Abstract
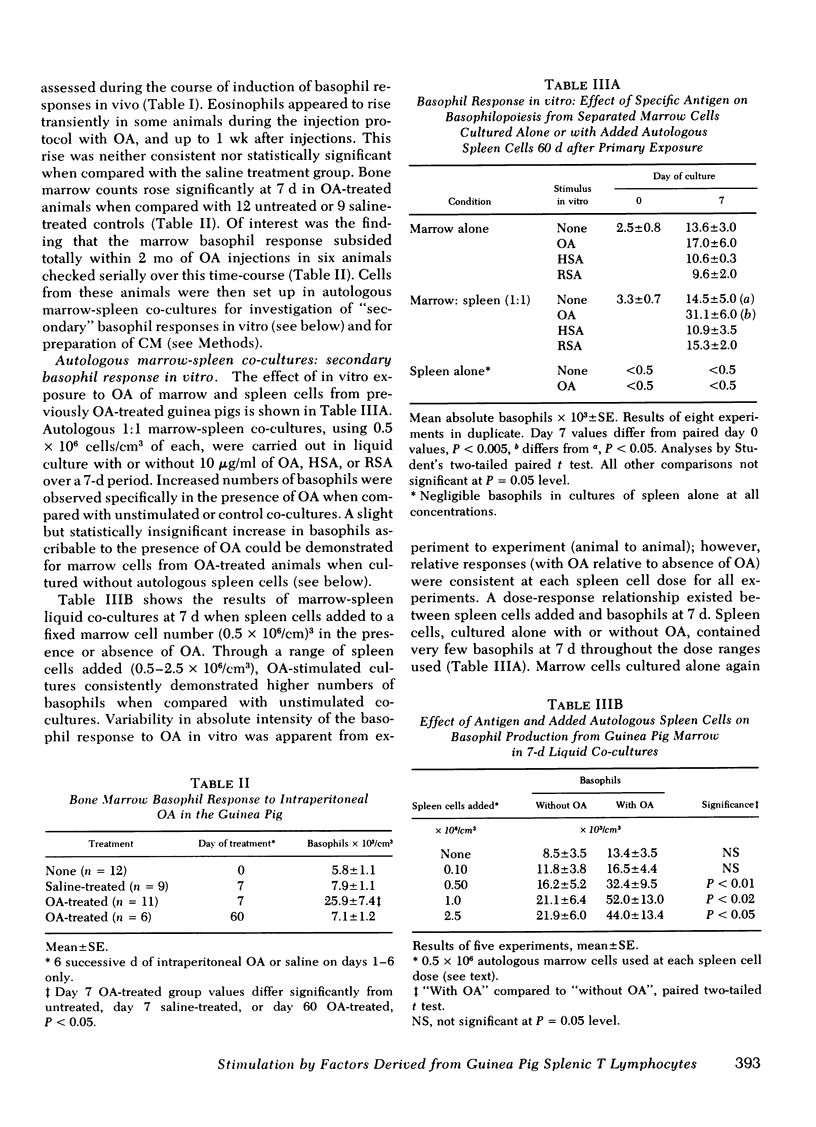
Factors influencing basophil production from the bone marrow of ovalbumin (OA)-sensitized guinea pigs have been examined in vitro. Autologous co-cultures of marrow and spleen cells from OA-immune animals contained significantly higher numbers of basophils after 7 d of liquid culture in the presence of OA, compared with control co-cultures or with marrow cultures alone (P < 0.005).
