Abstract
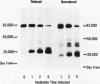
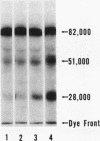
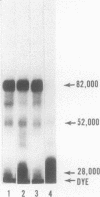
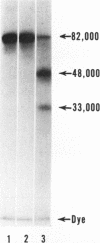
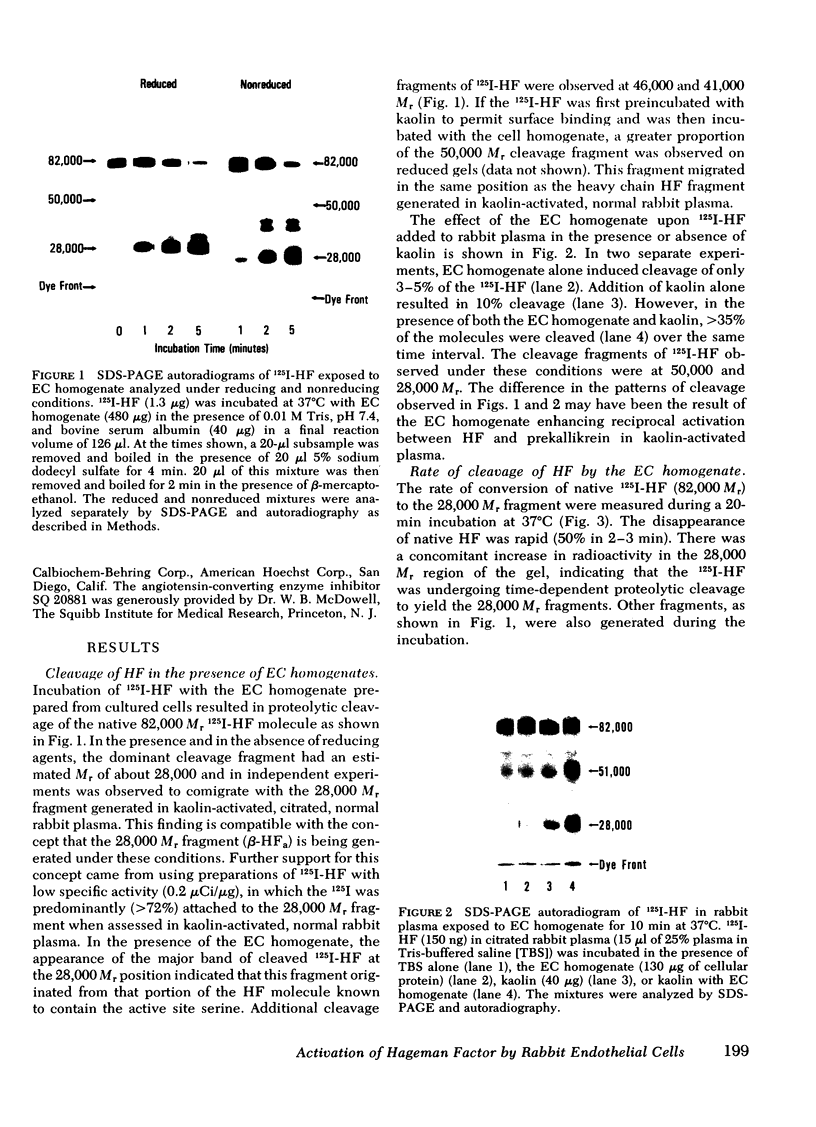
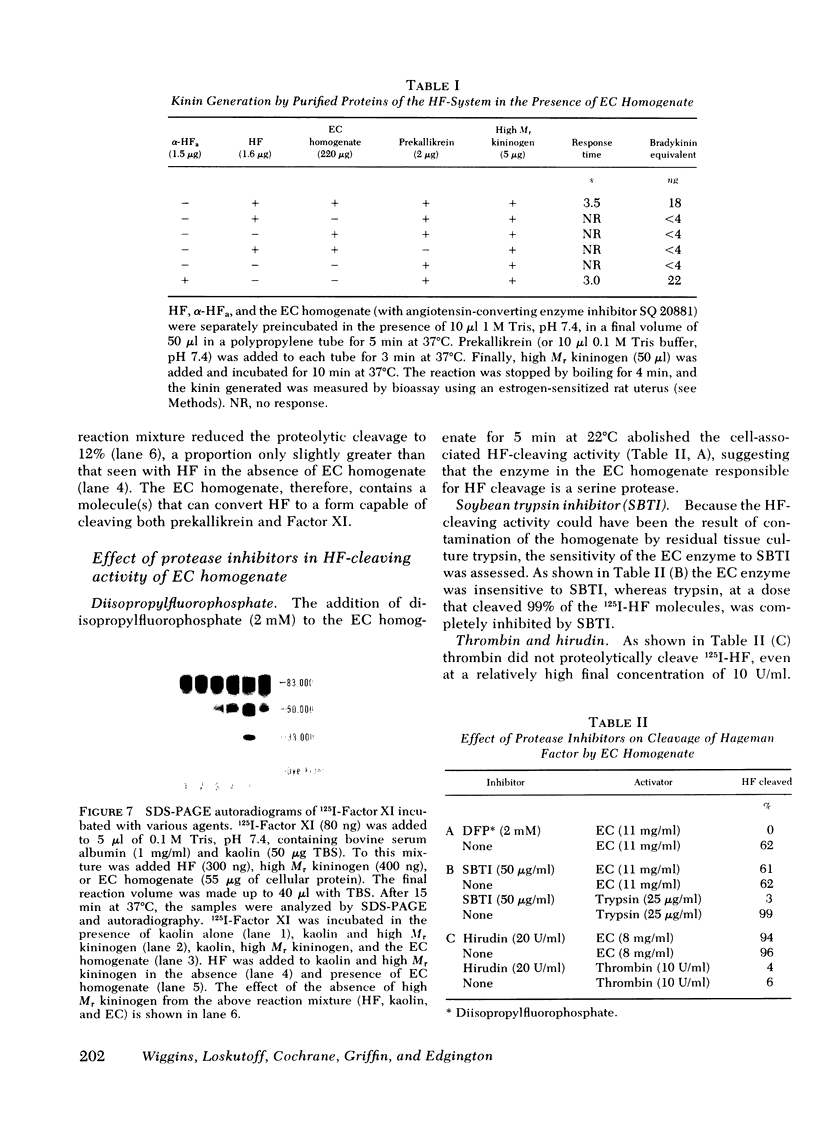
Rabbit Hageman factor was proteolytically cleaved and activated by a homogenate prepared from cultured rabbit endothelial cells. Cleavage of radiolabeled Hageman factor was monitored by polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate. Endothelial cell-mediated cleavage of Hageman factor was demonstrated both in a purified system and in plasma, was time and concentration dependent, and was associated with formation of the characteristic 28,000 Mr form of active Hageman factor. The rate of cleavage of Hageman factor was not affected by Triton X-100 (Rohm and Haas, Co., Philadelphia, Pa.), hexadimethrine bromide (Polybrene, Aldrich Chemical Co., Inc., Milwaukee, Wis.), hirudin, soybean trypsin inhibitor, or antisera to plasminogen or prekallikrein. However, cleavage was enhanced by kaolin, and was inhibited by diisopropyl-fluorophosphate. The enzyme responsible for cleavage of Hageman factor was localized to the 100,000-g-sedimentable, subcellular fraction of the endothelial cell homogenate and was relatively specific, because neither radiolabeled rabbit Factor XI nor rabbit prekallikrein were themselves proteolytically cleaved by the endothelial cell homogenate. However, when these molecules were incubated with the homogenate in the presence of Hageman factor, both Factor XI and prekallikrein were cleaved, demonstrating that Hageman factor had been activated by the endothelial cell homogenate. Furthermore, the kallikrein generated by endothelial cell homogenate-activated Hageman factor was capable of liberating kinin from high molecular weight kininogen as measured by bioassay. Cultured rabbit endothelial cells, therefore, possess the capacity to activate Hageman factor by proteolysis. This may be one mechanism for Hageman factor activation in vivo.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouma B. N., Griffin J. H. Deficiency of factor XII-dependent plasminogen proactivator in prekallikrein-deficient plasma. J Lab Clin Med. 1978 Jan;91(1):148–155. [PubMed] [Google Scholar]
- Bouma B. N., Griffin J. H. Human blood coagulation factor XI. Purification, properties, and mechanism of activation by activated factor XII. J Biol Chem. 1977 Sep 25;252(18):6432–6437. [PubMed] [Google Scholar]
