle leptin-infused rats (LL) for 12 days in experiment 1. Data are means ± SEM for 9 rats. Data for a specific parameter that do not share a common superscript are significantly different at P < .05.
Experiment 2: Rats infused centrally with low doses of leptin into both the third and fourth ventricles for 12 days show differential responses to leptin at days 6 and 12
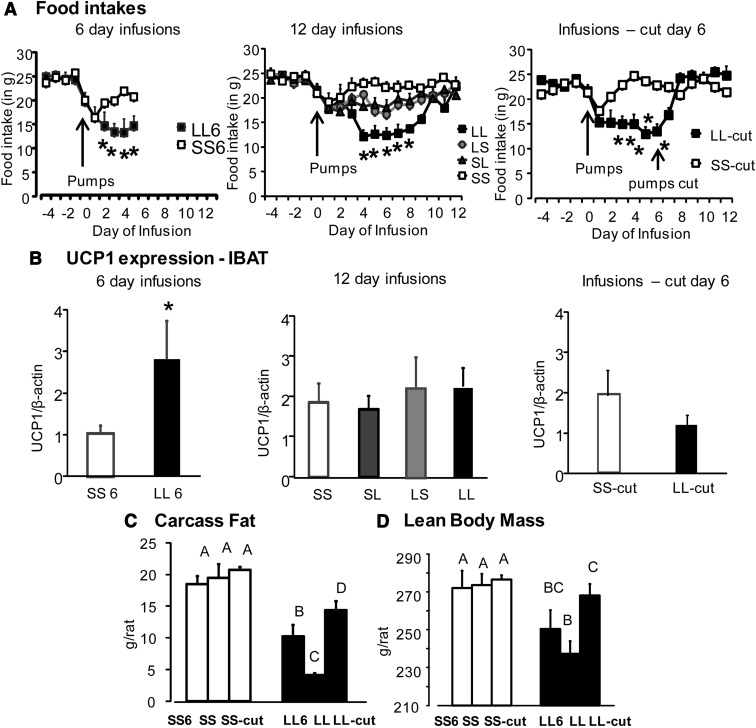
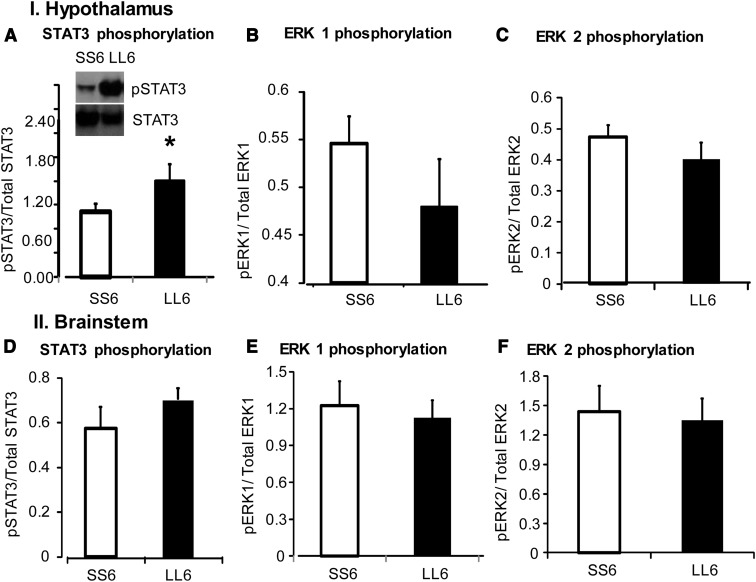
On day 6, there was a significant decrease in body weight, body fat, and lean body mass in rats receiving low-dose leptin infusions into both third and fourth ventricles (LL6) compared with controls (SS6) (Table 2). This was accompanied by a decrease in food intake in these rats (Figure 4A). Leptin reduced the size of all fat depots weighed in LL6 rats compared with SS6 rats (Table 2). There was a significant depletion of energy stores of the liver on day 6 of infusion when liver weights, liver lipid, and liver glycogen were all significantly lower in LL6 rats compared with SS6 rats. There was no difference in serum leptin, insulin, or glucose levels between the 2 groups (Table 2). Expression of UCP1 protein in IBAT of LL6 rats was increased compared with SS6 rats (Figure 4B) (P < .001). Expression of pSTAT3 in the hypothalamus of LL6 rats was increased compared with SS6 rats (Figure 5A; P < .05). There were no differences in expression of other leptin signaling proteins in the hypothalamus and no changes in leptin signaling proteins in the brainstem (Figure 5, B–F). There were no differences in expression of hypothalamic ObRb or short-form ObR between groups (data not shown). There was not enough protein to measure ObR expression in the brainstem. Endogenous leptin mRNA expression in inguinal fat determined by real-time PCR was unchanged at day 6 despite the significant reduction in fat depot weight (data not shown).
