Abstract
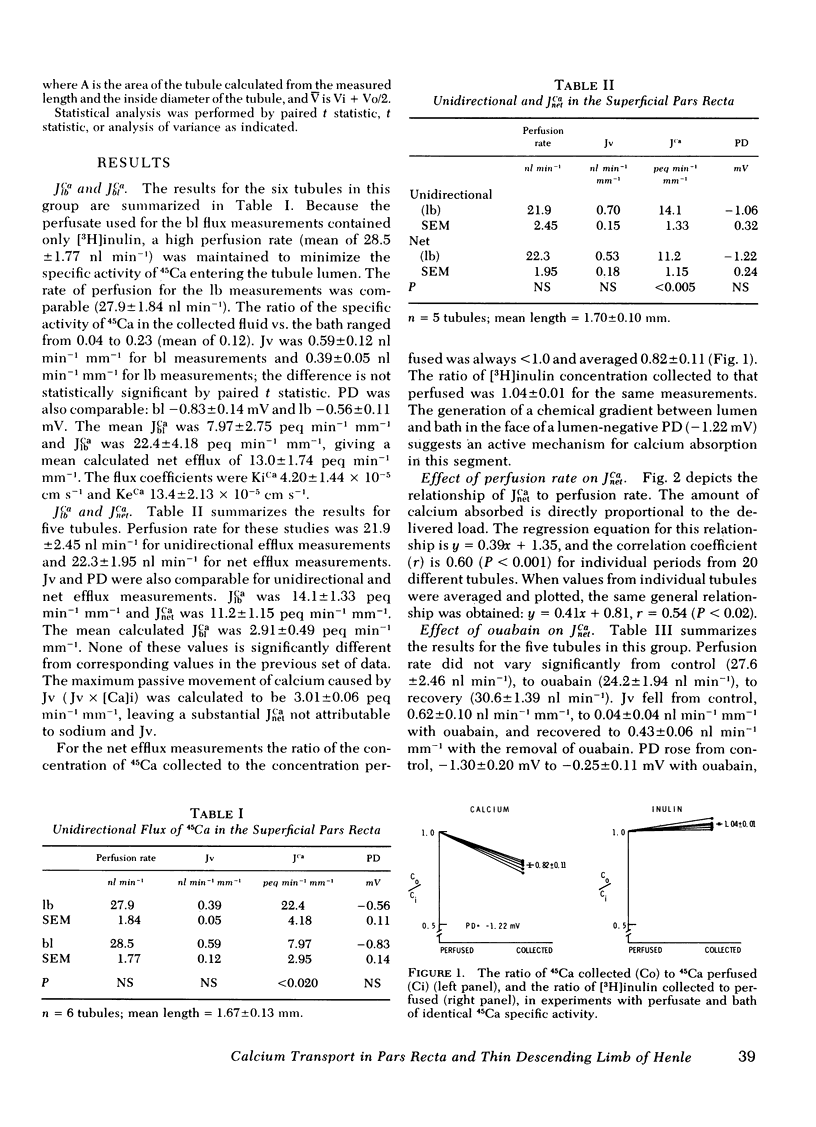
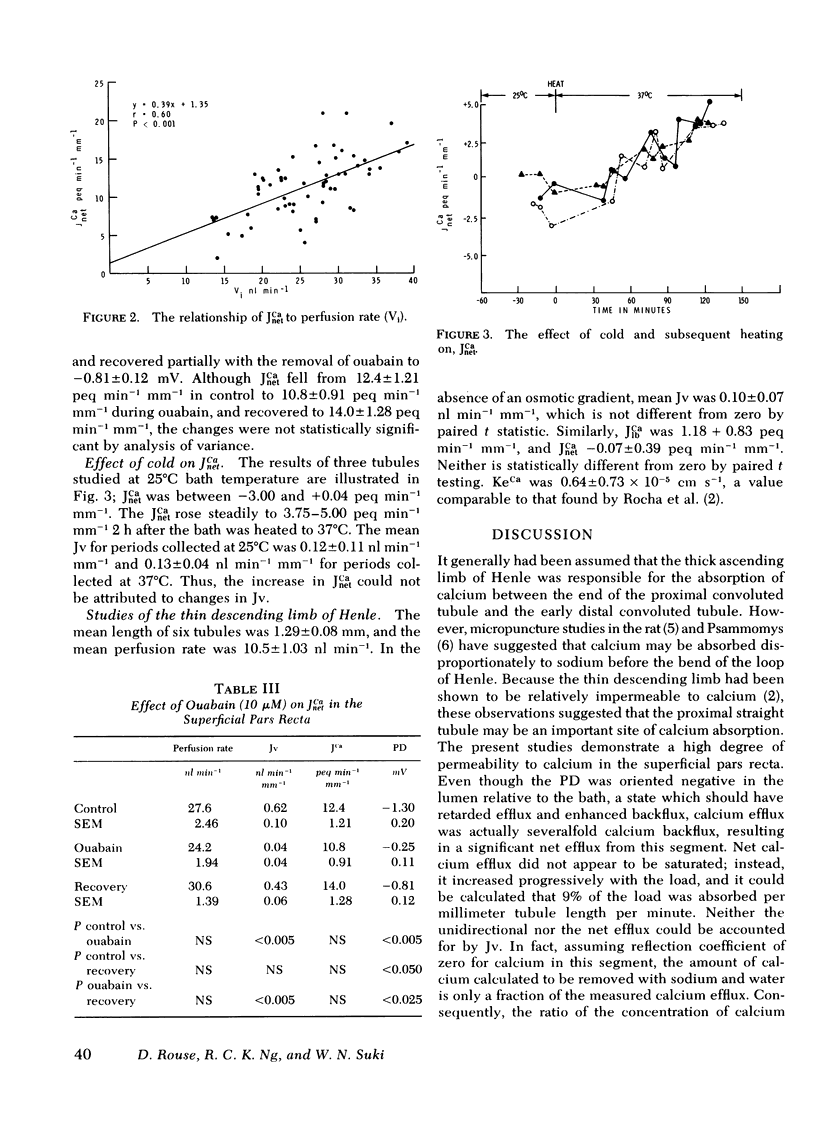
Unidirectional calcium flux (JCa) in the superficial pars recta and thin descending limb of Henle (DLH) was examined by the isolated tubule microperfusion technic using 45Ca as the isotopic tracer. In the pars recta sequential measurements of lumen-to-bath flux (JlbCa) and bath-to-lumen flux (JblCa) revealed: JlbCa 22.4 +/- 4.18, JblCa 7.97 +/- 1.95, and calculated net efflux of calcium (JnetCa 13.0 +/- 1.74 peq min-1 mm-u. To measure JnetCa directly, 45Ca of identical specific activity was used to bathe and perfuse the tubule. These studies revealed: JlbCa 14.1 +/- 1.33, JnetCa 11.2 +/- 1.15, and calculated JblCa 2.91 +/- 0.49 peq min-1 mm-1. The addition of ouabain (10 microM) resulted in a rise in potential difference and a fall in water absorption, but not a statistically significant change in JnetCa. Tubules studies at 25 degrees C bath temperature, showed no significant JnetCa, and upon heating the bath to 37 degrees C, showed JnetCa of 3.75--5.00 peg min-1 mm-1. Unidirectional and net efflux studies in six DLH showed no significant transport of calcium. These studies demonstrate substantial active absorption of calcium by the superficial pars recta, which is not inhibitable by ouabain but is inhibited by lowering bath temperature to 25 degrees C. No significant calcium transport was found in the DLH using identical technics.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaustein M. P. The interrelationship between sodium and calcium fluxes across cell membranes. Rev Physiol Biochem Pharmacol. 1974;70:33–82. doi: 10.1007/BFb0034293. [DOI] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Imai M. Calcium transport across the rabbit thick ascending limb of Henle's loop perfused in vitro. Pflugers Arch. 1978 May 31;374(3):255–263. doi: 10.1007/BF00585603. [DOI] [PubMed] [Google Scholar]
