Abstract
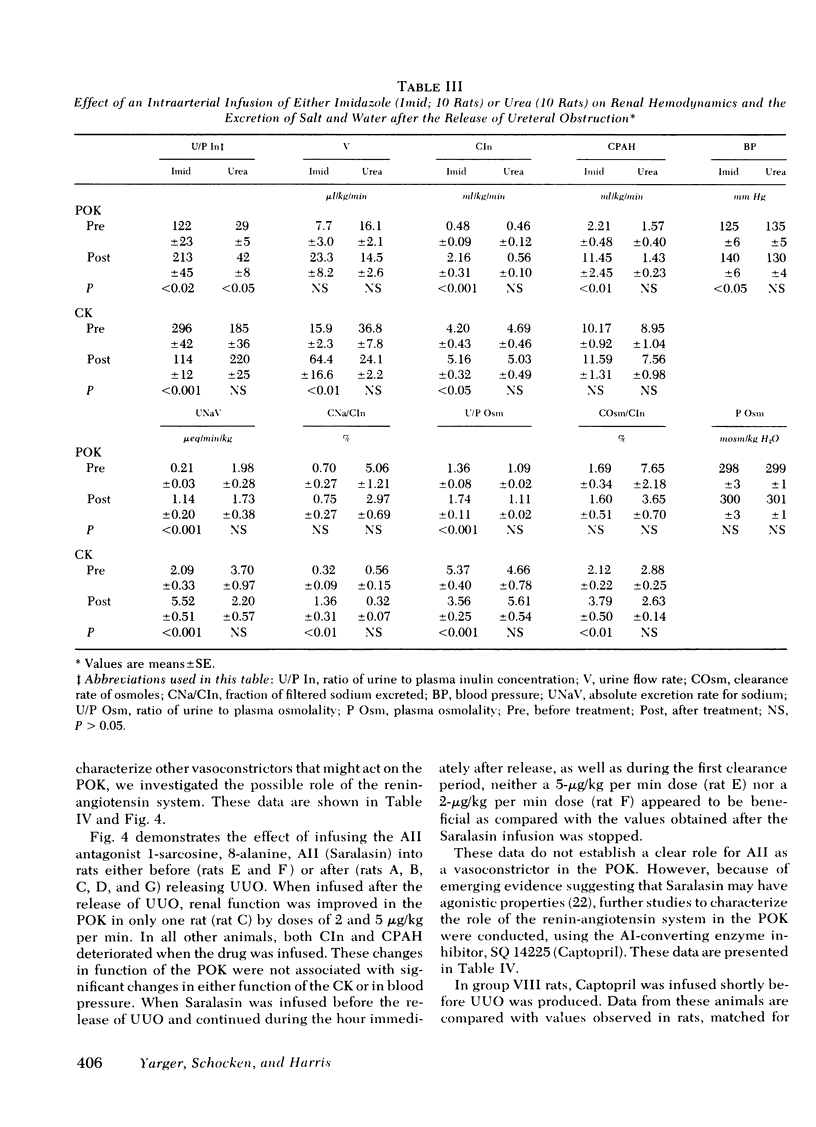
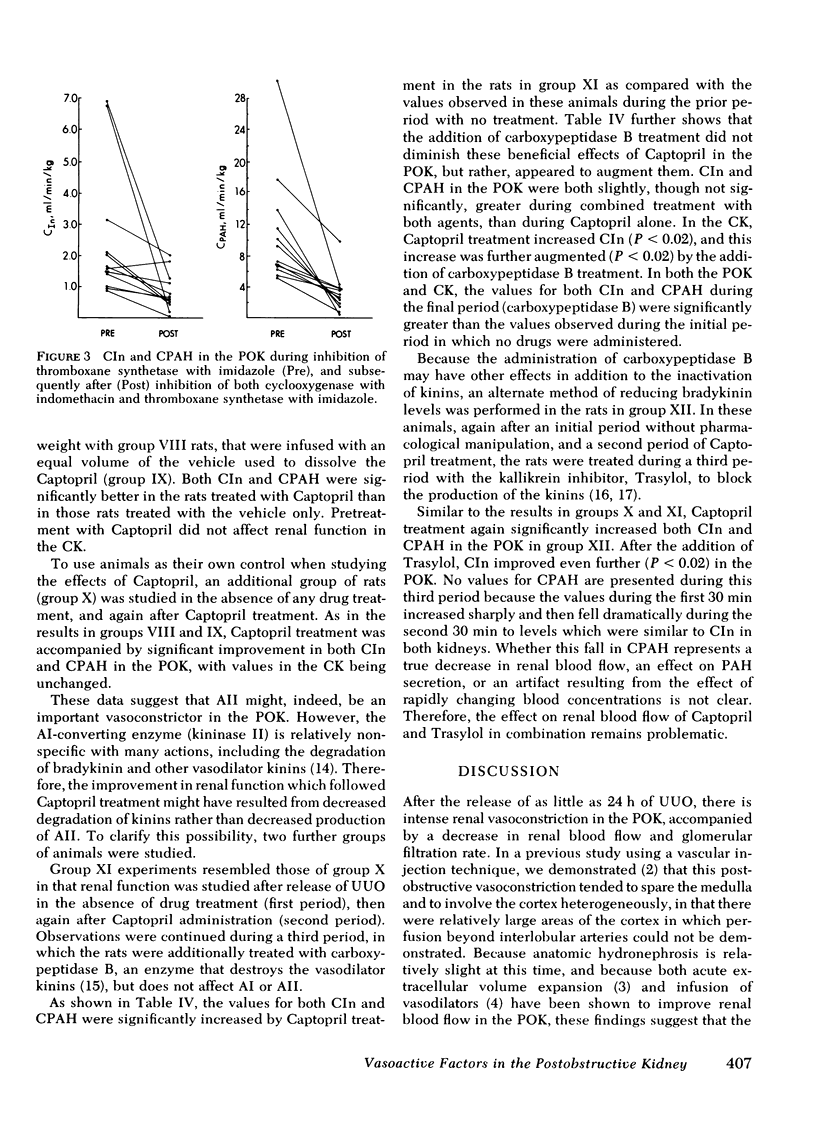
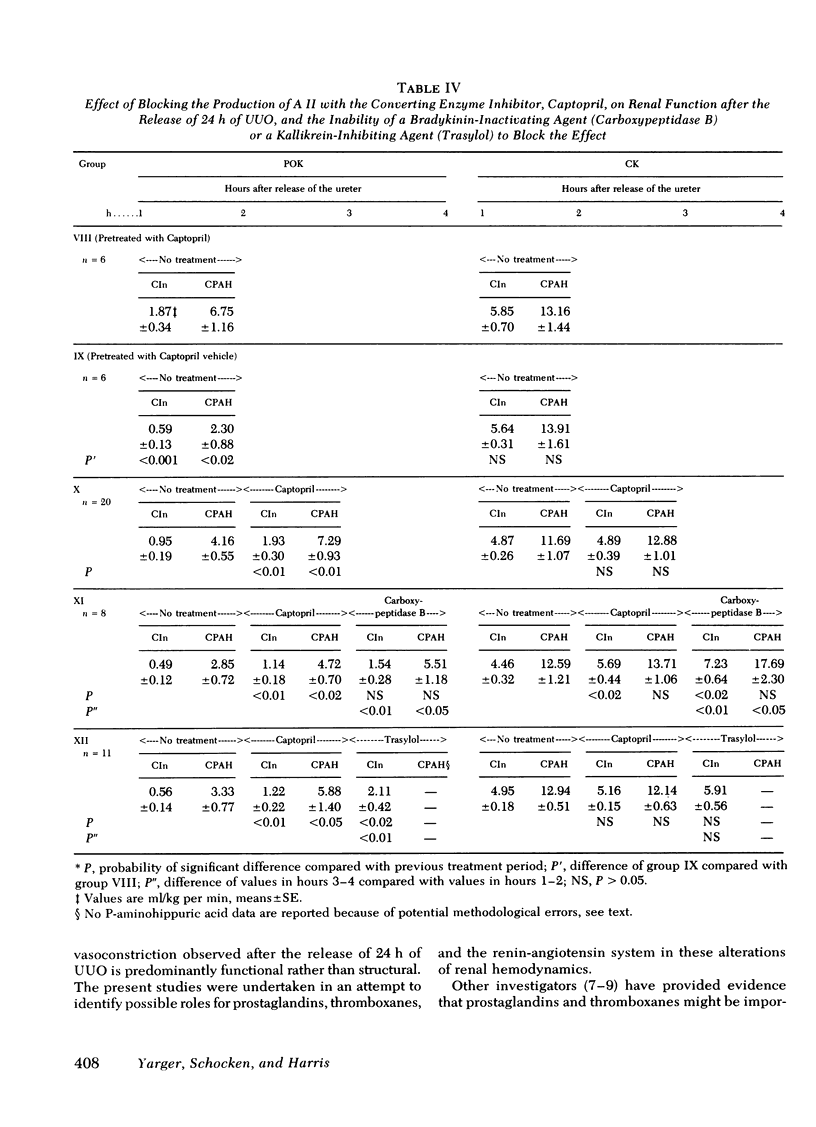
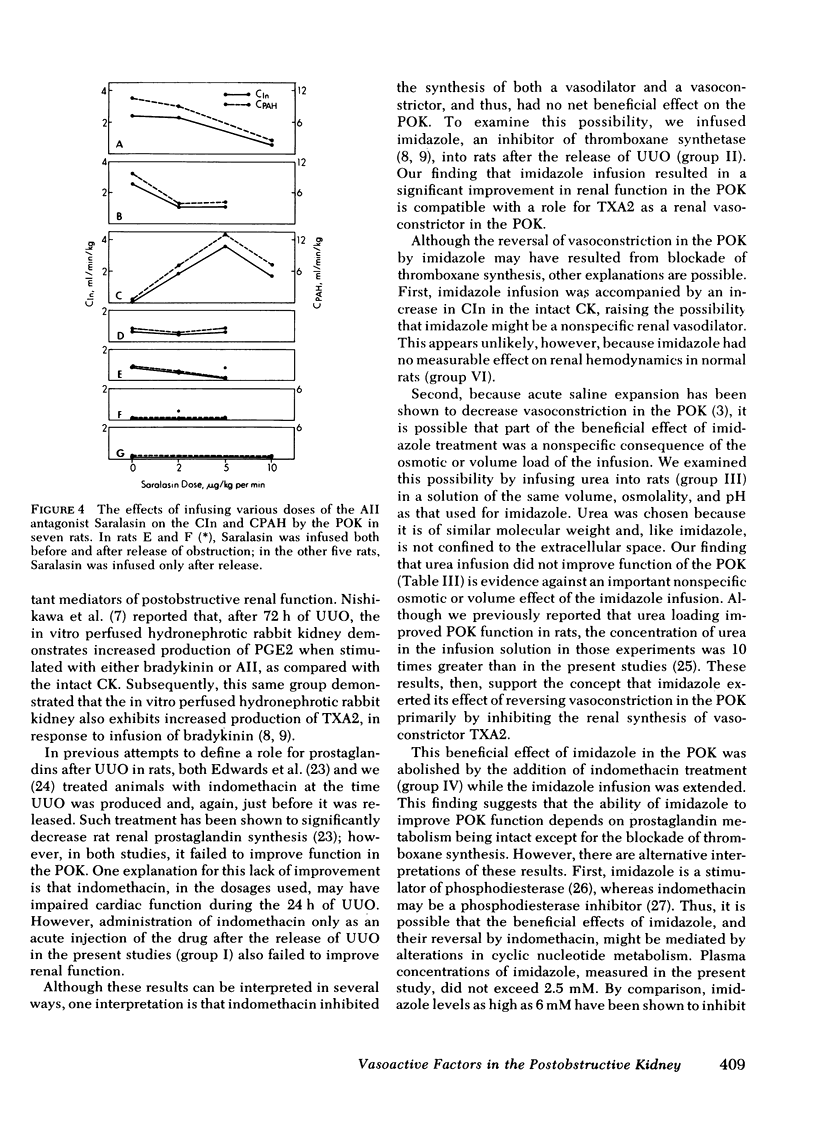
Relief of unilateral ureteral obstruction (UUO) of 24 h duration in rats is followed by severe renal vasoconstriction in the postobstructive kidney (POK). The present study examined possible roles of renal prostaglandins (PG) and thromboxanes (TX), as well as the renin-angiotensin system, in this vasoconstriction. Administration of the cyclooxygenase inhibitor indomethacin, which blocks both PG and TX production, failed to improve POK hemodynamics in UUO rats. To explore the possible role of the TX compounds, which include the potent vasoconstrictor thromboxane A2 (TXA2), UUO rats were infused with imidazole, an agent that blocks synthesis of TX, but not of PG. Imidiazole led to two- to threefold increases in the clearance of both inulin and rho-aminohippuric acid by the POK. This effect of imidazole was abolished by indomethacin, suggesting that the amelioration of POK vasoconstriction by imidazole was a result of inhibition of vasoconstrictor TX synthesis (e.g. TXA2), with PG vasodilators (e.g. PGE2 or PG12) still active. Urea, infused in a solution whose osmolality and volume were identical to the imidazole infusion, failed to improve hemodynamics in the POK, making it unlikely that nonspecific effects of volume expansion or osmotic diuresis mediated the beneficial effect of imidazole. Further studies examined the possible role of the renin-angiotensin systems in the vasoconstriction of the POK. UUO rats infused with the angiotensin II antagonist, Saralasin, exhibited no significant