Table 2.
Body Weight, Body Composition, Energy Stores, and Serum Hormone Levels of Rats From Experiment 2
| SS6 | LL6 | SS-cut | LL-cut | |
|---|---|---|---|---|
| Start weight, g | 350 ± 8 | 347 ± 4 | 344 ± 11 | 352 ± 6 |
| End weight, g | 353 ± 10 | 312 ± 13b | 365 ± 9 | 347 ± 9 |
| Body fat, g | 19 ± 1 | 10 ± 2b | 21 ± 1 | 14 ± 2b |
| Lean body mass, g | 272 ± 10 | 250 ± 10b | 277 ± 7 | 268 ± 6 |
| Liver weight, g | 12 ± 5 | 9 ± 1b | 12 ± 4 | 12 ± 4 |
| Liver lipid, mg | 485 ± 9 | 351 ± 40b | 502 ± 23 | 437 ± 15b |
| Liver glycogen, mg | 97 ± 31 | 38 ± 8b | 103 ± 20 | 109 ± 23 |
| Fat depots | ||||
| Inguinal, g | 4.30 ± 1.8 | 2.60 ± 0.4b | 4.80 ± 1.6 | 3.40 ± 1.1b |
| Epididymal, g | 2.84 ± 1.2 | 2.77 ± 0.83 | 3.04 ± 1.01 | 2.28 ± 0.7b |
| Retroperitoneal, g | 1.22 ± 0.5 | 0.50 ± 0.14b | 1.39 ± 0.46 | 0.89 ± 0.3b |
| Mesenteric, g | 1.30 ± 0.53 | 0.57 ± 0.18b | 1.50 ± 0.5 | 0.96 ± 0.3b |
| Serum measures | ||||
| Leptin, ng/mL | 1.36 ± 0.13 | 2.27 ± 0.77 | 2.27 ± 0.27 | 3.69 ± 2.1 |
| Insulin, ng/mL | 0.88 ± 0.28 | 0.94 ± 0.3 | 0.72 ± 0.12 | 0.66 ± 0.13 |
| Range | 0.23–1.88 | 0.30–1.92 | 0.34–1.36 | 0.28–1.35 |
| Glucose, mg/dL | 143 ± 10 | 124 ± 7 | 133 ± 8 | 112 ± 7 |
| Range | 121–181 | 101–152 | 99–160 | 73–134 |
Data are means ± SEM for groups of 8 to 9 rats. SS6 and LL6 measures were made after 6 days of infusion. Infusions were stopped at day 6 for LL6 and SS6 rats, and all measures for these groups were made at day 12.
Significant difference between SS and LL rats at P < .05.
Figure 4.
Food intake, Western blot analysis of UCP1 in brown fat, body fat, and lean body mass of rats from experiment 2 infused with saline, low-dose leptin in either third or fourth ventricles (LS or SL), or both ventricles (LL) for 6 or 12 days. Data are means ± SEM for 7 to 11 rats. *, Significant difference between saline and LL rats at P < .05. Values for body fat and lean body mass that do not share a common superscript are significantly different at P < .05.
Figure 5.
Western blot analysis of total STAT3 expression and phosphorylation (p) of leptin signaling proteins STAT3 and ERK1 and -2 in hypothalamic and brainstem tissue of saline-infused (SS6) and double ventricle leptin-infused rats (LL6) for 6 days in experiment 2. Data are means ± SEM for 8 rats. *, Significant difference between saline and LL rats at P < .05.
