Abstract
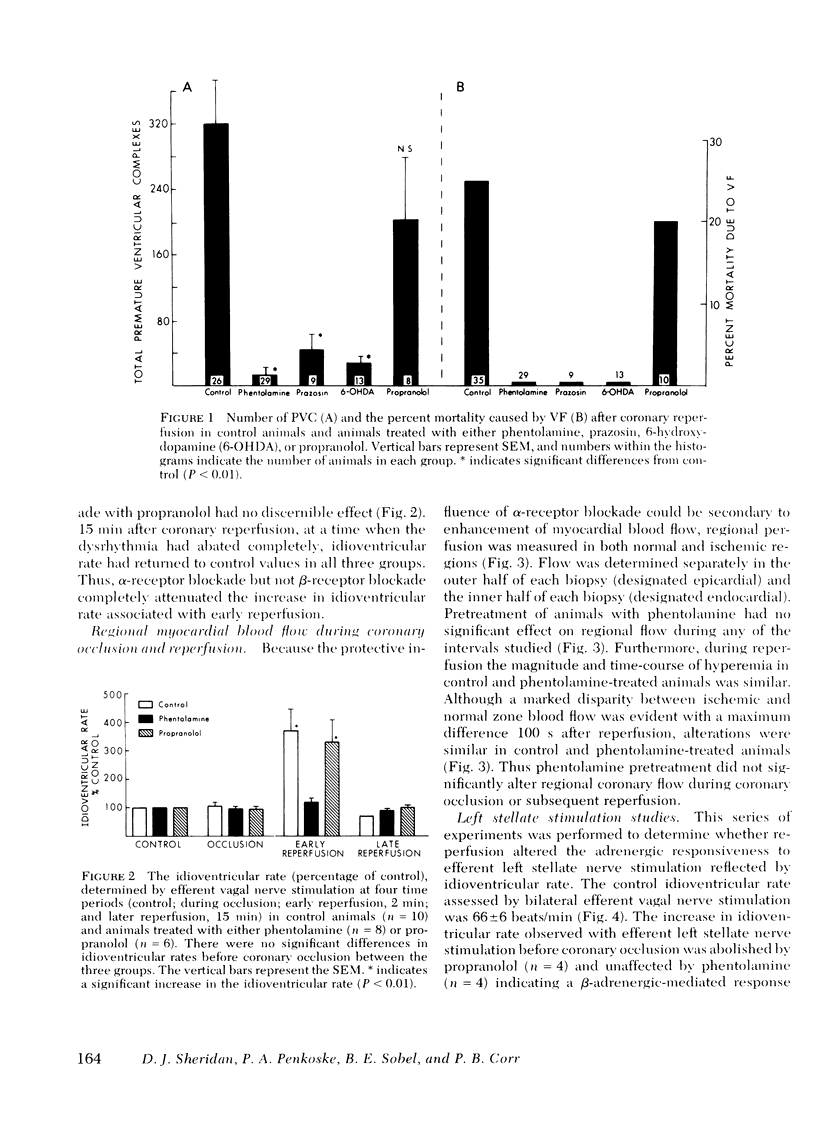
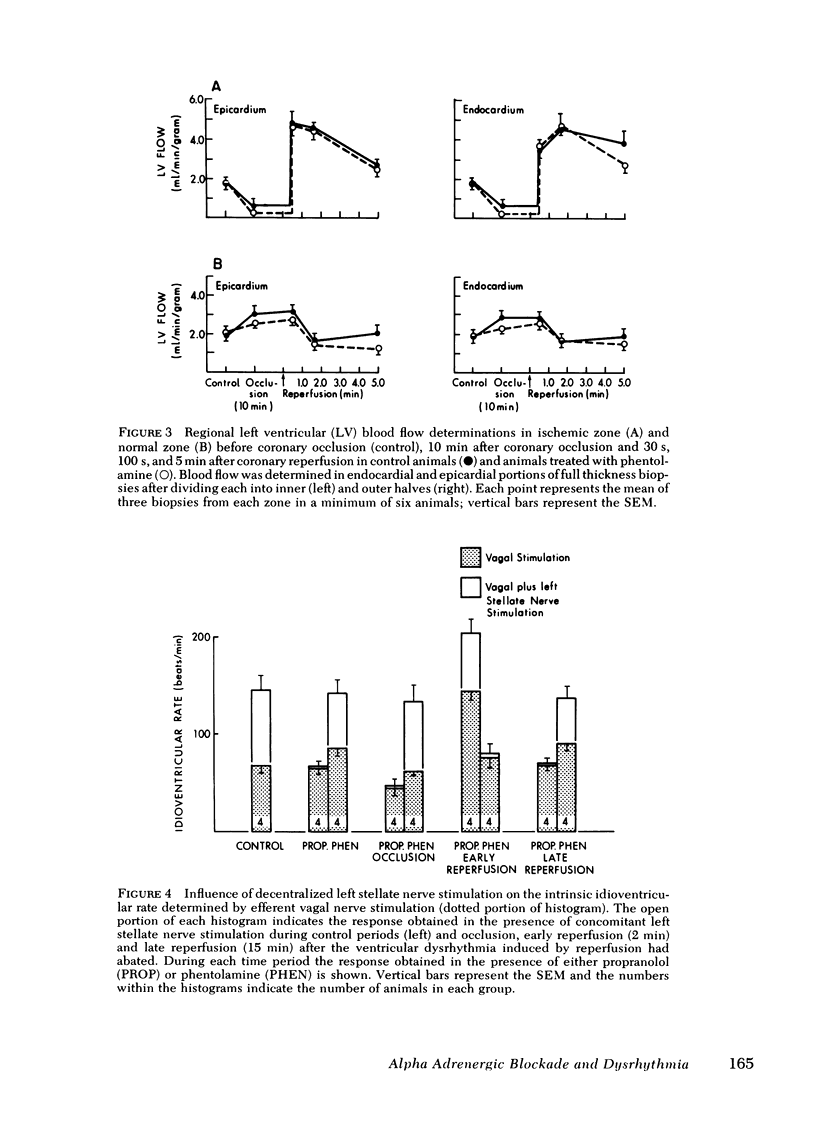
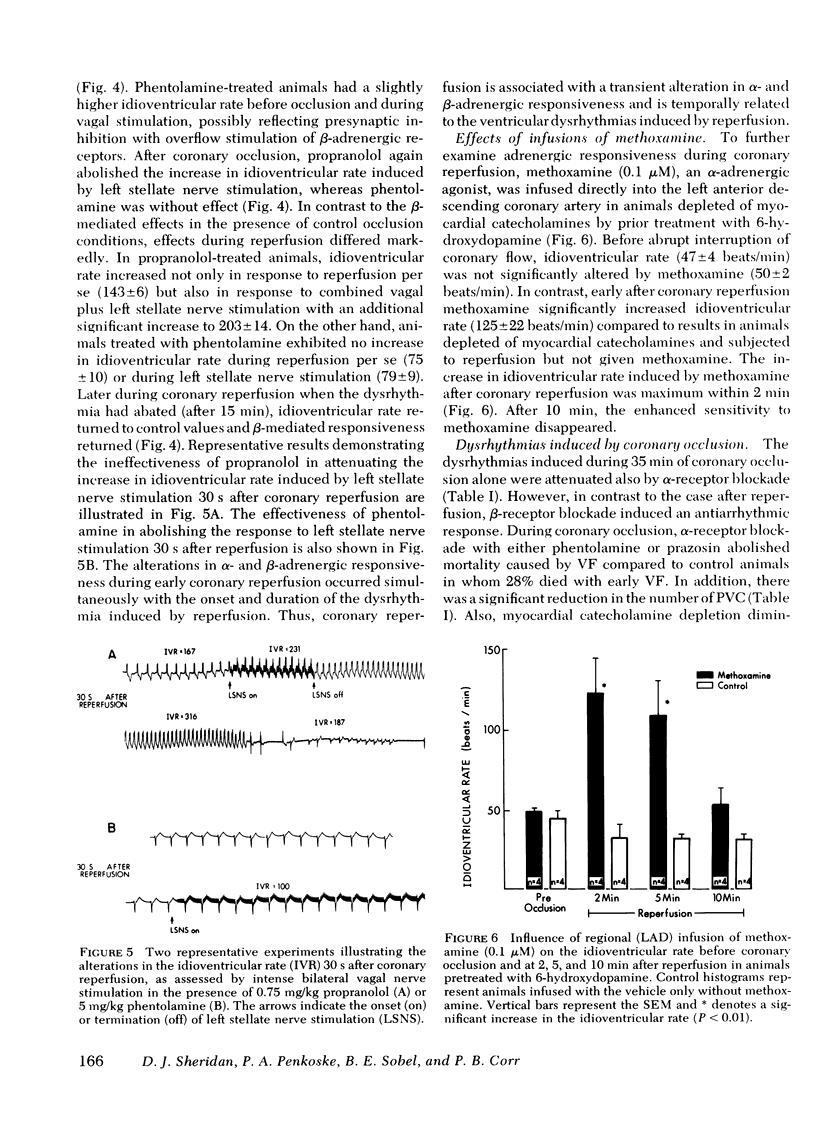
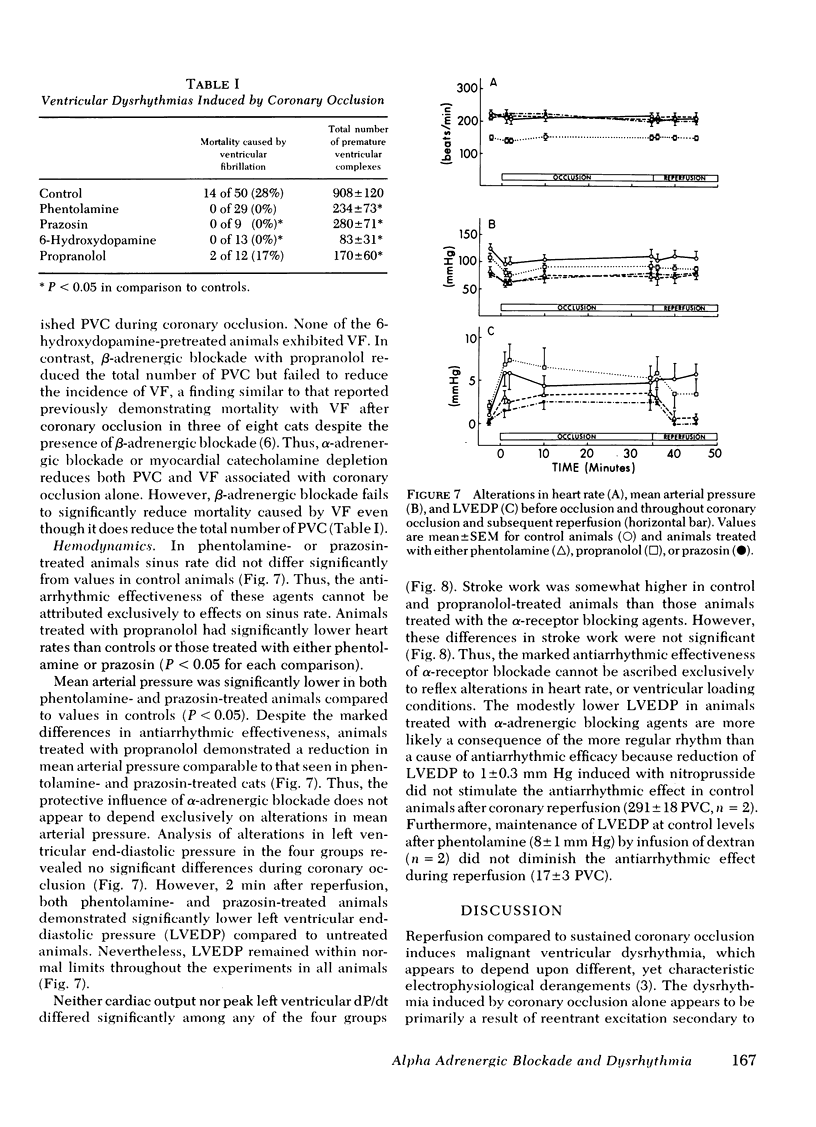
Alpha compared to beta adrenergic contributions to dysrhythmias induced by left anterior descending coronary occlusion and by reperfusion were assessed in chloralose-anesthetized cats (n = 96). Alpha receptor blockade with either phentolamine or prazosin significantly reduced the number of premature ventricular complexes during coronary reperfusion (321 +/- 62-14 +/- 10 premature ventricular complexes, P less than 0.001), abolished early ventricular fibrillation (from 25% in controls to 0%), and prevented the increase in idioventricular rate seen with coronary reperfusion. However, beta-receptor blockade was without effect. Ventricular dysrhythmias induced by coronary occlusion alone (without reperfusion) were attenuated markedly by alpha-receptor blockade under conditions in which perfusion (measured with radiolabeled microspheres) within ischemic zones was not affected. Alternative sympatholytic interventions including pretreatment with 6-hydroxydopamine to deplete myocardial norepinephrine from 8.8 +/- 1.4 to 0.83 +/- 0.2 ng/mg protein and render the heart unresponsive to tyramine (120 microgram/kg) attenuated dysrhythmias induced by both coronary occlusion and reperfusion in a fashion identical to that seen with alpha-receptor blockade. Although efferent sympathetic activation induced by left stellate nerve stimulation increased idioventricular rate from 66 +/- 6 to 144+/- 7 beats/min (P less than 0.01) before coronary occlusion, this response was blocked by propranolol but not by phentolamine. In contrast, during reperfusion the increase in idioventricular rate induced by left stellate nerve stimulation (to 203 +/- 14) was not inhibited by propranolol but was abolished by phentolamine (79 +/- 