Abstract
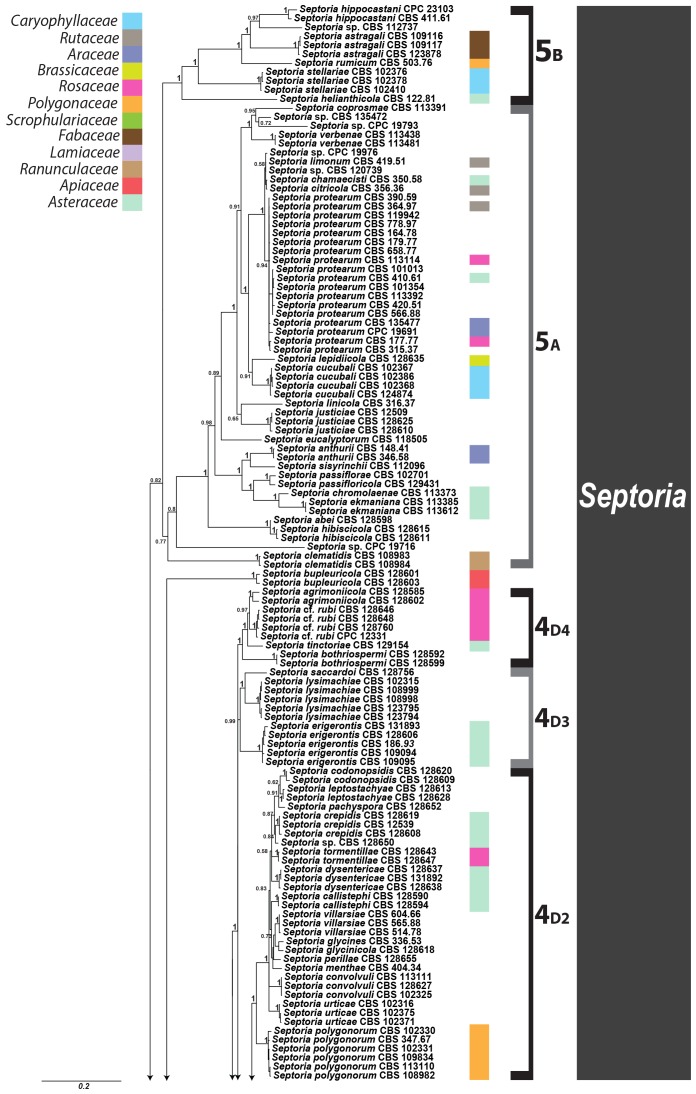
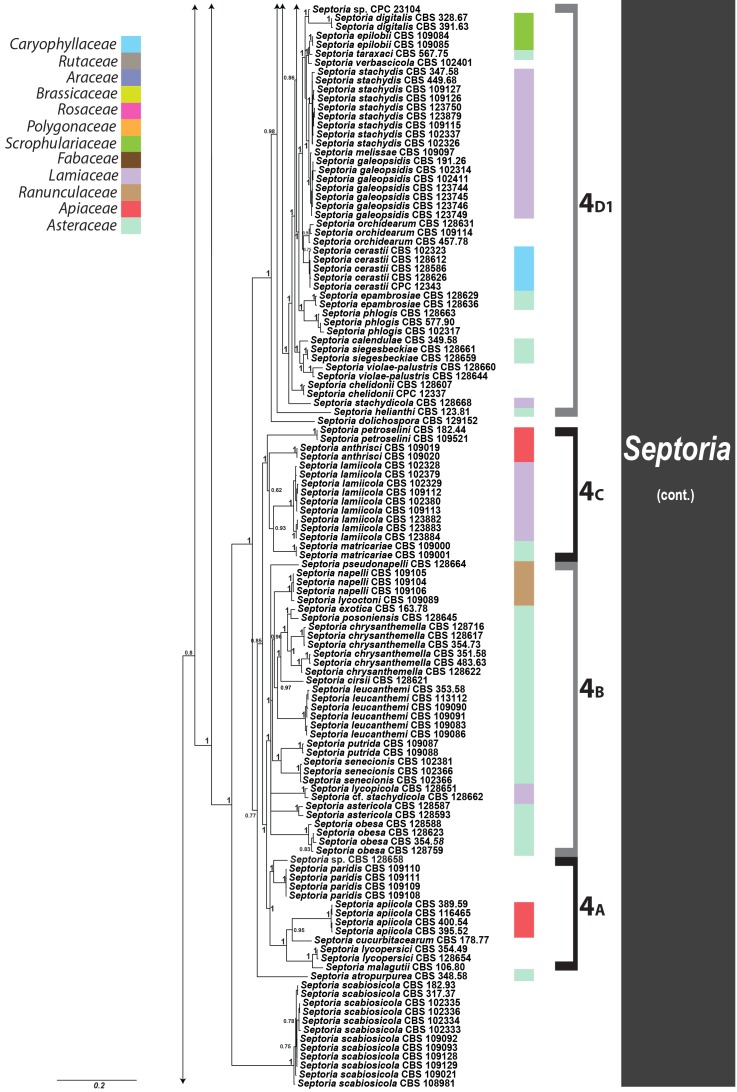
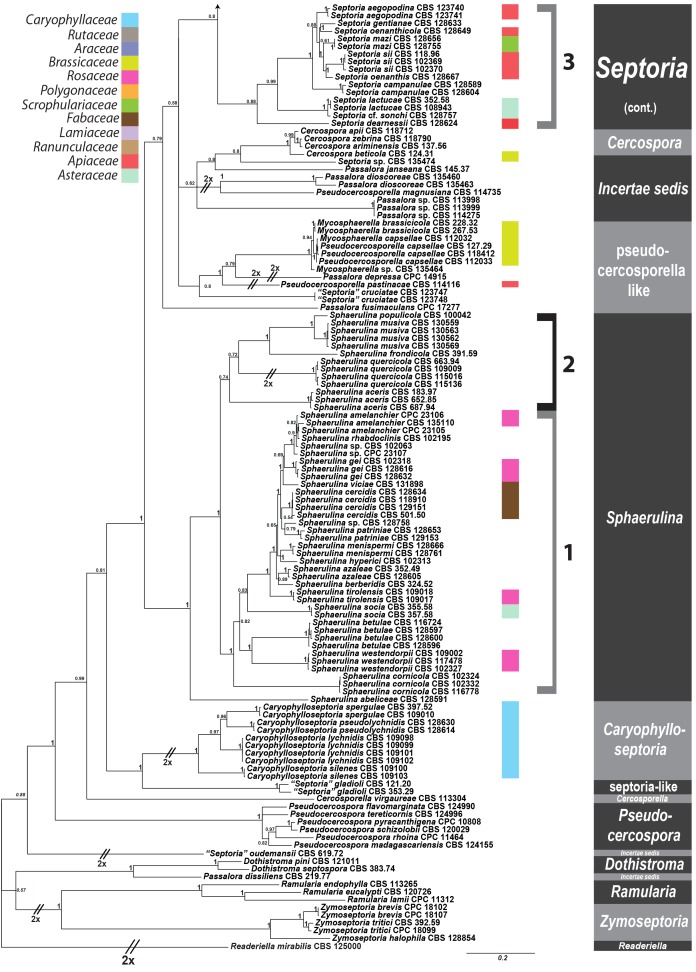
Septoria is a large genus of asexual morphs of Ascomycota causing leaf spot diseases of many cultivated and wild plants. Host specificity has long been a decisive criterium in species delimitation in Septoria, mainly because of the paucity of useful morphological characters and the high level of variation therein. This study aimed at improving the species delimitation of Septoria by adopting a polyphasic approach, including multilocus DNA sequencing and morphological analyses on the natural substrate and in culture. To this end 365 cultures preserved in CBS, Utrecht, The Netherlands, among which many new isolates obtained from fresh field specimens were sequenced. Herbarium material including many types was also studied. Full descriptions of the morphology in planta and in vitro are provided for 57 species. DNA sequences were generated for seven loci, viz. nuclear ITS and (partial) LSU ribosomal RNA genes, RPB2, actin, calmodulin, Btub, and EF. The robust phylogeny inferred showed that the septoria-like fungi are distributed over three main clades, establishing the genera Septoria s. str., Sphaerulina, and Caryophylloseptoria gen. nov. Nine new combinations and one species, Sphaerulina tirolensis sp. nov. were proposed. It is demonstrated that some species have wider host ranges than expected, including hosts from more than one family. Septoria protearum, previously only associated with Proteaceae was found to be also associated with host plants from six additional families of phanerogams and cryptogams. To our knowledge this is the first study to provide DNA-based evidence that multiple family-associations occur for a single species in Septoria. The distribution of host families over the phylogenetic tree showed a highly dispersed pattern for 10 host plant families, providing new insight into the evolution of these fungi. It is concluded that trans-family host jumping is a major force driving the evolution of Septoria and Sphaerulina.
Taxonomic novelties:
New genus - Caryophylloseptoria Verkley, Quaedvlieg & Crous; New species - Sphaerulina tirolensis Verkley, Quaedvlieg & Crous; New combinations - Caryophylloseptoria lychnidis (Desm.) Verkley, Quaedvlieg & Crous, Caryophylloseptoria silenes (Westend.) Verkley, Quaedvlieg & Crous, Caryophylloseptoria spergulae (Westend.) Verkley, Quaedvlieg & Crous, Sphaerulina aceris (Lib.) Verkley, Quaedvlieg & Crous, Sphaerulina cornicola (DC.: Fr.) Verkley, Quaedvlieg & Crous, Sphaerulina gei (Roberge ex Desm.) Verkley, Quaedvlieg & Crous, Sphaerulina hyperici (Roberge ex Desm.) Verkley, Quaedvlieg & Crous, Sphaerulina frondicola (Fr.) Verkley, Quaedvlieg & Crous, Sphaerulina socia (Pass.) Quaedvlieg, Verkley & Crous; Epitypifications (basionyms) - Ascochyta lysimachiae Lib., Septoria astragali Roberge ex Desm., Septoria cerastii Roberge ex Desm., Septoria clematidis Roberge ex Desm., Septoria cruciatae Roberge ex Desm., Septoria spergulae Westend., Septoria epilobii Westend., Septoria galeopsidis Westend., Septoria gei Roberge ex Desm., Septoria hyperici Roberge ex Desm., Septoria rubi Westend., Septoria senecionis Westend., Septoria urticae Roberge ex Desm.
Key words: Evolution, host jumping, host specificity, Multilocus Sequence Typing (MLST), Mycosphaerella, Mycosphaerellaceae, new genus, new species, Pleosporales, Phloeospora, Septoria, Sphaerulina, taxonomy, systematics
INTRODUCTION
Fungi classified in the genus Septoria Sacc. are asexual morphs of Ascomycota causing leaf spot diseases on many cultivated and wild plants. Some 3000 Septoria names have been described in literature (Verkley et al. 2004a, b). Sexual morphs are unknown for most taxa, but those reported were mostly classified in Mycosphaerella and Sphaerulina (Von Arx 1983, Sutton & Hennebert 1994, Crous et al. 2000, Verkley & Priest 2000, Crous et al. 2001, Aptroot 2006). Several overviews of the taxonomic work done on these fungi have been provided in the literature (Shin & Sameva 2004, Priest 2006, Quaedvlieg et al. 2013). Priest (2006) discussed the complex nomenclatural history of Septoria. The type species of Septoria, S. cytisi, is a fungus occurring on the woody legume Cytisus laburnum (= Laburnum anagyroides) and several other, mostly herbaceous Fabaceae (Farr 1992, Muthumary 1999). The phylogenetic position of this species for which no cultures are available has for long been uncertain. However, using well-identified herbarium material, Quaedvlieg et al. (2011) were able to extract DNA and successfully amplify and sequence nuclear ribosomal RNA genes to determine its position in a comprehensive phylogeny inferred for Mycosphaerellaceae.
Most taxonomists adopted a generic concept of Septoria that included fungi forming pycnidial conidiomata with holoblastic, hyaline, smooth-walled conidiogenous cells with sympodial and/or percurrent proliferation and hyaline, smooth, filiform to cylindrical multi-septate conidia (Sutton 1980, Constantinescu 1984, Sutton & Pascoe 1987, 1989, Farr 1991, 1992). Similar fungi forming acervular conidiomata were classified in Phloeospora, with Phloeospora ulmi as the type species, yet some researchers adopted a broader concept to include Phloeospora in Septoria (Jørstad 1965, Von Arx 1983, Andrianova 1987, Braun 1995). Recent DNA-sequencing studies have shown that the morphological characters that were used to delimit coelomycete genera in the past, in particular those pertaining to conidiomatal structure and conidiogenesis, did not correlate well with the sequence-inferred phylogenies (Crous et al. 2001, Verkley et al. 2004a, b). Quaedvlieg et al. (2013) present in their broad-scope study the results of an in-depth morphological and multi-gene sequence analyses of the septoria-like genera based on numerous isolates (including S. cytisi). In their study, they resolve the affinities and settle the nomenclature of all important septoria-like genera in the Dothideales and Pleosporales.
Host specificity has long been a decisive criterium in species delimitation in Septoria, mainly because of the paucity of useful morphological characters and the high level of variation therein. Traditionally, species of Septoria that were morphologically very similar but found on plants of different host families, were regarded as distinct taxa. Material from the same genus or from closely related host genera from the same plant family that could be distinguished by features such as conidial length and/or width and septation were usually also considered to belong to separate species. Most taxonomists revising Septoria lacked facilities to thoroughly investigate host ranges. A number of economically important Septoria species and species complexes have been subjected to infection experiments on various hosts, viz. the pathogens of Apium (Cochran 1932, Sheridan 1968) and cultivated Chrysanthemum (Waddell & Weber 1963, Punithalingam & Wheeler 1965). The results of these studies largely seemed to confirm the general belief that Septoria species have host ranges that are limited to a single genus of plants and in relatively few cases, also include a few closely related genera from the same plant family (Priest 2006). Molecular phylogenetic studies on Septoria species infecting Asteraceae (Verkley & Starink-Willemse 2004) and woody perennials (Feau et al. 2006) showed that species that are capable of infecting hosts of the same plant family do not (always) cluster in monophyletic groups, which is indicative of disjunct evolutionary patterns of these pathogens and their hosts. To explain these patterns, it has been postulated that “host jumping” occurs from typical (susceptible) hosts to “non-host” plants through asymptomatic tissue infection and subsequent exploration of new susceptible hosts. Examples of this were found in certain Mycosphaerella species and their Acacia hosts (Crous et al. 2004b, Crous & Groenewald 2005), but the mechanisms driving host jumping are not yet understood. With our study in which we investigate the phylogenetic relationships of species from a wider spectrum of host families we hope to provide more insight into the evolution of these fungal pathogens and their host plants and to contribute to understanding such mechanisms.
Early molecular phylogenetic studies have confirmed the relationships of septoria-like fungi with sexual morphs within Mycosphaerellaceae, and that the septoria-like fungi are of poly- and paraphyletic origins (Stewart et al. 1999, Crous et al. 2001, Goodwin et al. 2001, Verkley et al. 2004a, b, Verkley & Starink-Willemse, 2004). The ITS and/or LSU nrDNA sequence data used in those studies did not provide sufficient phylogenetic information to discriminate closely related species nor resolve most of the internal nodes in the trees. Verkley et al. (2004a, b) already concluded that groups within the then known “Mycosphaerella clade” showed no correlation to conidiomatal structure or conidiogenesis, confirming the conclusions drawn by Crous et al. (2001). Feau et al. (2006) sequenced the ITS, partial β-tubulin gene, and a proportion of the mitochondrial small subunit ribosomal gene (mtSSU) to infer a phylogeny for Septoria associated with diseases of woody perennials (many of which are here transferred to Sphaerulina). Although their inferred trees provided improved resolution, it was clear that even more DNA loci would be needed to fully resolve closely related species and species complexes within Septoria s. str.
The primary goal of our work was to improve the taxonomy of Septoria by adopting a polyphasic approach to taxon delimitation. To this end we studied cultures preserved in CBS, Utrecht, the Netherlands and material freshly collected in the field, did a full characterisation of the morphology in planta and in vitro, and sequenced seven DNA loci, viz. nuclear ITS and (partial) LSU ribosomal RNA genes, and RPB2, actin (Act), calmodulin (Cal), β-tubulin (Btub), and translation elongation factor 1-alpha (EF) genes. The obtained datasets of the seven loci were also evaluated for PCR amplification success rates and barcode gaps in order to determine which individual, or combination of loci, would be best suited for fast and reliable species resolution and identification.
Most students of Septoria have focused on material on the natural substrate and did not isolate and deposit cultures in public culture collections. Of all material we were able to successfully isolate, cultures were deposited in CBS-KNAW Fungal Biodiversity Centre (CBS) in Utrecht, The Netherlands. To assess the nomenclature this material was compared to type material as far as it could be obtained for study. Where useful new material and associated pure cultures were designated as epitypes, to facilitate future work. This study supplements the work of Quaedvlieg et al. (2013), who attain a broader perspective and address the complicated taxonomy and polyphyly of septoria-like fungi, proposing several new genera for taxa that are distantly related to Septoria cytisi and allied species.
MATERIAL AND METHODS
Collecting, isolating and morphological comparison
Infected plant material was collected in the field and taken to the laboratory. Leaves were examined directly under a stereomicroscope to observe sporulating structures, or when insufficiently developed, incubated in a Petri-dish with wetted filter paper for 1-2 d to enhance the development of fruiting bodies. Cirrhi of spores were removed and mounted in tapwater for the microscopic examination of conidia. Isolates were obtained by either transferring cirrhi directly onto 3 % malt extract agar (MEA, Oxoid) plates with 50 ppm penicillin and streptomycin, and streaked over the agar surface with an inoculation loop and some sterile water. Sometimes conidia in water from slide preparations were taken with a loop and streaked directly onto a plate. After 1-3 d at room temperature, germinated conidia were transferred on to fresh media without antibiotics. New isolates were deposited in the CBS. Cultures taken from the CBS Collection were activated from lyophilised or cryopreserved material and inoculated on oatmeal (OA) and MEA plates. A complete overview of the material used in this study is presented in Table 1.
Table 1.