improvement in POK function, a finding that might be at least partly attributable to agonist/vasoconstrictor properties of Saralasin. In other experiments, treatment of UUO rats with the angiotensin-converting enzyme blocker SQ 14225 (Captopril), in order to inhibit angiotensin II formation, led to at least twofold increases in the clearance of both inulin and rho-aminohippuric acid in the POK. It is unlikely that Captopril exerted this beneficial effect by potentiating the vasodilator kinins, because the effect was not diminished by administration of either carboxypeptidase B (which destroys the kinins) or Trasylol (which blocks kinin synthesis). Thus, these results suggest that both angiotensin II, as well as metabolites of the PG-TX system, may be important determinants of postobstructive renal hemodynamics in the rat.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. M., Blackwell G. J., Flower R. J., McGiff J. C., Mullane K. M., Vane J. R. Genetic hypertension in rats is accompanied by a defect in renal prostaglandin catabolism. Nature. 1976 Apr 15;260(5552):582–586. doi: 10.1038/260582a0. [DOI] [PubMed] [Google Scholar]
- BROWN J. J., DAVIES D. L., LEVER A. F., PARKER R. A., ROBERTSON J. I. ASSAY OF RENIN IN SINGLE GLOMERULI RENIN DISTRIBUTION IN THE NORMAL RABBIT KIDNEY. Lancet. 1963 Sep 28;2(7309):668–668. doi: 10.1016/s0140-6736(63)90459-8. [DOI] [PubMed] [Google Scholar]
- Boucher R., Ménard J., Genest J. A micromethod for measurement of renin in the plasma and kidney of rats. Can J Physiol Pharmacol. 1967 Sep;45(5):881–890. doi: 10.1139/y67-103. [DOI] [PubMed] [Google Scholar]
