Abstract
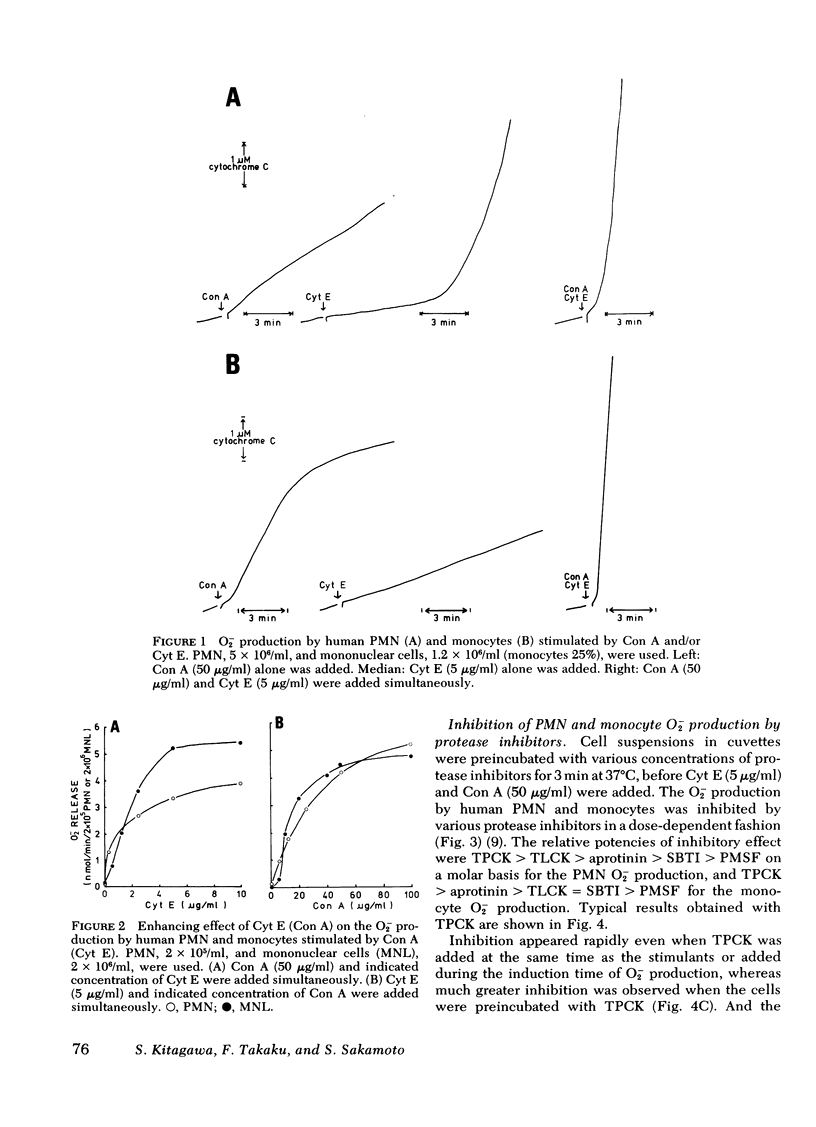
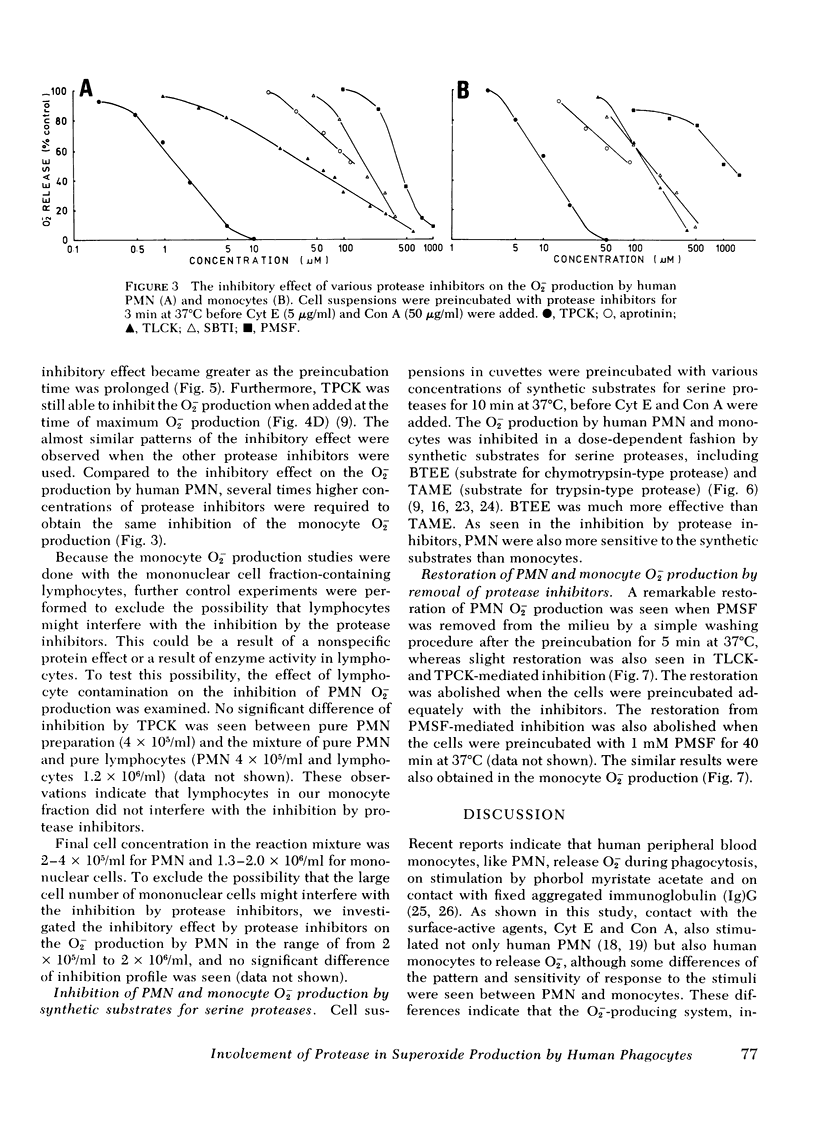
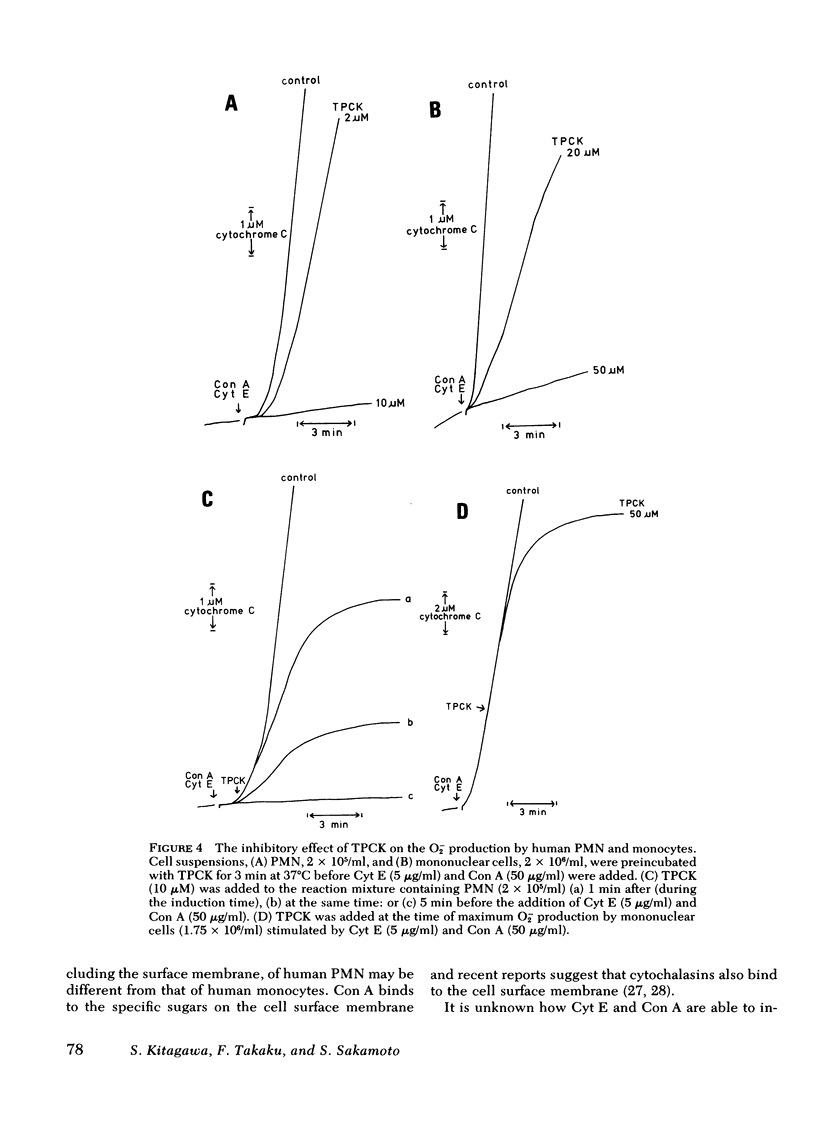
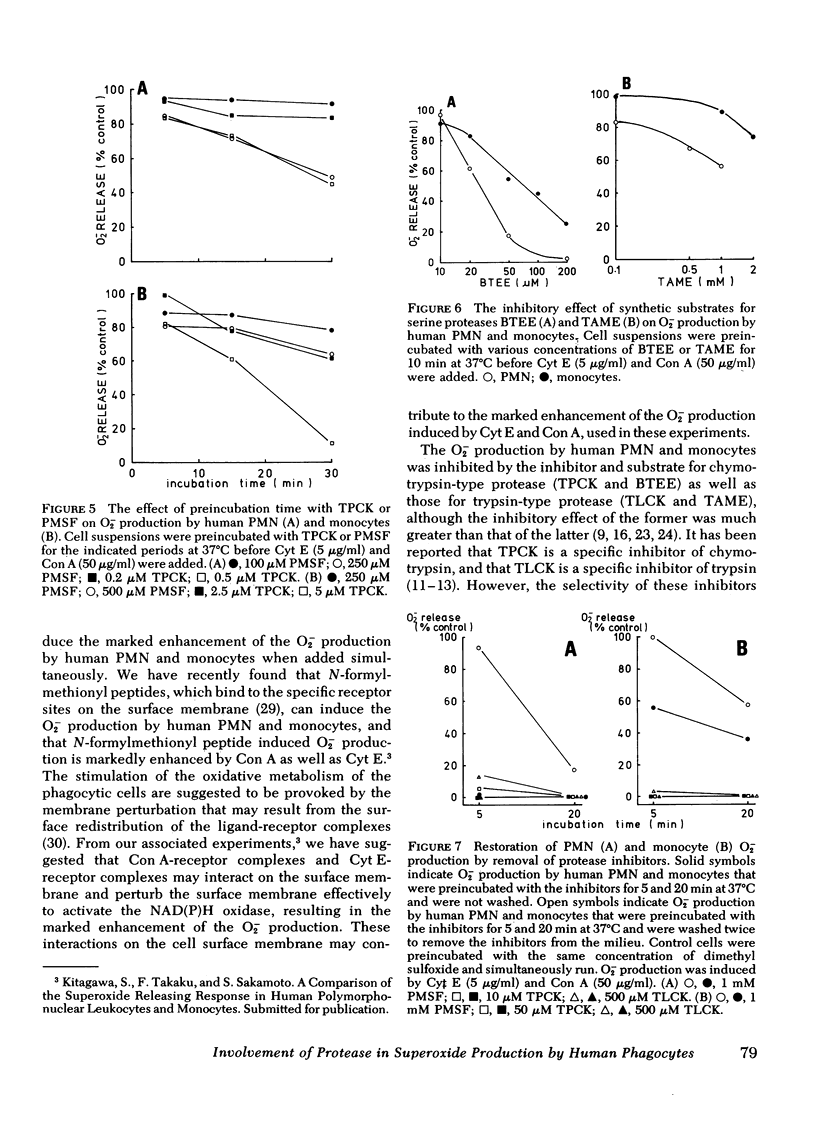
The possible participation of proteases in superoxide (O2-) production by human polymorphonuclear leukocytes (PMN) and monocytes was explores using various protease inhibitors and substrates. Protease inhibitors of serine proteases and synthetic inhibitors that modify the active site of serine proteases. Substrates used were synthetic substrates of the chymotrypsin type as well as trypsin type of protease. All these inhibitors and substrates inhibited O2- oroduction by human PMN and monocytes induced by cytochalasin E and concanavalin A, though PMN were more sensitive to these inhibitors and substrates than monocytes. Inhibition appeared rapidly even when the inhibitors were added at the same time as the stimulants, during the "induction time of O2-production" or at the time of maximum O2- production, whereas much greater inhibition was observed when the cells were preincubated with the inhibitors. These observations suggest that enzymatically active serine proteases are essential for these phagocytic cells to initiate and maintain the O2- production in response to the stimuli. The inhibitory effect of the inhibitor and substrate for chymotrypsin type protease was greater than that of those substances for trypsin-type protease. Macromolecular inhibitors also inhibited the O2- production. These findings suggest that the serine proteases involved in the O2- production by human PMN and monocytes are similar to chymotrypsin rather than trypsin, and are possibly located at the cell surface membrane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aswanikumar S., Corcoran B., Schiffmann E., Day A. R., Freer R. J., Showell H. J., Becker E. L. Demonstration of a receptor on rabbit neutrophils for chemotactic peptides. Biochem Biophys Res Commun. 