- Cochrane C. G., Revak S. D., Wuepper K. D. Activation of Hageman factor in solid and fluid phases. A critical role of kallikrein. J Exp Med. 1973 Dec 1;138(6):1564–1583. doi: 10.1084/jem.138.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosne A. M., Legrand C., Bauvois B., Bodevin E., Caen J. P. Comparative degradation of adenylnucleotides by cultured endothelial cells and fibroblasts. Biochem Biophys Res Commun. 1978 Nov 14;85(1):183–189. doi: 10.1016/s0006-291x(78)80027-8. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Alexander R. W. Angiotensin II stimulation of prostaglandin production in cultured human vascular endothelium. Science. 1975 Jul 18;189(4198):219–220. doi: 10.1126/science.1138377. [DOI] [PubMed] [Google Scholar]
- Griffin J. H., Cochrane C. G. Human factor XII (Hageman factor). Methods Enzymol. 1976;45:56–65. doi: 10.1016/s0076-6879(76)45009-7. [DOI] [PubMed] [Google Scholar]
- Griffin J. H. Role of surface in surface-dependent activation of Hageman factor (blood coagulation factor XII). Proc Natl Acad Sci U S A. 1978 Apr;75(4):1998–2002. doi: 10.1073/pnas.75.4.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y. N., Kato H., Iwanaga S., Suzuki T. Primary structure of bovine plasma high-molecular-weight kininogen. The amino acid sequence of a glycopeptide portion (fragment 1) following the C-terminus ot the bradykinin moiety. J Biochem. 1976 Jun;79(6):1201–1222. doi: 10.1093/oxfordjournals.jbchem.a131175. [DOI] [PubMed] [Google Scholar]
- KELLERMEYER R. W., BRECKENRIDGE R. T. THE INFLAMMATORY PROCESS IN ACUTE GOUTY ARTHRITIS. I. ACTIVATION OF HAGEMAN FACTOR BY SODIUM URATE CRYSTALS. J Lab Clin Med. 1965 Feb;65:307–315. [PubMed] [Google Scholar]
- Kaplan A. P., Austen K. F. A prealbumin activator of prekallikrein. II. Derivation of activators of prekallikrein from active Hageman factor by digestion with plasmin. J Exp Med. 1971 Apr 1;133(4):696–712. doi: 10.1084/jem.133.4.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisiel W., Fujikawa K., Davie E. W. Activation of bovine factor VII (proconvertin) by factor XIIa (activated Hageman factor). Biochemistry. 1977 Sep 20;16(19):4189–4194. doi: 10.1021/bi00638a009. [DOI] [PubMed] [Google Scholar]
