Abstract
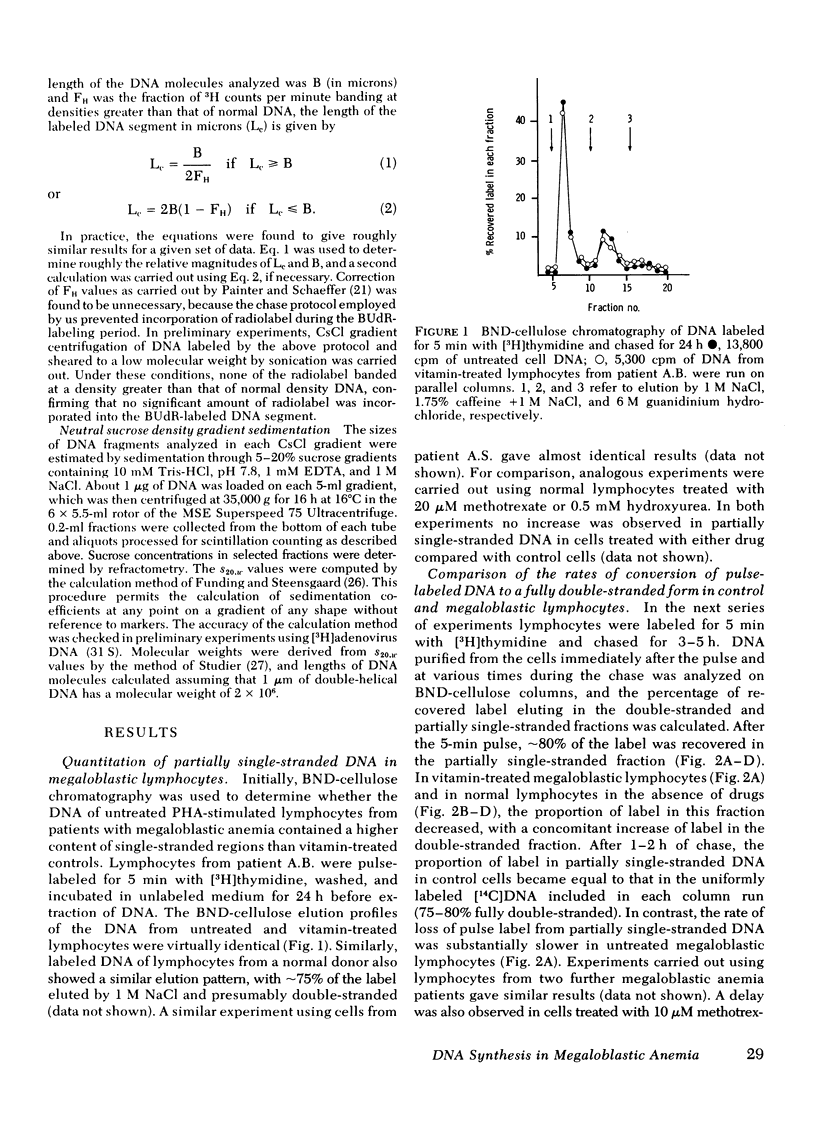
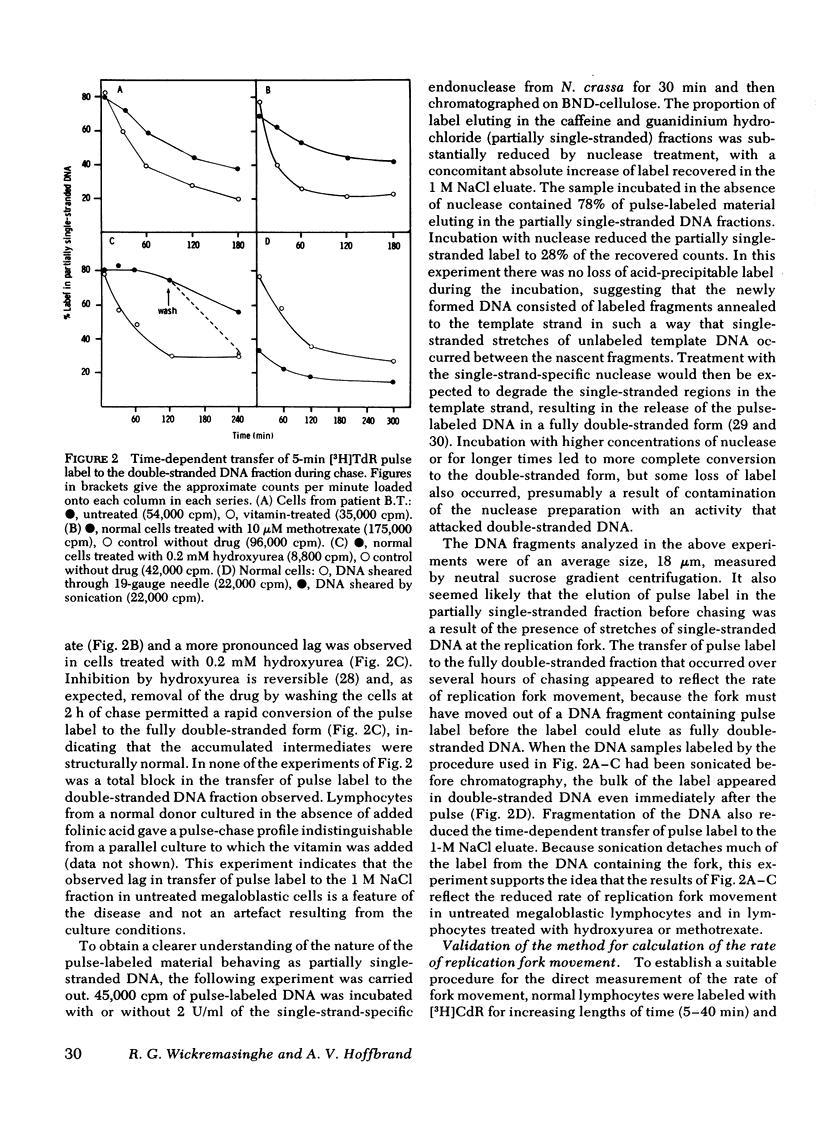
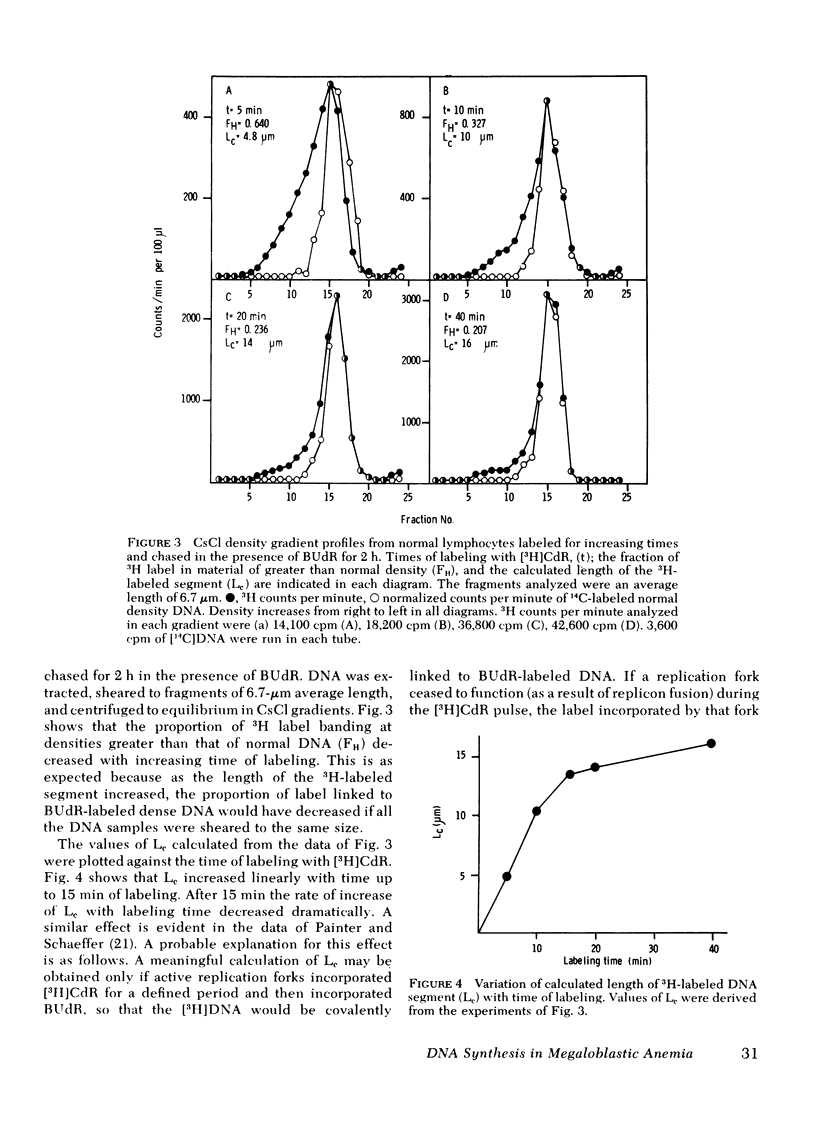
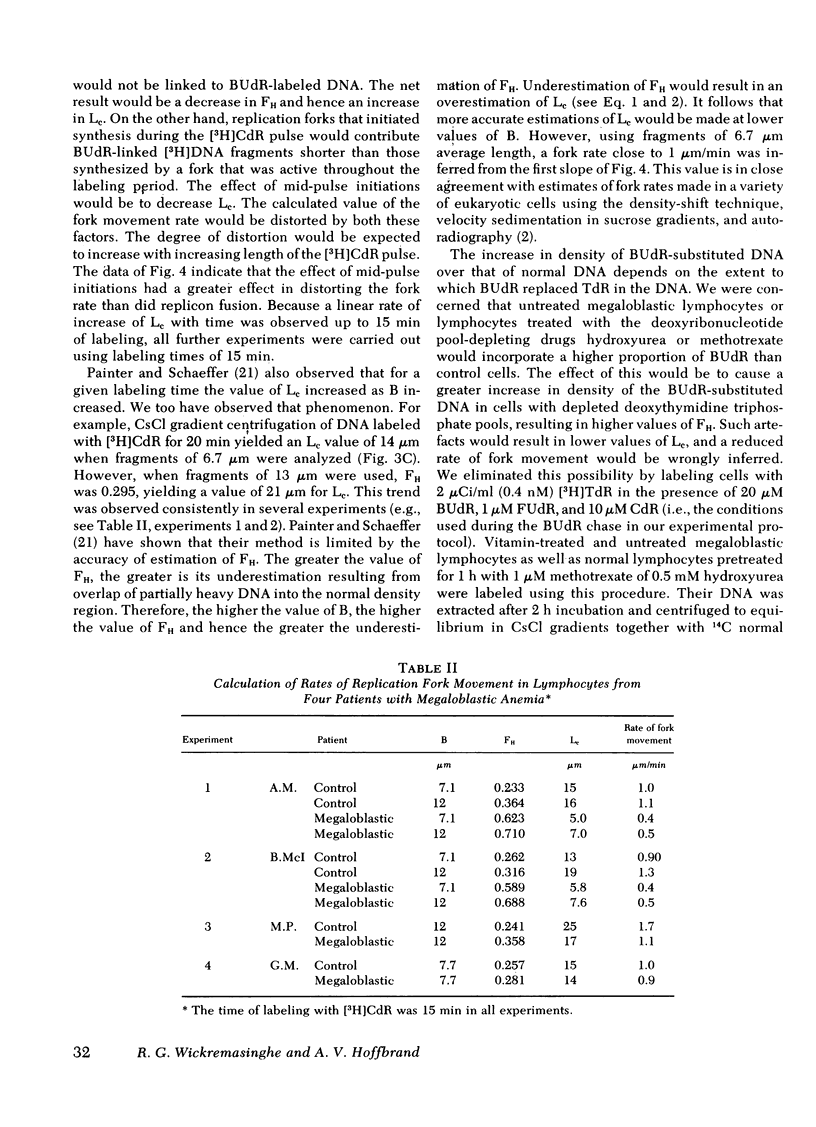
Chromatography on benzoylated naphthoylated DEAE-cellulose has been used to fractionate fully double-stranded from partially single-stranded DNA molecules. DNA was extracted from phytohemagglutinin-stimulated lymphocytes from patients with megaloblastic anemia resulting from vitamin B12 or folate deficiency after pulse-labeling the cells with [3H]thymidine for 5 min and chasing in unlabeled medium for 24 h. No gross accumulation of partially single-stranded material was observed in the DNA of these cells when compared with DNA from similarly labeled control cells obtained by the addition of 5-formyl tetrahydrofolic acid to the culture medium. When DNA from lymphocytes labeled with a 5-min pulse of [3H]thymidine and sheared to fragments of an average length of 18 micrometer was chromatographed on benzoylated naphthoylated DEAE-cellulose, approximately 80% of the label was recovered in the partially single-stranded fraction. After chasing in unlabeled medium the label was progressively transferred to the double-stranded fraction over a period of 2--3 h. The rate of transfer was slower in megaloblastic lymphocytes than in controls. The difference in rate suggested a slower rate of replication fork movement in megaloblastic lymphocytes and so the density shift technique of Painter and schaeffer (J. Mol. Biol. 45: 467--479, 1969) was used to measure the fork rate directly. [3H]Deoxycytidine was used as the labeled nucleoside to avoid possible complications arising from [3H]thymidine labeling of megaloblastic cells. Investigations on the lymphocytes from four patients showed that the