10). Intracoronary methoxamine (0.1 microM) in animals depleted of myocardial catecholamines by 6-hydroxydopamine pretreatment did not affect idioventricular rate before coronary occlusion. However, early after coronary reperfusion, methoxamine increased idioventricular rate from 33 +/- 7 to 123 +/- 21 beats/min (P less than 0.01). Thus, enhanced alpha-adrenergic responsiveness occurs during myocardial ischemia and appears to be primary mediator of the electrophysiological derangements and resulting malignant dysrhythmias induced by catecholamines during myocardial ischemia and reperfusion.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENFEY B. G., GREEFF K. Interactions of sympathomimetic drugs and their antagonists on the isolated atrium. Br J Pharmacol Chemother. 1961 Oct;17:232–235. doi: 10.1111/j.1476-5381.1961.tb01283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD H., BURNSTOCK G., CAMPBELL G., JOWETT A., O'SHEA J., WOOD M. The cholinergic blocking action of adrenergic blocking agents in the pharmacological analysis of autonomic innervation. Br J Pharmacol Chemother. 1963 Jun;20:418–435. doi: 10.1111/j.1476-5381.1963.tb01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambridge D., Davey M. J., Massingham R. The pharmacology of antihypertensive drugs with special reference to vasodilators, alpha-adrenergic blocking agents and prazosin. Med J Aust. 1977 Aug 20;2(1 Suppl):2–6. doi: 10.5694/j.1326-5377.1977.tb107750.x. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Gillis R. A. Autonomic neural influences on the dysrhythmias resulting from myocardial infarction. Circ Res. 1978 Jul;43(1):1–9. doi: 10.1161/01.res.43.1.1. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Pearle D. L., Hinton J. R., Roberts W. C., Gillis R. A. Site of myocardial infarction. A determinant of the cardiovascular changes induced in the cat by coronary occlusion. Circ Res. 1976 Dec;39(6):840–847. doi: 10.1161/01.res.39.6.840. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Witkowski F. X., Sobel B. E. Mechanisms contributing to malignant dysrhythmias induced by ischemia in the cat. J Clin Invest. 1978 Jan;61(1):109–119. doi: 10.1172/JCI108908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranefield P. F., Hoffman B. F., Wit A. L. Block of conduction in partially depolarized cardiac purkinje fibres induced by an -adrenergic agent. Nat New Biol. 1971 Dec 1;234(48):159–160. doi: 10.1038/newbio234159a0. [DOI] [PubMed] [Google Scholar]
- DiMicco J. A., Prestel T., Pearle D. L., Gillis R. A. Mechanism of cardiovascular changes produced in cats by activation of the central nervous system with picrotoxin. Circ Res. 1977 Oct;41(4):446–451. doi: 10.1161/01.res.41.4.446. [DOI] [PubMed] [Google Scholar]
- Dowdy E. G., Kaya K. Studies of the mechanism of cardiovascular responses to CI-581. Anesthesiology. 1968 Sep-Oct;29(5):931–943. doi: 10.1097/00000542-196809000-00014. [DOI] [PubMed] [Google Scholar]
- ELIAKIM M., BELLET S., TAWIL E., MULLER O. Effect of vagal stimulation and acetylcholine on the ventricle. Studies in dogs with complete atrioventricular block. Circ Res. 1961 Nov;9:1372–1379. [PubMed] [Google Scholar]
- Ebert P. A., Vanderbeek R. B., Allgood R. J., Sabiston D. C., Jr Effect of chronic cardiac denervation on arrhythmias after coronary artery ligation. Cardiovasc Res. 1970 Apr;4(2):141–147. doi: 10.1093/cvr/4.2.141. [DOI] [PubMed] [Google Scholar]
- Elharrar V., Watanabe A. M., Molello J., Besch H. R., Jr, Zipes D. P. Adrenergaically mediated ventricular fibrillation in probucol-treated dogs: roles of alpha and beta adrenergic receptors. Pacing Clin Electrophysiol. 1979 Jul;2(4):435–443. doi: 10.1111/j.1540-8159.1979.tb05219.x. [DOI] [PubMed] [Google Scholar]