1977 Jan 24;74(2):810–817. doi: 10.1016/0006-291x(77)90375-8. [DOI] [PubMed] [Google Scholar]
- Aswanikumar S., Schiffmann E., Corcoran B. A., Wahl S. M. Role of a peptidase in phagocyte chemotaxis. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2439–2442. doi: 10.1073/pnas.73.7.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J. P., Parker C. W. Cytochalasin binding to macrophages. Cell Immunol. 1978 Nov;41(1):103–121. doi: 10.1016/s0008-8749(78)80031-8. [DOI] [PubMed] [Google Scholar]
- Becker E. L., Koza E. P., Sigman M. Organophosphorus inhibition of lysosomal enzyme secretion from polymorphonuclear leucocytes. Evidence of a lack of a requirement for esterase activation. Immunology. 1978 Aug;35(2):373–380. [PMC free article] [PubMed] [Google Scholar]
- Becker E. L., Showell H. J. The ability of chemotactic factors to induce lysosomal enzyme release. II. The mechanism of release. J Immunol. 1974 Jun;112(6):2055–2062. [PubMed] [Google Scholar]
- Berlin R. D., Oliver J. M. Analogous ultrastructure and surface properties during capping and phagocytosis in leukocytes. J Cell Biol. 1978 Jun;77(3):789–804. doi: 10.1083/jcb.77.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs R. T., Drath D. B., Karnovsky M. L., Karnovsky M. J. Localization of NADH oxidase on the surface of human polymorphonuclear leukocytes by a new cytochemical method. J Cell Biol. 1975 Dec;67(3):566–586. doi: 10.1083/jcb.67.