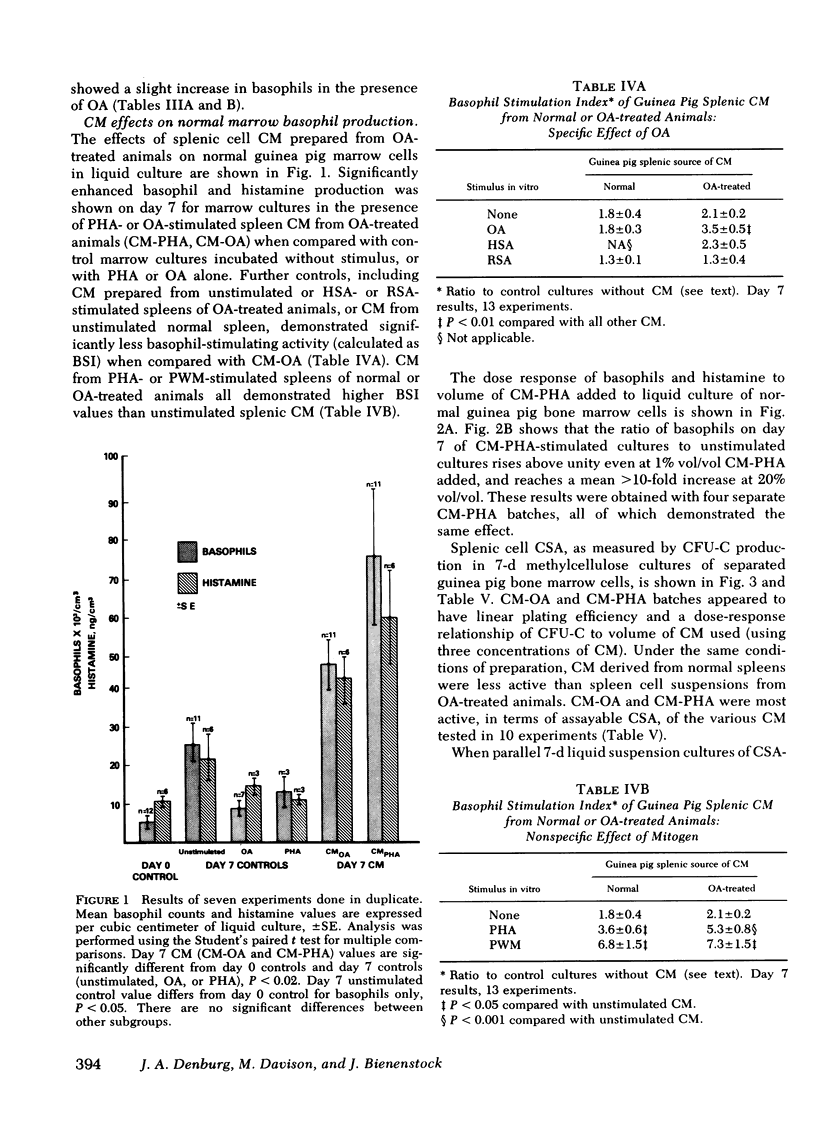
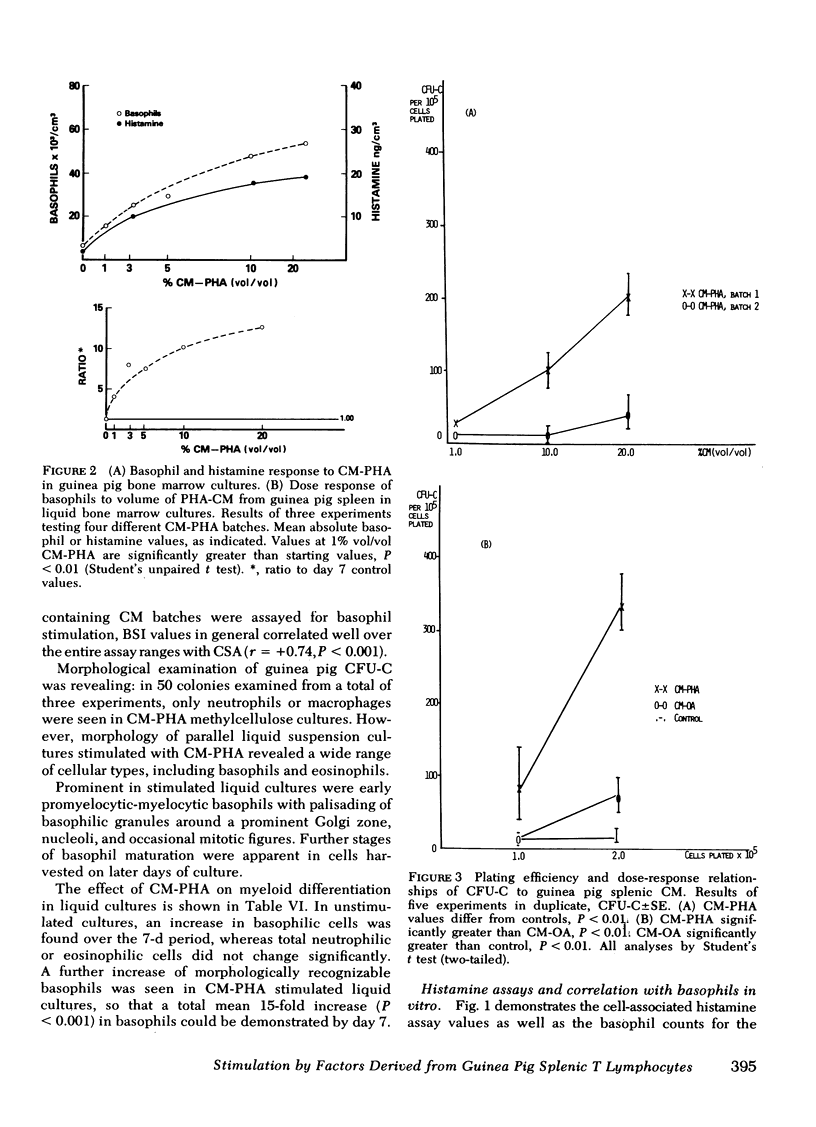
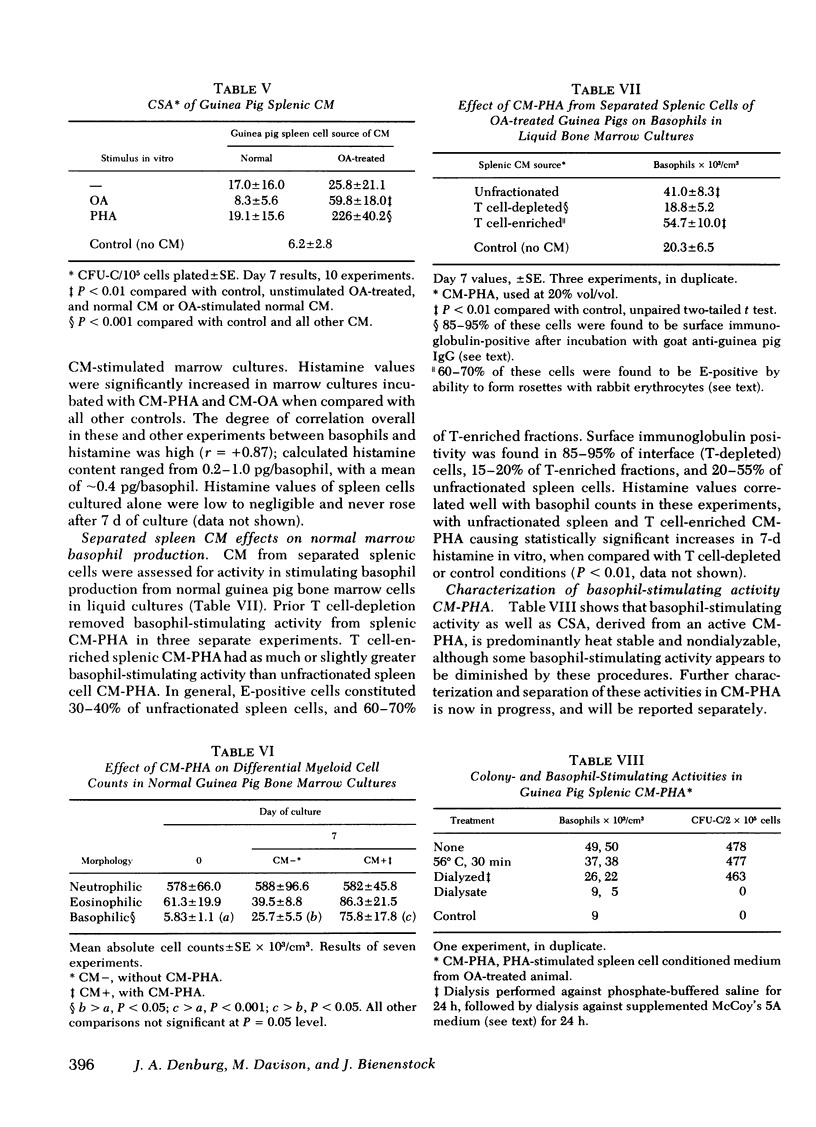
Basophils increased in co-culture as the number of spleen cells added to a fixed number of marrow cells was increased from 0.10 to 2.5 × 106/ml; at each spleen cell concentration, the presence of OA significantly enhanced basophil production in vitro when compared with unstimulated co-cultures. There was no basophil production from spleen cell suspensions cultured in the absence of autologous marrow cells. Conditioned media (CM) prepared from OA-stimulated spleen cells of OA-treated animals (CM-OA) caused a specific stimulation of basophil production from normal guinea pig bone marrow cells in liquid cultures (P < 0.01). Phytohemagglutinin (PHA)- and pokeweed mitogen-stimulated CM (CM-PHA, CM-pokeweed mitogen) nonspecifically enhanced normal basophilopoiesis, causing dose-dependent increases in basophils and histamine in vitro. CM-OA and CM-PHA also preferentially stimulated formation of neutrophil-macrophage colony-forming units in semisolid methylcellulose cultures.