In rats infused for 12 days, LL rats showed a significant decrease in body weight gain, body fat, and lean body mass (P < .05) compared with LS and SL rats and controls (SS). LS and SL rats showed no differences in body weight gain or lean body mass compared with control SS rats; however, their body fat was lower than SS rats and yet higher than LL rats (Table 3). Food intake of LL rats was significantly lower than for other groups of rats from days 4 to 8. Their food intake gradually increased over time, and by day 9 was not different from control SS rats, confirming the results from experiment 1 (Figure 4A). Leptin reduced the size of all fat depots and depleted the energy stores of the liver in LL rats when compared with SS rats. Liver glycogen of LL rats was also lower than in the single infused SL group but was not significantly different from that of LS rats (P < .06) (Table 3). On day 12, the expression of UCP1 protein in IBAT of LL rats (infused for 12 days) was no different from that of saline-infused SS rats (Figure 4B).
Table 3.
Body Weight, Body Composition, Energy Stores, and Serum Hormone Levels of Rats From Experiment 2 Receiving Third or Fourth Ventricle Infusions for 12 Days
| SS | LS | SL | LL | |
|---|---|---|---|---|
| Start weight, g | 350 ± 5 | 352 ± 6 | 350 ± 5 | 347 ± 9 |
| End weight, g | 359 ± 9b | 325 ± 19b | 343 ± 9b | 300 ± 8c |
| Body fat, g | 19 ± 2b | 11 ± 3c | 13 ± 2c | 4 ± 1d |
| Lean body mass, g | 274 ± 12b | 254 ± 15b,c | 271 ± 7b | 245 ± 9c |
| Liver weight, g | 11 ± 4b | 9 ± 3b,c | 10 ± 3b | 8 ± 2c |
| Liver lipid, mg | 456 ± 27b | 391 ± 48b | 397 ± 30b | 269 ± 45c |
| Liver glycogen, mg | 66 ± 9b | 60 ± 13b,c | 81 ± 16b | 26 ± 3c |
| Fat depots | ||||
| Inguinal, g | 4.20 ± 1.5b | 2.70 ± 0.8c | 2.90 ± 0.9b,c | 1.21 ± 0.4d |
| Epididymal, g | 2.97 ± 1.05b | 1.61 ± 0.49c | 1.88 ± 0.57c | 0.30 ± 0.1d |
| Retroperitoneal, g | 1.16 ± 0.41b | 0.68 ± 0.2b,c | 0.48 ± 0.1c,d | 0.07 ± 0.02d |
| Mesenteric, g | 1.27 ± 0.45b | 0.77 ± 0.23b,c | 0.59 ± 0.18c,d | 0.20 ± 0.06d |
| Serum measures | ||||
| Leptin, ng/mL | 1.63 ± 0.21 | 1.18 ± 0.21 | 0.76 ± 0.10 | 0.90 ± 0.21 |
| Insulin, ng/mL | 1.58 ± 0.43 | 1.05 ± 0.27 | 0.70 ± 0.19 | 1.03 ± 0.5 |
| Range | 0.27–3.97 | 0.53–1.73 | 0.23–1.80 | 0.19–2.47 |
| Glucose, mg/dL | 123 ± 11 | 96 ± 15 | 126 ± 6 | 104 ± 12 |
| Range | 72–170 | 90–180 | 101–171 | 100–146 |
a Data are means ± SEM for groups of 7 to 11 rats. All measures were made after 12 days of infusion.
Values for a specific parameter that do not share a common superscript are significantly different at P < .05.
In the rats where infusions were stopped on day 6 by cutting the tubing, LL-cut rats regained weight from days 7 to 12. The body weight gain and food intake of LL-cut rats were no different from SS-cut rats after day 7 (Table 2 and Figure 4A). Lean body mass was not different between SS-cut and LL-cut rats on day 12; however, carcass fat was significantly lower in LL-cut rats compared with SS-cut rats (Table 2). Leptin reduced the size of all fat depots in LL-cut rats compared with SS-cut rats. There was no difference in serum leptin, insulin, or glucose between the two groups. Liver weights and liver glycogen content were not different, but liver lipid was significantly lower in LL-cut rats than SS-cut rats (Table 2). There was no difference in UCP1 expression in IBAT of LL-cut rats compared with SS-cut rats (Figure 4B). There was no difference in inguinal fat UCP1 expression as well as in IBAT mitochondrial cytochrome c oxidase (MTCO1) expression across treatments (data not shown).