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Dewald B., Baggiolini M., Curnutte J. T., Babior B. M. Subcellular localization of the superoxide-forming enzyme in human neutrophils. J Clin Invest. 1979 Jan;63(1):21–29. doi: 10.1172/JCI109273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Cerqueira M., Lind S., Kaplan H. B. Evidence that the superoxide-generating system of human leukocytes is associated with the cell surface. J Clin Invest. 1977 Feb;59(2):249–254. doi: 10.1172/JCI108635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- INAGAMI T., STURTEVANT J. M. Nonspecific catalyses by alpha-chymotrypsin and trypsin. J Biol Chem. 1960 Apr;235:1019–1023. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E., Guthrie L. A. Generation of superoxide anion and chemiluminescence by human monocytes during phagocytosis and on contact with surface-bound immunoglobulin G. J Exp Med. 1976 Jun 1;143(6):1551–1556. doi: 10.1084/jem.143.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa S., Takaku F., Sakamoto S. Possible involvement of proteases in superoxide production by human polymorphonuclear leukocytes. FEBS Lett. 1979 Mar 15;99(2):275–278. doi: 10.1016/0014-5793(79)80971-0. [DOI] [PubMed] [Google Scholar]
- Lin S., Lin D. C., Flanagan M. D. Specificity of the effects of cytochalasin B on transport and motile processes. Proc Natl Acad Sci U S A. 1978 Jan;75(1):329–333. doi: 10.1073/pnas.75.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musson R. A., Becker E. L. The role of an activatable esterase in immune-dependent phagocytosis by human neutrophils. J Immunol. 1977 Apr;118(4):1354–1365. [PubMed] [Google Scholar]