CM-PHA prepared from T cell-enriched splenic cell suspensions contained basophil-stimulating activity, whereas T cell-depleted CM-PHA activity did not exceed control values (P < 0.01). Preliminary characterization of CM-PHA revealed that basophil-stimulating activity was predominantly heat stable and nondialyzable.
These results demonstrate OA-specific, as well as mitogen-dependent T-cell regulation of guinea pig basophilopoiesis in vitro. The data are compatible with the existence of a specific “basophilopoietin” in CM derived from guinea pig splenic T cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askenase P. W. Role of basophils, mast cells, and vasoamines in hypersensitivity reactions with a delayed time course. Prog Allergy. 1977;23:199–320. [PubMed] [Google Scholar]
- Basten A., Beeson P. B. Mechanism of eosinophilia. II. Role of the lymphocyte. J Exp Med. 1970 Jun 1;131(6):1288–1305. doi: 10.1084/jem.131.6.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaven M. A., Jacobsen S., Horáková Z. Modification of the enzymatic isotopic assay of histamine and its application to measurement of histamine in tissues, serum and urine. Clin Chim Acta. 1972 Mar;37:91–103. doi: 10.1016/0009-8981(72)90419-6. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Golde D. W. Production of colony-stimulating activity by human lymphocytes. Nature. 1974 Apr 19;248(5450):703–704. doi: 10.1038/248703a0. [DOI] [PubMed] [Google Scholar]
- Colley D. G. Eosinophils and immune mechanisms. IV. Culture conditions, antigen requirements, production kinetics and immunologic specificity of the lymphokine eosinophil stimulation promoter. Cell Immunol. 1976 Jun 15;24(2):328–335. doi: 10.1016/0008-8749(76)90216-1. [DOI] [PubMed] [Google Scholar]
- Dao C., Metcalf D., Bilski-Pasquier G. Eosinophil and neutrophil colony-forming cells in culture. Blood. 1977 Nov;50(5):833–839. [PubMed] [Google Scholar]
- Day R. P., Singal D. P., Bienenstock J. Presence of thymic antigen on rabbit basophils. J Immunol. 1975 Apr;114(4):1333–1336. [PubMed] [Google Scholar]
- Dresch C., Johnson G. R., Metcalf D. Eosinophil colony formation in semisolid cultures of human bone marrow cells. Blood. 1977 May;49(5):835–844. [PubMed] [Google Scholar]
- Dvorak H. F., Selvaggio S. S., Dvorak A. M., Colvin R. B., Lean D. B., Rypysc J. Purification of basophilic leukocytes from guinea pig blood and bone marrow. J Immunol. 1974 Dec;113(6):1694–1702. [PubMed] [Google Scholar]
- Eccleston E., Leonard B. J., Lowe J. S., Welford H. J. Basophilic leukaemia in the albino rat and a demonstration of the basopoietin. Nat New Biol. 1973 Jul 18;244(133):73–76. doi: 10.1038/newbio244073b0. [DOI] [PubMed] [Google Scholar]
- Galli S. J., Galli A. S., Dvorak A. M., Dvorak H. F. Metabolic studies of guinea pig basophilic leukocytes in short-term tissue culture. I. Measurement of histaminesynthesizing capacity by using an isotopic-thin layer chromatographic assay. J Immunol. 1976 Oct;117(4):1085–1092. [PubMed] [Google Scholar]
- Greene B. M., Colley D. G. Eosinophils and immune mechanisms. III. Production of the lymphokine eosinophil stimulation promoter by mouse T lymphocytes. J Immunol. 1976 Apr;116(4):1078–1083. [PubMed] [Google Scholar]