Isolates used during this study.
| Species | Old name | Isolate no1 | Host | Location | Collector |
GenBank Accession no2 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EF | Tub | RPB2 | LSU | ITS | Act | Cal | ||||||
| Caryophylloseptoria lychnidis | Septoria lychnidis | CBS 109098 | Silene pratensis | Austria | G.J.M. Verkley | KF253234 | KF252768 | KF252292 | KF251790 | KF251286 | KF253595 | KF253949 |
| Septoria lychnidis | CBS 109099 | Silene pratensis | Austria | G.J.M. Verkley | KF253235 | KF252769 | KF252293 | KF251791 | KF251287 | KF253596 | KF253950 | |
| Septoria lychnidis | CBS 109101 | Silene pratensis | Austria | G.J.M. Verkley | KF253236 | KF252770 | KF252294 | KF251792 | KF251288 | KF253597 | KF253951 | |
| Septoria lychnidis | CBS 109102 | Silene pratensis | Austria | G.J.M. Verkley | KF253237 | KF252771 | KF252295 | KF251793 | KF251289 | KF253598 | KF253952 | |
| Car. pseudolychnidis | Septoria lychnidis | CBS 128614 | Lychnis cognata | South Korea | H.D. Shin | KF253238 | KF252772 | KF252296 | KF251794 | KF251290 | KF253599 | KF253953 |
| Septoria lychnidis | CBS 128630 | Lychnis cognata | South Korea | H.D. Shin | KF253239 | KF252773 | KF252297 | KF251795 | KF251291 | KF253600 | KF253954 | |
| Car. silenes | Septoria silenes | CBS 109100 | Silene nutans | Austria | G.J.M. Verkley | KF253240 | KF252774 | KF252298 | KF251796 | KF251292 | KF253601 | KF253955 |
| Septoria silenes | CBS 109103 | Silene pratensis | Austria | G.J.M. Verkley | KF253241 | KF252775 | KF252299 | KF251797 | KF251293 | KF253602 | KF253956 | |
| Car. spergulae | Septoria sp. | CBS 109010 | Spergula morisonii | Netherlands | A. Aptroot | KF253242 | KF252776 | KF252300 | KF251798 | KF251294 | KF253603 | KF253957 |
| Septoria dianthi | CBS 397.52 | Dianthus caryophyllus | Netherlands | Schouten | KF253243 | KF252777 | KF252301 | KF251799 | KF251295 | KF253604 | KF253958 | |
| Cercospora apii | – | CBS 118712 | – | Fiji | P. Tyler | KF253244 | KF252778 | KF252302 | KF251800 | KF251296 | KF253605 | KF253959 |
| Cer. ariminensis | – | CBS 137.56 | Hedysarum coronarium | Italy | M. Ribaldi | KF253245 | KF252779 | KF252303 | KF251801 | KF251297 | KF253606 | KF253960 |
| Cer. beticola | – | CBS 124.31 | – | Romania | E.W. Schmidt | KF253246 | KF252780 | KF252304 | KF251802 | KF251298 | KF253607 | KF253961 |
| Cercospora sp. | – | CBS 112737 | Rhus typhina | Canada | K.A. Seifert | KF253247 | KF252781 | – | KF251803 | KF251299 | KF253608 | KF253962 |
| Cer. zebrina | – | CBS 118790 | Trifolium subterraneum | Australia | M.J. Barbetti | KF253248 | KF252782 | KF252305 | KF251804 | KF251300 | KF253609 | KF253963 |
| Cercosporella virgaureae | – | CBS 113304 | Erigeron annuus | South Korea | H.D. Shin | KF253249 | – | KF252306 | KF251805 | KF251301 | KF253610 | KF253964 |
| Dothistroma pini | – | CBS 121011 | Pinus palassiana | Ukraine | A.C. Usichenko | KF253250 | – | KF252307 | KF251806 | KF251302 | KF253611 | KF253965 |
| Dot. septosporum | – | CBS 383.74 | Pinus coulteri | France | M. Morelet | KF253251 | – | KF252308 | KF251807 | KF251303 | KF253612 | KF253966 |
| Mycosphaerella brassicicola | – | CBS 228.32 | Brassica oleracea | Denmark | C.A. Jörgensen | KF253252 | KF252783 | KF252309 | KF251808 | KF251304 | KF253613 | KF253967 |
| – | CBS 267.53 | Brassica oleracea | Netherlands | F. Quak | KF253253 | KF252784 | KF252310 | KF251809 | KF251305 | KF253614 | KF253968 | |
| Myc. capsellae | – | CBS 112033 | Brassica sp. | UK | R. Evans | KF253254 | KF252785 | KF252311 | KF251810 | KF251306 | KF253615 | KF253969 |
| Mycosphaerella sp. | CBS 135464; CPC 11677 | Brassica sp. | UK | R. Evans | – | KF252786 | KF252312 | KF251811 | KF251307 | KF253616 | KF253970 | |
| Passalora depressa | – | CPC 14915 | Angelica gigas | South Korea | H.D. Shin | KF253256 | KF252788 | KF252314 | KF251813 | KF251309 | – | KF253972 |
| Pas. dioscoreae | – | CBS 135460;CPC 10855 | Dioscorea tokora | South Korea | H.D. Shin | KF253257 | KF252789 | KF252315 | KF251814 | KF251310 | KF253618 | – |
| – | CBS 135463; CPC 11513 | Dioscorea tenuipes | South Korea | H.D. Shin | KF253258 | KF252790 | KF252316 | KF251815 | KF251311 | KF253619 | – | |
| Pas. dissiliens | – | CBS 219.77 | Vitis vinifera | Iraq | M.S.A. Al-Momen | KF253259 | KF252791 | KF252317 | KF251816 | KF251312 | KF253620 | – |
| Pas. fusimaculans | – | CPC 17277 | Agrostis sp. | Thailand | Pheng Pheng | KF253260 | KF252792 | KF252318 | KF251817 | KF251313 | KF253621 | KF253973 |
| Pas. janseana | – | CBS 145.37 | – | – | E.C. Tullis | KF253261 | KF252793 | – | KF251818 | KF251314 | KF253622 | KF253974 |
| Passalora sp. | – | CBS 113998 | Cajanus cajan | South Africa | L. van Jaarsveld | KF253262 | KF252794 | KF252319 | KF251819 | KF251315 | KF253623 | – |
| Passalora sp. | – | CBS 113999 | Cajanus cajan | South Africa | L. van Jaarsveld | KF253263 | KF252795 | KF252320 | KF251820 | KF251316 | KF253624 | – |
| Passalora sp. | – | CBS 114275 | Cajanus cajan | South Africa | L. van Jaarsveld | KF253264 | KF252796 | KF252321 | KF251821 | KF251317 | – | – |
| Pseudocercospora madagascariensis | – | CBS 124155 | Eucalyptus camaldulensis | Madagascar | M.J. Wingfield | KF253265 | – | KF252322 | KF251822 | KF251318 | KF253625 | – |
| Pse. pyracanthae | – | CPC 10808 | Pyracantha angustifolia | South Korea | H.D. Shin | KF253266 | – | KF252323 | KF251823 | KF251319 | KF253626 | – |
| Pse. pyracanthigena | – | CBS 112032 | Pyracantha angustifolia | South Korea | M.J. Park | KF253267 | KF252797 | KF252324 | KF251824 | KF251320 | KF253627 | KF253975 |
| Pse. rhoina | – | CPC 11464 | Rhus chinensis | South Korea | H.D. Shin | KF253268 | – | KF252325 | KF251825 | KF251321 | – | – |
| Pse. schizolobii | – | CBS 120029 | Schizolobium parahybum | Ecuador | M.J. Wingfield | KF253269 | KF252798 | KF252326 | KF251826 | KF251322 | KF253628 | – |
| – | CBS 124990 | Eucalyptus camaldulensis | Thailand | W. Himaman | KF253270 | – | KF252327 | KF251827 | KF251323 | KF253629 | – | |
| Pse. tereticornis | – | CBS 124996 | Eucalyptus nitens | Australia | A.J. Cargenie | KF253271 | KF252799 | KF252328 | KF251828 | KF251324 | KF253630 | KF253976 |
| Pseudocercosporella capsellae | – | CBS 118412 | Brassica sp. | New Zealand | C.F. Hill | KF253272 | KF252800 | KF252329 | KF251829 | KF251325 | KF253631 | KF253977 |
| – | CBS 127.29 | – | – | K. Togashi | KF253273 | KF252801 | KF252330 | KF251830 | KF251326 | KF253632 | KF253978 | |
| Pella. magnusiana | – | CBS 114735 | Geranium silvaticum | Sweden | E. Gunnerbeck | KF253274 | KF252802 | – | KF251831 | KF251327 | – | KF253979 |
| Pella. pastinacae | – | CBS 114116 | Laserpitium latifolium | Sweden | K. & L. Holm | KF253275 | KF252803 | KF252331 | KF251832 | KF251328 | KF253633 | KF253980 |
| Ramularia endophylla | – | CBS 113265 | Quercus robur | Netherlands | G.J.M. Verkley | KF253276 | – | KF252332 | KF251833 | KF251329 | KF253634 | KF253981 |
| Ram. eucalypti | – | CBS 120726 | Eucalyptus grandiflora | Italy | W. Gams | KF253277 | – | KF252333 | KF251834 | KF251330 | KF253635 | KF253982 |
| Ram. lamii | – | CPC 11312 | Leonurus sibiricus | South Korea | H.D. Shin | KF253278 | – | KF252334 | KF251835 | KF251331 | KF253636 | KF253983 |
| Readeriella mirabilis | – | CBS 125000 | Eucalyptus globulus | Australia | I.W. Smith | KF253279 | KF252804 | KF252335 | KF251836 | KF251332 | KF253637 | KF253984 |
| Septoria abei | – | CBS 128598 | Hibiscus syriacus | South Korea | H.D. Shin | KF253280 | KF252805 | KF252336 | KF251837 | KF251333 | KF253638 | KF253985 |
| Sep. aegopodina | – | CBS 123740 | Aegopodium podagraria | Czech Republic | G.J.M. Verkley | KF253281 | KF252806 | – | KF251838 | KF251334 | KF253639 | KF253986 |
| – | CBS 123741 | Aegopodium podagraria | Czech Republic | G.J.M. Verkley | KF253282 | KF252807 | – | KF251839 | KF251335 | KF253640 | KF253987 | |
| Sep. agrimoniicola | – | CBS 128585 | Agrimonia pilosa | South Korea | H.D. Shin | KF253283 | KF252808 | KF252337 | KF251840 | KF251336 | KF253641 | KF253988 |
| – | CBS 128602 | Agrimonia pilosa | South Korea | H.D. Shin | KF253284 | KF252809 | KF252338 | KF251841 | KF251337 | – | KF253989 | |
| Sep. anthrisci | – | CBS 109019 | Anthriscus sp. | Austria | G.J.M. Verkley | KF253285 | KF252810 | KF252339 | KF251842 | KF251338 | KF253642 | KF253990 |
| – | CBS 109020 | Anthriscus sp. | Austria | G.J.M. Verkley | KF253286 | KF252811 | KF252340 | KF251843 | KF251339 | KF253643 | KF253991 | |
| Sep. anthurii | – | CBS 148.41 | Anthurium sp. | – | P. Kotthoff | KF253287 | KF252812 | KF252341 | KF251844 | KF251340 | KF253644 | KF253992 |
| – | CBS 346.58 | Anthurium sp. | Germany | R. Schneider | KF253288 | KF252813 | KF252342 | KF251845 | KF251341 | KF253645 | KF253993 | |
| Sep. apiicola | – | CBS 116465 | Apium graveolens | Netherlands | R. Munning | KF253289 | KF252814 | KF252343 | KF251846 | KF251342 | KF253646 | KF253994 |
| – | CBS 389.59 | Apium graveolens | Italy | M. Ribaldi | KF253290 | KF252815 | KF252344 | KF251847 | KF251343 | KF253647 | KF253995 | |
| – | CBS 395.52 | Apium sp. | Netherlands | G. van den Ende | KF253291 | KF252816 | KF252345 | KF251848 | KF251344 | KF253648 | KF253996 | |
| – | CBS 400.54 | Apium graveolens | Netherlands | J.A. von Arx | KF253292 | KF252817 | KF252346 | KF251849 | KF251345 | KF253649 | KF253997 | |
| Sep. astericola | – | CBS 128587 | Aster tataricus | South Korea | H.D. Shin | KF253293 | KF252818 | KF252347 | KF251850 | KF251346 | KF253650 | KF253998 |
| – | CBS 128593 | Aster yomena | South Korea | H.D. Shin | KF253294 | KF252819 | KF252348 | KF251851 | KF251347 | KF253651 | KF253999 | |
| Sep. astragali | – | CBS 109117 | Astragalus glycyphyllos | Austria | G.J.M. Verkley | KF253296 | KF252821 | KF252350 | KF251853 | KF251349 | KF253653 | KF254001 |
| – | CBS 123878 | Astragalus glycyphyllos | Czech Republic | G.J.M. Verkley | KF253297 | KF252822 | KF252351 | KF251854 | KF251350 | KF253654 | KF254002 | |
| – | CBS 109116 | Astragalus glycyphyllos | Austria | G.J.M. Verkley | KF253298 | KF252823 | KF252352 | KF251855 | KF251351 | KF253655 | KF254003 | |
| Sep. atropurpurea | – | CBS 348.58 | Aster canus | Germany | R. Schneider | KF253299 | KF252824 | KF252353 | KF251856 | KF251352 | KF253656 | KF254004 |
| Sep. bothriospermi | – | CBS 128592 | Bothriospermum tenellum | South Korea | H.D. Shin | KF253300 | KF252825 | KF252354 | KF251857 | KF251353 | KF253657 | KF254005 |
| – | CBS 128599 | Bothriospermum tenellum | South Korea | H.D. Shin | KF253301 | KF252826 | KF252355 | KF251858 | KF251354 | KF253658 | KF254006 | |
| Sep. bupleuricola | – | CBS 128601 | Bupleurum longiradiatum | South Korea | H.D. Shin | KF253302 | KF252827 | KF252356 | KF251859 | KF251355 | KF253659 | KF254007 |
| – | CBS 128603 | Bupleurum falcatum | South Korea | H.D. Shin | KF253303 | KF252828 | KF252357 | KF251860 | KF251356 | KF253660 | KF254008 | |
| Sep. calendulae | – | CBS 349.58 | Calendula arvensis | Italy | R. Schneider | KF253304 | KF252829 | KF252358 | KF251861 | KF251357 | KF253661 | KF254009 |
| Sep. callistephi | – | CBS 128590 | Callistephus chinensis | South Korea | H.D. Shin | KF253305 | KF252830 | KF252359 | KF251862 | KF251358 | KF253662 | KF254010 |
| – | CBS 128594 | Callistephus chinensis | South Korea | H.D. Shin | KF253306 | KF252831 | KF252360 | KF251863 | KF251359 | KF253663 | KF254011 | |
| Sep. campanulae | – | CBS 128589 | Campanula takesimana | South Korea | H.D. Shin | KF253307 | KF252832 | KF252361 | KF251864 | KF251360 | KF253664 | KF254012 |
| – | CBS 128604 | Campanula takesimana | South Korea | H.D. Shin | KF253308 | KF252833 | KF252362 | KF251865 | KF251361 | KF253665 | KF254013 | |
| Sep. cerastii | – | CBS 102323 | Cerastium fontanum | Netherlands | G.J.M. Verkley | KF253309 | KF252834 | KF252363 | KF251866 | KF251362 | KF253666 | KF254014 |
| – | CBS 128586 | Cerastium holosteoides | South Korea | H.D. Shin | KF253310 | KF252835 | KF252364 | KF251867 | KF251363 | KF253667 | KF254015 | |
| – | CBS 128612 | Cerastium holosteoides | South Korea | H.D. Shin | KF253311 | KF252836 | KF252365 | KF251868 | KF251364 | KF253668 | KF254016 | |
| – | CBS 128626 | Cerastium holosteoides | South Korea | H.D. Shin | KF253312 | KF252837 | KF252366 | KF251869 | KF251365 | KF253669 | KF254017 | |
| – | CPC 12343 | Cerastium holosteoides | South Korea | H.D. Shin | KF253313 | KF252838 | KF252367 | KF251870 | KF251366 | KF253670 | KF254018 | |
| Sep. cf. rubi | Septoria sp. | CPC 12331 | Rubus crataegifolius | South Korea | H.D. Shin | KF253317 | KF252842 | KF252371 | KF251874 | KF251370 | KF253674 | KF254022 |
| Septoria rubi | CBS 128646 | Rubus crataegifolius | South Korea | H.D. Shin | KF253314 | KF252839 | KF252368 | KF251871 | KF251367 | KF253671 | KF254019 | |
| Septoria rubi | CBS 128648 | Rubus crataegifolius | South Korea | H.D. Shin | KF253315 | KF252840 | KF252369 | KF251872 | KF251368 | KF253672 | KF254020 | |
| Septoria rubi | CBS 128760 | Rubus crataegifolius | South Korea | H.D. Shin | KF253316 | KF252841 | KF252370 | KF251873 | KF251369 | KF253673 | KF254021 | |
| Sep. cf. sonchi | – | CBS 128757 | Sonchus asper | South Korea | H.D. Shin | KF253500 | KF253020 | KF252546 | KF252057 | KF251552 | KF253855 | KF254204 |
| Sep. cf. stachydicola | Septoria lycopicola | CBS 128662 | Stachys riederi | South Korea | H.D. Shin | KF253513 | KF253034 | KF252559 | KF252071 | KF251566 | KF253867 | KF254218 |
| Sep. chamaecisti | – | CBS 350.58 | Helianthemum hybridum | Germany | R. Schneider | KF253318 | KF252843 | KF252372 | KF251875 | KF251371 | KF253675 | KF254023 |
| Sep. chelidonii | – | CBS 128607 | Chelidonium majus | South Korea | H.D. Shin | KF253319 | KF252844 | KF252373 | KF251876 | KF251372 | KF253676 | KF254024 |
| – | CPC 12337 | Chelidonium majus | South Korea | H.D. Shin | KF253320 | KF252845 | KF252374 | KF251877 | KF251373 | KF253677 | KF254025 | |
| Sep. chromolaenae | – | CBS 113373 | Chromolaena odorata | Cuba | S. Neser | KF253321 | KF252846 | KF252375 | KF251878 | KF251374 | KF253678 | KF254026 |
| Sep. chrysanthemella | – | CBS 128617 | Chrysanthemum morifolium | South Korea | H.D. Shin | KF253322 | KF252847 | KF252376 | KF251879 | KF251375 | KF253679 | KF254027 |
| – | CBS 128622 | Chrysanthemum boreale | South Korea | H.D. Shin | KF253323 | KF252848 | KF252377 | KF251880 | KF251376 | KF253680 | KF254028 | |
