Abstract
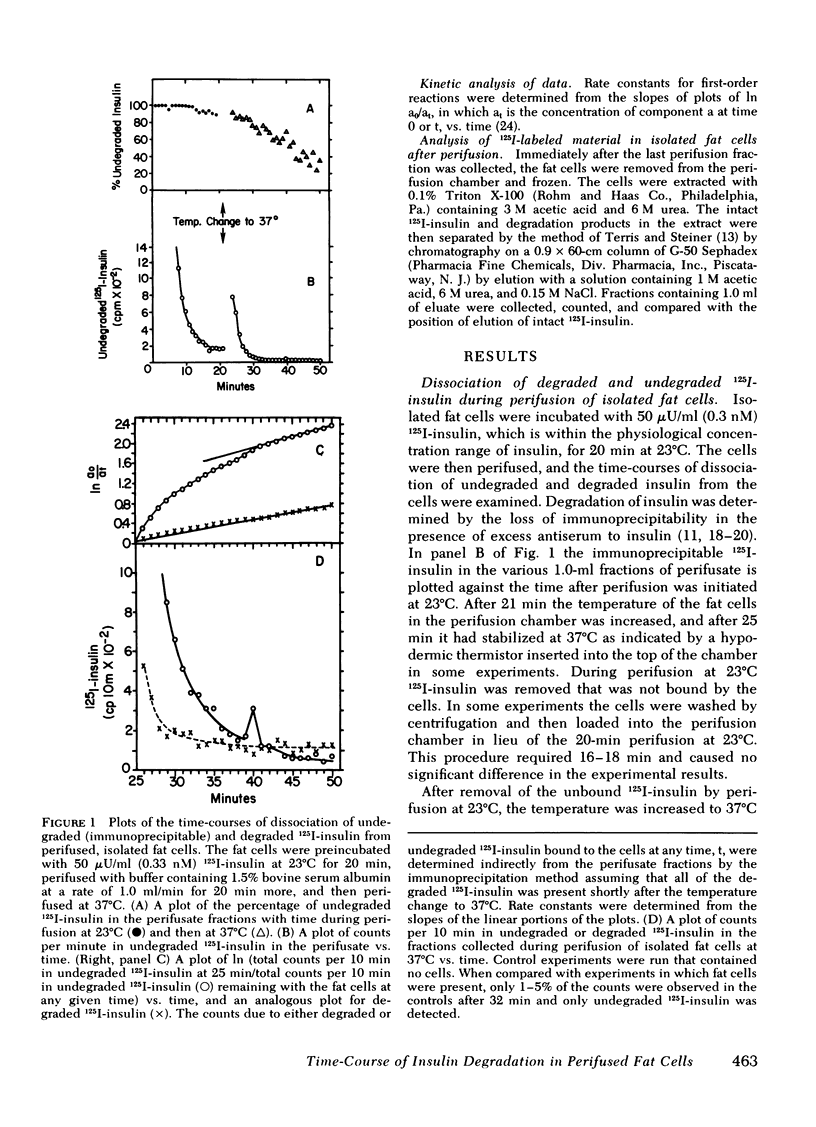
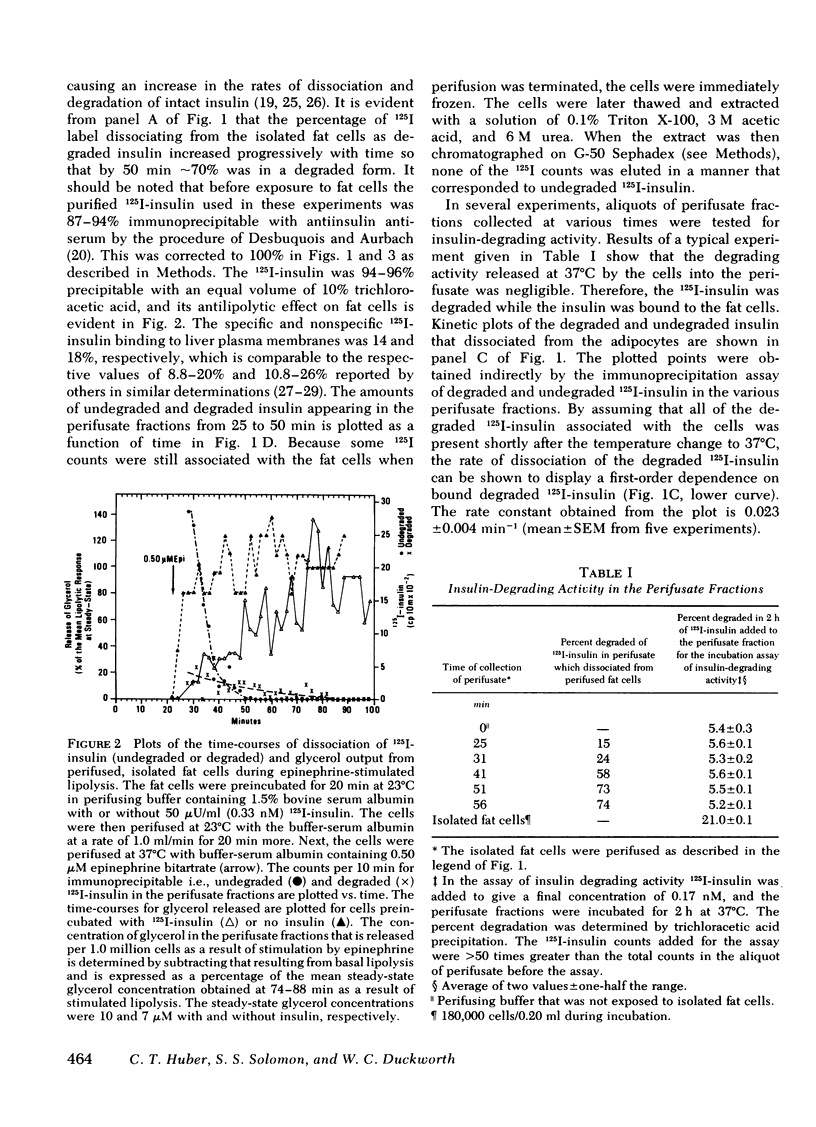
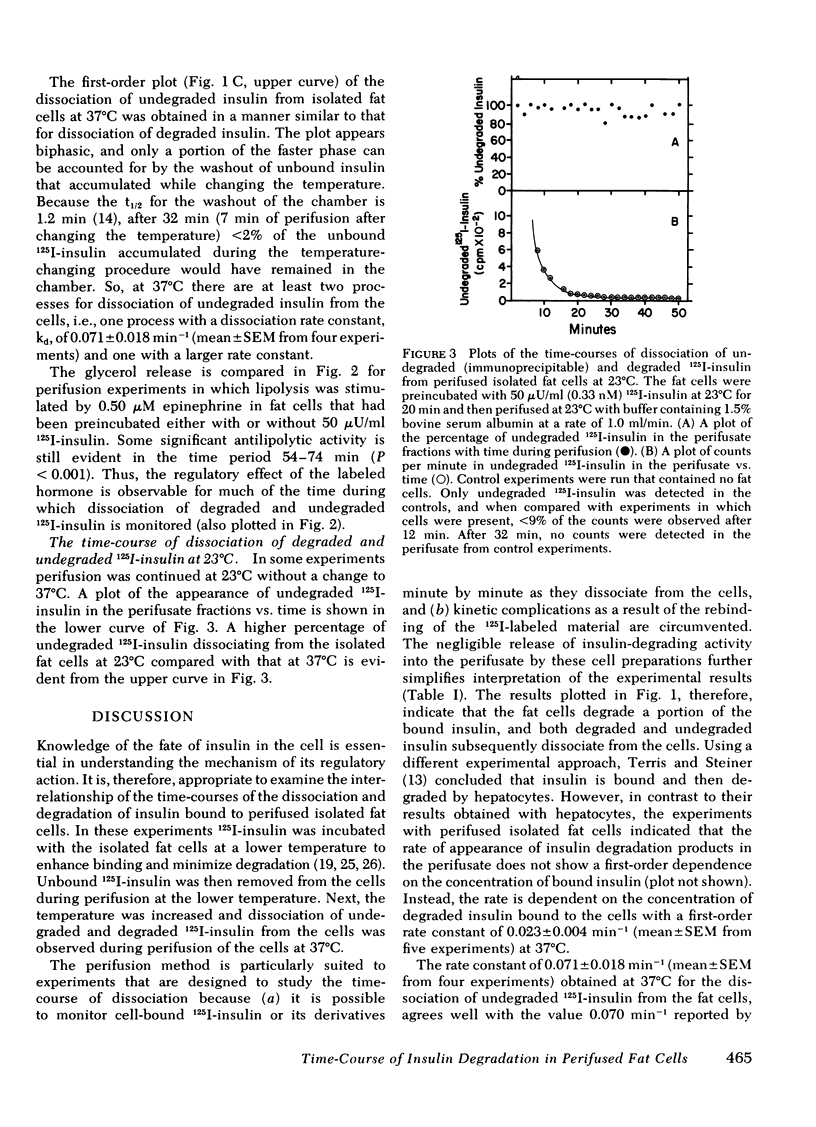
Isolated fat cells from rat epididymal adipose tissue were preincubated with 50 microU/ml (0.33 nM) 125I-insulin at 23 degrees C to enhance binding while retarding degradation. The fat cells were then perifused at that temperature to remove unbound 125I-insulin, and fractions of perifusate were collected each minute. The temperature of the cells in the perifusion chamber was then rapidly increased to 37 degrees C, and perifusion was continued. The fat cells degraded a portion of the bound 125I-insulin measured by loss of immunoprecipitability with excess antisera to insulin. The percentage of degraded 125I-insulin dissociating from the fat cells increased progressively with time at 37 degrees C, and the rateof dissociation of 125I-insulin degradation products showed a first-order dependence on the amount of degraded 125I-insulin bound to the cells. To explain this first-order dependence it is necessary to postulate a "processing" step after binding and before degradation. The first-order rate constant at 37 degrees C is 0.023 +/- 0.004 min-1. Fast and slow dissociating components can be resolved from kinetic plots of the dissociation of undegraded 125I-insulin (immunoprecipitable) from the isolated fat cells. The antilipolytic activity of the 125I-insulin on epinephrine-stimulated lipolysis is evident over much of the time-course of dissociation. A model for the degradation of insulin bound to isolated fat cells is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. O., Largis E. E., Miller E. A., Ashmore J. Continuous monitoring of lipolytic rates in perifused isolated fat cells. J Appl Physiol. 1973 Jan;34(1):125–127. doi: 10.1152/jappl.1973.34.1.125. [DOI] [PubMed] [Google Scholar]