- Jamison R. L., Frey N. R., Lacy F. B. Calcium reabsorption in the thin loop of Henle. Am J Physiol. 1974 Sep;227(3):745–751. doi: 10.1152/ajplegacy.1974.227.3.745. [DOI] [PubMed] [Google Scholar]
- Kawamura S., Imai M., Seldin D. W., Kukko J. P. Characteristics of salt and water transport in superficial and juxtamedullary straight segments of proximal tubules. J Clin Invest. 1975 Jun;55(6):1269–1277. doi: 10.1172/JCI108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S., Kokko J. P. Urea secretion by the straight segment of the proximal tubule. J Clin Invest. 1976 Sep;58(3):604–612. doi: 10.1172/JCI108507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinne-Saffran E., Kinne R. Localization of a calcium-stimulated ATPase in the basal-lateral plasma membranes of the proximal tubule of rat kidney cortex. J Membr Biol. 1974 Jul 12;17(3):263–274. doi: 10.1007/BF01870187. [DOI] [PubMed] [Google Scholar]
- Kokko J. P. Proximal tubule potential difference. Dependence on glucose on glucose, HCO 3 , and amino acids. J Clin Invest. 1973 Jun;52(6):1362–1367. doi: 10.1172/JCI107308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha A. S., Kokko J. P. Sodium chloride and water transport in the medullary thick ascending limb of Henle. Evidence for active chloride transport. J Clin Invest. 1973 Mar;52(3):612–623. doi: 10.1172/JCI107223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha A. S., Magaldi J. B., Kokko J. P. Calcium and phosphate transport in isolated segments of rabbit Henle's loop. J Clin Invest. 