| – | CBS 483.63 | Chrysanthemum sp. | Netherlands | H.A. van der Aa | KF253324 | KF252849 | KF252378 | KF251881 | KF251377 | KF253681 | KF254029 | |
| – | CBS 128716 | – | South Africa | E. Oh | KF253325 | KF252850 | KF252379 | KF251882 | KF251378 | KF253682 | KF254030 | |
| – | CBS 351.58 | Chrysanthemum indicum | Germany | R. Schneider | KF253326 | KF252851 | KF252380 | KF251883 | KF251379 | KF253683 | KF254031 | |
| – | CBS 354.73 | Chrysanthemum morifolium | New Zealand | G.F. Laundon | KF253327 | KF252852 | KF252381 | KF251884 | KF251380 | KF253684 | KF254032 | |
| Sep. cirsii | – | CBS 128621 | Cirsium setidens | South Korea | H.D. Shin | KF253328 | KF252853 | KF252382 | KF251885 | KF251381 | KF253685 | KF254033 |
| Sep. citri (= protearum complex) | ||||||||||||
| Septoria orchidearum | CBS 101013 | Masdevallia sp. | Netherlands | W. Veenbaas-Rijks | KF253457 | KF252978 | KF252504 | KF252013 | KF251508 | KF253812 | KF254161 | |
| Septoria sp. | CBS 101354 | Gevuina avellana | New Zealand | S. Ganev | KF253458 | KF252979 | KF252505 | KF252014 | KF251509 | KF253813 | KF254162 | |
| Septoria lobeliae | CBS 113392 | Lobelia erinus | – | S. Wolcon | KF253460 | KF252981 | KF252507 | KF252016 | KF251511 | KF253815 | KF254164 | |
| Septoria aciculosa | CBS 177.77 | Fragaria sp. | New Zealand | H.J. Boesewinkel | KF253463 | KF252984 | KF252509 | KF252019 | KF251514 | KF253818 | KF254167 | |
| Septoria citri | CBS 315.37 | – | – | L.L. Huillier | KF253465 | – | KF252511 | KF252021 | KF251516 | KF253820 | KF254169 | |
| Septoria gerberae | CBS 410.61 | Gerbera jamesonii | Italy | W. Gerlach | KF253468 | KF252988 | KF252514 | KF252024 | KF251519 | KF253823 | KF254172 | |
| Septoria hederae | CBS 566.88 | Hedera helix | France | H.A. van der Aa | KF253470 | KF252990 | KF252515 | KF252026 | KF251521 | KF253825 | KF254174 | |
| Sep. citricola | – | CBS 356.36 | Citrus sinensis | Italy | G. Ruggieri | KF253329 | KF252854 | KF252383 | KF251886 | KF251382 | KF253686 | KF254034 |
| Sep. clematidis | – | CBS 108983 | Clematis vitalba | Germany | G.J.M. Verkley | KF253330 | KF252855 | KF252384 | KF251887 | KF251383 | KF253687 | KF254035 |
| – | CBS 108984 | Clematis vitalba | Germany | G.J.M. Verkley | KF253331 | KF252856 | KF252385 | KF251888 | KF251384 | KF253688 | KF254036 | |
| Sep. codonopsidis | – | CBS 128609 | Codonopsis lanceolata | South Korea | H.D. Shin | KF253332 | KF252857 | KF252386 | KF251889 | KF251385 | KF253689 | KF254037 |
| – | CBS 128620 | Codonopsis lanceolata | South Korea | H.D. Shin | KF253333 | KF252858 | KF252387 | KF251890 | KF251386 | KF253690 | KF254038 | |
| Sep. convolvuli | – | CBS 102325 | Calystegia sepium | Netherlands | G.J.M. Verkley | KF253334 | KF252859 | KF252388 | KF251891 | KF251387 | KF253691 | KF254039 |
| – | CBS 113111 | Calystegia sepium | New Zealand | G.J.M. Verkley | KF253335 | KF252860 | KF252389 | KF251892 | KF251388 | KF253692 | KF254040 | |
| – | CBS 128627 | Calystegia soldanella | South Korea | H.D. Shin | KF253336 | KF252861 | KF252390 | KF251893 | KF251389 | KF253693 | KF254041 | |
| Sep. coprosmae | – | CBS 113391 | Coprosma robusta | New Zealand | G.J.M. Verkley | KF253255 | KF252787 | KF252313 | KF251812 | KF251308 | KF253617 | KF253971 |
| Sep. crepidis | – | CPC 12539 | Crepis japonica | South Korea | H.D. Shin | KF253339 | KF252864 | KF252393 | KF251896 | KF251392 | KF253696 | KF254044 |
| – | CBS 128608 | Youngia japonica | South Korea | H.D. Shin | KF253337 | KF252862 | KF252391 | KF251894 | KF251390 | KF253694 | KF254042 | |
| – | CBS 128619 | Youngia japonica | South Korea | H.D. Shin | KF253338 | KF252863 | KF252392 | KF251895 | KF251391 | KF253695 | KF254043 | |
| Sep. cruciatae | Septoria sp. | CBS 123747 | Galium odoratum | Czech Republic | G.J.M. Verkley | KF253340 | KF252865 | KF252394 | KF251897 | KF251393 | KF253697 | KF254045 |
| Septoria sp. | CBS 123748 | Galium odoratum | Czech Republic | G.J.M. Verkley | KF253341 | KF252866 | KF252395 | KF251898 | KF251394 | KF253698 | KF254046 | |
| Sep. cucubali | – | CBS 102367 | Cucubalus baccifer | Netherlands | G.J.M. Verkley | KF253342 | KF252867 | KF252396 | KF251899 | KF251395 | KF253699 | KF254047 |
| – | CBS 102368 | Cucubalus baccifer | Netherlands | G.J.M. Verkley | KF253343 | KF252868 | KF252397 | KF251900 | KF251396 | KF253700 | KF254048 | |
| – | CBS 102386 | Saponaria officinalis | Netherlands | G.J.M. Verkley | KF253344 | KF252869 | KF252398 | KF251901 | KF251397 | KF253701 | KF254049 | |
| Septoria sp. | CBS 124874 | Fagus sylvatica | Germany | M. Unterseher | KF253345 | KF252870 | KF252399 | KF251902 | KF251398 | KF253702 | KF254050 | |
| Sep. cucurbitacearum | – | CBS 178.77 | Cucurbita maxima | New Zealand | H.J. Boesewinkel | KF253346 | – | KF252400 | KF251903 | KF251399 | KF253703 | KF254051 |
| Sep. dearnessii | – | CBS 128624 | Angelica dahurica | South Korea | H.D. Shin | KF253347 | KF252871 | KF252401 | KF251904 | KF251400 | KF253704 | KF254052 |
| Sep. digitalis | – | CBS 328.67 | Digitalis lanata | Netherlands | H.A. van der Aa | KF253348 | KF252872 | KF252402 | KF251905 | KF251401 | KF253705 | KF254053 |
| – | CBS 391.63 | Digitalis lanata | Czech Republic | V. Holubová | KF253349 | KF252873 | KF252403 | KF251906 | KF251402 | KF253706 | KF254054 | |
| Sep. dolichospora | – | CBS 129152 | Solidago virgaurea | South Korea | H.D. Shin | KF253350 | KF252874 | – | KF251907 | KF251403 | KF253707 | KF254055 |
| Sep. dysentericae | – | CBS 128637 | Inula britannica | South Korea | H.D. Shin | KF253351 | KF252875 | KF252404 | KF251908 | KF251404 | KF253708 | KF254056 |
| – | CBS 128638 | Inula britannica | South Korea | H.D. Shin | KF253352 | KF252876 | KF252405 | KF251909 | KF251405 | KF253709 | KF254057 | |
| – | CBS 131892; CPC 12328 | Inula britannica | South Korea | H.D. Shin | KF253353 | KF252877 | KF252406 | KF251910 | KF251406 | KF253710 | KF254058 | |
| Sep. ekmaniana | – | CBS 113385 | Chromolaena odorata | Mexico | M.J. Morris | KF253354 | KF252878 | – | KF251911 | KF251407 | KF253711 | KF254059 |
| – | CBS 113612 | Chromolaena odorata | Mexico | M.J. Morris | KF253355 | KF252879 | – | KF251912 | KF251408 | KF253712 | KF254060 | |
| Sep. epambrosiae | – | CBS 128629 | Ambrosia trifida | South Korea | H.D. Shin | KF253356 | KF252880 | KF252407 | KF251913 | KF251409 | KF253713 | KF254061 |
| – | CBS 128636 | Ambrosia trifida | South Korea | H.D. Shin | KF253357 | KF252881 | KF252408 | KF251914 | KF251410 | KF253714 | KF254062 | |
| Sep. epilobii | – | CBS 109084 | Epilobium fleischeri | Austria | G.J.M. Verkley | KF253358 | KF252882 | KF252409 | KF251915 | KF251411 | KF253715 | KF254063 |
| – | CBS 109085 | Epilobium fleischeri | Austria | G.J.M. Verkley | KF253359 | KF252883 | KF252410 | KF251916 | KF251412 | KF253716 | KF254064 | |
| Sep. erigerontis | – | CBS 109094 | Erigeron annuus | Austria | G.J.M. Verkley | KF253360 | KF252884 | KF252411 | KF251917 | KF251413 | KF253717 | KF254065 |
| – | CBS 109095 | Erigeron annuus | Austria | G.J.M. Verkley | KF253361 | KF252885 | KF252412 | KF251918 | KF251414 | KF253718 | KF254066 | |
| – | CBS 128606 | Erigeron annuus | South Korea | H.D. Shin | KF253362 | KF252886 | KF252413 | KF251919 | KF251415 | KF253719 | KF254067 | |
| – | CBS 131893; CPC 12340 | Erigeron annuus | South Korea | H.D. Shin | KF253363 | KF252888 | KF252414 | KF251920 | KF251416 | KF253720 | KF254068 | |
| Septoria schnabliana | CBS 186.93 | Erigeron annuus | Italy | M. Vurro | KF253364 | KF252887 | KF252537 | KF252048 | KF251543 | KF253893 | KF254244 | |
| Sep. eucalyptorum | – | CBS 118505 | Eucalyptus sp. | India | W. Gams | KF253365 | KF252889 | KF252415 | KF251921 | KF251417 | KF253721 | KF254069 |
| Sep. exotica | – | CBS 163.78 | Hebe speciosa | New Zealand | H.J. Boesewinkel | KF253366 | KF252890 | KF252416 | KF251922 | KF251418 | KF253722 | KF254070 |
| Sep. galeopsidis | – | CBS 123744 | Galeopsis sp. | Czech Republic | G.J.M. Verkley | KF253367 | KF252891 | KF252417 | KF251923 | KF251419 | KF253723 | KF254071 |
| – | CBS 123746 | Galeopsis sp. | Czech Republic | G.J.M. Verkley | KF253368 | KF252892 | KF252418 | KF251924 | KF251420 | KF253724 | KF254072 | |
| – | CBS 123749 | Galeopsis sp. | Czech Republic | G.J.M. Verkley | KF253369 | KF252893 | KF252419 | KF251925 | KF251421 | KF253725 | KF254073 | |
| – | CBS 191.26 | Galeopsis sp. | – | C. Killian | KF253370 | KF252894 | KF252420 | KF251926 | KF251422 | KF253726 | KF254074 | |
| – | CBS 102314 | Galeopsis tetrahit | Netherlands | G.J.M. Verkley | KF253371 | KF252895 | KF252421 | KF251927 | KF251423 | KF253727 | KF254075 | |
| – | CBS 102411 | Galeopsis tetrahit | Netherlands | G.J.M. Verkley | KF253372 | KF252896 | KF252422 | KF251928 | KF251424 | KF253728 | KF254076 | |
| – | CBS 123745 | Galeopsis sp. | Czech Republic | G.J.M. Verkley | KF253373 | KF252897 | KF252423 | KF251929 | KF251425 | KF253729 | KF254077 | |
| Sep. gentianae | – | CBS 128633 | Gentiana scabra | South Korea | H.D. Shin | KF253374 | KF252898 | KF252424 | KF251930 | KF251426 | KF253730 | KF254078 |
| Sep. gladioli | – | CBS 121.20 | – | – | – | KF253375 | KF252899 | KF252425 | KF251931 | KF251427 | KF253731 | KF254079 |
| – | CBS 353.29 | – | Netherlands | J.C. Went | KF253376 | KF252900 | KF252426 | KF251932 | KF251428 | KF253732 | KF254080 | |
| Sep. glycines | – | CBS 336.53 | – | Japan | H. Kurata | KF253377 | KF252901 | – | KF251933 | KF251429 | KF253733 | KF254081 |
| Sep. glycinicola | – | CBS 128618 | Glycine max | South Korea | H.D. Shin | KF253378 | KF252902 | KF252427 | KF251934 | KF251430 | KF253734 | KF254082 |
| Sep. helianthi | – | CBS 123.81 | Helianthus annuus | – | M. Muntañola | KF253379 | KF252903 | KF252428 | KF251935 | KF251431 | KF253735 | KF254083 |
| Sep. helianthicola | – | CBS 122.81 | Helianthus annuus | – | M. Muntañola | KF253380 | KF252904 | KF252429 | KF251936 | KF251432 | KF253736 | KF254084 |
| Sep. hibiscicola | – | CBS 128611 | Hibiscus syriacus | South Korea | H.D. Shin | KF253381 | KF252905 | KF252430 | KF251937 | KF251433 | KF253737 | KF254085 |
| – | CBS 128615 | Hibiscus syriacus | South Korea | H.D. Shin | KF253382 | KF252906 | KF252431 | KF251938 | KF251434 | KF253738 | KF254086 | |
| Sep. hippocastani | – | CBS 411.61 | Aesculus hippocastanum | Germany | W. Gerlach | KF253383 | KF252907 | KF252432 | KF251939 | KF251435 | KF253739 | KF254087 |
| Sep. justiciae | – | CPC 12509 | Justicia procumbens | South Korea | H.D. Shin | KF253386 | KF252910 | KF252435 | KF251942 | KF251438 | KF253742 | KF254090 |
| – | CBS 128610 | Justicia procumbens | South Korea | H.D. Shin | KF253384 | KF252908 | KF252433 | KF251940 | KF251436 | KF253740 | KF254088 | |
| – | CBS 128625 | Justicia procumbens | South Korea | H.D. Shin | KF253385 | KF252909 | KF252434 | KF251941 | KF251437 | KF253741 | KF254089 | |
| Sep. lactucae | – | CBS 108943 | Lactuca sativa | Netherlands | P. Grooteman | KF253387 | KF252911 | KF252436 | KF251943 | KF251439 | KF253743 | KF254091 |
| – | CBS 352.58 | Lactuca sativa | Germany | G. Sörgel | KF253388 | KF252912 | KF252437 | KF251944 | KF251440 | KF253744 | KF254092 | |
| Sep. lamiicola | – | CBS 102328 | Lamium album | Netherlands | G.J.M. Verkley | KF253389 | KF252913 | KF252438 | KF251945 | KF251441 | KF253745 | KF254093 |
| – | CBS 102329 | Lamium album | Netherlands | G.J.M. Verkley | KF253390 | KF252914 | KF252439 | KF251946 | KF251442 | KF253746 | KF254094 | |
| – | CBS 102379 | Lamium sp. | Netherlands | G.J.M. Verkley | KF253391 | KF252915 | KF252440 | KF251947 | KF251443 | KF253747 | KF254095 | |
| – | CBS 102380 | Lamium sp. | Netherlands | G.J.M. Verkley | KF253392 | KF252916 | KF252441 | KF251948 | KF251444 | KF253748 | KF254096 | |
| – | CBS 109112 | Lamium album | Austria | G.J.M. Verkley | KF253393 | KF252917 | KF252442 | KF251949 | KF251445 | KF253749 | KF254097 | |
| – | CBS 109113 | Lamium album | Austria | G.J.M. Verkley | KF253394 | KF252918 | KF252443 | KF251950 | KF251446 | KF253750 | KF254098 | |
| – | CBS 123882 | Lamium sp. | Czech Republic | G.J.M. Verkley | KF253395 | KF252919 | KF252444 | KF251951 | KF251447 | KF253751 | KF254099 | |
| – | CBS 123883 | Lamium sp. | Czech Republic | G.J.M. Verkley | KF253396 | KF252920 | KF252445 | KF251952 | KF251448 | KF253752 | KF254100 | |
| – | CBS 123884 | Lamium sp. | Czech Republic | G.J.M. Verkley | KF253397 | KF252921 | KF252446 | KF251953 | KF251449 | KF253753 | KF254101 | |
| Sep. lepidiicola | – | CBS 128635 | Lepidium virginicum | South Korea | H.D. Shin | KF253398 | KF252922 | KF252447 | KF251954 | KF251450 | KF253754 | KF254102 |
| Sep. leptostachyae | – | CBS 128613 | Phryma leptostachya | South Korea | H.D. Shin | KF253399 | KF252923 | KF252448 | KF251955 | KF251451 | KF253755 | KF254103 |
| – | CBS 128628 | Phryma leptostachya | South Korea | H.D. Shin | KF253400 | KF252924 | KF252449 | KF251956 | KF251452 | KF253756 | KF254104 | |
| Sep. leucanthemi | – | CBS 109083 | Chrysanthemum leucanthemum | Austria | G.J.M. Verkley | KF253401 | KF252925 | KF252450 | KF251957 | KF251453 | KF253757 | KF254105 |
| – | CBS 109086 | Chrysanthemum leucanthemum | Austria | G.J.M. Verkley | KF253402 | KF252926 | KF252451 | KF251958 | KF251454 | KF253758 | KF254106 | |
| – | CBS 109090 | Chrysanthemum leucanthemum | Austria | G.J.M. Verkley | KF253403 | KF252927 | KF252452 | KF251959 | KF251455 | KF253759 | KF254107 | |
| – | CBS 109091 | Chrysanthemum leucanthemum | Austria | G.J.M. Verkley | KF253404 | KF252928 | KF252453 | KF251960 | KF251456 | KF253760 | KF254108 | |
| – | CBS 113112 | Chrysanthemum leucanthemum | New Zealand | G.J.M. Verkley | KF253405 | KF252929 | KF252454 | KF251961 | KF251457 | KF253761 | KF254109 | |
| – | CBS 353.58 | Chrysanthemum maximum | Germany | R. Schneider | KF253406 | KF252930 | KF252455 | KF251962 | KF251458 | KF253762 | KF254110 | |
| Sep. limonum | – | CBS 419.51 | Citrus limonium | Italy | G. Goidánich | KF253407 | KF252931 | KF252456 | KF251963 | KF251459 | KF253763 | KF254111 |
| Sep. linicola | – | CBS 316.37 | Linum usitatissimum | – | H.W. Hollenweber | KF253408 | KF252932 | KF252457 | KF251964 | KF251460 | KF253764 | KF254112 |
| Sep. lycoctoni | – | CBS 109089 | Aconitum vulparia | Austria | G.J.M. Verkley | KF253409 | KF252933 | KF252458 | KF251965 | KF251461 | KF253765 | KF254113 |