- Bergeron J. J., Posner B. I., Josefsberg Z., Sikstrom R. Intracellular polypeptide hormone receptors. The demonstration of specific binding sites for insulin and human growth hormone in Golgi fractions isolated from the liver of female rats. J Biol Chem. 1978 Jun 10;253(11):4058–4066. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Amherdt M., Van Obberghen E., Kahn C. R., Orci L. 125I-insulin binding to cultured human lymphocytes. Initial localization and fate of hormone determined by quantitative electron microscopic autoradiography. J Clin Invest. 1978 Apr;61(4):1057–1070. doi: 10.1172/JCI109005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. J., Jacobs S., Cuatrecasas P. Quantitative aspects of hormone-receptor interactions of high affinity. Effect of receptor concentration and measurement of dissociation constants of labeled and unlabeled hormones. Biochim Biophys Acta. 1975 Oct 6;406(2):294–303. doi: 10.1016/0005-2736(75)90011-5. [DOI] [PubMed] [Google Scholar]
- Crofford O. B., Rogers N. L., Russell W. G. The effect of insulin on fat cells. An insulin degrading system extracted from plasma membranes of insulin responsive cells. Diabetes. 1972;21(2 Suppl):403–413. doi: 10.2337/diab.21.2.s403. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Hollenberg M. D. Binding of insulin and other hormones to non-receptor materials: saturability, specificity and apparent "negative cooperativity". Biochem Biophys Res Commun. 1975 Jan 6;62(1):31–41. doi: 10.1016/s0006-291x(75)80401-3. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Insulin--receptor interactions in adipose tissue cells: direct measurement and properties. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1264–1268. doi: 10.1073/pnas.68.6.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Properties of the insulin receptor of isolated fat cell membranes. J Biol Chem. 1971 Dec 10;246(23):7265–7274. [PubMed] [Google Scholar]