replication fork rate in vitamin-treated control lyphocytes was about 1 micrometer/min. The fork rates in the corresponding untreated cells were invariably lower and rates ranging from 40 to 92% of those of controls were observed. Normal lymphocytes treated with the deoxynucleotide pool-depleting drugs methotrexate or hydroxyurea displayed defects in DNA synthesis similar to those of untreated megaloblastic lymphocytes. We propose that the delayed DNA replication fork movement in cells of patients with megaloblastic anemia results from impaired biosynthesis of DNA precursors.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B., Sternglanz R. Recent excitement in the DNA replication problem. Nature. 1977 Oct 20;269(5630):655–661. doi: 10.1038/269655a0. [DOI] [PubMed] [Google Scholar]
- Bernheim J. L., Dorian R. E., Mendelsohn J. DNA synthesis and proliferation of human lymphocytes in vitro. I. Cell kinetics of response to phytohemagglutinin. J Immunol. 1978 Mar;120(3):955–962. [PubMed] [Google Scholar]
- Bernheim J. L., Mendelsohn J. DNA synthesis and proliferation of human lymphocytes in vitro. II. Characterization of the DNA newly synthesized after phytohemagglutinin stimulation. J Immunol. 1978 Mar;120(3):963–970. [PubMed] [Google Scholar]
- Chan A. C., Walker I. G. DNA synthesis in L-cells in the presence of 5-fluorodeoxyuridine. Biochim Biophys Acta. 1975 Jul 23;395(4):422–432. doi: 10.1016/0005-2787(75)90066-0. [DOI] [PubMed] [Google Scholar]
- Das K. C., Hoffbrand A. V. Lymphocyte transformation in megaloblastic anaemia: morphology and DNA synthesis. Br J Haematol. 1970 Oct;19(4):459–468. doi: 10.1111/j.1365-2141.1970.tb06973.x. [DOI] [PubMed] [Google Scholar]