| Sep. lycopersici | – | CBS 128654 | Lycopersicon esculentum | South Korea | H.D. Shin | KF253410 | KF252934 | KF252459 | KF251966 | KF251462 | KF253766 | KF254114 |
| – | CBS 354.49 | Lycopersicon esculentum | Canada | B.H. MacNeil | KF253411 | KF252935 | KF252460 | KF251967 | KF251463 | KF253767 | KF254115 | |
| Sep. lycopicola | – | CBS 128651 | Lycopus ramosissimus | South Korea | H.D. Shin | KF253412 | KF252936 | KF252461 | KF251968 | KF251464 | KF253768 | KF254116 |
| Sep. lysimachiae | – | CBS 102315 | Lysimachia vulgaris | Netherlands | G.J.M. Verkley | KF253413 | KF252937 | KF252462 | KF251969 | KF251465 | KF253769 | KF254117 |
| – | CBS 108998 | Lysimachia vulgaris | Netherlands | G.J.M. Verkley | KF253414 | KF252938 | KF252463 | KF251970 | KF251466 | KF253770 | KF254118 | |
| – | CBS 108999 | Lysimachia vulgaris | Netherlands | G.J.M. Verkley | KF253415 | KF252939 | KF252464 | KF251971 | KF251467 | KF253771 | KF254119 | |
| – | CBS 123794 | Lysimachia sp. | Czech Republic | G.J.M. Verkley | KF253416 | KF252940 | KF252465 | KF251972 | KF251468 | KF253772 | KF254120 | |
| – | CBS 123795 | Lysimachia sp. | Czech Republic | G.J.M. Verkley | KF253417 | KF252941 | KF252466 | KF251973 | KF251469 | KF253773 | KF254121 | |
| Sep. malagutii | – | CBS 106.80 | Solanum sp. | Peru | G.H. Boerema | KF253418 | – | KF252467 | KF251974 | KF251470 | KF253774 | KF254122 |
| Sep. matricariae | – | CBS 109000 | Matricaria discoidea | Netherlands | G.J.M. Verkley | KF253419 | KF252942 | KF252468 | KF251975 | KF251471 | KF253775 | KF254123 |
| – | CBS 109001 | Matricaria discoidea | Netherlands | G.J.M. Verkley | KF253420 | KF252943 | KF252469 | KF251976 | KF251472 | KF253776 | KF254124 | |
| Sep. mazi | – | CBS 128656 | Mazus japonicus | South Korea | H.D. Shin | KF253421 | KF252944 | KF252470 | KF251977 | KF251473 | KF253777 | KF254125 |
| – | CBS 128755 | Mazus japonicus | South Korea | H.D. Shin | KF253422 | KF252945 | KF252471 | KF251978 | KF251474 | KF253778 | KF254126 | |
| Sep. melissae | – | CBS 109097 | Melissa officinalis | Netherlands | H.A. van der Aa | KF253423 | KF252946 | KF252472 | KF251979 | KF251475 | KF253779 | KF254127 |
| Sep. menthae | – | CBS 404.34 | – | Japan | T. Hemmi | KF253424 | KF252947 | – | KF251980 | KF251476 | KF253780 | KF254128 |
| Sep. napelli | – | CBS 109104 | Aconitum napellus | Austria | G.J.M. Verkley | KF253425 | KF252948 | KF252473 | KF251981 | KF251477 | KF253781 | KF254129 |
| – | CBS 109105 | Aconitum napellus | Austria | G.J.M. Verkley | KF253426 | KF252949 | KF252474 | KF251982 | KF251478 | KF253782 | KF254130 | |
| – | CBS 109106 | Aconitum napellus | Austria | G.J.M. Verkley | KF253427 | KF252950 | KF252475 | KF251983 | KF251479 | KF253783 | KF254131 | |
| Sep. obesa | Septoria artimisiae | CBS 128588 | Artemisia lavandulaefolia | South Korea | H.D. Shin | KF253428 | KF252951 | KF252476 | KF251984 | KF251480 | KF253784 | KF254132 |
| Septoria chrysanthemella | CBS 128623 | Chrysanthemum indicum | South Korea | H.D. Shin | KF253429 | KF252952 | KF252477 | KF251985 | KF251481 | KF253785 | KF254133 | |
| – | CBS 128759 | Chrysanthemum morifolium | South Korea | H.D. Shin | KF253430 | – | KF252478 | KF251986 | KF251482 | KF253786 | KF254134 | |
| – | CBS 354.58 | Chrysantemum indicum | Germany | R. Schneider | KF253431 | – | KF252479 | KF251987 | KF251483 | KF253787 | KF254135 | |
| Sep. oenanthis | – | CBS 128667 | Cicuta virosa | South Korea | H.D. Shin | KF253432 | KF252953 | KF252481 | KF251989 | KF251485 | KF253788 | KF254136 |
| Sep. oenanthicola | Septoria oenanthis | CBS 128649 | Oenanthe javanica | South Korea | H.D. Shin | KF253433 | KF252954 | KF252480 | KF251988 | KF251484 | KF253789 | KF254137 |
| Sep. orchidearum | Septoria cyclaminis | CBS 128631 | Cyclamen fatrense | South Korea | H.D. Shin | KF253434 | KF252955 | KF252482 | KF251990 | KF251486 | KF253790 | KF254138 |
| – | CBS 457.78 | Listera ovata | France | H.A. van der Aa | KF253435 | KF252956 | KF252483 | KF251991 | KF251487 | KF253791 | KF254139 | |
| Sep. oudemansii | – | CBS 619.72 | Poa pratensis | Germany | R. Schneider | KF253436 | KF252957 | KF252484 | KF251992 | KF254299 | – | KF254140 |
| Sep. pachyspora | – | CBS 128652 | Zyathoxylum schinifolium | South Korea | H.D. Shin | KF253437 | KF252958 | KF252485 | KF251993 | KF251488 | KF253792 | KF254141 |
| Sep. paridis | – | CBS 109111 | Paris quadrifolia | Austria | G.J.M. Verkley | KF253438 | KF252959 | KF252486 | KF251994 | KF251489 | KF253793 | KF254142 |
| – | CBS 109110 | Paris quadrifolia | Austria | G.J.M. Verkley | KF253439 | KF252960 | KF252487 | KF251995 | KF251490 | KF253794 | KF254143 | |
| Septoria violae-palustris | CBS 109108 | Viola sp. | Austria | G.J.M. Verkley | KF253440 | KF252961 | KF252488 | KF251996 | KF251491 | KF253795 | KF254144 | |
| Septoria violae-palustris | CBS 109109 | Viola sp. | Austria | G.J.M. Verkley | KF253441 | KF252962 | KF252489 | KF251997 | KF251492 | KF253796 | KF254145 | |
| Sep. passifloricola | Sep. passiflorae | CBS 102701 | Passiflora edulis | New Zealand | C.F. Hill | KF253442 | KF252963 | KF252490 | KF251998 | KF251493 | KF253797 | KF254146 |
| – | CBS 129431 | Passiflora edulis | South Korea | H.D. Shin | KF253443 | KF252964 | – | KF251999 | KF251494 | KF253798 | KF254147 | |
| Sep. perillae | – | CBS 128655 | Perilla frutescens | South Korea | H.D. Shin | KF253444 | KF252965 | KF252491 | KF252000 | KF251495 | KF253799 | KF254148 |
| Sep. petroselini | – | CBS 109521 | – | Netherlands | H.A. van der Aa | KF253445 | KF252966 | KF252492 | KF252001 | KF251496 | KF253800 | KF254149 |
| – | CBS 182.44 | Petroselinum sativum | Netherlands | S.D. de Wit | KF253446 | KF252967 | KF252493 | KF252002 | KF251497 | KF253801 | KF254150 | |
| Sep. phlogis | – | CBS 102317 | Phlox sp. | Netherlands | G.J.M. Verkley | KF253447 | KF252968 | KF252494 | KF252003 | KF251498 | KF253802 | KF254151 |
| – | CBS 128663 | Phlox paniculata | South Korea | H.D. Shin | KF253448 | KF252969 | KF252495 | KF252004 | KF251499 | KF253803 | KF254152 | |
| – | CBS 577.90 | Phlox sp. | Netherlands | H.A. van der Aa | KF253449 | KF252970 | KF252496 | KF252005 | KF251500 | KF253804 | KF254153 | |
| Sep. polygonorum | – | CBS 102330 | Polygonum persicaria | Netherlands | G.J.M. Verkley | KF253450 | KF252971 | KF252497 | KF252006 | KF251501 | KF253805 | KF254154 |
| – | CBS 102331 | Polygonum persicaria | Netherlands | G.J.M. Verkley | KF253451 | KF252972 | KF252498 | KF252007 | KF251502 | KF253806 | KF254155 | |
| – | CBS 108982 | Polygonum persicaria | Germany | G.J.M. Verkley | KF253452 | KF252973 | KF252499 | KF252008 | KF251503 | KF253807 | KF254156 | |
| – | CBS 109834 | Polygonum persicaria | Netherlands | G.J.M. Verkley | KF253453 | KF252974 | KF252500 | KF252009 | KF251504 | KF253808 | KF254157 | |
| – | CBS 113110 | Polygonum persicaria | New Zealand | C.F. Hill | KF253454 | KF252975 | KF252501 | KF252010 | KF251505 | KF253809 | KF254158 | |
| – | CBS 347.67 | Polygonum persicaria | Netherlands | H.A. van der Aa | KF253455 | KF252976 | KF252502 | KF252011 | KF251506 | KF253810 | KF254159 | |
| Sep. posoniensis | – | CBS 128645 | Chrysosplenium japonicum | South Korea | H.D. Shin | KF253456 | KF252977 | KF252503 | KF252012 | KF251507 | KF253811 | KF254160 |
| Sep. protearum | Septoria sp. | CPC 19691 | Zanthedeschia aethiopica | South Africa | P.W. Crous | KF253474 | KF252994 | KF252519 | KF252030 | KF251525 | KF253829 | KF254178 |
| Septoria sp. | CBS 113114 | Geum sp. | New Zealand | G.J.M. Verkley | KF253459 | KF252980 | KF252506 | KF252015 | KF251510 | KF253814 | KF254163 | |
| Septoria sp. | CBS 119942 | Asplenium ruta-muraria | Germany | G.J.M. Verkley | KF253461 | KF252982 | – | KF252017 | KF251512 | KF253816 | KF254165 | |
| Septoria sp. | CBS 135477; CPC 19675 | Zanthedeschia aethiopica | South Africa | P.W. Crous | KF253473 | KF252993 | KF252518 | KF252029 | KF251524 | KF253828 | KF254177 | |
| Septoria sp. | CBS 164.78 | Nephrolepis sp. | New Zealand | H.J. Boesewinkel | KF253462 | KF252983 | KF252508 | KF252018 | KF251513 | KF253817 | KF254166 | |
| Septoria sp. | CBS 179.77 | Myosotis sp. | New Zealand | H.J. Boesewinkel | KF253464 | KF252985 | KF252510 | KF252020 | KF251515 | KF253819 | KF254168 | |
| Septoria sp. | CBS 364.97 | Skimmia sp. | Netherlands | J. de Gruyter | KF253466 | KF252986 | KF252512 | KF252022 | KF251517 | KF253821 | KF254170 | |
| Septoria ligustri | CBS 390.59 | Ligustrum vulgare | Italy | M. Ribaldi | KF253467 | KF252987 | KF252513 | KF252023 | KF251518 | KF253822 | KF254171 | |
| Septoria pistaciae | CBS 420.51 | Pistacia vera | Italy | G. Goidánich | KF253469 | KF252989 | – | KF252025 | KF251520 | KF253824 | KF254173 | |
| Septoria sp. | CBS 658.77 | Boronia denticulata | New Zealand | H.J. Boesewinkel | KF253471 | KF252991 | KF252516 | KF252027 | KF251522 | KF253826 | KF254175 | |
| – | CBS 778.97 | Protea cynaroides | South Africa | L. Viljoen | KF253472 | KF252992 | KF252517 | KF252028 | KF251523 | KF253827 | KF254176 | |
| Sep. pseudonapelli | Septoria napelli | CBS 128664 | Aconitum pseudolaeve | South Korea | H.D. Shin | KF253475 | KF252995 | KF252520 | KF252031 | KF251526 | KF253830 | KF254179 |
| Sep. putrida | – | CBS 109087 | Senecio nemorensis | Austria | G.J.M. Verkley | KF253476 | KF252996 | KF252521 | KF252032 | KF251527 | KF253831 | KF254180 |
| – | CBS 109088 | Senecio nemorensis | Austria | G.J.M. Verkley | KF253477 | KF252997 | KF252522 | KF252033 | KF251528 | KF253832 | KF254181 | |
| Sep. rumicum | Septoria acetosae | CBS 503.76 | Rumex acetosa | France | H.A. van der Aa | KF253478 | KF252998 | KF252523 | KF252034 | KF251529 | KF253833 | KF254182 |
| Sep. saccardoi | – | CBS 128756 | Lysimachia vulgaris | South Korea | H.D. Shin | KF253479 | KF252999 | KF252524 | KF252035 | KF251530 | KF253834 | KF254183 |
| Sep. scabiosicola | – | CBS 102333 | Knautia arvensis | Netherlands | G.J.M. Verkley | KF253480 | KF253000 | KF252525 | KF252036 | KF251531 | KF253835 | KF254184 |
| – | CBS 102334 | Knautia arvensis | Netherlands | G.J.M. Verkley | KF253481 | KF253001 | KF252526 | KF252037 | KF251532 | KF253836 | KF254185 | |
| – | CBS 102335 | Knautia arvensis | Netherlands | G.J.M. Verkley | KF253482 | KF253002 | KF252527 | KF252038 | KF251533 | KF253837 | KF254186 | |
| – | CBS 102336 | Knautia arvensis | Netherlands | G.J.M. Verkley | KF253483 | KF253003 | KF252528 | KF252039 | KF251534 | KF253838 | KF254187 | |
| – | CBS 108981 | Knautia arvensis | Germany | G.J.M. Verkley | KF253484 | KF253004 | KF252529 | KF252040 | KF251535 | KF253839 | KF254188 | |
| – | CBS 109021 | Knautia arvensis | Austria | G.J.M. Verkley | KF253485 | KF253005 | KF252530 | KF252041 | KF251536 | KF253840 | KF254189 | |
| – | CBS 109092 | Knautia dipsacifolia | Austria | G.J.M. Verkley | KF253486 | KF253006 | KF252531 | KF252042 | KF251537 | KF253841 | KF254190 | |
| – | CBS 109093 | Knautia dipsacifolia | Austria | G.J.M. Verkley | KF253487 | KF253007 | KF252532 | KF252043 | KF251538 | KF253842 | KF254191 | |
| – | CBS 109128 | Knautia dipsacifolia | Austria | G.J.M. Verkley | KF253488 | KF253008 | KF252533 | KF252044 | KF251539 | KF253843 | KF254192 | |
| – | CBS 109129 | Knautia dipsacifolia | Austria | G.J.M. Verkley | KF253489 | KF253009 | KF252534 | KF252045 | KF251540 | KF253844 | KF254193 | |
| – | CBS 182.93 | Succissa pratensis | France | H.A. van der Aa | KF253490 | KF253010 | KF252535 | KF252046 | KF251541 | KF253845 | KF254194 | |
| – | CBS 317.37 | – | – | – | KF253491 | KF253011 | KF252536 | KF252047 | KF251542 | KF253846 | KF254195 | |
| Sep. senecionis | – | CBS 102366 | Senecio fluviatilis | Netherlands | G.J.M. Verkley | KF253492 | KF253012 | KF252538 | KF252049 | KF251544 | KF253847 | KF254196 |
| – | CBS 102381 | Senecio fluviatilis | Netherlands | G.J.M. Verkley | KF253493 | KF253013 | KF252539 | KF252050 | KF251545 | KF253848 | KF254197 | |
| Sep. siegesbeckiae | – | CBS 128659 | Siegesbeckia glabrescens | South Korea | H.D. Shin | KF253494 | KF253014 | KF252540 | KF252051 | KF251546 | KF253849 | KF254198 |
| – | CBS 128661 | Siegesbeckia pubescens | South Korea | H.D. Shin | KF253495 | KF253015 | KF252541 | KF252052 | KF251547 | KF253850 | KF254199 | |
| Sep. sii | – | CBS 102369 | Berula erecta | Netherlands | G.J.M. Verkley | KF253496 | KF253016 | KF252542 | KF252053 | KF251548 | KF253851 | KF254200 |
| – | CBS 102370 | Berula erecta | Netherlands | G.J.M. Verkley | KF253497 | KF253017 | KF252543 | KF252054 | KF251549 | KF253852 | KF254201 | |
| – | CBS 118.96 | Berula erecta | Netherlands | H.A. van der Aa | KF253498 | KF253018 | KF252544 | KF252055 | KF251550 | KF253853 | KF254202 | |
| Sep. sisyrinchii | – | CBS 112096 | Sysirinchium sp. | New Zealand | C.F. Hill | KF253499 | KF253019 | KF252545 | KF252056 | KF251551 | KF253854 | KF254203 |
| Septoria sp. | Pseudocercospora sp. | CPC 19976 | Feijoa sellowiana | Italy | G. Polizzy | KF253509 | KF253030 | – | KF252067 | KF251562 | KF253863 | KF254214 |
| Septoria sp. | – | CPC 23104 | – | Italy | E. van Agtmaal | KF253511 | KF253032 | KF252557 | KF252069 | KF251564 | KF253865 | KF254216 |
| Septoria sp. | – | CBS 109114 | Campanula glomerata | Austria | G.J.M. Verkley | KF253501 | KF253021 | KF252547 | KF252058 | KF251553 | KF253856 | KF254205 |
| Septoria sp. | – | CBS 120739 | Eucalyptus sp. | Italy | W. Gams | KF253503 | KF253023 | KF252549 | KF252060 | KF251555 | KF253858 | KF254207 |
| Septoria sp. | Septoria taraxaci | CBS 128650 | Taraxacum officinale | South Korea | H.D. Shin | KF253504 | KF253024 | KF252550 | KF252061 | KF251556 | KF253859 | KF254208 |
| Septoria sp. | Septoria posoniensis | CBS 128658 | Chrysoplenium japonicum | South Korea | H.D. Shin | KF253505 | KF253025 | KF252551 | KF252062 | KF251557 | KF253860 | KF254209 |
| Septoria sp. | – | CBS 135472; CPC 19304 | Vigna unguiculata ssp. sesquipedalis | Austria | P.W. Crous | KF253506 | KF253026 | KF252552 | KF252063 | KF251558 | KF253861 | KF254210 |
| Septoria sp. | – | CBS 135474; CPC 19485 | Conyza canadensis | Brazil | R.W. Barreto | KF253507 | KF253027 | KF252553 | KF252064 | KF251559 | KF253862 | KF254211 |
| Septoria sp. | – | CBS 135478; CPC 19716 | Searsia laevigatum | South Africa | A. Wood | KF253508 | KF253028 | KF252554 | KF252065 | KF251560 | – | KF254212 |
| Septoria sp. | – | CBS 135479; CPC 19793 | Syzygium cordatum | South Africa | P.W. Crous | – | KF253029 | KF252555 | KF252066 | KF251561 | – | KF254213 |