- Czech M. P. Molecular basis of insulin action. Annu Rev Biochem. 1977;46:359–384. doi: 10.1146/annurev.bi.46.070177.002043. [DOI] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Duckworth W. C. Insulin and glucagon binding and degradation by kidney cell membranes. Endocrinology. 1978 Jun;102(6):1766–1774. doi: 10.1210/endo-102-6-1766. [DOI] [PubMed] [Google Scholar]
- Duckworth W. C. Insulin and glucagon degradation by the kidney. I. Subcellular distribution under different assay condition. Biochim Biophys Acta. 1976 Jul 21;437(2):518–530. doi: 10.1016/0304-4165(76)90020-9. [DOI] [PubMed] [Google Scholar]
- Duckworth W. C., Stentz F. B., Heinemann M., Kitabchi A. E. Initial site of insulin cleavage by insulin protease. Proc Natl Acad Sci U S A. 1979 Feb;76(2):635–639. doi: 10.1073/pnas.76.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freychet P., Kahn R., Roth J., Neville D. M., Jr Insulin interactions with liver plasma membranes. Independence of binding of the hormone and its degradation. J Biol Chem. 1972 Jun 25;247(12):3953–3961. [PubMed] [Google Scholar]
- Freychet P., Kahn R., Roth J., Neville D. M., Jr Insulin interactions with liver plasma membranes. Independence of binding of the hormone and its degradation. J Biol Chem. 1972 Jun 25;247(12):3953–3961. [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- Gammeltoft S., Gliemann J. Binding and degradation of 125I-labelled insulin by isolated rat fat cells. Biochim Biophys Acta. 1973 Aug 17;320(1):16–32. doi: 10.1016/0304-4165(73)90161-x. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Gammeltoft S., Vinten J. Time course of insulin-receptor binding and insulin-induced lipogenesis in isolated rat fat cells. J Biol Chem. 1975 May 10;250(9):3368–3374. [PubMed] [Google Scholar]
- Goldfine I. D., Smith G. J. Binding of insulin to isolated nuclei. Proc Natl Acad Sci U S A. 1976 May;73(5):1427–1431. doi: 10.1073/pnas.73.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Atherosclerosis: the low-density lipoprotein receptor hypothesis. Metabolism. 1977 Nov;26(11):1257–1275. doi: 10.1016/0026-0495(77)90119-6. [DOI] [PubMed] [Google Scholar]