- Das K. C., Hoffbrand A. V. Studies of folate uptake by phytohaemagglutinin-stimulated lymphocytes. Br J Haematol. 1970 Aug;19(2):203–221. doi: 10.1111/j.1365-2141.1970.tb01617.x. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Huberman J. A. Eukaryotic chromosome replication. Annu Rev Genet. 1975;9:245–284. doi: 10.1146/annurev.ge.09.120175.001333. [DOI] [PubMed] [Google Scholar]
- Eliasson R., Reichard P. Primase initiates Okazaki pieces during polyoma DNA synthesis. Nature. 1978 Mar 9;272(5649):184–185. doi: 10.1038/272184a0. [DOI] [PubMed] [Google Scholar]
- Eliasson R., Reichard P. Replication of polyoma DNA in isolated nuclei. Synthesis and distribution of initiator RNA. J Biol Chem. 1978 Oct 25;253(20):7469–7475. [PubMed] [Google Scholar]
- Fakan S., Turner G. N., Pagano J. S., Hancock R. Sites of replication of chromosomal DNA in a eukaryotic cell. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2300–2305. doi: 10.1073/pnas.69.8.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R. M., Mendelsohn J., Barbosa E., Goulian M. RNA in nascent DNA from cultured human lymphocytes. Nat New Biol. 1973 Oct 24;245(147):234–237. doi: 10.1038/newbio245234a0. [DOI] [PubMed] [Google Scholar]
- Fridland A. Effect of methotrexate on deoxyribonucleotide pools and DNA synthesis in human lymphocytic cells. Cancer Res. 1974 Aug;34(8):1883–1888. [PubMed] [Google Scholar]
- Ganeshaguru K., Hoffbrand A. V. The effect of deoxyuridine, vitamin B12, folate and alcohol on the uptake of thymidine and on the deoxynucleoside triphosphate concentrations in normal and megaloblastic cells. Br J Haematol. 1978 Sep;40(1):29–41. doi: 10.1111/j.1365-2141.1978.tb03636.x. [DOI] [PubMed] [Google Scholar]
- Gautschi J. R., Kern R. M. DNA replication in mammalian cells in the presence of cycloheximide. Exp Cell Res. 1973 Jul;80(1):15–26. doi: 10.1016/0014-4827(73)90270-x. [DOI] [PubMed] [Google Scholar]