| Septoria sp. | – | CPC 23103; MP11 | Aesculus sp. | Netherlands | S.I.R. Videira | KF253510 | KF253031 | KF252556 | KF252068 | KF251563 | KF253864 | KF254215 |
| Sep. stachydicola | – | CBS 128668 | Stachys riederi | South Korea | H.D. Shin | KF253512 | KF253033 | KF252558 | KF252070 | KF251565 | KF253866 | KF254217 |
| Sep. stachydis | – | CBS 109115 | Campanula glomerata | Austria | G.J.M. Verkley | KF253502 | KF253022 | KF252548 | KF252059 | KF251554 | KF253857 | KF254206 |
| – | CBS 102326 | Stachys sylvatica | Netherlands | G.J.M. Verkley | KF253514 | KF253035 | KF252560 | KF252072 | KF251567 | KF253868 | KF254219 | |
| – | CBS 102337 | Stachys sylvatica | Netherlands | G.J.M. Verkley | KF253515 | KF253036 | KF252561 | KF252073 | KF251568 | KF253869 | KF254220 | |
| – | CBS 109126 | Stachys sylvatica | Austria | G.J.M. Verkley | KF253516 | KF253037 | KF252562 | KF252074 | KF251569 | KF253870 | KF254221 | |
| – | CBS 109127 | Stachys sylvatica | Austria | G.J.M. Verkley | KF253517 | KF253038 | KF252563 | KF252075 | KF251570 | KF253871 | KF254222 | |
| – | CBS 123750 | Stachys sp. | Czech Republic | G.J.M. Verkley | KF253518 | KF253039 | KF252564 | KF252076 | KF251571 | KF253872 | KF254223 | |
| – | CBS 123879 | Stachys sp. | Czech Republic | G.J.M. Verkley | KF253519 | KF253040 | KF252565 | KF252077 | KF251572 | KF253873 | KF254224 | |
| – | CBS 449.68 | Stachys sylvatica | Netherlands | H.A. van der Aa | KF253520 | KF253041 | KF252566 | KF252078 | KF251573 | KF253874 | KF254225 | |
| Sep. astericola | CBS 347.58 | Aster canus | Germany | R. Schneider | KF253295 | KF252820 | KF252349 | KF251852 | KF251348 | KF253652 | KF254000 | |
| Sep. stellariae | – | CBS 102376 | Stellaria media | Netherlands | G.J.M. Verkley | KF253521 | KF253042 | KF252567 | KF252079 | KF251574 | KF253875 | KF254226 |
| – | CBS 102378 | Stellaria media | Netherlands | G.J.M. Verkley | KF253522 | KF253043 | KF252568 | KF252080 | KF251575 | KF253876 | KF254227 | |
| – | CBS 102410 | Stellaria media | Netherlands | G.J.M. Verkley | KF253523 | KF253044 | KF252569 | KF252081 | KF251576 | KF253877 | KF254228 | |
| Sep. taraxaci | – | CBS 567.75 | Taraxacum sp. | Armenia | H.A. van der Aa | KF253524 | KF253045 | KF252570 | KF252082 | KF251577 | KF253878 | KF254229 |
| Sep. tinctoriae | – | CBS 129154 | Serratula coronata | South Korea | H.D. Shin | KF253525 | KF253046 | KF252571 | KF252083 | KF251578 | KF253879 | KF254230 |
| Sep. tormentillae | – | CBS 128643 | Potentilla fragarioides | South Korea | H.D. Shin | KF253526 | KF253047 | KF252572 | KF252084 | KF251579 | KF253880 | KF254231 |
| – | CBS 128647 | Potentilla fragarioides | South Korea | H.D. Shin | KF253527 | KF253048 | KF252573 | KF252085 | KF251580 | KF253881 | KF254232 | |
| Sep. urticae | Septoria glechomatis | CBS 102316 | Glechoma hederacea | Netherlands | G.J.M. Verkley | KF253528 | KF253049 | KF252574 | KF252086 | KF251581 | KF253882 | KF254233 |
| – | CBS 102371 | Urtica dioica | Netherlands | G.J.M. Verkley | KF253529 | KF253050 | KF252575 | KF252087 | KF251582 | KF253883 | KF254234 | |
| – | CBS 102375 | Urtica dioica | Netherlands | G.J.M. Verkley | KF253530 | KF253051 | KF252576 | KF252088 | KF251583 | KF253884 | KF254235 | |
| Sep. verbascicola | – | CBS 102401 | Verbascum nigrum | Netherlands | G.J.M. Verkley | KF253531 | KF253052 | KF252577 | KF252089 | KF251584 | KF253885 | KF254236 |
| Sep. verbenae | – | CBS 113438 | Verbena officinalis | New Zealand | G.J.M. Verkley | KF253532 | KF253053 | KF252578 | KF252090 | KF251585 | KF253886 | KF254237 |
| – | CBS 113481 | Verbena officinalis | New Zealand | G.J.M. Verkley | KF253533 | KF253054 | KF252579 | KF252091 | KF251586 | KF253887 | KF254238 | |
| Sep. villarsiae | – | CBS 514.78 | Nymphoides peltata | Netherlands | H.A. van der Aa | KF253534 | KF253055 | KF252580 | KF252092 | KF251587 | KF253888 | KF254239 |
| – | CBS 565.88 | Nymphoides peltata | Netherlands | H.A. van der Aa | KF253535 | KF253056 | KF252581 | KF252093 | KF251588 | KF253889 | KF254240 | |
| – | CBS 604.66 | Nymphoides peltata | Netherlands | L. Marvanová | KF253536 | KF253057 | KF252582 | KF252094 | KF251589 | KF253890 | KF254241 | |
| Sep. violae-palustris | – | CBS 128644 | Viola selkirkii | South Korea | H.D. Shin | KF253537 | KF253058 | KF252583 | KF252095 | KF251590 | KF253891 | KF254242 |
| – | CBS 128660 | Viola yedoensis | South Korea | H.D. Shin | KF253538 | KF253059 | KF252584 | KF252096 | KF251591 | KF253892 | KF254243 | |
| Sphaerulina abeliceae | Septoria abeliceae | CBS 128591 | Zelkova serrata | South Korea | H.D. Shin | KF253539 | – | KF252585 | KF252097 | KF251592 | KF253894 | KF254245 |
| Sphaerulina aceris | Mycosphaerella latebrosa | CBS 183.97 | Acer pseudoplatanus | Netherlands | H.A. van der Aa | KF253540 | – | KF252586 | KF252098 | KF251593 | KF253895 | KF254246 |
| Mycosphaerella latebrosa | CBS 652.85 | Acer pseudoplatanus | Netherlands | H.A. van der Aa | KF253541 | KF253060 | KF252587 | KF252099 | KF251594 | KF253896 | KF254300 | |
| Mycosphaerella latebrosa | CBS 687.94 | Acer pseudoplatanus | Netherlands | G.J.M. Verkley | KF253542 | KF253061 | KF252588 | KF252100 | KF251595 | KF253897 | KF254247 | |
| Sphaerulina amelanchier | – | CBS 135110 | Amelanchier sp. | Netherlands | S.I.R. Videira | KF253543 | KF253062 | KF252589 | KF252101 | KF251596 | KF253898 | KF254248 |
| Septoria sp. | CPC 23107; MP9 | Betula sp. | Netherlands | S.I.R. Videira | KF253583 | KF253098 | KF252626 | KF252139 | KF251634 | KF253937 | KF254288 | |
| Septoria sp. | CPC 23105; MP22 | Quercus sp. | Netherlands | S.I.R. Videira | KF253544 | KF253063 | KF252590 | KF252102 | KF251597 | KF253899 | KF254249 | |
| – | CPC 23106; MP7 | Castanea sp. | Netherlands | S.I.R. Videira | KF253545 | KF253064 | KF252591 | KF252103 | KF251598 | KF253900 | KF254250 | |
| Sphaerulina azaleae | Septoria azaleae | CBS 128605 | Rhododendron sp. | South Korea | H.D. Shin | KF253546 | KF253065 | KF252592 | KF252104 | KF251599 | KF253901 | KF254251 |
| Septoria azaleae | CBS 352.49 | Rhododendron sp. | Belgium | J. van Holder | KF253547 | KF253066 | KF252593 | KF252105 | KF251600 | KF253902 | KF254252 | |
| Sphaerulina berberidis | Mycosphaerella berberidis | CBS 324.52 | Berberis vulgaris | Switzerland | E. Müller | KF253548 | KF253067 | KF252594 | KF252106 | KF251601 | KF253903 | KF254253 |
| Sphaerulina betulae | Septoria betulae | CBS 116724 | Betula pubescens | Scotland | S. Green | KF253549 | KF253068 | KF252595 | KF252107 | KF251602 | KF253904 | KF254254 |
| Septoria betulae | CBS 128596 | Betula platyphylla | South Korea | H.D. Shin | KF253550 | KF253069 | KF252596 | KF252108 | KF251603 | KF253905 | KF254255 | |
| Septoria betulae | CBS 128597 | Betula schmidtii | South Korea | H.D. Shin | KF253551 | KF253070 | KF252597 | KF252109 | KF251604 | KF253906 | KF254256 | |
| Septoria betulae | CBS 128600 | Betula platyphylla | South Korea | H.D. Shin | KF253552 | KF253071 | KF252598 | KF252110 | KF251605 | KF253907 | KF254257 | |
| Sphaerulina cercidis | Septoria provencialis | CBS 118910 | Eucalyptus sp. | France | P.W. Crous | KF253553 | KF253072 | KF252602 | KF252114 | KF251609 | KF253908 | KF254258 |
| Septoria cercidis | CBS 128634 | Cercis siliquastrum | South Korea | H.D. Shin | KF253554 | KF253073 | KF252599 | KF252111 | KF251606 | KF253909 | KF254259 | |
| Septoria cercidis | CBS 129151 | Cercis siliquastrum | South Korea | H.D. Shin | KF253555 | KF253074 | KF252600 | KF252112 | KF251607 | KF253910 | KF254260 | |
| Septoria cercidis | CBS 501.50 | Cercis siliquastrum | Netherlands | G. van den Ende | KF253556 | KF253075 | KF252601 | KF252113 | KF251608 | KF253911 | KF254261 | |
| Sphaerulina cornicola | Septoria cornicola | CBS 102324 | Cornus sp. | Netherlands | A. van Iperen | KF253557 | KF253076 | KF252603 | KF252115 | KF251610 | KF253912 | KF254262 |
| Septoria comicola | CBS 102332 | Cornus sp. | Netherlands | A. van Iperen | KF253558 | KF253077 | KF252604 | KF252116 | KF251611 | KF253913 | KF254263 | |
| Septoria cornicola | CBS 116778 | Cornus sanguinea | USA | A.Y. Rossman | KF253559 | KF253078 | – | KF252117 | KF251612 | KF253914 | KF254264 | |
| Sphaerulina frondicola | Septoria populi | CBS 391.59 | Populus pyramidalis | Germany | R. Schneider | KF253572 | – | KF252617 | KF252130 | KF251625 | KF253927 | KF254277 |
| Sphaerulina gei | Septoria gei | CBS 102318 | Geum urbanum | Netherlands | G.J.M. Verkley | KF253560 | KF253079 | KF252605 | KF252118 | KF251613 | KF253915 | KF254265 |
| Septoria gei | CBS 128616 | Geum japonicum | South Korea | H.D. Shin | KF253561 | KF253080 | KF252606 | KF252119 | KF251614 | KF253916 | KF254266 | |
| Septoria gei | CBS 128632 | Geum japonicum | South Korea | H.D. Shin | KF253562 | KF253081 | KF252607 | KF252120 | KF251615 | KF253917 | KF254267 | |
| Sphaerulina hyperici | Septoria hyperici | CBS 102313 | Hypericum sp. | Netherlands | G.J.M. Verkley | KF253563 | KF253082 | KF252608 | KF252121 | KF251616 | KF253918 | KF254268 |
| Sphaerulina menispermi | Septoria menispermi | CBS 128666 | Menispermum dauricum | South Korea | H.D. Shin | KF253564 | KF253083 | KF252609 | KF252122 | KF251617 | KF253919 | KF254269 |
| Septoria menispermi | CBS 128761 | Menispermum dauricum | South Korea | H.D. Shin | KF253565 | KF253084 | KF252610 | KF252123 | KF251618 | KF253920 | KF254270 | |
| Sphaerulina musiva | Septoria musiva | CBS 130559 | Populus sp. | Canada | J. LeBoldus | KF253566 | – | KF252611 | KF252124 | KF251619 | KF253921 | KF254271 |
| Septoria musiva | CBS 130562 | Populus sp. | Canada | J. LeBoldus | KF253567 | KF253085 | KF252612 | KF252125 | KF251620 | KF253922 | KF254272 | |
| Septoria musiva | CBS 130563 | Populus deltoides × P. balsamifera | Canada | J. LeBoldus | KF253568 | – | KF252613 | KF252126 | KF251621 | KF253923 | KF254273 | |
| Septoria musiva | CBS 130569 | Populus deltoides | Canada | J. LeBoldus | KF253569 | KF253086 | KF252614 | KF252127 | KF251622 | KF253924 | KF254274 | |
| Sphaerulina patriniae | Septoria patriniae | CBS 128653 | Patrinia scabiosaefolia | South Korea | H.D. Shin | KF253570 | KF253087 | KF252615 | KF252128 | KF251623 | KF253925 | KF254275 |
| Septoria patriniae | CBS 129153 | Patrinia villosa | South Korea | H.D. Shin | KF253571 | KF253088 | KF252616 | KF252129 | KF251624 | KF253926 | KF254276 | |
| Sphaerulina populicola | Mycosphaerella populicola | CBS 100042 | Populus trichocarpa | USA | G. Newcombe | KF253573 | – | KF252618 | KF252131 | KF251626 | KF253928 | KF254278 |
| Sphaerulina quercicola | Septoria quercicola | CBS 109009 | Quercus rubra | Netherlands | G.J.M. Verkley | KF253574 | KF253089 | KF252619 | KF252132 | KF251627 | KF253929 | KF254279 |
| Septoria quercicola | CBS 115016 | Quercus robur | Netherlands | G.J.M. Verkley | KF253575 | KF253090 | KF252620 | KF252133 | KF251628 | KF253930 | KF254280 | |
| Septoria quercicola | CBS 115136 | Quercus robur | Netherlands | G.J.M. Verkley | KF253576 | KF253091 | KF252621 | KF252134 | KF251629 | KF253931 | KF254281 | |
| Septoria quercicola | CBS 663.94 | Quercus robur | Netherlands | H.A. van der Aa | KF253577 | KF253092 | KF252622 | KF252135 | KF251630 | KF253932 | KF254282 | |
| Sphaerulina rhabdoclinis | Dothistroma rhabdoclinis | CBS 102195 | Pseudotsuga menziesii | Germany | H. Butin | KF253578 | KF253093 | KF252623 | KF252136 | KF251631 | – | KF254283 |
| Sphaerulina socia | Septoria rosae | CBS 355.58 | Rosa sp. | – | – | KF253579 | KF253094 | KF252624 | KF252137 | KF251632 | KF253933 | KF254284 |
| Septoria socia | CBS 357.58 | Chrysanthemum leucanthemum | Germany | R. Schneider | KF253580 | KF253095 | KF252625 | KF252138 | KF251633 | KF253934 | KF254285 | |
| Sphaerulina sp. | Septoria sp. | CBS 102063 | Actinidia deliciosa | New Zealand | C.F. Hill | KF253581 | KF253096 | KF252627 | KF252140 | KF251635 | KF253935 | KF254286 |
| Sphaerulina sp. | Septoria lysimachiae | CBS 128758 | Lysimachia clethroides | South Korea | H.D. Shin | KF253582 | KF253097 | KF252628 | KF252141 | KF251636 | KF253936 | KF254287 |
| Sphaerulina tirolensis | Septoria rubi | CBS 109017 | Rubus idaeus | Austria | G.J.M. Verkley | KF253584 | KF253099 | KF252629 | KF252142 | KF251637 | KF253938 | KF254289 |
| Mycosphaerella rubi | CBS 109018 | Rubus idaeus | Austria | G.J.M. Verkley | KF253585 | KF253100 | KF252630 | KF252143 | KF251638 | KF253939 | KF254290 | |
| Sphaerulina viciae | – | CBS 131898 | Vicia amurense | South Korea | H.D. Shin | KF253586 | KF253101 | KF252631 | KF252144 | KF251639 | KF253940 | KF254291 |
| Sphaerulina westendorpii | Septoria rubi | CBS 102327 | Rubus sp. | Netherlands | G.J.M. Verkley | KF253587 | KF253102 | KF252632 | KF252145 | KF251640 | KF253941 | KF254292 |
| Mycosphaerella rubi | CBS 109002 | Rubus sp. | Netherlands | G.J.M. Verkley | KF253588 | KF253103 | KF252633 | KF252146 | KF251641 | KF253942 | KF254293 | |
| Septoria rubi | CBS 117478 | Rubus fruticosus | Netherlands | G.J.M. Verkley | KF253589 | KF253104 | KF252634 | KF252147 | KF251642 | KF253943 | KF254294 | |
| Zymoseptoria brevis | – | CPC 18102 | Phalaris paradoxa | Iran | M. Razavi | KF253590 | – | KF252635 | KF252148 | KF251643 | KF253944 | KF254295 |
| – | CPC 18107 | Phalaris minor | Iran | M. Razavi | KF253591 | – | KF252636 | KF252149 | KF251644 | KF253945 | KF254296 | |
| Zymoseptoria halophila | – | CBS 128854 | Hordeum glaucum | Iran | M. Razavi | KF253592 | – | – | KF252150 | KF251645 | KF253946 | KF254297 |
| Zymoseptoria tritici | – | CPC 18099 | Aegilops tauschii | Iran | M. Razavi | KF253594 | – | KF252638 | KF252152 | KF251647 | KF253948 | KF254299 |
| – | CBS 392.59 | Triticum aestivum | Switzerland | E. Becker | KF253593 | – | KF252637 | KF252151 | KF251646 | KF253947 | KF254298 | |
CBS: CBS Fungal Biodiversity Centre, Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; CPC: Collection Pedro Crous, housed at CBS; S: William Quaedvlieg working collection (will be merged into the CPC collection); MP: Sandra Isabel Rodrigues Videira working collection (will be merged into the CPC collection).
Act:: Actin, Cal: Calmodulin, EF: Translation elongation factor 1-alpha, RPB2: RNA polymerase II second largest subunit, Btub: β-tubulin LSU: 28S large subunit of the nrRNA gene and ITS: internal transcribed spacer regions of the nrDNA operon.