A separate set of statistical analyses were carried out to understand the differences between LL and SS groups infused for different periods of time. Rats receiving leptin infusion in both third and fourth ventricles for 6 days (LL6 rats) had significantly less body fat compared with controls, and this was further decreased in rats that received leptin infusions for 12 days (LL). Rats in which leptin infusions were stopped at day 6 (LL-cut) had more body fat than LL-6 or LL rats killed at day 12 but continued to have less fat than controls (SS6, SS-cut, and SS) (Figure 4C). The lean body mass of LL6 rats, LL rats, and LL-cut rats was less than that of controls (SS6, SS, and SS-cut). LL rats infused for 12 days showed a nonsignificant decrease in lean body mass compared with LL6 rats. LL-cut rats showed a significant increase in lean tissue compared with LL rats infused for 12 days (Figure 4D).
Discussion
Activation of hypothalamic ObRs has been shown to inhibit food intake and weight gain (10, 11). Brainstem receptors have also been identified as mediators of similar leptin-induced changes (13, 19). The objective of the studies described here was to determine whether leptin signals from the forebrain and hindbrain integrate to maintain body fat and regulate energy balance. The results show that when ObRs in both areas are activated, there is a synergistic effect to inhibit food intake and breakdown of fat and lean tissue.
Rats infused with subthreshold doses of leptin in both ventricles (LL) demonstrated a sharp decrease in food intake as compared with rats infused in a single ventricle (LS and SL) and controls (SS) during the first 5 days of infusions. In this phase of low food intake, RER was low, indicating that the rats oxidized primarily endogenous fat and protein for energy, resulting in a decrease in both body fat and lean tissue. The drop in food intake of LL rats was reversed from day 6, and this could be due to a depletion of endogenous energy stores, indicated by reduced body fat and liver glycogen. In this situation, metabolic signals of energy depletion overrode the hypophagic response to leptin. Similar results have been published by others (20). Phosphorylated ERK1/2 in the hypothalamus has been demonstrated as a mediator of the anorectic effect of leptin (7), and hypothalamic ERK is modified by multiple factors associated with feeding and energy balance (21, 22). The unexpectedly low phospho-ERK1/2 seen in our LL rats may represent the integrated response to these factors and contributed to the increase in food intake after day 6 in these rats (23). It is important to note that at no point did these rats attempt to overeat, and it is possible that the activated ERK1/2 measured in tissue blocks was not exclusively located in cells that expressed ObR. A previous study using a single ip or intracerebroventricular injection of leptin found no increase in ERK1/2 phosphorylation in the brainstem (7). Our data are consistent with these observations in that there were no differences in brainstem ERK1/2 between LL rats and SS rats, and although there was a trend for ERK1/2 activation to decrease in the brainstem of LS and SL rats in experiment 1, these differences were not statistically significant.
In experiment 1, all of the rats showed an initial weight loss of approximately 20 g over 2 days, and this was attributed to the stress of isoflurane anesthesia, surgery to attach pumps to infusion cannula, placement of iButtons on IBAT, and the initiation of ventricular infusions. These effects were nonspecific and uniform across treatment groups because the surgeries were performed by the same person and all treatment groups were represented in each cohort of rats. This initial weight loss was soon reversed in the SS, LS, and SL control groups, whereas the LL rats showed a dramatic and sustained loss of body weight throughout the infusion period, reflected in a 50% loss in body fat and a 16% decrease in lean body mass. Leptin reduced the size of all fat pads weighed except mesenteric fat. The reduction in size of epididymal and retroperitoneal depots was greater than that of the inguinal depot, suggesting that the sc fat depot was less sensitive to activation of central ObR. Others have shown variability in sensitivity of fat pads to the lipolytic effects of leptin (11), sympathetic innervation (24), and cellularity (25) of fat depots, implying that the variable responses of the different fat pads is not exclusive to the experiments described here. The decrease in body fat and lean body mass seen in LL6 rats infused for 6 days was associated with an inhibition of food intake and an increased activation of STAT3 in the hypothalamus. A reversal of the inhibition of food intake on day 6 prevented any further loss of lean tissue in rats infused for 12 days confirming that this loss was secondary to the leptin-induced hypophagia. In addition, the activation of STAT3 in the hypothalamus of these rats (LL) was unchanged from controls and LS and SL rats. However, body fat of the LL rats continued to decrease, suggesting that they were still leptin-responsive. The energy expenditure of LL rats remained at control levels throughout the 12 days, implying that the further reduction in fat observed despite reversal in food intake in LL rats could be secondary to an inappropriately high energy expenditure. There were no differences in expression of both the short-form (ObR) as well as long-form (ObRb) ObRs between treatment groups at either 6 or 12 days of the experiment, suggesting that these leptin responses were not due to an increased expression of functional ObRs.