1977 May;59(5):975–983. doi: 10.1172/JCI108720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer J. A., Andreoli T. E. Anion transport processes in the mammalian superficial proximal straight tubule. J Clin Invest. 1976 Aug;58(2):500–513. doi: 10.1172/JCI108494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer J. A., Troutman S. L., Andreoli T. E. Volume reabsorption, transepithelial potential differences, and ionic permeability properties in mammalian superficial proximal straight tubules. J Gen Physiol. 1974 Nov;64(5):582–607. doi: 10.1085/jgp.64.5.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer J. A., Troutman S. L., Watkins M. L., Andreoli T. E. Volume absorption in the pars recta. I. "Simple" active Na+ transport. Am J Physiol. 1978 Apr;234(4):F332–F339. doi: 10.1152/ajprenal.1978.234.4.F332. [DOI] [PubMed] [Google Scholar]
- Shareghi G. R., Stoner L. C. Calcium transport across segments of the rabbit distal nephron in vitro. Am J Physiol. 1978 Oct;235(4):F367–F375. doi: 10.1152/ajprenal.1978.235.4.F367. [DOI] [PubMed] [Google Scholar]
- Tune B. M., Burg M. B. Glucose transport by proximal renal tubules. Am J Physiol. 1971 Aug;221(2):580–585. doi: 10.1152/ajplegacy.1971.221.2.580. [DOI] [PubMed] [Google Scholar]
- Tune B. M., Burg M. B., Patlak C. S. Characteristics of p-aminohippurate transport in proximal renal tubules. Am J Physiol. 1969 Oct;217(4):1057–1063. doi: 10.1152/ajplegacy.1969.217.4.1057. [DOI] [PubMed] [Google Scholar]
- de Rouffignac C., Morel F., Moss N., Roinel N. Micropuncture study of water and electrolyte movements along the loop of Henle in psammomys with special reference to magnesium, calcium and phosphorus. Pflugers Arch. 1973 Nov 30;344(4):309–326. doi: 10.1007/BF00592784. [DOI] [PubMed] [Google Scholar]


