Abstract
Previous studies showed hyperre-sponsiveness to human growth hormone (hGH) in men with myotonic or limb girdle dystrophies (MMD or LGD). Because polyamines may mediate some actions of hGH, we have now investigated polyamine metabolism in these and other dystrophies.
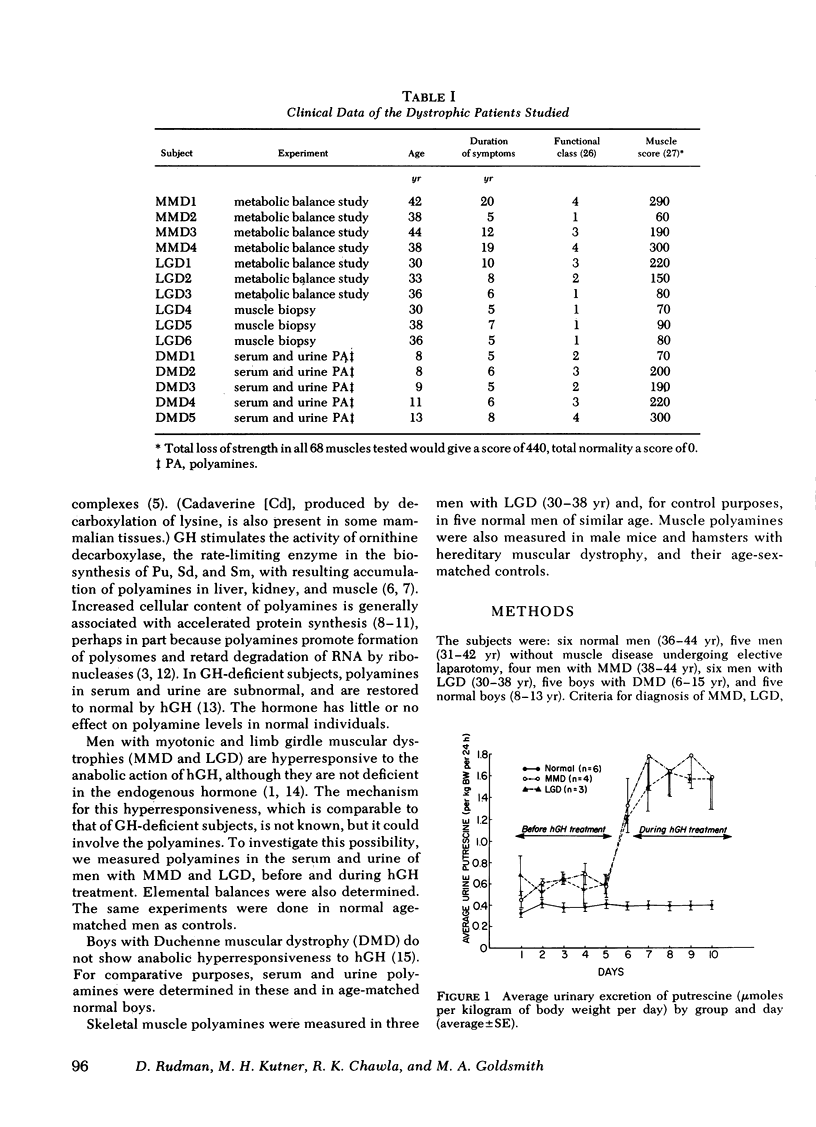
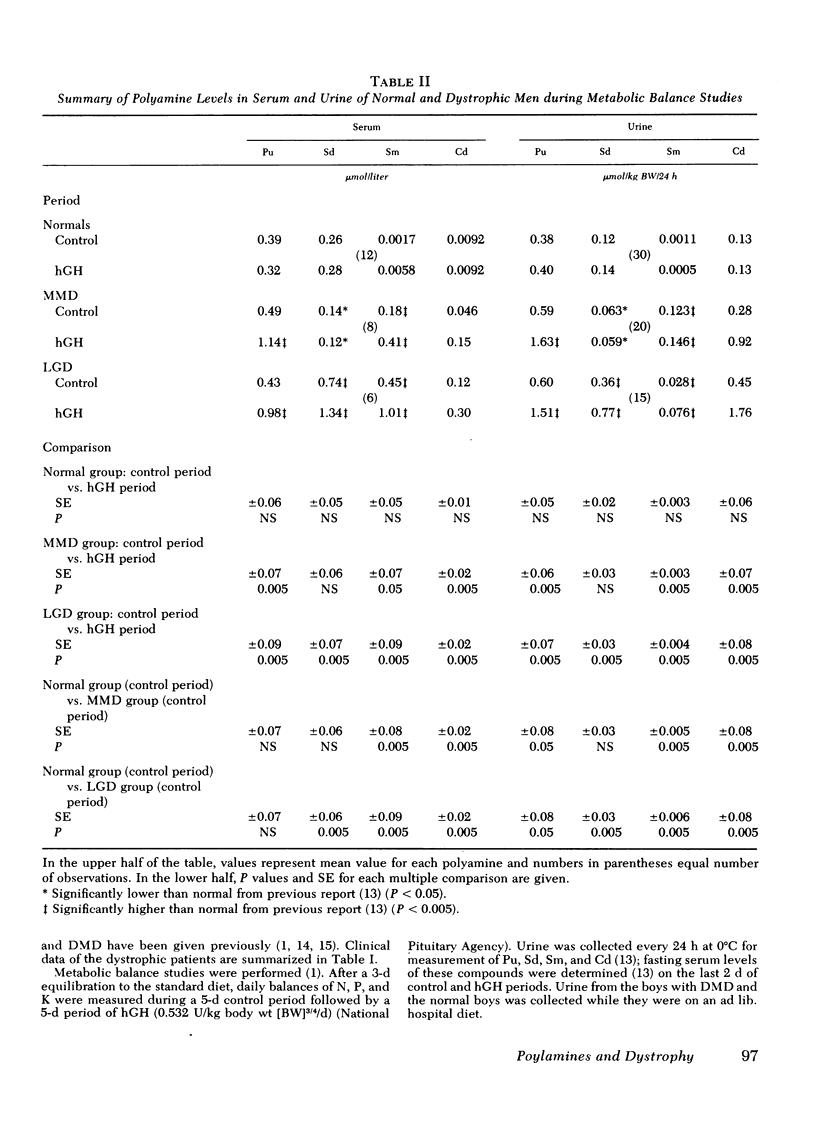
Under metabolic balance study conditions, serum and urine levels of putrescine (Pu), spermidine (Sd), spermine (Sm), and cadaverine (Cd) were measured in six normal men (36-44 yr), four men with MMD (38-44 yr), and three men with LGD (30-36 yr), before and during treatment with 0.532 U/kg body wt ¾/d of hGH. Daily balances of N, P, and K were also monitored. In the normal subjects, hGH did not influence elemental balances or serum and urine polyamines. In MMD, hGH caused significant retention of N, P, and K (P < 0.005). Basal levels of Sm and Cd were significantly elevated above normal (P < 0.005), and Pu, Sm, and Cd increased two- to fourfold above basal during hGH treatment (P < 0.005). In LGD, hGH also caused retention of N, P, and K. Basal levels of nearly all the polyamines (not serum Pu) were significantly above normal in serum and urine (P < 0.05). During hGH treatment, all four polyamines rose significantly above basal (P < 0.005).
Serum and urine polyamine levels in five boys with Duchenne muscular dystrophy, age 8-13, did not differ from those in five age-matched normal boys.