- Metcalf D., MacDonald H. R., Odartchenko N., Sordat B. Growth of mouse megakaryocyte colonies in vitro. Proc Natl Acad Sci U S A. 1975 May;72(5):1744–1748. doi: 10.1073/pnas.72.5.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. M., McGarry M. P. A diffusible stimulator of eosinophilopoiesis produced by lymphoid cells as demonstrated with diffusion chambers. Blood. 1976 Aug;48(2):293–300. [PubMed] [Google Scholar]
- Miyoshi I., Uchida H., Tsubota T., Kubonish I., Hiraki S., Kitajima K. Basophilic differentiation of chronic myelogenous leukaemia cells in vitro. Scand J Haematol. 1977 Oct;19(4):321–326. doi: 10.1111/j.1600-0609.1977.tb01481.x. [DOI] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Nathan D. G., Chess L., Hillman D. G., Clarke B., Breard J., Merler E., Housman D. E. Human erythroid burst-forming unit: T-cell requirement for proliferation in vitro. J Exp Med. 1978 Feb 1;147(2):324–339. doi: 10.1084/jem.147.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish W. E., Luckhurst E., Cowan S. I. Eosinophilia. V. Delayed hypersensitivity, blood and bone marrow eosinophilia, induced in normal guinea-pigs by adoptive transfer of lymphocytes from syngeneic donors. Clin Exp Immunol. 1977 Jul;29(1):75–83. [PMC free article] [PubMed] [Google Scholar]
- Pearce F. L., Behrendt H., Blum U., Poblete-Freundt G., Pult P., Stang-Voss C., Schmutzler W. Isolation and study of functional mast cells from lung and mesentery of the guinea pig. Agents Actions. 1977 Mar;7(1):45–56. doi: 10.1007/BF01964880. [DOI] [PubMed] [Google Scholar]
- Ruscetti F. W., Allalunis J., Chervenick P. A. Granulocyte colony stimulating activity from lymphocytes: separation from lymphokines by cytochalasin B. Cell Immunol. 1978 Mar 15;36(2):388–392. doi: 10.1016/0008-8749(78)90283-6. [DOI] [PubMed] [Google Scholar]
- Ruscetti F. W., Chervenick P. A. Release of colony-stimulating activity from thymus-derived lymphocytes. J Clin Invest. 1975 Mar;55(3):520–527. doi: 10.1172/JCI107958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti F. W., Cypess R. H., Chervenick P. A. Specific release of neutrophillic- and eosinophilic-stimulating factors from sensitized lymphocytes. Blood. 1976 May;47(5):757–765. [PubMed] [Google Scholar]
- Schulte-Wissermann H., Borzy M. S., Albrecht R., Hong R. Functional relationship of macrophages and basophils to the thymus gland. Scand J Immunol. 1979;9(1):45–52. doi: 10.1111/j.1365-3083.1979.tb02705.x. [DOI] [PubMed] [Google Scholar]
- Shah R. G., Caporale L. H., Moore M. A. Characterization of colony-stimulating activity produced by human monocytes and phytohemagglutinin-stimulated lymphocytes. Blood. 1977 Nov;50(5):811–821. [PubMed] [Google Scholar]
- Shortman K., Williams N., Adams P. The separation of different cell classes from lymphoid organs. V. Simple procedures for the removal of cell debris. Damaged cells and erythroid cells from lymphoid cell suspensions. J Immunol Methods. 1972 May;1(3):273–287. doi: 10.1016/0022-1759(72)90005-1. [DOI] [PubMed] [Google Scholar]
- Weller P. F., Dvorak J. A., Whitehouse W. C. Human eosinophil stimulation promoter lymphokine: production by antigen stimulated lymphocytes and assay with a new electro-optical technique. Cell Immunol. 1978 Sep 15;40(1):91–102. doi: 10.1016/0008-8749(78)90318-0. [DOI] [PubMed] [Google Scholar]
- Wilson A. B., Gurner B. W. Increased affinity of guinea pig thymocytes and thymus-dependent lymphocytes for papain-treated rabbit erythrocytes compared to untreated erythrocytes. J Immunol Methods. 1975 Jun;7(2-3):163–168. doi: 10.1016/0022-1759(75)90013-7. [DOI] [PubMed] [Google Scholar]