- Nagai K., Nakamura T., Koyama J. Characterization of macrophage proteases involved in the ingestion of antigen-antibody complexes by the use of protease inhibitors. FEBS Lett. 1978 Aug 15;92(2):299–302. doi: 10.1016/0014-5793(78)80774-1. [DOI] [PubMed] [Google Scholar]
- Nakagawara A., Ferdais Nabi B. Z., Minakami S. An improved procedure for the diagnosis of chronic granulomatous disease, using concanavalin A and cytochalasin E. Clin Chim Acta. 1977 Jan 17;74(2):173–176. doi: 10.1016/0009-8981(77)90219-4. [DOI] [PubMed] [Google Scholar]
- Nakagawara A., Kakinuma K., Shin H., Miyazaki S., Minakami S. Lack of cytochalasin E-induced superoxide release by polymorphonuclear leucocytes of patients with chronic granulomatous disease: a new diagnostic test. Clin Chim Acta. 1976 Jul 1;70(1):133–137. doi: 10.1016/0009-8981(76)90015-2. [DOI] [PubMed] [Google Scholar]
- Nakagawara A., Minakami S. Generation of superoxide anions by leukocytes treated with cytochalasin E. Biochem Biophys Res Commun. 1975 May 19;64(2):760–767. doi: 10.1016/0006-291x(75)90385-x. [DOI] [PubMed] [Google Scholar]
- Oliver J. M., Ukena T. E., Berlin R. D. Effects of phagocytosis and colchicine on the distribution of lectin-binding sites on cell surfaces. Proc Natl Acad Sci U S A. 1974 Feb;71(2):394–398. doi: 10.1073/pnas.71.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman D. S., Ward P. A., Becker E. L. The requirement of serine esterase function in complement-dependent erythrophagocytosis. J Exp Med. 1969 Oct 1;130(4):745–764. doi: 10.1084/jem.130.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo D., Zabucchi G., Berton G., Schneider C. Metabolic stimulation of polymorphonuclear leucocytes: effects of tetravalent and divalent concanavalin A. J Membr Biol. 1978 Dec 29;44(3-4):321–330. doi: 10.1007/BF01944227. [DOI] [PubMed] [Google Scholar]