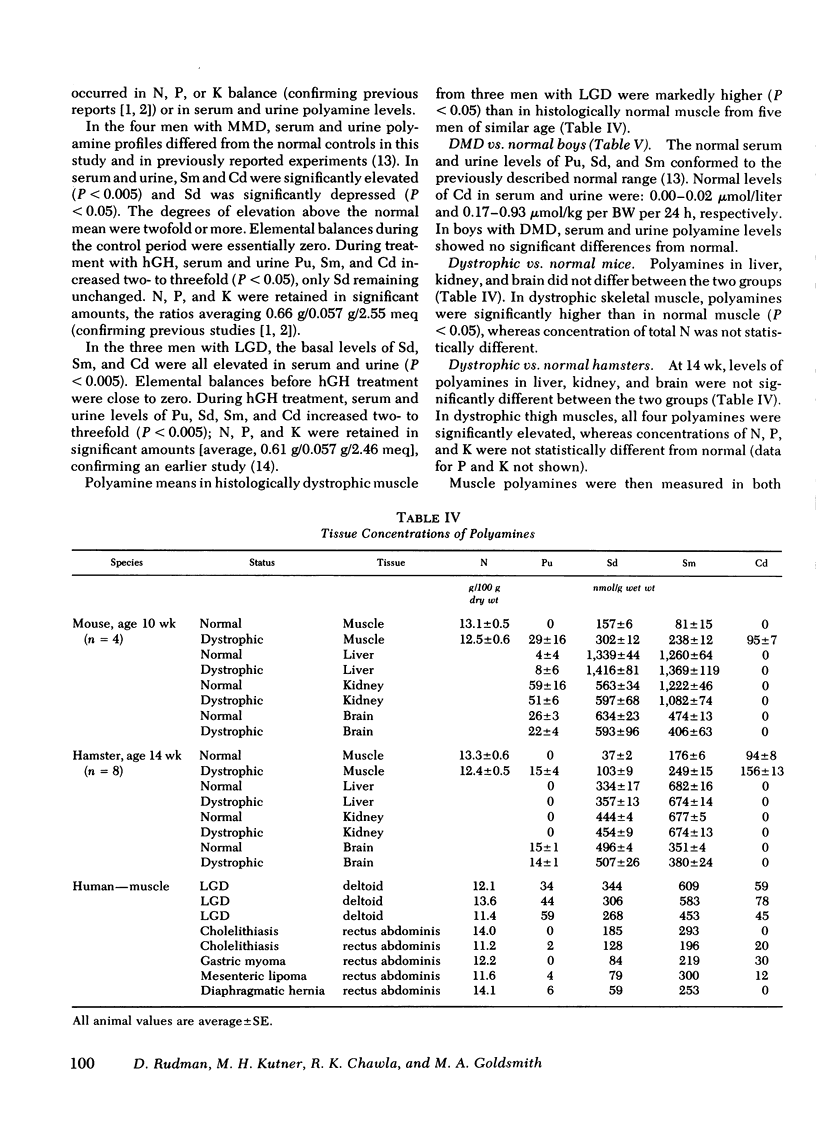
Skeletal muscle polyamines were measured in five men (31-40 yr) without muscle disease and in three men with LGD (30-38 yr). Average concentrations of Pu, Sd, Sm, and Cd were 46, 306, 548, and 61 nmol/g wet wt in LGD and 1, 121, 245, and 14 in the normal subjects, respectively (P < 0.05 in each instance).