- Koide T., Kato H., Davie E. W. Isolation and characterization of bovine factor XI (plasma thromboplastin antecedent). Biochemistry. 1977 May 17;16(10):2279–2286. doi: 10.1021/bi00629a037. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laake K., Osterud B. Activation of purified plasma factor VII by human plasmin, plasma kallikrein, and activated components of the human intrinsic blood coagulation system. Thromb Res. 1974 Dec;5(6):759–772. doi: 10.1016/0049-3848(74)90119-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loskutoff D. J., Edgington T. E. Synthesis of a fibrinolytic activator and inhibitor by endothelial cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3903–3907. doi: 10.1073/pnas.74.9.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandle R. J., Colman R. W., Kaplan A. P. Identification of prekallikrein and high-molecular-weight kininogen as a complex in human plasma. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4179–4183. doi: 10.1073/pnas.73.11.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandle R., Jr, Kaplan A. P. Hageman factor substrates. Human plasma prekallikrein: mechanism of activation by Hageman factor and participation in hageman factor-dependent fibrinolysis. J Biol Chem. 1977 Sep 10;252(17):6097–6104. [PubMed] [Google Scholar]
- Mason R. G., Sharp D., Chuang H. Y., Mohammad S. F. The endothelium: roles in thrombosis and hemostasis. Arch Pathol Lab Med. 1977 Feb;101(2):61–64. [PubMed] [Google Scholar]
- Maynard J. R., Dreyer B. E., Stemerman M. B., Pitlick F. A. Tissue-factor coagulant activity of cultured human endothelial and smooth muscle cells and fibroblasts. Blood. 1977 Sep;50(3):387–396. [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Cochrane C. G. Direct evidence for Hageman factor (factor XII) activation by bacterial lipopolysaccharides (endotoxins). J Exp Med. 1974 Sep 1;140(3):797–811. doi: 10.1084/jem.140.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPAPORT S. I., AAS K., OWREN P. A. The effect of glass upon the activity of the various plasma clotting factors. J Clin Invest. 1955 Jan;34(1):9–19. doi: 10.1172/JCI103067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATNOFF O. D., DAVIE E. W., MALLETT D. L. Studies on the action of Hageman factor: evidence that activated Hageman factor in turn activates plasma thromboplastin antecedent. J Clin Invest. 