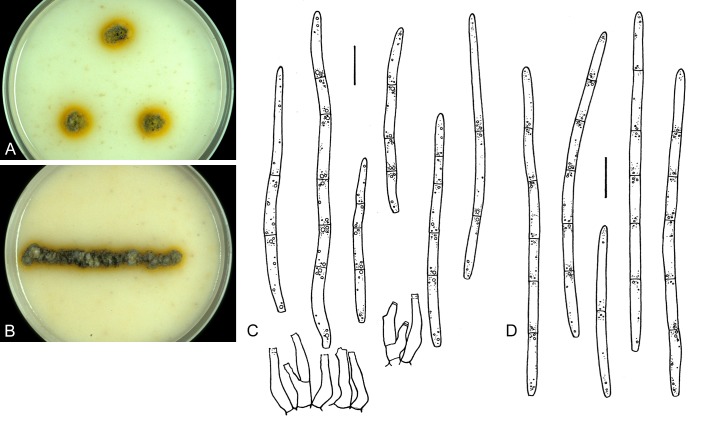
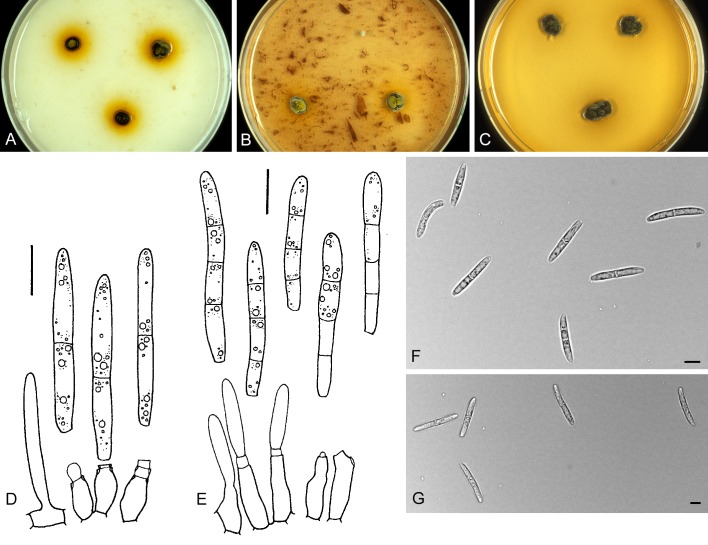
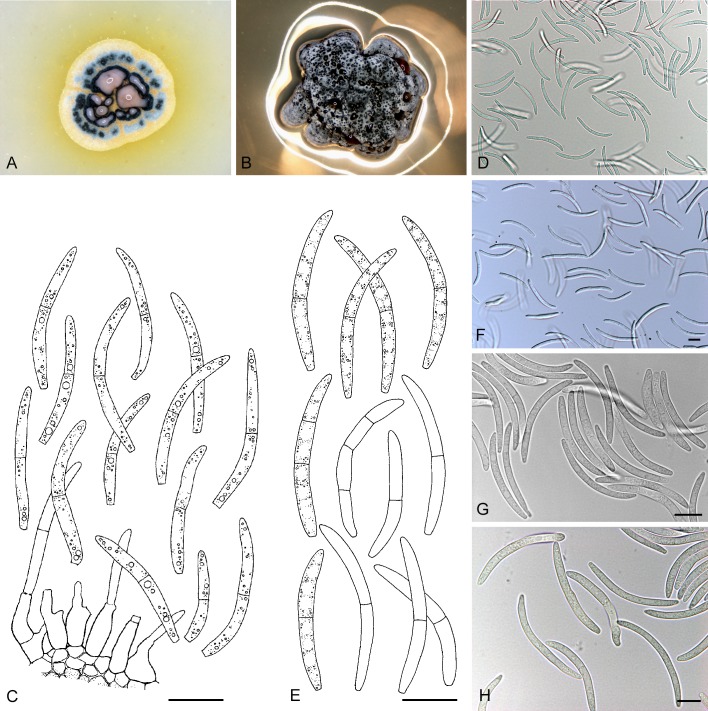
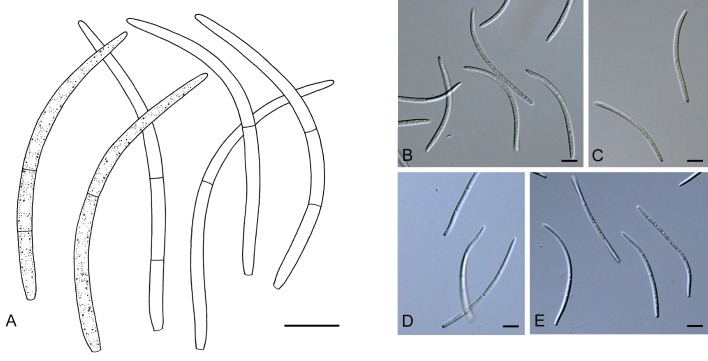
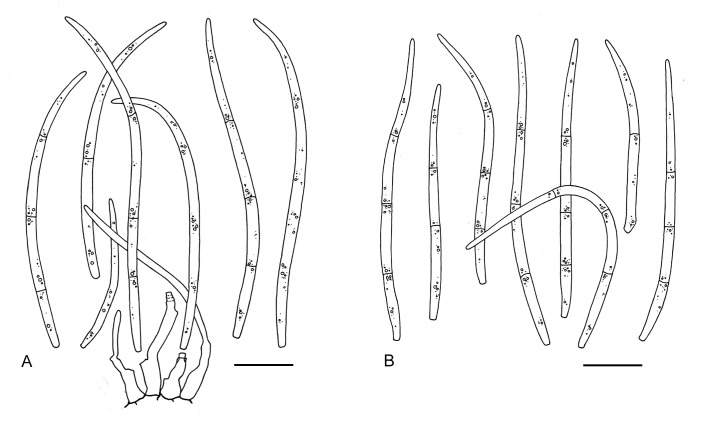
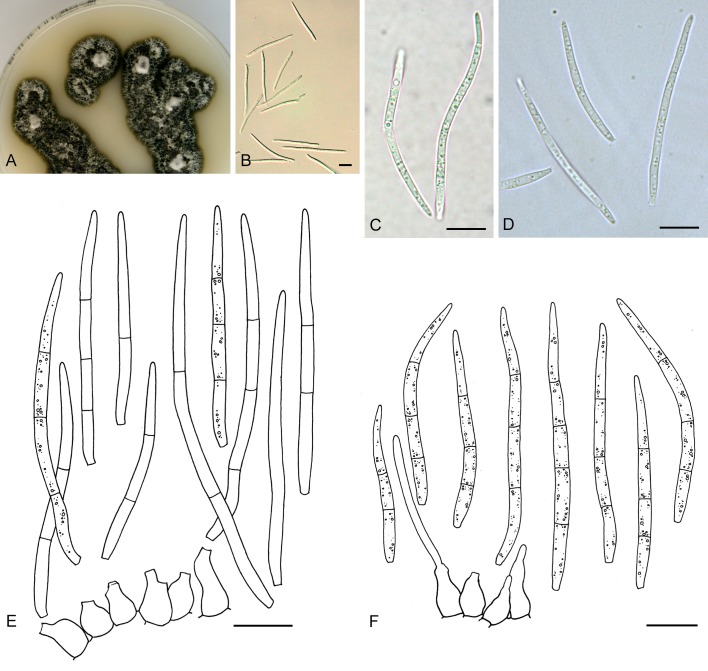
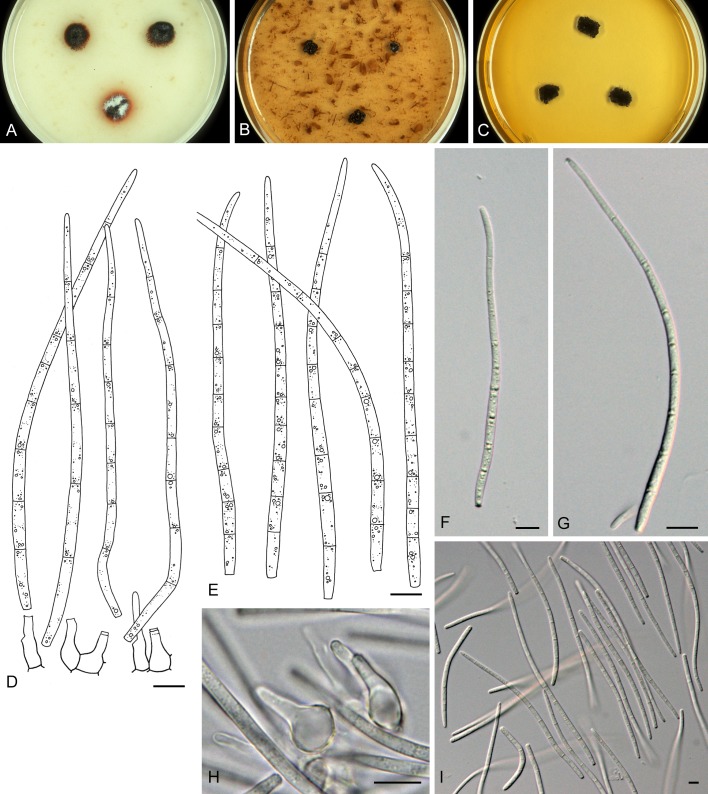
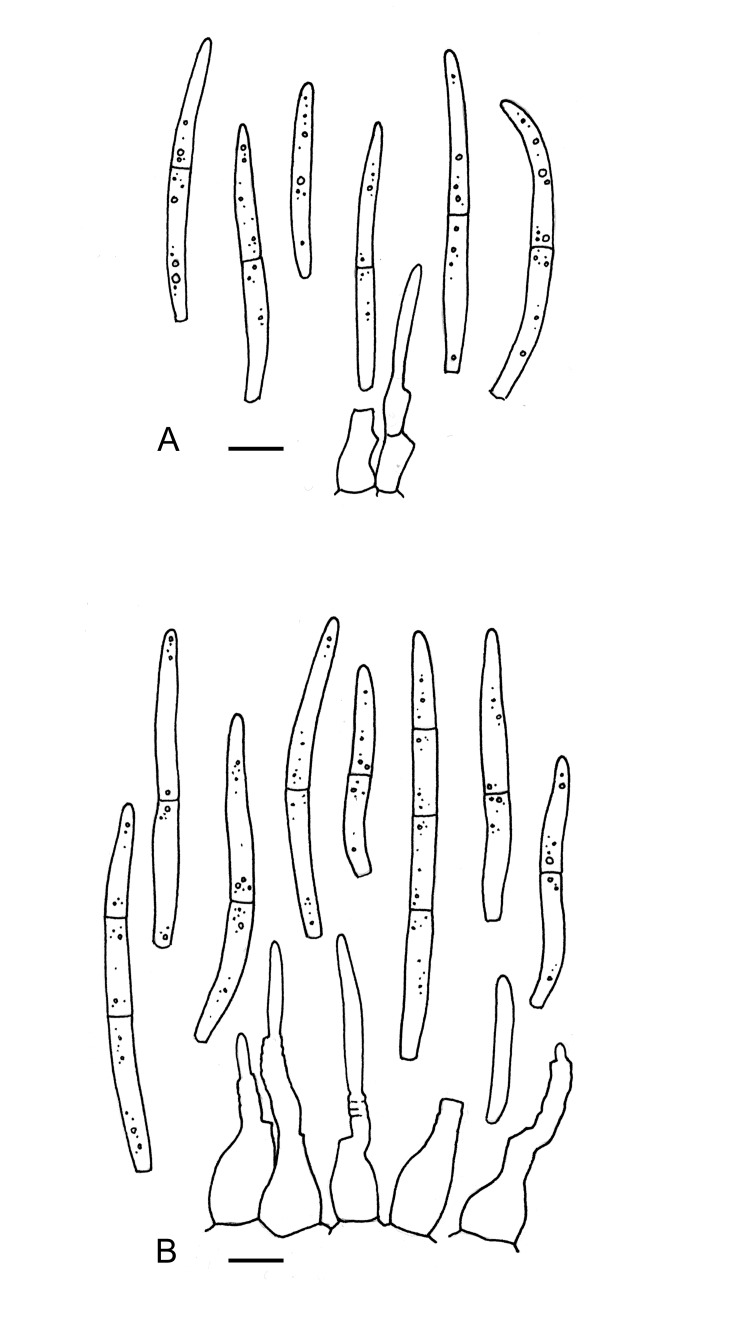
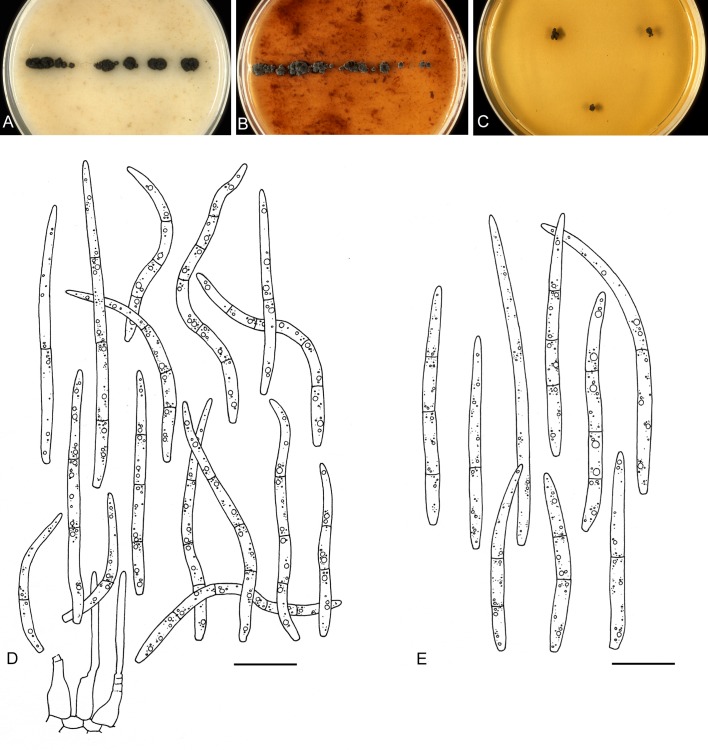
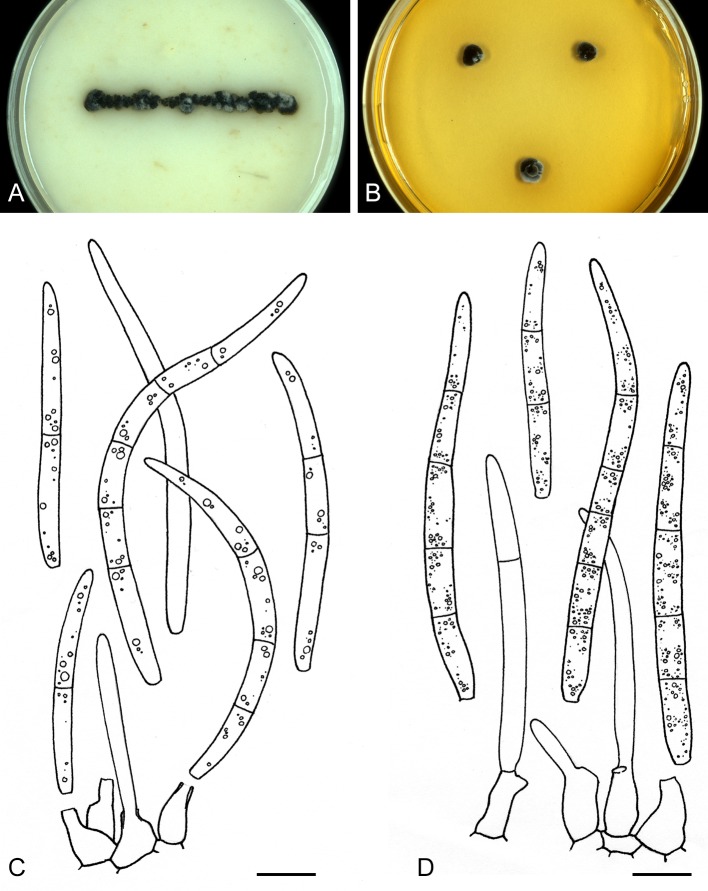
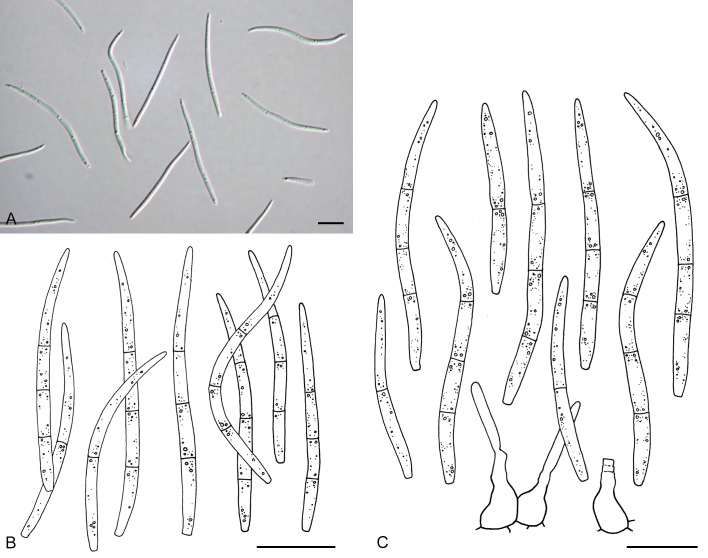
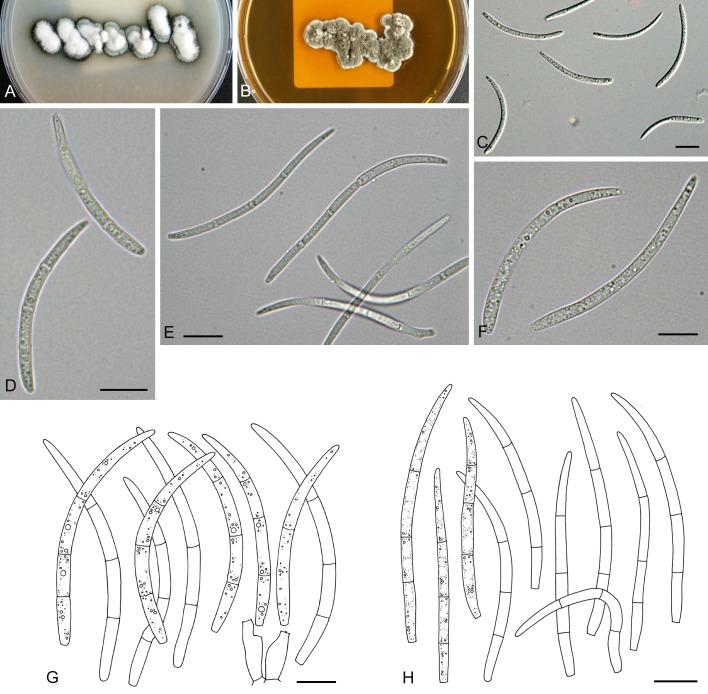
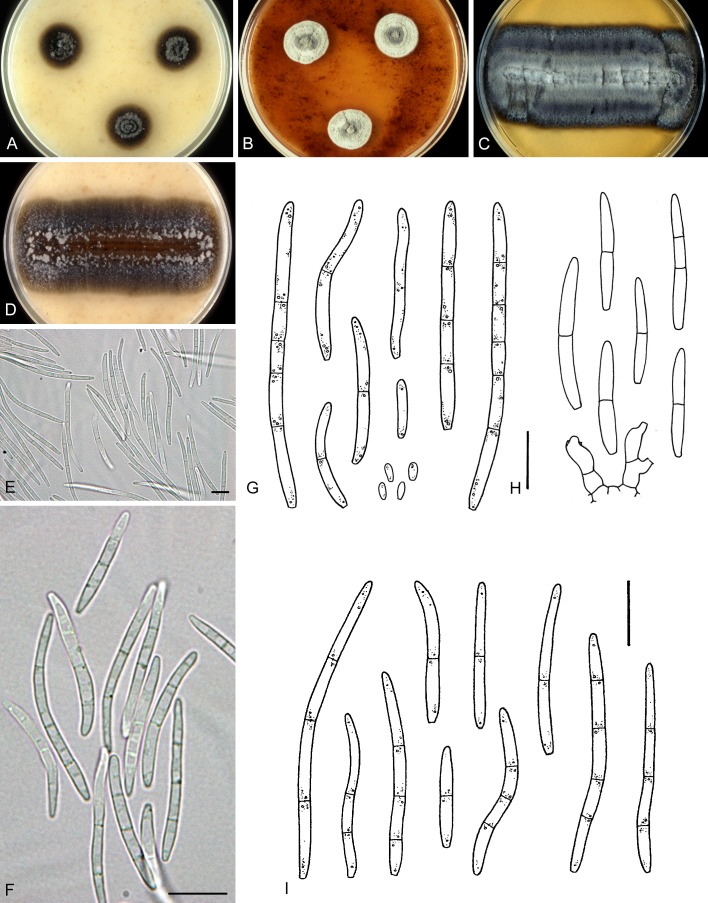
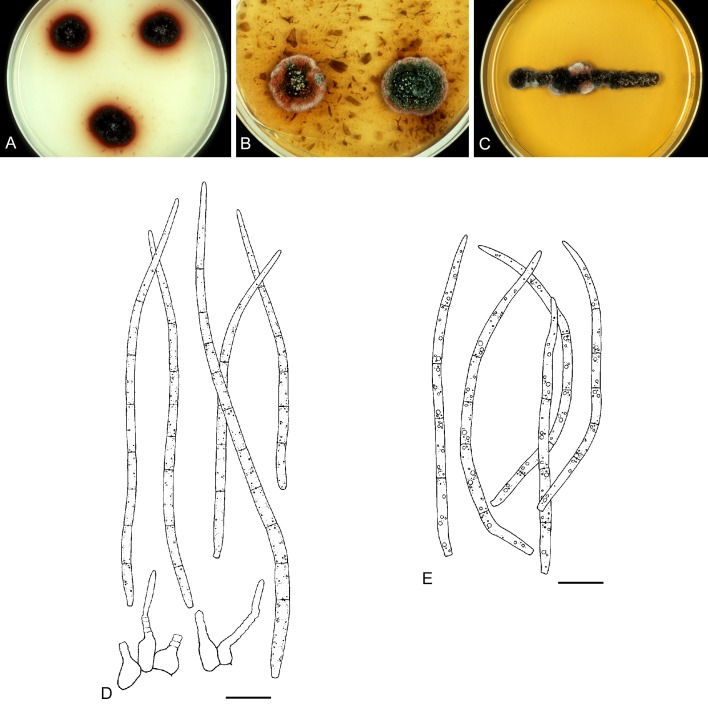
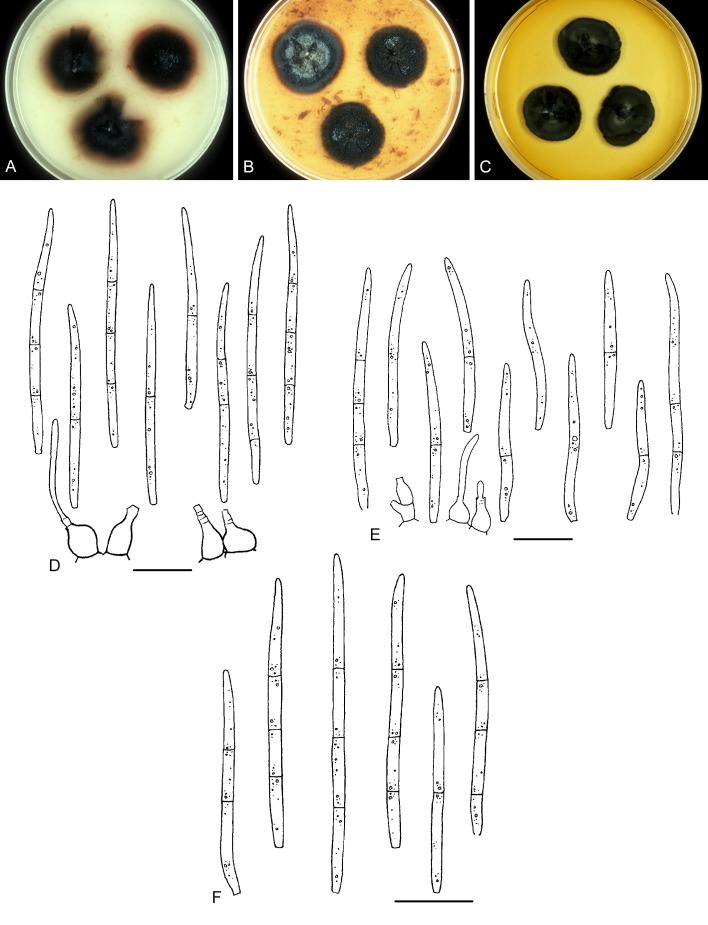
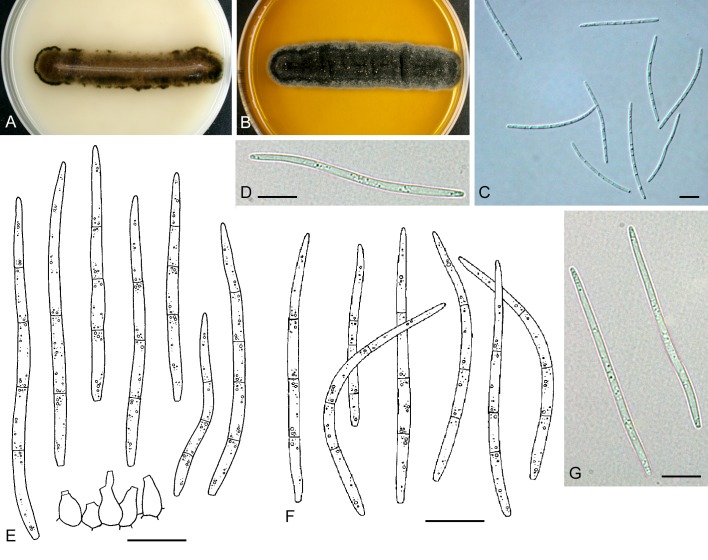
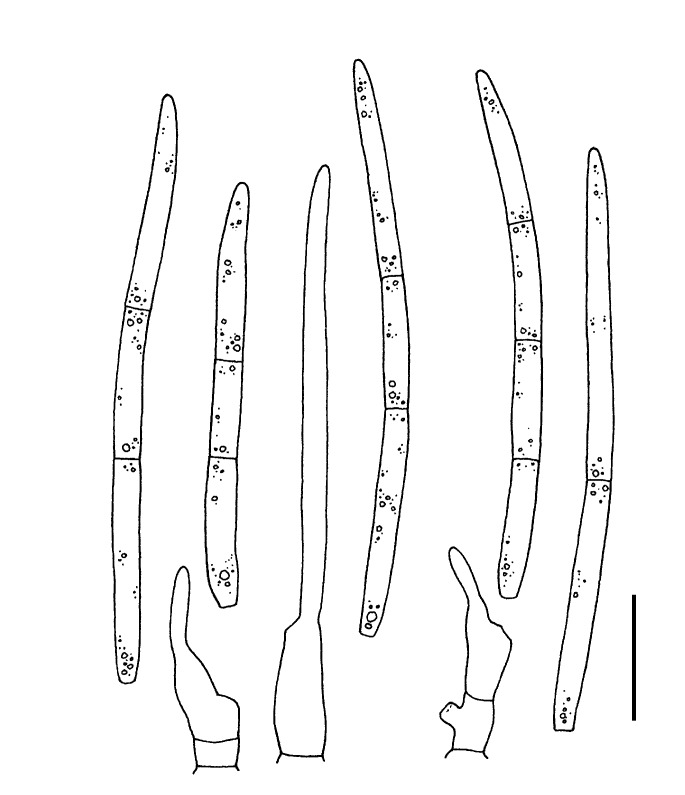
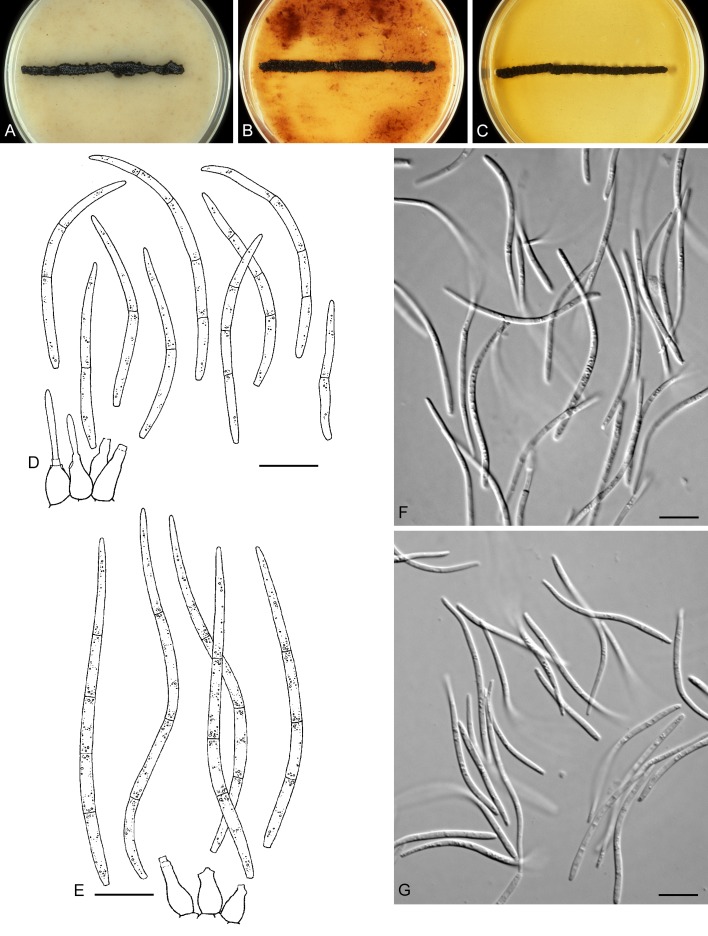
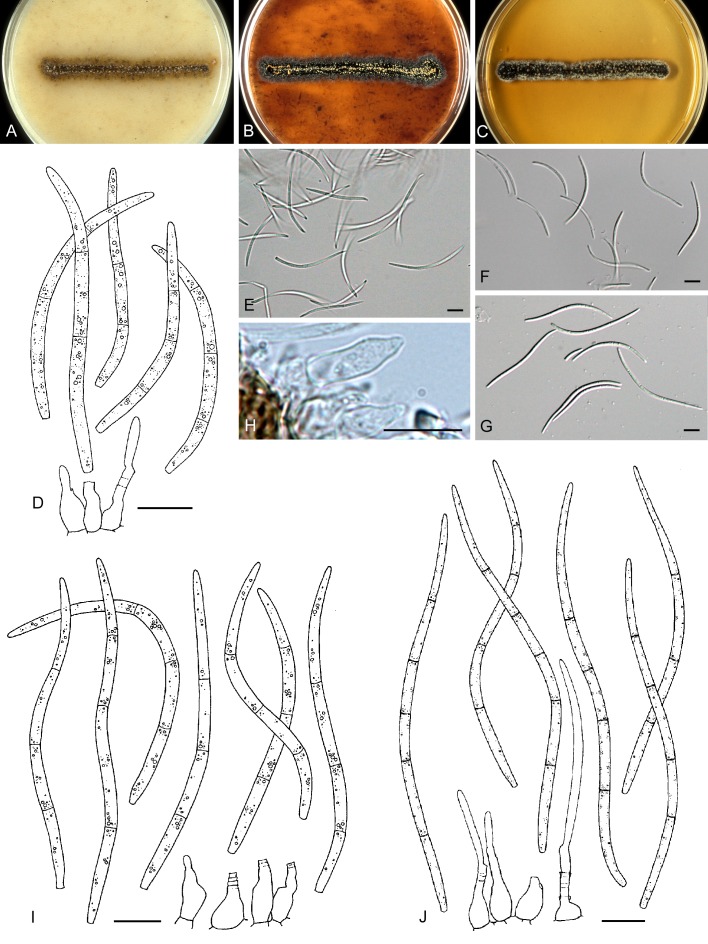
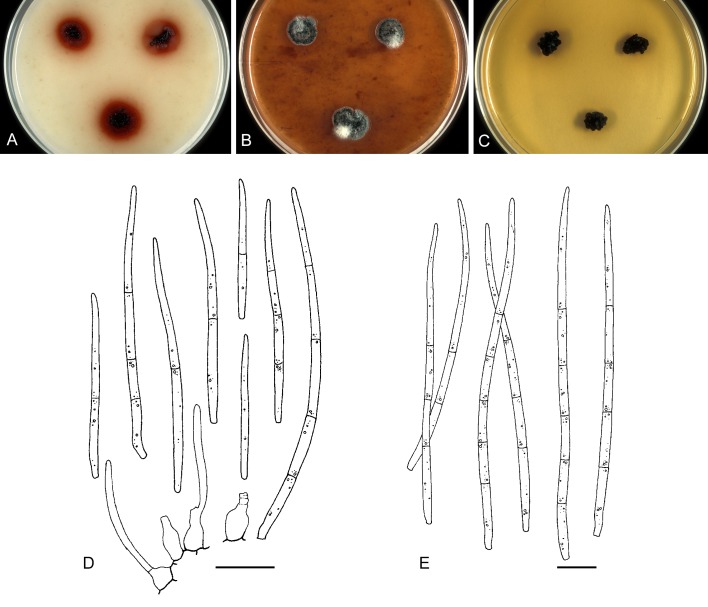
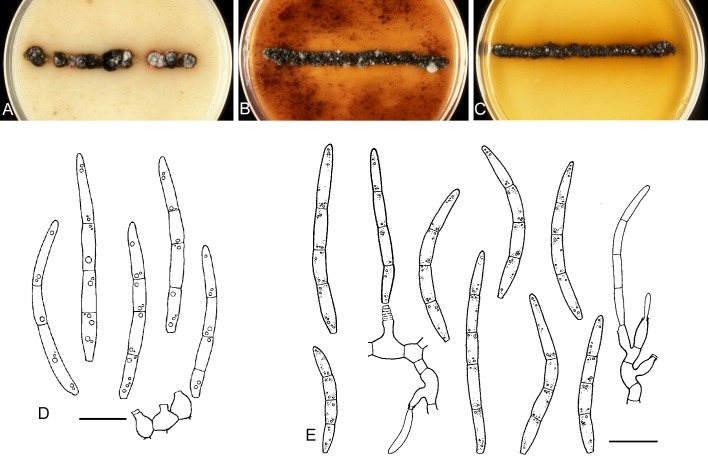
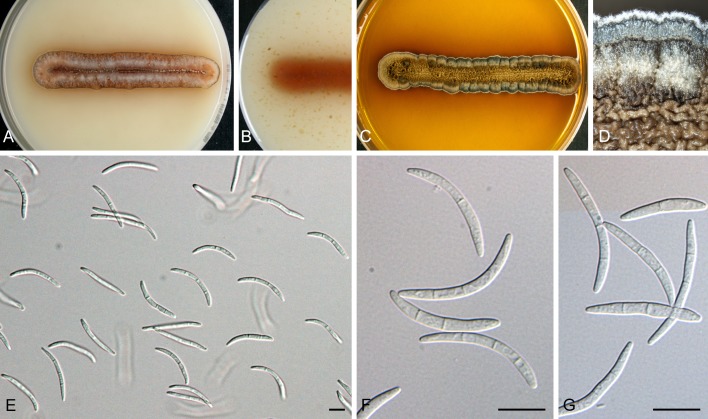
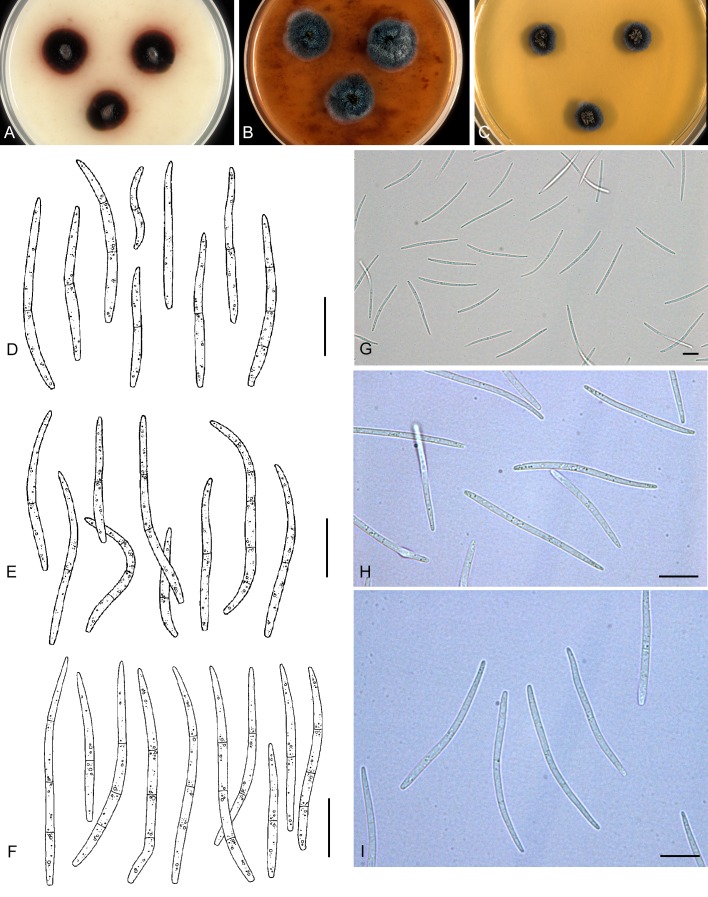
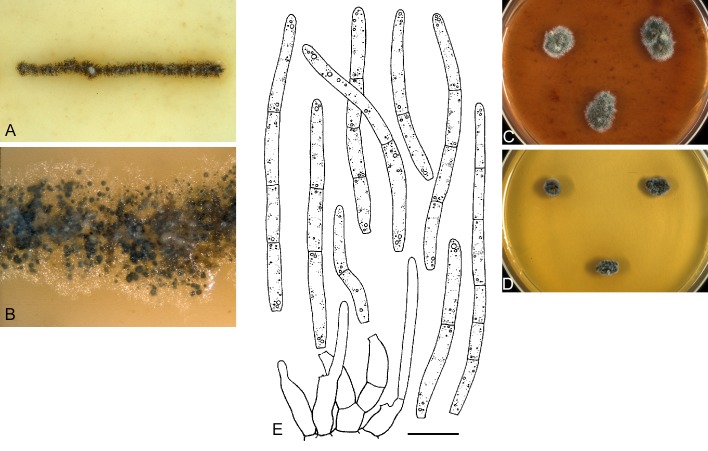
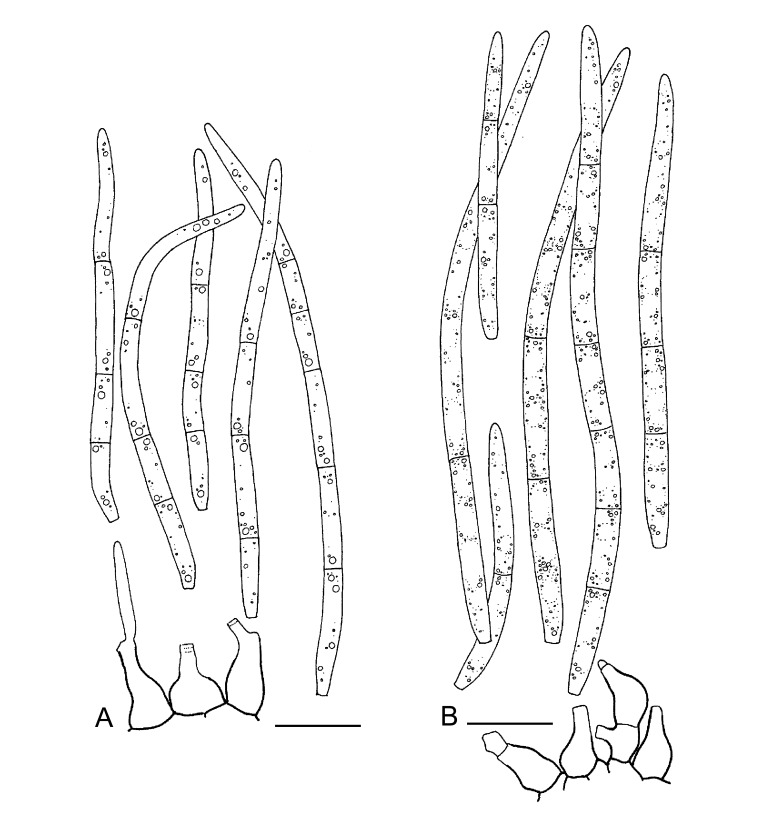
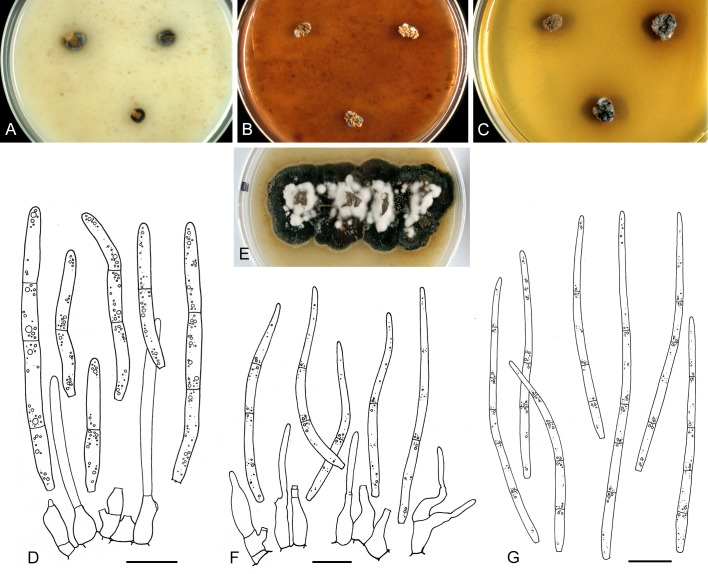
For the morphological study in planta hand sections were made from infected leaves, mounted in water and examined under an Olympus BX 50 microscope equipped with bright field and differential interference contrast (DIC) objectives, and photographed using a mounted Nikon Digital Sight DS-5M camera. Conidial masses were mounted in water and 30 spores measured. For culture studies, 7-14-d-old cultures were transferred to fresh OA, MEA and cherry decoction agar (CHA) plates and placed in an incubator under n-UV light (12 h light, 12 h dark) at 15 °C to promote sporulation (if otherwise, this is indicated in the descriptions). Media were prepared according to Crous et al. (2009). Colony colours were described according to Rayner (1970). Sporulating structures obtained from cultures were used for the morphological description in vitro. Photographs of culture plates were taken after 2-3 wk on a photo stand with daylight tubes with a Pentax K110 D digital camera. Cultures were incubated up to 40 d to observe sporulation and other features.