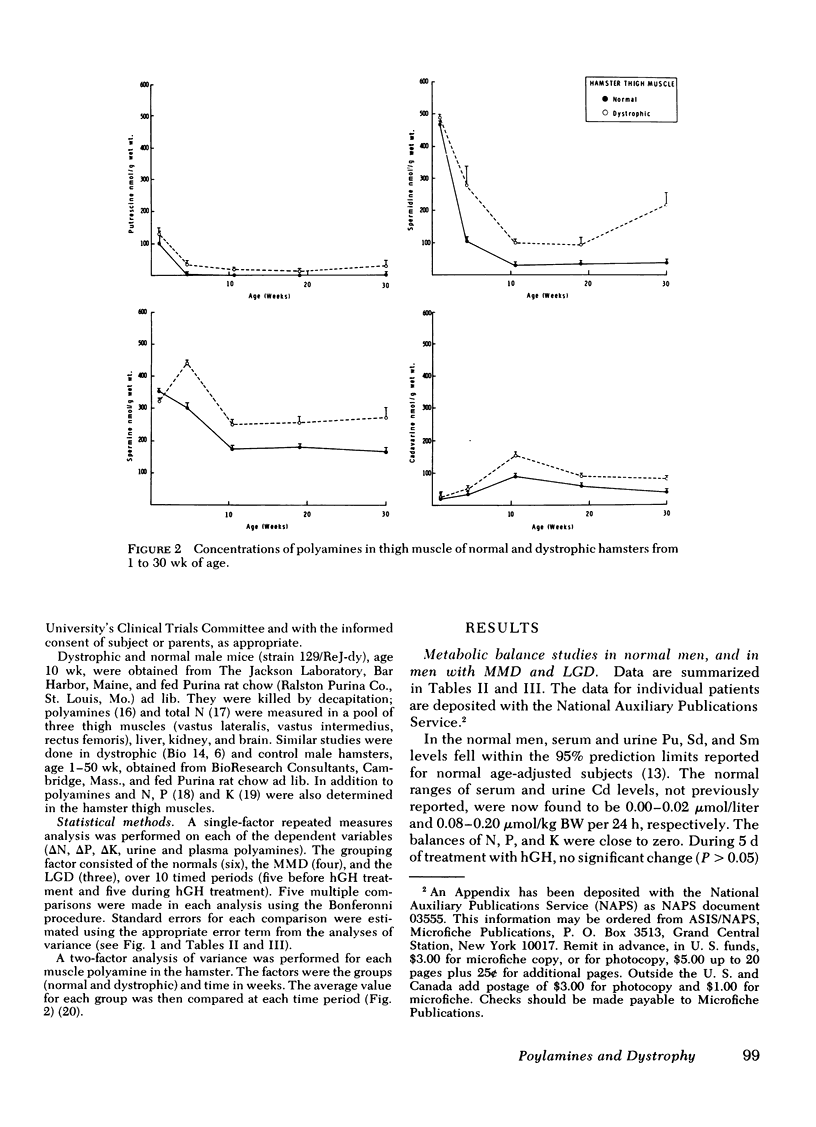
Polyamines were determined in skeletal muscle, liver, kidney, and brain of male mice with hereditary muscular dystrophy and in age- and sex-matched normal controls. Pu, Sd, Sm, and Cd levels were two to three times higher than normal in muscle, but did not differ in liver, kidney, and brain. Similar findings were made in male hamsters with hereditary dystrophy and in their controls. The abnormality in hamster muscle polyamines appeared between 1 and 6 wk of age and persisted or intensified until 30 wk.