- Giotti A., Ledda F., Mannaioni P. F. Effects of noradrenaline and isoprenaline, in combination with - and -receptor blocking substances, on the action potential of cardiac Purkinje fibres. J Physiol. 1973 Feb;229(1):99–113. doi: 10.1113/jphysiol.1973.sp010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould L., Gomprecht R. F., Zahir M. Oral phentolamine for treatment of ventricular premature contractions. Br Heart J. 1971 Jan;33(1):101–104. doi: 10.1136/hrt.33.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. S. Potassium and experimental coronary occlusion. Am Heart J. 1966 Jun;71(6):797–802. doi: 10.1016/0002-8703(66)90601-6. [DOI] [PubMed] [Google Scholar]
- Hsueh W., Isakson P. C., Needleman P. Hormone selective lipase activation in the isolated rabbit heart. Prostaglandins. 1977 Jun;13(6):1073–1091. doi: 10.1016/0090-6980(77)90135-6. [DOI] [PubMed] [Google Scholar]
- Kostrzewa R. M., Jacobowitz D. M. Pharmacological actions of 6-hydroxydopamine. Pharmacol Rev. 1974 Sep;26(3):199–288. [PubMed] [Google Scholar]
- Limbird L. E., Lefkowitz R. J. Adenylate cyclase-coupled beta adrenergic receptors effect of membrane lipid-perturbing agents on receptor binding and enzyme stimulation by catecholamines. Mol Pharmacol. 1976 Jul;12(4):559–567. [PubMed] [Google Scholar]
- Miura Y., Inui J., Imamura H. Alpha-adrenoceptor-mediated restoration of calcium-dependent potential in the partially depolarized rabbit papillary muscle. Naunyn Schmiedebergs Arch Pharmacol. 1978 Jan-Feb;301(3):201–205. doi: 10.1007/BF00507038. [DOI] [PubMed] [Google Scholar]
- Pappano A. J. Propranolol-insensitive effects of epinephrine on action potential repolarization in electrically driven atria of the guinea pig. J Pharmacol Exp Ther. 1971 Apr;177(1):85–95. [PubMed] [Google Scholar]
- Passon P. G., Peuler J. D. A simplified radiometric assay for plasma norepinephrine and epinephrine. Anal Biochem. 1973 Feb;51(2):618–631. doi: 10.1016/0003-2697(73)90517-4. [DOI] [PubMed] [Google Scholar]