It is well documented that food-restricted rats have low energy expenditure (26, 27); however, hypophagic LL rats maintained an energy expenditure comparable to the control SS rats. Similar data have been shown by others (23). The maintenance of energy expenditure in LL rats correlated with a small, but statistically significant, increase in average daytime IBAT temperature in experiment 1 and an increase in thermogenic UCP1 protein expression at day 6 in experiment 2, both of which are suggestive of increased thermogenesis. This was confirmed by an increase in STAT3 activation in the brainstem of LL rats, which has been shown to regulate thermogenic responses via melanocortin receptor stimulation (28). A pair-fed group of rats was not included in this study but would have confirmed that integrated effects of leptin are not solely limited to an inhibition of food intake. Future studies will incorporate this control group.
Leptin is released in proportion to white fat mass, and it activates sympathetic nervous system outflow to white adipose tissue, promoting lipolysis and inhibiting its own expression (29–31). Interestingly, LL rats with small fat pads had serum leptin comparable to other treatment groups. In experiment 2, endogenous leptin mRNA expression in LL rats was unchanged compared with controls at day 6, suggesting that the maintenance of serum leptin concentrations was a result of maintaining peripheral leptin expression rather than leakage from cerebrospinal fluid into the circulation.
Several groups have reported that activation of either hypothalamic or brainstem ObR with acute leptin injections in the third or fourth ventricle cause weight loss (10, 11, 13). In our study, we found that although low doses of leptin infused in either the third or fourth ventricle alone had no effect on energy balance, when infused simultaneously, they produced a response similar to that reported for injections of higher doses of leptin into either the third or fourth ventricle. There are two major differences between previous studies investigating the role of leptin in energy balance regulation and the ones described here. The first is the method of leptin administration. We used chronic infusions compared with single acute injections. The second is the dose of leptin. The amount of leptin used in injection studies was 10- to 15-fold higher (13, 32) than the doses used for infusion in the experiments described here. To determine whether there was an integration of leptin signals in the forebrain and hindbrain, we chose low doses of leptin that did not elicit a significant leptin response when each infusion was done independently. These low doses of leptin were selected based on the results of a previous dose-response study (14). They were deliberately selected to be doses that did not have a significant effect on body weight when leptin was infused into only one ventricle. In addition, because cerebrospinal fluid flows from the third to the fourth ventricle, our use of 0.6 μg/d in the fourth ventricle, which was 6 times higher than the 0.1 μg/d infused into the third ventricle, made it unlikely that our observations of a possible interaction were due to drainage of leptin from the third to the fourth ventricle.
The studies described in this paper show that low concentrations of leptin infused in either the forebrain or hindbrain have no significant effect on energy balance; however, leptin signals from both regions of the brain, when integrated, induce a state of negative energy balance. The response does not appear to be additive because there was no effect of infusing leptin in either ventricle alone. It is important to consider that low-dose leptin when infused into the third ventricle could possibly slowly drain into the fourth ventricle and that low-dose leptin when infused into the fourth ventricle could slowly drain into the subarachnoid space adjacent to the hypothalamus. The leptin drained into these spaces during single infusions is not sufficient to produce a response strong enough to cause an inhibition in food intake or breakdown of body fat by itself (below threshold for a response). However, one cannot exclude the possibility that when leptin infusions in both the third and fourth ventricle are combined, these subthreshold responses produce an above-threshold response and effectively be the cause of a decrease in food intake and reduce body fat and lean tissue. If this was the case, it would be more likely for 0.6 μg leptin draining from the fourth ventricle to have a significant additive effect in the third ventricle, rather than drainage of 0.1 μg leptin from the third ventricle to significantly raise leptin in the fourth ventricle.
It is also possible that the low doses of leptin infused into the fourth ventricle potentiate activation of ObRs in the hypothalamus as described by Ruiter et al (19), who found that injection of leptin into either the fourth ventricle or directly into the NTS increased phosphorylation of STAT3 in the hypothalamus. By contrast, chronic forebrain ObR activation could also potentiate hindbrain ObR activation, which is consistent with an increase in phospho-STAT3 levels in the brainstem seen in LL rats. Another possible mechanism that we cannot rule out is that an unidentified region in the brain receives projections either directly or indirectly from both the forebrain and hindbrain (33), and if input is received from both sites, then this area of integration inhibits food intake. Examination of the neuroanatomic evidence for connections between the hypothalamus and hindbrain (33) and immunohistochemical evidence for sites that express ObR (34) provide obvious candidates for sites of neural communication between the forebrain and hindbrain that include the arcuate nucleus and nucleus of the solitary tract. There are, however, likely to be other nuclei in the forebrain, hindbrain, and midbrain that mediate the interaction of leptin signaling across the neuroaxis, and additional studies are required for their identification.
