Abstract
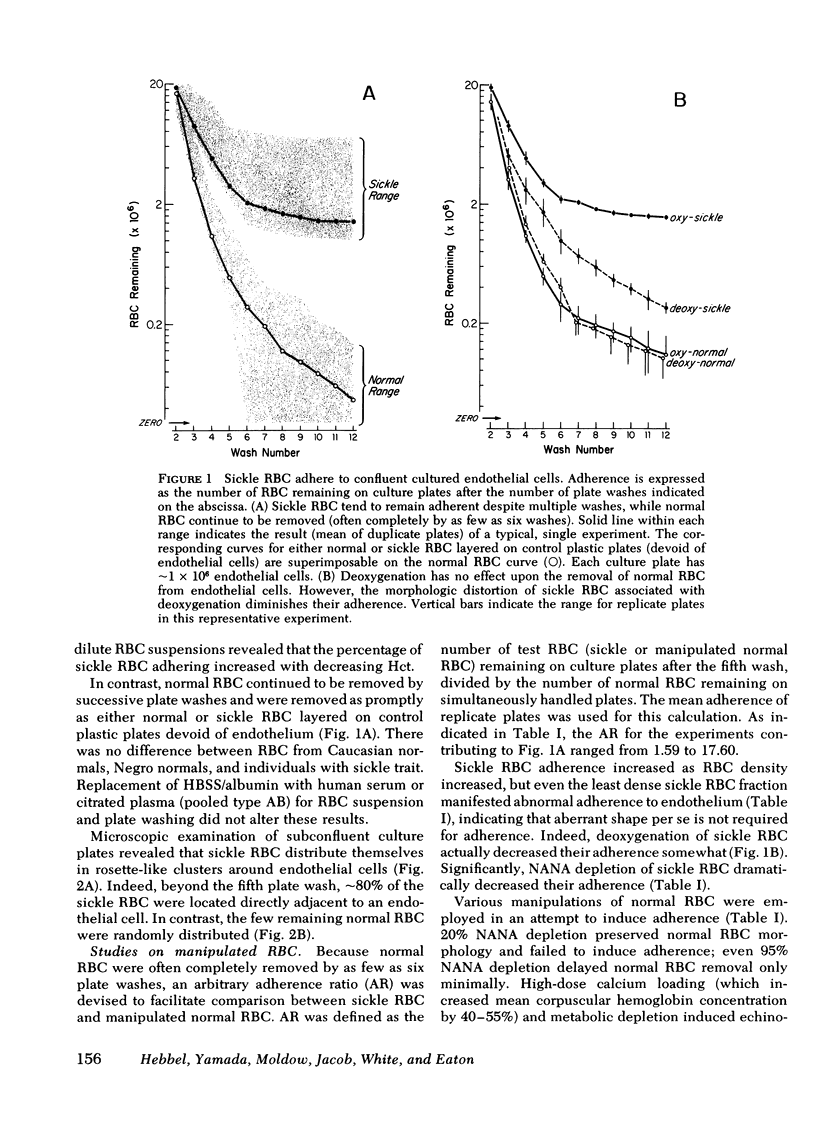
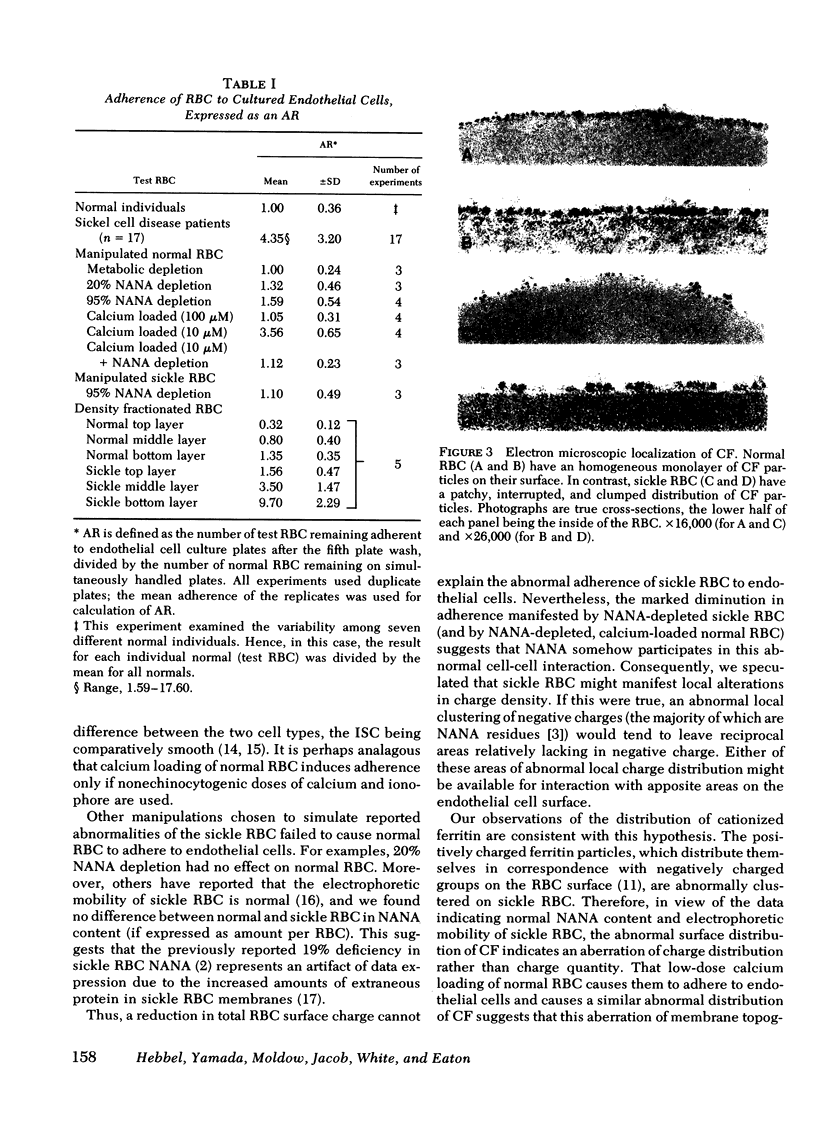
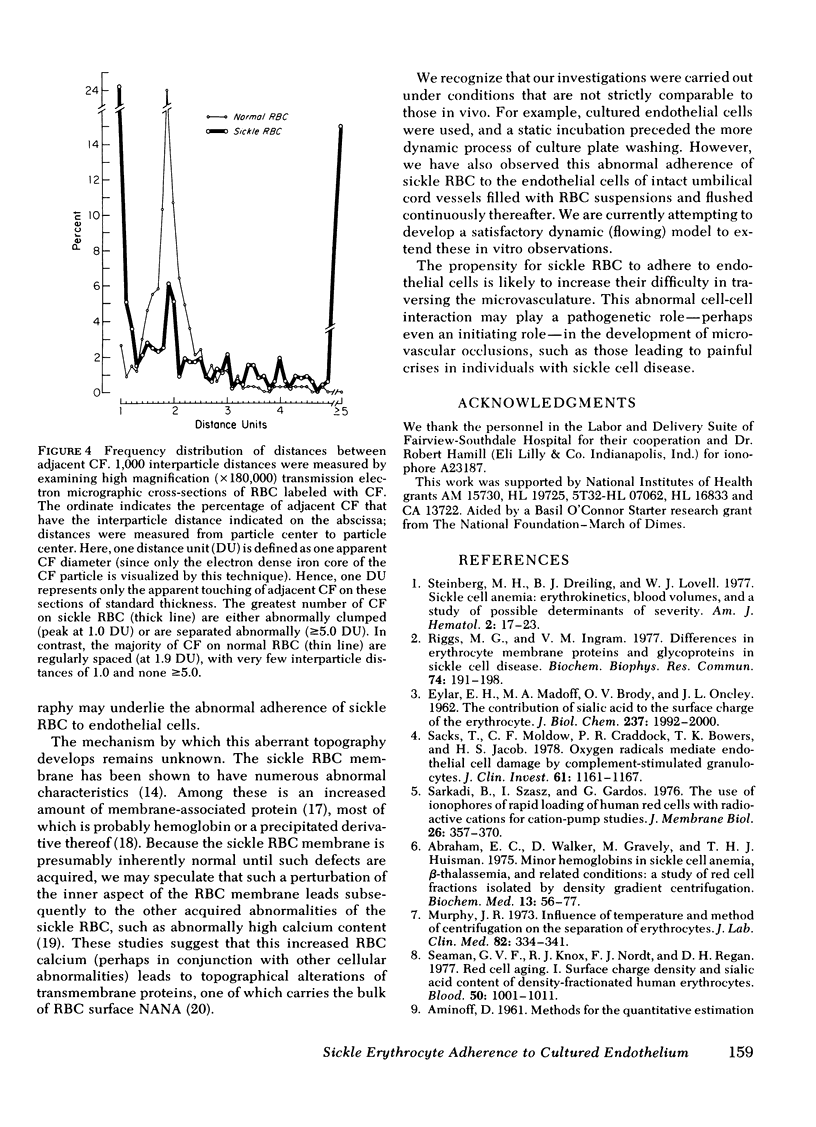
The abnormal shape and poor deformability of the sickled erythrocyte (RBC) have generally been held responsible for the microvascular occlusions of sickle cell disease. However, there is no correlation between the clinical severity of this disease and the presence of sickled RBC. In searching for additional factors that might contribute to the pathophysiology of sickle cell disease, we have investigated the possibility that sickle RBC might be less than normally repulsive of the vascular endothelium. After RBC suspensions are allowed to settle onto plates of cultured human endothelial cells, normal RBC are completely removed by as few as six washes. In contrast, sickle RBC remain adherent despite multiple washes. On subconfluent culture plates, normal RBC are distributed randomly, whereas sickle RBC cluster around endothelial cells. Sickle RBC adherence is not enhanced by deoxygenation but does increase with increasing RBC density. The enzymatic removal of membrane sialic acid greatly diminishes the adherence of sickle RBC to endothelial cells, suggesting that sialic acid participates in this abnormal cell-cell interaction. Although net negative charge appears normal, sickle RBC mainfest an abnormal clumping of negative surface charge as demonstrated by localization of cationized ferritin. These abnormalities are reproduced in normal RBC loaded with nonechinocytogenic amounts of calcium. We conclude that sickle RBC adhere to vascular endothelial cells in vitro, perhaps caused by a calcium-induced aberration of membrane topography. This adherence may be a pathogenetic factor in the microvascular