- Penkoske P. A., Sobel B. E., Corr P. B. Disparate electrophysiological alterations accompanying dysrhythmia due to coronary occlusion and reperfusion in the cat. Circulation. 1978 Dec;58(6):1023–1035. doi: 10.1161/01.cir.58.6.1023. [DOI] [PubMed] [Google Scholar]
- Posner P., Farrar E. L., Lambert C. R. Inhibitory effects of catecholamines in canine cardiac Purkinje fibers. Am J Physiol. 1976 Nov;231(5 Pt 1):1415–1420. doi: 10.1152/ajplegacy.1976.231.5.1415. [DOI] [PubMed] [Google Scholar]
- Powell J. R., Feigl E. O. Carotid sinus reflex coronary vasoconstriction during controlled myocardial oxygen metabolism in the dog. Circ Res. 1979 Jan;44(1):44–51. doi: 10.1161/01.res.44.1.44. [DOI] [PubMed] [Google Scholar]
- Rosen M. R., Gelband H., Hoffman B. F. Effects of phentolamine on electrophysiologic properties of isolated canine purkinje fibers. J Pharmacol Exp Ther. 1971 Dec;179(3):586–593. [PubMed] [Google Scholar]
- Rosen M. R., Hordof A. J., Ilvento J. P., Danilo P., Jr Effects of adrenergic amines on electrophysiological properties and automaticity of neonatal and adult canine Purkinje fibers: evidence for alpha- and beta-adrenergic actions. Circ Res. 1977 Apr;40(4):390–400. doi: 10.1161/01.res.40.4.390. [DOI] [PubMed] [Google Scholar]
- Schaffer W. A., Cobb L. A. Recurrent ventricular fibrillation and modes of death in survivors of out-of-hospital ventricular fibrillation. N Engl J Med. 1975 Aug 7;293(6):259–262. doi: 10.1056/NEJM197508072930601. [DOI] [PubMed] [Google Scholar]
- Sharma V. K., Banerjee S. P. alpha-adrenergic receptor in rat heart. Effects of thyroidectomy. J Biol Chem. 1978 Aug 10;253(15):5277–5279. [PubMed] [Google Scholar]
- Sommers H. M., Jennings R. B. Ventricular fibrillation and myocardial necrosis after transient ischemia. Effect of treatment with oxygen, procainamide, reserpine, and propranolol. Arch Intern Med. 1972 May;129(5):780–789. [PubMed] [Google Scholar]