- Hammond J. M., Jarett L. Insulin degradation by isolated fat cells and their subcellular fractions. Diabetes. 1975 Nov;24(11):1011–1019. doi: 10.2337/diab.24.11.1011. [DOI] [PubMed] [Google Scholar]
- Huber C. T., Duckworth W. C., Solomon S. S. A continuous automated assay of lipolysis during perifusion of isolated fat cells. Anal Biochem. 1978 Mar;85(1):239–250. doi: 10.1016/0003-2697(78)90295-6. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Cuatrecasas P. The mobile receptor hypothesis and "cooperativity" of hormone binding. Application to insulin. Biochim Biophys Acta. 1976 May 21;433(3):482–495. doi: 10.1016/0005-2736(76)90275-3. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Baird K. The fate of insulin bound to adipocytes. Evidence for compartmentalization and processing. J Biol Chem. 1978 Jul 25;253(14):4900–4906. [PubMed] [Google Scholar]
- Kahn C. R., Freychet P., Roth J., Neville D. M., Jr Quantitative aspects of the insulin-receptor interaction in liver plasma membranes. J Biol Chem. 1974 Apr 10;249(7):2249–2257. [PubMed] [Google Scholar]
- Knight M., Klee W. A. The relationship between enkephalin degradation and opiate receptor occupancy. J Biol Chem. 1978 Jun 10;253(11):3843–3847. [PubMed] [Google Scholar]
- Kono T., Robinson F. W., Sarver J. A., Vega F. V., Pointer R. H. Actions of insulin in fat cells. Effects of low temperature, uncouplers of oxidative phosphorylation, and respiratory inhibitors. J Biol Chem. 1977 Apr 10;252(7):2226–2233. [PubMed] [Google Scholar]
- Neumann E., Bernhardt J. Physical chemistry of excitable biomembranes. Annu Rev Biochem. 1977;46:117–141. doi: 10.1146/annurev.bi.46.070177.001001. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Isolation of an organ specific protein antigen from cell-surface membrane of rat liver. Biochim Biophys Acta. 1968 Apr 9;154(3):540–552. doi: 10.1016/0005-2795(68)90014-7. [DOI] [PubMed] [Google Scholar]