occlusions characteristic of sickle cell disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham E. C., Walker D., Gravely M., Huisman T. H. Minor hemoglobins in sickle cell anemia, beta-thalassemia, and related conditions: a study of red cell fractions isolated by density gradient centrifugation. Biochem Med. 1975 May;13(1):56–77. doi: 10.1016/0006-2944(75)90140-4. [DOI] [PubMed] [Google Scholar]
- Asakura T., Minakata K., Adachi K., Russell M. O., Schwartz E. Denatured hemoglobin in sickle erythrocytes. J Clin Invest. 1977 Apr;59(4):633–640. doi: 10.1172/JCI108681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertles J. F., Milner P. F. Irreversibly sickled erythrocytes: a consequence of the heterogeneous distribution of hemoglobin types in sickle-cell anemia. J Clin Invest. 1968 Aug;47(8):1731–1741. doi: 10.1172/JCI105863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon D., Goldstein L., Marikovsky Y., Skutelsky E. Use of cationized ferritin as a label of negative charges on cell surfaces. J Ultrastruct Res. 1972 Mar;38(5):500–510. doi: 10.1016/0022-5320(72)90087-1. [DOI] [PubMed] [Google Scholar]
- Durocher J. R., Glader B. E., Conrad M. E. Effect of cyanate on erythrocyte membrane surface charge. Proc Soc Exp Biol Med. 1973 Oct 1;144(1):249–251. doi: 10.3181/00379727-144-37566. [DOI] [PubMed] [Google Scholar]
- EYLAR E. H., MADOFF M. A., BRODY O. V., ONCLEY J. L. The contribution of sialic acid to the surface charge of the erythrocyte. J Biol Chem. 1962 Jun;237:1992–2000. [PubMed] [Google Scholar]
- Eaton J. W., Jacob H. S., White J. G. Membrane abnormalities of irreversibly sickled cells. Semin Hematol. 1979 Jan;16(1):52–64. [PubMed] [Google Scholar]