- Spear J. F., Moore E. N. Influence of brief vagal and stellate nerve stimulation on pacemaker activity and conduction within the atrioventricular conduction system of the dog. Circ Res. 1973 Jan;32(1):27–41. doi: 10.1161/01.res.32.1.27. [DOI] [PubMed] [Google Scholar]
- VASSALLE M., GREENSPAN K., JOMAIN S., HOFFMAN B. F. EFFECTS OF POTASSIUM ON AUTOMATICITY AND CONDUCTION OF CANINE HEARTS. Am J Physiol. 1964 Aug;207:334–340. doi: 10.1152/ajplegacy.1964.207.2.334. [DOI] [PubMed] [Google Scholar]
- Vassalle M., Knob R. E., Cummins M., Lara G. A., Castro C., Stuckey J. H. An analysis of fast idioventricular rhythm in the dog. Circ Res. 1977 Aug;41(2):218–226. doi: 10.1161/01.res.41.2.218. [DOI] [PubMed] [Google Scholar]
- Vassalle M., Knob R. E., Lara G. A., Stuckey J. H. The effect of adrenergic enhancement on overdrive excitation. J Electrocardiol. 1976;9(4):335–343. doi: 10.1016/s0022-0736(76)80026-x. [DOI] [PubMed] [Google Scholar]
- Vassalle M., Musso E. On the mechanisms underlying digitalis toxicity in cardiac Purkinje fibers. Recent Adv Stud Cardiac Struct Metab. 1976;9:355–376. [PubMed] [Google Scholar]
- Vassalle M., Stuckey J. H., Levine M. J. Sympathetic control of ventricular automaticity: role of the adrenal medulla. Am J Physiol. 1969 Oct;217(4):930–937. doi: 10.1152/ajplegacy.1969.217.4.930. [DOI] [PubMed] [Google Scholar]
- Whalen D. A., Jr, Hamilton D. G., Ganote C. E., Jennings R. B. Effect of a transient period of ischemia on myocardial cells. I. Effects on cell volume regulation. Am J Pathol. 1974 Mar;74(3):381–397. [PMC free article] [PubMed] [Google Scholar]
- Williams L. T., Lefkowitz R. J., Watanabe A. M., Hathaway D. R., Besch H. R., Jr Thyroid hormone regulation of beta-adrenergic receptor number. J Biol Chem. 1977 Apr 25;252(8):2787–2789. [PubMed] [Google Scholar]