- Olefsky J. M., Chang H. Insulin binding to adipocytes. Evidence for functionally distinct receptors. Diabetes. 1978 Sep;27(9):946–958. doi: 10.2337/diab.27.9.946. [DOI] [PubMed] [Google Scholar]
- Pollet R. J., Standaert M. L., Haase B. A. Insulin binding to the human lymphocyte receptor. Evaluation of the negative cooperativity model. J Biol Chem. 1977 Aug 25;252(16):5828–5834. [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodoyez J. C., Sodoyez-Goffaux F., Goff M. M., Zimmerman A. E., Arquilla E. R. [127-I]- or carrier-free [125-I]monoiodoinsulin. J Biol Chem. 1975 Jun 10;250(11):4268–4277. [PubMed] [Google Scholar]
- Solomon S. S., Duckworth W. C. Effect of antecedent hormone administration on lipolysis in the perifused isolated fat cell. J Lab Clin Med. 1976 Dec;88(6):984–994. [PubMed] [Google Scholar]
- Steiner D. F., Terris S., Chan S. J., Rubenstein A. H. Chemical and biological aspects of insulin and proinsulin. Acta Med Scand Suppl. 1976;601:55–107. [PubMed] [Google Scholar]
- Terris S., Steiner D. F. Retention and degradation of 125I-insulin by perfused livers from diabetic rats. J Clin Invest. 1976 Apr;57(4):885–896. doi: 10.1172/JCI108365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin B. P., Duckworth W. C., Solomon S. S. Perifusion of isolated rat adipose cells. Modulation of lipolysis by adenosine. J Clin Invest. 1977 Aug;60(2):442–448. doi: 10.1172/JCI108794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. T., Lefkowitz R. J. Slowly reversible binding of catecholamine to a nucleotide-sensitive state of the beta-adrenergic receptor. J Biol Chem. 1977 Oct 25;252(20):7207–7213. [PubMed] [Google Scholar]
- de Meyts P., Roth J., Neville D. M., Jr, Gavin J. R., 3rd, Lesniak M. A. Insulin interactions with its receptors: experimental evidence for negative cooperativity. Biochem Biophys Res Commun. 1973 Nov 1;55(1):154–161. doi: 10.1016/s0006-291x(73)80072-5. [DOI] [PubMed] [Google Scholar]