- Gautschi J. R. Letter: Effects of puromycin on DNA chain elongation in mammalian cells. J Mol Biol. 1974 Mar 25;84(1):223–229. doi: 10.1016/0022-2836(74)90224-1. [DOI] [PubMed] [Google Scholar]
- HERBERT V., ZALUSKY R. Interrelations of vitamin B12 and folic acid metabolism: folic acid clearance studies. J Clin Invest. 1962 Jun;41:1263–1276. doi: 10.1172/JCI104589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. The presence of single-stranded regions in mammalian DNA. J Mol Biol. 1978 Mar 15;119(4):487–506. doi: 10.1016/0022-2836(78)90198-5. [DOI] [PubMed] [Google Scholar]
- Hoffbrand A. V., Tripp E. Unbalanced deoxyribonucleotide synthesis caused by methotrexate. Br Med J. 1972 Apr 15;2(5806):140–142. doi: 10.1136/bmj.2.5806.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman J. A., Horwitz H. Discontinuous DNA synthesis in mammalian cells. Cold Spring Harb Symp Quant Biol. 1974;38:233–238. doi: 10.1101/sqb.1974.038.01.026. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Riggs A. D. On the mechanism of DNA replication in mammalian chromosomes. J Mol Biol. 1968 Mar 14;32(2):327–341. doi: 10.1016/0022-2836(68)90013-2. [DOI] [PubMed] [Google Scholar]
- Iyer V. N., Rupp W. D. Usefulness of benzoylated naphthoylated DEAE-cellulose to distinguish and fractionate double-stranded DNA bearing different extents of single-stranded regions. Biochim Biophys Acta. 1971 Jan 1;228(1):117–126. doi: 10.1016/0005-2787(71)90551-x. [DOI] [PubMed] [Google Scholar]
- Khym J. X., Jones M. H., Lee W. H., Regan J. D., Volkin E. On the question of compartmentalization of the nucleotide pool. J Biol Chem. 1978 Dec 25;253(24):8741–8746. [PubMed] [Google Scholar]