These data reveal abnormalities of polyamine metabolism in men with MMD, in men with LGD, and in mice or hamsters with hereditary muscular dystrophy. The polyamine disorder could be related to dystrophic patients' hyperresponsiveness to hGH.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Heymsfield S. B., Bethel R. A., Rudman D. Hyperresponsivness of patients with clinical and premyopathic myotonic dystrophy to human growth hormone. J Clin Endocrinol Metab. 1977 Jul;45(1):147–158. doi: 10.1210/jcem-45-1-147. [DOI] [PubMed] [Google Scholar]
- JAENNE J., RAINA A., SIIMES M. SPERMIDINE AND SPERMINE IN RAT TISSUES AT DIFFERENT AGES. Acta Physiol Scand. 1964 Dec;62:352–358. doi: 10.1111/j.1748-1716.1964.tb10433.x. [DOI] [PubMed] [Google Scholar]
- Kostyo J. L. Changes in polyamine content of rat liver following hypophysectomy and treatment with growth hormone. Biochem Biophys Res Commun. 1966 Apr 19;23(2):150–155. doi: 10.1016/0006-291x(66)90520-1. [DOI] [PubMed] [Google Scholar]
- LILIENFELD A. M., JACOBS M., WILLIS M. A study of the reproducibility of muscle testing and certain other aspects of muscle scoring. Phys Ther Rev. 1954 Jun;34(6):279–289. doi: 10.1093/ptj/34.6.279. [DOI] [PubMed] [Google Scholar]
- Levy C. C., Mitch W. E., Schmukler M. Effect of polyamines on a ribonuclease which hydrolyzes ribonucleic acid at uridylic acid residues. J Biol Chem. 1973 Aug 25;248(16):5712–5719. [PubMed] [Google Scholar]
- Liang T., Mezzetti G., Chen C., Liao S. Selective polyamine-binding proteins. Spermine binding by an androgen-sensitive phosphoprotein. Biochim Biophys Acta. 1978 Sep 6;542(3):430–441. doi: 10.1016/0304-4165(78)90374-4. [DOI] [PubMed] [Google Scholar]
- Marton L. J., Russell D. H., Levy C. C. Measurement of putrescine, spermidine, and spermine in physiological fluids by use of an amino acid analyzer. Clin Chem. 1973 Aug;19(8):923–926. [PubMed] [Google Scholar]
- Mills J. B., Reagan C. R., Rudman D., Kostyo J. L., Zachariah P., Wilhelmi A. E. Metabolic effects of plasmin digests of human growth hormone in the rat and man. J Clin Invest. 1973 Nov;52(11):2941–2951. doi: 10.1172/JCI107491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina A., Jänne J. Physiology of the natural polyamines putrescine, spermidine and spermine. Med Biol. 1975 Jun;53(3):121–147. [PubMed] [Google Scholar]
- Raina A., Jänne J. Polyamines and the accumulation of RNA in mammalian systems. Fed Proc. 1970 Jul-Aug;29(4):1568–1574. [PubMed] [Google Scholar]
- Rudman D., Chyatte S. B., Gerron G. G., O'Beirne I., Barlow J. Hyper-responsiveness of patients with limb-girdle dystrophy to human growth hormone. J Clin Endocrinol Metab. 1972 Aug;35(2):256–260. doi: 10.1210/jcem-35-2-256. [DOI] [PubMed] [Google Scholar]
- Rudman D., Chyatte S. B., Patterson J. H., Gerron G. G., O'Beirne I., Barlow J., Ahmann P., Jordan A., Mosteller R. C. Observations on the responsiveness of human subjects to human growth hormone. Effects of endogenous growth hormone deficiency and myotinic dystrophy. J Clin Invest. 1971 Sep;50(9):1941–1949. doi: 10.1172/JCI106686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudman D., Chyatte S. B., Patterson J. H., Gerron G. G., O'Beirne I., Barlow J., Jordan A., Shavin J. S. Metabolic effects of human growth hormone and of estrogens in boys with Duchenne muscular dystrophy. J Clin Invest. 1972 May;51(5):1118–1124. doi: 10.1172/JCI106904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. H., Snyder S. H. Amine synthesis in regenerating rat liver: effect of hypophysectomy and growth hormone on ornithine decarboxylase. Endocrinology. 1969 Feb;84(2):223–228. doi: 10.1210/endo-84-2-223. [DOI] [PubMed] [Google Scholar]
- SWINYARD C. A., DEAVER G. G., GREENSPAN L. Gradients of functional ability of importance in rehabilitation of patients with progressive muscular and neuromuscular diseases. Arch Phys Med Rehabil. 1957 Sep;38(9):574–579. [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Biosynthesis and metabolism of 1,4-diaminobutane, spermidine, spermine, and related amines. Adv Enzymol Relat Areas Mol Biol. 1972;36:203–268. doi: 10.1002/9780470122815.ch7. [DOI] [PubMed] [Google Scholar]