- ERDOES E. G., WOHLER J. R., LEVINE M. I. BLOCKING OF THE IN VIVO EFFECTS OF BRADYKININ AND KALLIDIN WITH CARBOXYPEPTIDASE B. J Pharmacol Exp Ther. 1963 Dec;142:327–334. [PubMed] [Google Scholar]
- Flores A. G., Sharp G. W. Endogenous prostaglandins and osmotic water flow in the toad bladder. Am J Physiol. 1972 Dec;223(6):1392–1397. doi: 10.1152/ajplegacy.1972.223.6.1392. [DOI] [PubMed] [Google Scholar]
- Gerber J. G., Nies A. S. The hemodynamic effects of prostaglandins in the rat. Evidence for important species variation in renovascular responses. Circ Res. 1979 Mar;44(3):406–410. doi: 10.1161/01.res.44.3.406. [DOI] [PubMed] [Google Scholar]
- Harris R. H., Yarger W. E. Renal function after release of unilateral ureteral obstruction in rats. Am J Physiol. 1974 Oct;227(4):806–815. doi: 10.1152/ajplegacy.1974.227.4.806. [DOI] [PubMed] [Google Scholar]
- Harris R. H., Yarger W. E. The pathogenesis of post-obstructive diuresis. The role of circulating natriuretic and diuretic factors, including urea. J Clin Invest. 1975 Oct;56(4):880–887. doi: 10.1172/JCI108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa I., Brenner B. M. Local intrarenal vasoconstrictor-vasodilator interactions in mild partial ureteral obstruction. Am J Physiol. 1979 Feb;236(2):F131–F140. doi: 10.1152/ajprenal.1979.236.2.F131. [DOI] [PubMed] [Google Scholar]
- Maddox D. A., Price D. C., Rector F. C., Jr Effects of surgery on plasma volume and salt and water excretion in rats. Am J Physiol. 1977 Dec;233(6):F600–F606. doi: 10.1152/ajprenal.1977.233.6.F600. [DOI] [PubMed] [Google Scholar]
- Malik K. U., McGiff J. C. Modulation by prostaglandins of adrenergic transmission in the isolated perfused rabbit and rat kidney. Circ Res. 1975 May;36(5):599–609. doi: 10.1161/01.res.36.5.599. [DOI] [PubMed] [Google Scholar]