In summary, our observations establish that the chronic activation of both forebrain and hindbrain ObR is required to produce a leptin response comparable to that seen with high-dose acute leptin injections into either the third or the fourth ventricle. These results suggest that under near-physiological conditions, the forebrain and hindbrain integrate their leptin signals to either potentiate each other or to inhibit feeding neurons in other areas of the brain via neuronal projections to a common site. Although it is not possible to identify the exact nature of communication from the studies described here, our findings demonstrate that under experimental conditions, it is necessary for both forebrain and hindbrain ObR to be activated (using low doses of exogenous leptin) to decrease food intake and body weight and that this outcome is a result of integrated leptin signals from the two brain areas. It is important to note that the doses used in our experiment are very low (0.1 μg/d for third ventricle and 0.6 μg/d for fourth ventricle), which re-emphasizes the relevance of our finding of essential forebrain-hindbrain communication in the metabolic regulation of energy balance in physiological systems. Future studies will involve localization experiments to identify potential areas in the forebrain and/or hindbrain essential for this integrated response that reduces food intake and body weight.
Acknowledgments
This work was supported by National Institutes of Health Grant DK 053903 awarded to R.B.S.H.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- IBAT
- intracapsular brown adipose tissue
- LL
- leptin-leptin
- LS
- leptin-saline
- ObR
- leptin receptor
- ObRb
- long isoform of the leptin receptor
- PI3K
- phosphoinositide-3-kinase
- PTP1B
- protein-tyrosine phosphatase
- RER
- respiratory exchange ratio
- SOCS3
- suppressor of cytokine signaling-3
- SL
- saline-leptin
- SS
- saline-saline
- STAT3
- signal transducer and activator of transcription-3
- UCP1
- uncoupling protein 1.
References
- 1. Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432 [DOI] [PubMed] [Google Scholar]
- 2. Harris RB. Leptin: much more than a satiety signal. Annu Rev Nutr. 2000;20:45–75 [DOI] [PubMed] [Google Scholar]
- 3. Hwa JJ, Fawzi AB, Graziano MP, et al. Leptin increases energy expenditure and selectively promotes fat metabolism in ob/ob mice. Am J Physiol. 1997;272:R1204–R1209 [DOI] [PubMed] [Google Scholar]
- 4. Harris RB, Bartness TJ, Grill HJ. Leptin responsiveness in chronically decerebrate rats. Endocrinology. 2007;148:4623–4633 [DOI] [PubMed] [Google Scholar]
- 5. Bates SH, Stearns WH, Dundon TA, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859 [DOI] [PubMed] [Google Scholar]
- 6. Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795 [DOI] [PubMed] [Google Scholar]
- 7. Rahmouni K, Sigmund CD, Haynes WG, Mark AL. Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes. 2009;58:536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Myers MG., Jr Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res. 2004;59:287–304 [DOI] [PubMed] [Google Scholar]
- 9. Bence KK, Delibegovic M, Xue B, et al. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12:917–924 [DOI] [PubMed] [Google Scholar]
- 10. Maness LM, Kastin AJ, Farrell CL, Banks WA. Fate of leptin after intracerebroventricular injection into the mouse brain. Endocrinology. 1998;139:4556–4562 [DOI] [PubMed] [Google Scholar]
- 11. Penn DM, Jordan LC, Kelso EW, Davenport JE, Harris RB. Effects of central or peripheral leptin administration on norepinephrine turnover in defined fat depots. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1613–R1621 [DOI] [PubMed] [Google Scholar]
- 12. Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143:239–246 [DOI] [PubMed] [Google Scholar]