- LINN S., LEHMAN I. R. AN ENDONUCLEASE FROM NEUROSPORA CRASSA SPECIFIC FOR POLYNUCLEOTIDES LACKING AN ORDERED STRUCTURE. II. STUDIES OF ENZYME SPECIFICITY. J Biol Chem. 1965 Mar;240:1294–1304. [PubMed] [Google Scholar]
- Laipis P. J., Levine A. J. DNA replication in SV40-infected cells. IX. The inhibition of a gap-filling step during discontinuous synthesis of SV40 DNA. Virology. 1973 Dec;56(2):580–594. doi: 10.1016/0042-6822(73)90059-7. [DOI] [PubMed] [Google Scholar]
- Magnusson G. Hydroxyurea-induced accumulation of short fragments during polyoma DNA replication. I. Characterization of fragments. J Virol. 1973 Sep;12(3):600–608. doi: 10.1128/jvi.12.3.600-608.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. F., Radford I., Pardee M. Accumulation of short DNA fragments in hydroxyurea treated mouse L-cells. Biochem Biophys Res Commun. 1977 Jan 10;74(1):9–15. doi: 10.1016/0006-291x(77)91368-7. [DOI] [PubMed] [Google Scholar]
- Nuzzo F., Brega A., Falaschi A. DNA replication in mammalian cells. I. The size of newly synthesized helices. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1017–1024. doi: 10.1073/pnas.65.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R. B., Schaefer A. W. Rate of synthesis along replicons of different kinds of mammalian cells. J Mol Biol. 1969 Nov 14;45(3):467–479. doi: 10.1016/0022-2836(69)90306-4. [DOI] [PubMed] [Google Scholar]
- Perlman D., Huberman J. A. Asymmetric Okazaki piece synthesis during replication of simian virus 40 DNA in vivo. Cell. 1977 Dec;12(4):1029–1043. doi: 10.1016/0092-8674(77)90167-2. [DOI] [PubMed] [Google Scholar]
- Reddy G. P., Mathews C. K. Functional compartmentation of DNA precursors in T4 phage-infected bacteria. J Biol Chem. 1978 May 25;253(10):3461–3467. [PubMed] [Google Scholar]