- Mimran A., Hinrichs K. J., Hollenberg N. K. Characterization of smooth muscle receptors for angiotensin: studies with an antagonist. Am J Physiol. 1974 Jan;226(1):185–190. doi: 10.1152/ajplegacy.1974.226.1.185. [DOI] [PubMed] [Google Scholar]
- Morrison A. R., Nishikawa K., Needleman P. Thromboxane A2 biosynthesis in the ureter obstructed isolated perfused kidney of the rabbit. J Pharmacol Exp Ther. 1978 Apr;205(1):1–8. [PubMed] [Google Scholar]
- Morrison A. R., Nishikawa K., Needleman P. Unmasking of thromboxane A2 synthesis by ureteral obstruction in the rabbit kidney. Nature. 1977 May 19;267(5608):259–260. doi: 10.1038/267259a0. [DOI] [PubMed] [Google Scholar]
- Needleman P., Raz A., Ferrendelli J. A., Minkes M. Application of imidazole as a selective inhibitor thromboxane synthetase in human platelets. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1716–1720. doi: 10.1073/pnas.74.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K., Morrison A., Needleman P. Exaggerated prostaglandin biosynthesis and its influence on renal resistance in the isolated hydronephrotic rabbit kidney. J Clin Invest. 1977 Jun;59(6):1143–1150. doi: 10.1172/JCI108738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolwerth A. C., Morrison A. R., Yates J., Klahr S. Effect of imidazole on renal gluconeogenesis. Biochim Biophys Acta. 1976 Oct 22;444(3):674–684. doi: 10.1016/0304-4165(76)90314-7. [DOI] [PubMed] [Google Scholar]
- Shenasky J. H., 2nd, Gillenwater J. Y., Graham S. D., Jr, Wooster L. D. Effects of vasoactive drugs on renal vascular resistance in obstructive disease. J Urol. 1971 Sep;106(3):355–359. doi: 10.1016/s0022-5347(17)61287-4. [DOI] [PubMed] [Google Scholar]