- 13. Skibicka KP, Grill HJ. Hypothalamic and hindbrain melanocortin receptors contribute to the feeding, thermogenic, and cardiovascular action of melanocortins. Endocrinology. 2009;150:5351–5361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harris RB. Leptin-induced increase in body fat content of rats. Am J Physiol Endocrinol Metab. 2012;304(3):E267–E281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haring SJ, Harris RB. The relation between dietary fructose, dietary fat and leptin responsiveness in rats. Physiol Behav. 2011;104:914–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris RB, Apolzan JW. Changes in glucose tolerance and leptin responsiveness of rats offered a choice of lard, sucrose, and chow. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1327–R1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harris RB. Factors influencing energy intake of rats fed either a high-fat or a fat mimetic diet. Int J Obes Relat Metab Disord. 1994;18:632–640 [PubMed] [Google Scholar]
- 18. Harris RB. Growth measurements in Sprague-Dawley rats fed diets of very low fat concentration. J Nutr. 1991;121:1075–1080 [DOI] [PubMed] [Google Scholar]
- 19. Ruiter M, Duffy P, Simasko S, Ritter RC. Increased hypothalamic signal transducer and activator of transcription 3 phosphorylation after hindbrain leptin injection. Endocrinology. 2010;151:1509–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pal R, Sahu A. Leptin signaling in the hypothalamus during chronic central leptin infusion. Endocrinology. 2003;144:3789–3798 [DOI] [PubMed] [Google Scholar]
- 21. Thakur V, Pritchard MT, McMullen MR, Nagy LE. Adiponectin normalizes LPS-stimulated TNF-α production by rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2006;290:G998–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bitner RS, Nikkel AL, Otte S, et al. Dopamine D4 receptor signaling in the rat paraventricular hypothalamic nucleus: evidence of natural coupling involving immediate early gene induction and mitogen activated protein kinase phosphorylation. Neuropharmacology. 2006;50:521–531 [DOI] [PubMed] [Google Scholar]
- 23. Zauner C, Schneeweiss B, Kranz A, et al. Resting energy expenditure in short-term starvation is increased as a result of an increase in serum norepinephrine. Am J Clin Nutr. 2000;71:1511–1515 [DOI] [PubMed] [Google Scholar]
- 24. Youngstrom TG, Bartness TJ. White adipose tissue sympathetic nervous system denervation increases fat pad mass and fat cell number. Am J Physiol. 1998;275:R1488–R1493 [DOI] [PubMed] [Google Scholar]
- 25. DiGirolamo M, Fine JB, Tagra K, Rossmanith R. Qualitative regional differences in adipose tissue growth and cellularity in male Wistar rats fed ad libitum. Am J Physiol. 1998;274:R1460–R1467 [DOI] [PubMed] [Google Scholar]
- 26. Harris RB, Kelso EW, Flatt WP, Bartness TJ, Grill HJ. Energy expenditure and body composition of chronically maintained decerebrate rats in the fed and fasted condition. Endocrinology. 2006;147:1365–1376 [DOI] [PubMed] [Google Scholar]
- 27. Ramsey JJ, Hagopian K. Energy expenditure and restriction of energy intake: could energy restriction alter energy expenditure in companion animals? J Nutr. 2006;136:1958S–1966S [DOI] [PubMed] [Google Scholar]
- 28. Skibicka KP, Grill HJ. Energetic responses are triggered by caudal brainstem melanocortin receptor stimulation and mediated by local sympathetic effector circuits. Endocrinology. 2008;149:3605–3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klein S, Coppack SW, Mohamed-Ali V, Landt M. Adipose tissue leptin production and plasma leptin kinetics in humans. Diabetes. 1996;45:984–987 [DOI] [PubMed] [Google Scholar]
- 30. Collins S, Kuhn CM, Petro AE, Swick AG, Chrunyk BA, Surwit RS. Role of leptin in fat regulation. Nature. 1996;380:677. [DOI] [PubMed] [Google Scholar]
- 31. Commins SP, Watson PM, Levin N, Beiler RJ, Gettys TW. Central leptin regulates the UCP1 and ob genes in brown and white adipose tissue via different β-adrenoceptor subtypes. J Biol Chem. 2000;275:33059–33067 [DOI] [PubMed] [Google Scholar]
- 32. Huo L, Maeng L, Bjørbaek C, Grill HJ. Leptin and the control of food intake: neurons in the nucleus of the solitary tract are activated by both gastric distension and leptin. Endocrinology. 2007;148:2189–2197 [DOI] [PubMed] [Google Scholar]
- 33. Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982;257:275–325 [DOI] [PubMed] [Google Scholar]
- 34. Elmquist JK, Bjørbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547 [PubMed] [Google Scholar]