- Reichard P. Control of deoxyribonucleotide synthesis in vitro and in vivo. Adv Enzyme Regul. 1972;10:3–16. doi: 10.1016/0065-2571(72)90003-9. [DOI] [PubMed] [Google Scholar]
- Reichard P. From deoxynucleotides to DNA synthesis. Fed Proc. 1978 Jan;37(1):9–14. [PubMed] [Google Scholar]
- Rogers J. C., Boldt D., Kornfeld S., Skinner A., Valeri C. R. Excretion of deoxyribonucleic acid by lymphocytes stimulated with phytohemagglutinin or antigen. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1685–1689. doi: 10.1073/pnas.69.7.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Salzman N. P., Thoren M. M. Inhibition in the joining of DNA intermediates to growing simian virus 40 chains. J Virol. 1973 May;11(5):721–729. doi: 10.1128/jvi.11.5.721-729.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma D. S., Zubroff J. Synthesis and fragmentation of DNA in phytohaemagglutinin-stimulated human peripheral blood lymphocytes. Immunol Commun. 1973;2(3):277–285. doi: 10.3109/08820137309022799. [DOI] [PubMed] [Google Scholar]
- Scudiero D., Henderson E., Norin A., Strauss B. The measurement of chemically-induced DNA repair synthesis in human cells by BND-cellulose chromatography. Mutat Res. 1975 Sep;29(3):473–488. doi: 10.1016/0027-5107(75)90066-4. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe S. N., Longland J. E. Assessment of deoxyuridine suppression test in diagnosis of vitamin B12 or folate deficiency. Br Med J. 1974 Jul 20;3(5924):148–150. doi: 10.1136/bmj.3.5924.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickremasinghe R. G., Hoffbrand A. V. Defective DNA synthesis in megaloblastic anaemia. Studies employing velocity sedimentation in alkaline sucrose gradients. Biochim Biophys Acta. 1979 Jun 20;563(1):46–58. doi: 10.1016/0005-2787(79)90006-6. [DOI] [PubMed] [Google Scholar]