1961 May;40:803–819. doi: 10.1172/JCI104314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revak S. D., Cochrane C. G., Bouma B. N., Griffin J. H. Surface and fluid phase activities of two forms of activated Hageman factor produced during contact activation of plasma. J Exp Med. 1978 Mar 1;147(3):719–729. doi: 10.1084/jem.147.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revak S. D., Cochrane C. G. The relationship of structure and function in human Hageman factor. The association of enzymatic and binding activities with separate regions of the molecule. J Clin Invest. 1976 Apr;57(4):852–860. doi: 10.1172/JCI108361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba S. R., Mason R. G. Studies of an activity from endothelial cells that inhibits platelet aggregation, serotonin release, and clot retraction. Thromb Res. 1974 Dec;5(6):747–757. doi: 10.1016/0049-3848(74)90118-2. [DOI] [PubMed] [Google Scholar]
- Soltay M. J., Movat H. Z., Ozge-Anwar A. H. The kinin system of human plasma. V. The probable derivation of prekallikrein activator from activated Hageman factor (XIIa). Proc Soc Exp Biol Med. 1971 Dec;138(3):952–958. doi: 10.3181/00379727-138-36026. [DOI] [PubMed] [Google Scholar]
- Thompson R. E., Mandle R., Jr, Kaplan A. P. Association of factor XI and high molecular weight kininogen in human plasma. J Clin Invest. 1977 Dec;60(6):1376–1380. doi: 10.1172/JCI108898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN B. A. FIBRIONLYTIC ACTIVITY OF VASCULAR ENDOTHELIUM. Br Med Bull. 1964 Sep;20:213–214. doi: 10.1093/oxfordjournals.bmb.a070334. [DOI] [PubMed] [Google Scholar]
- Weksler B. B., Marcus A. J., Jaffe E. A. Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3922–3926. doi: 10.1073/pnas.74.9.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins R. C., Bouma B. N., Cochrane C. G., Griffin J. H. Role of high-molecular-weight kininogen in surface-binding and activation of coagulation Factor XI and prekallikrein. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4636–4640. doi: 10.1073/pnas.74.10.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins R. C., Cochrane C. G., Griffin J. H. Rabbit blood coagulation factor XI. Mechanism of activation of rabbit Hageman factor (factor XII). Thromb Res. 1979;15(3-4):487–495. doi: 10.1016/0049-3848(79)90154-3. [DOI] [PubMed] [Google Scholar]
- Wiggins R. C., Cochrane C. G., Griffin J. H. Rabbit blood coagulation factor XI. Purification and properties. Thromb Res. 1979;15(3-4):475–486. doi: 10.1016/0049-3848(79)90153-1. [DOI] [PubMed] [Google Scholar]