- Romeo D., Zabucchi G., Rossi F. Reversible metabolic stimulation of polymorphonuclear leukocytes and macrophages by concanavalin A. Nat New Biol. 1973 May 23;243(125):111–112. [PubMed] [Google Scholar]
- Ryan G. B., Borysenko J. Z., Karnovsky M. J. Factors affecting the redistribution of surface-bound concanavalin A on human polymorphonuclear leukocytes. J Cell Biol. 1974 Aug;62(2):351–365. doi: 10.1083/jcb.62.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOELLMANN G., SHAW E. Direct evidence for the presence of histidine in the active center of chymotrypsin. Biochemistry. 1963 Mar-Apr;2:252–255. doi: 10.1021/bi00902a008. [DOI] [PubMed] [Google Scholar]
- Sagone A. L., Jr, King G. W., Metz E. N. A comparison of the metabolic response to phagocytosis in human granulocytes and monocytes. J Clin Invest. 1976 May;57(5):1352–1358. doi: 10.1172/JCI108403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanaka K., O'Brien P. J. Mechanisms of H2O2 formation by leukocytes. Evidence for a plasma membrane location. Arch Biochem Biophys. 1975 Aug;169(2):428–435. doi: 10.1016/0003-9861(75)90184-8. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Becker E. L. Biochemical demonstration of the activatable esterase of the rabbit netrophil involved in the chemotactic response. J Immunol. 1970 Nov;105(5):1057–1067. [PubMed] [Google Scholar]
- Ward P. A., Becker E. L. Mechanisms of the inhibition of chemotaxis by phosphonate esters. J Exp Med. 1967 Jun 1;125(6):1001–1020. doi: 10.1084/jem.125.6.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]