- Terragno N. A., Terragno D. A., McGiff J. C. Contribution of prostaglandins to the renal circulation in conscious, anesthetized, and laparotomized dogs. Circ Res. 1977 Jun;40(6):590–595. doi: 10.1161/01.res.40.6.590. [DOI] [PubMed] [Google Scholar]
- Vaughan E. D., Jr, Sweet R. C., Gillenwater J. Y. Peripheral renin and blood pressure changes following complete unilateral ureteral occlusion. J Urol. 1970 Jul;104(1):89–92. doi: 10.1016/s0022-5347(17)61675-6. [DOI] [PubMed] [Google Scholar]
- Wells H., Lloyd W. Hypocalcemic effect of imidazole in rats. Endocrinology. 1968 Sep;83(3):521–529. doi: 10.1210/endo-83-3-521. [DOI] [PubMed] [Google Scholar]
- Werle E., Trautschold I., Haendle H., Fritz H. Physiologic, pharmacologic, and clinicalspects of proteinase inhibitors. Ann N Y Acad Sci. 1968 Jun 28;146(2):464–478. doi: 10.1111/j.1749-6632.1968.tb20306.x. [DOI] [PubMed] [Google Scholar]
- Wilson D. R. The influence of volume expansion on renal function after relief of chronic unilateral ureteral obstruction. Kidney Int. 1974 Jun;5(6):402–410. doi: 10.1038/ki.1974.58. [DOI] [PubMed] [Google Scholar]
- Yang H. Y., Erdös E. G., Levin Y. A dipeptidyl carboxypeptidase that converts angiotensin I and inactivates bradykinin. Biochim Biophys Acta. 1970 Aug 21;214(2):374–376. doi: 10.1016/0005-2795(70)90017-6. [DOI] [PubMed] [Google Scholar]
- Yarger W. E., Griffith L. D. Intrarenal hemodynamics following chronic unilateral ureteral obstruction in the dog. Am J Physiol. 1974 Oct;227(4):816–826. doi: 10.1152/ajplegacy.1974.227.4.816. [DOI] [PubMed] [Google Scholar]