- Eaton J. W., Skelton T. D., Swofford H. S., Kolpin C. E., Jacob H. S. Elevated erythrocyte calcium in sickle cell disease. Nature. 1973 Nov 9;246(5428):105–106. doi: 10.1038/246105a0. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T. Functional proteins of the human red blood cell membrane. Semin Hematol. 1979 Jan;16(1):3–20. [PubMed] [Google Scholar]
- Marikovsky Y., Khodadad J. K., Weinstein R. S. Influence of red cell shape on surface charge topography. Exp Cell Res. 1978 Oct 1;116(1):191–197. doi: 10.1016/0014-4827(78)90075-7. [DOI] [PubMed] [Google Scholar]
- Murphy J. R. Influence of temperature and method of centrifugation on the separation of erythrocytes. J Lab Clin Med. 1973 Aug;82(2):334–341. [PubMed] [Google Scholar]
- Riggs M. G., Ingram V. M. Differences in erythrocyte membrane proteins and glycoproteins in sickle cell disease. Biochem Biophys Res Commun. 1977 Jan 10;74(1):191–198. doi: 10.1016/0006-291x(77)91393-6. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi B., Szász I., Gárdos G. The use of ionophores of rapid loading of human red cells with radioactive cations for cation-pump studies. J Membr Biol. 1976 May;26(4):357–370. doi: 10.1007/BF01868883. [DOI] [PubMed] [Google Scholar]
- Seaman G. V., Knox R. J., Nordt F. J., Regan D. H. Red cell aging. I. Surface charge density and sialic acid content of density-fractionated human erythrocytes. Blood. 1977 Dec;50(6):1001–1011. [PubMed] [Google Scholar]
- Steinberg M. H., Dreiling B. J., Lovell W. J. Sickle cell anemia: erythrokinetics, blood volumes, and a study of possible determinants of severity. Am J Hematol. 1977;2(1):17–23. doi: 10.1002/ajh.2830020103. [DOI] [PubMed] [Google Scholar]
- White J. G. The fine structure of sickled hemoglobin in situ. Blood. 1968 May;31(5):561–579. [PubMed] [Google Scholar]