DNA isolation, PCR and sequencing
Genomic DNA was extracted from fungal mycelium growing on MEA, using the UltraClean® Microbial DNA Isolation Kit (Mo Bio Laboratories, Inc., Solana Beach, CA, USA). Strains (Table 1) were sequenced for seven loci: Actin (Act), calmodulin (Cal), β-tubulin (Btub), internal transcribed spacer (ITS), Translation elongation factor 1-alpha (EF) 28S nrDNA (LSU) and RNA polymerase II second largest subunit (RPB2); the primer sets listed in Table 2 were used. The PCR amplifications were performed in a total volume of 12.5 μL solution containing 10-20 ng of template DNA, 1 × PCR buffer, 0.7 μL DMSO (99.9 %), 2 mM MgCl2, 0.4 μM of each primer, 25 μM of each dNTP and 1.0 U Taq DNA polymerase (GoTaq, Promega). PCR amplification conditions were set as follows: an initial denaturation temperature of 96 °C for 2 min, followed by 40 cycles at the denaturation temperature of 96 °C for 45 s, primer annealing at the temperature stipulated in Table 2, primer extension at 72 °C for 90 s and a final extension step at 72 °C for 2 min. The resulting fragments were sequenced using the PCR primers together with a BigDye Terminator Cycle Sequencing Kit v. 3.1 (Applied Biosystems, Foster City, CA). Sequencing reactions were performed as described by Cheewangkoon et al. (2008). All novel sequences were deposited in NCBI’s GenBank database and alignments and phylogenetic trees in TreeBASE.
Table 2.
Primer combinations used during this study for generic amplification and sequencing.
| Locus | Primer | Primer sequence 5’ to 3’: |
Annealing
temperature (°C) |
Orientation | Reference |
|---|---|---|---|---|---|
| Translation elongation factor-1α | EF1-728F | CATCGAGAAGTTCGAGAAGG | 52 | Forward | Carbone & Kohn (1999) |
| EF-2 | GGARGTACCAGTSATCATGTT | 52 | Reverse | O’Donnell et al. (1998) | |
| β-tubulin | T1 | AACATGCGTGAGATTGTAAGT | 52 | Forward | O’Donnell & Cigelnik (1997) |
| β-Sandy-R | GCRCGNGGVACRTACTTGTT | 52 | Reverse | Stukenbrock et al. (2012) | |
| RNA polymerase II second largest subunit | fRPB2-5F | GAYGAYMGWGATCAYTTYGG | 49 | Forward | Liu et al. (1999) |
| fRPB2-414R | ACMANNCCCCARTGNGWRTTRTG | 49 | Reverse | Quaedvlieg et al. (2011) | |
| LSU | LSU1Fd | GRATCAGGTAGGRATACCCG | 52 | Forward | Crous et al. (2009a) |
| LR5 | TCCTGAGGGAAACTTCG | 52 | Reverse | Vilgalys & Hester (1990) | |
| ITS | ITS5 | GGAAGTAAAAGTCGTAACAAGG | 52 | Forward | White et al. (1990) |
| ITS4 | TCCTCCGCTTATTGATATGC | 52 | Reverse | White et al. (1990) | |
| Actin | ACT-512F | ATGTGCAAGGCCGGTTTCGC | 52 | Forward | Carbone & Kohn (1999) |
| ACT2Rd | ARRTCRCGDCCRGCCATGTC | 52 | Reverse | Groenewald et al. (2012) | |
| Calmodulin | CAL-235F | TTCAAGGAGGCCTTCTCCCTCTT | 50 | Forward | Quaedvlieg et al. (2012) |
| CAL2Rd | TGRTCNGCCTCDCGGATCATCTC | 50 | Reverse | Groenewald et al. (2012) |
Sequence alignement and phylogenetic analyses
A basic alignment of the obtained sequence data was first done using MAFFT v. 7 (http://mafft.cbrc.jp/alignment/server/index.html; Katoh et al. 2002) and if necessary, manually improved in BioEdit v. 7.0.5.2 (Hall 1999). To check the congruency of the multigene dataset, a 70 % neighbour-joining (NJ) reciprocal bootstrap method with maximum likelihood distance was performed (Mason-Gamer & Kellogg 1996, Lombard et al. 2010). Bayesian analyses (critical value for the topological convergence diagnostic set to 0.01) were performed on the concatenated loci using MrBayes v. 3.2.1 (Huelsenbeck & Ronquist 2001) as described by Crous et al. (2006a) using nucleotide substitution models that were selected using MrModeltest (Table 3) (Nylander 2004).
Table 3.
Amplification success, phylogenetic data and the substitution models used in the phylogenetic analysis, per locus.
| Locus | Act | Cal | EF1 | RPB2 | Btub | ITS | LSU |
|---|---|---|---|---|---|---|---|
| Amplification succes (%) | 99 | 100 | 100 | 97 | 100 | 100 | 100 |
| Number of characters | 304 | 601 | 619 | 354 | 565 | 574 | 853 |
| Unique site patterns | 234 | 407 | 507 | 198 | 380 | 261 | 147 |
| Substitution model used | GTR-I-gamma |
HKY-I-gamma |
GTR-I-gamma |
GTR-I-gamma |
HKY-I-gamma |
GTR-I-gamma |
GTR-I-gamma |
| Number of generations (1000×) | 10 197 | ||||||
| Total number of trees (n) | 22 962 | ||||||
| Sampled trees (n) | 17 222 |
Kimura-2-parameter values
The inter-and intraspecific distances for each individual dataset were calculated using MEGA v. 4.0 (Tamura et al. 2007) with the Kimura-2-parameter (pairwise deletion) model.
RESULTS
Identification of the best DNA barcode loci for Septoria species
Amplification success
The PCR amplification success rates were very high for all seven loci, varying from 97 % for RPB2 to 100 % for ITS and LSU (Table 3). Good amplification reactions of RPB2 required a 2-3 times higher DNA input then the other loci and this locus is therefore less favorable for easy identification. The other six loci amplified without problems.
Kimura-2-parameter values
The Kimura-2-parameter (K2P) distribution graphs are depicted in Fig. 1. They visualise the inter- and intraspecific distances per locus (barcoding gap). A good barcoding locus should have no overlap between the inter- and intraspecific K2P distances and should have an average interspecific distance that is at least 10 times as high as the average intraspecific distance of that locus (Hebert et al. 2003). The seven loci show a rather constant degree of intraspecific variation of 0.01 in their K2P distribution graphs, however their interspecific variations shows considerable differences. The average interspecific variation in both ITS and LSU datasets is very low (0.015) compared to their intraspecific variation (0.01), leading to a very low inter- to intraspecific variation ratios of 1.5: 1 for these two loci (Fig. 1). These low ratios are far below the required 10: 1 ratio, indicating a general lack of natural variation within these two loci, making them ill-suited for effective identification of the individual species used in this dataset. These low K2P results for ITS and LSU are consistent with previous results by Verkley et al. (2004a, b) which showed that both loci could not resolve the lower phylogenetic relationships between closely related Septoria species. Due to the presence of intron regions in the five remaining protein coding loci, these genes provide much higher interspecific variation than the more conserved ITS and LSU loci. These protein coding genes thus have (much) higher K2P inter- to intraspecific variation ratios: for Cal 14: 1, RPB2 17: 1, Act 23: 1, EF 26: 1 and for Btub 29: 1 (Fig. 1), making them all suitable for reliable species resolution throughout the range of septoria-like fungi. As the EF and Btub have the largest barcoding gap, these loci should give the highest species resolution and preferably be used for identifying species.
Fig. 1.
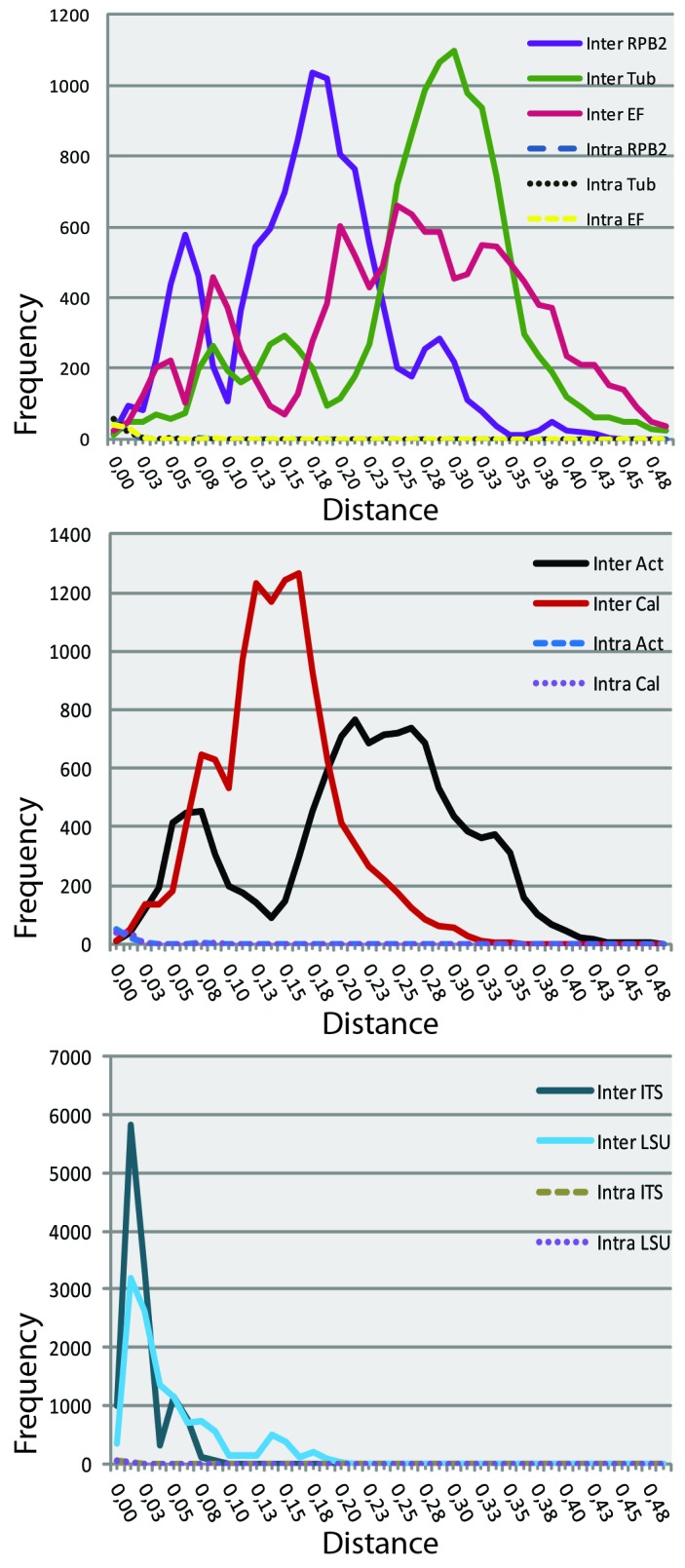
Frequency distributions of the Kimura-2-parameter distances (barcoding gaps) for the seven PCR loci.
Phylogeny
Basal to the seven-locus tree are the outgroup taxon Readeriella mirabilis (CBS 125000), and a monophyletic group comprising 11 strains, viz. Dothistroma pini (CBS 121011), D. septospora, (CBS 383.74), Passalora dissiliens (CBS 219.77), three Ramularia species (Mycosphaerella s. str., see Quaedvlieg et al. 2013) and three Zymoseptoria species, including its type species Z. tritici (syn. Mycosphaerella graminicola, Septoria tritici). The basal ingroup taxa include CBS 619.72 identified as Septoria oudemansii, a Pseudocercospora clade with six strains, and Cercosporella virgaureae (CBS 113304). A well-supported cluster of two basal lineages (bootstrap support 100 %) comprises a cluster (100 %) of two isolates identified as S. gladioli, and a second cluster (100 %) containing 10 strains representing four septoria-like species that are all associated with leaf spots on plants of the family Caryophyllaceae, and for which the new generic name Caryophylloseptoria is proposed below. These include C. silenes (CBS 109100, 109103), C. lychnidis (CBS 109098-109102), two isolates originating from Lychnis cognata in Korea for which the new species C. pseudolychnidis is proposed by Quaedvlieg et al. (2013) (CBS 128614, 128630), and two isolates of C. spergulae (CBS 397.52, 109010).
The remaining ingroup can be devided into a Sphaerulina clade (100 %, 51 strains including the basal strain of Sph. abeliceae, CBS 128591) and main Septoria clade (80 %, 259 strains) with, positioned in between smaller groups comprised of “Septoria” cruciatae (CBS 123747, 123748), a small pseudocercosporella-like clade comprising Passalora fusimaculans (CPC 17277), a clade with Passalora depressa (CPC 14915), “Mycosphaerella” brassicicola and affiliated taxa with Pseudocercosporella asexual morphs (100 %, 9 strains), and a miscellaneous clade containing “Passalora” sp. (100 %, CBS 113989, 113999, 114275), Passalora dioscoreae (CPC 10855, 11513), Pseudocercosporella magnusiana (CBS 114735), Passalora janseana (CBS 145.37), “Septoria erigerontis” (CPC 19485), and a Cercospora clade (100 %, 4 strains).
The Sphaerulina clade comprises the aforementioned CBS 128591 identified as S. abelicaea (from Zelkova serrata) and clades 1 and 2. Clade 1 (100 %, 37 strains) includes at its base three strains of Sph. cornicola, the sister taxa Sph. betulae and S. westendorpii (syn. S. rubi) on Rubus fruticosus (CBS 102327, 109002, 117478), and Sph. socia (CBS 355.58, CBS 357.58). The remainder of clade 1 contains a well-supported cluster of 25 strains with various species infecting herbaceous and woody hosts. CBS 109017 and 19018, originating from Rubus idaeus in Austria, represent a species for which Sphaerulina tirolensis sp. nov. is introduced below. Furthermore this cluster contains Sphaerulina berberidis (syn. Mycosphaerella berberidis, S. berberidis Niessl), Sph. azaleae, Sph. hyperici, Sph. menispermi, Sph. patriniae, Sph. cercidis, and Sph. gei. Clade 2 (74 %, 13 strains) of the Sphaerulina clade includes only species infecting tree, the poplar pathogens Sph. populicola (syn. Mycosphaerella populicola, CBS 100042), Sph. musiva (syn. Septoria musiva, four strains), and Sph. frondicola (syn. Mycosphaerella populi, S. populi, CBS 391.59), and furthermore Sphaerulina aceris (syn. Mycosphaerella latebrosa, Phloeospora aceris, asexual morph S. aceris, three strains), which causes leaf spot on Acer spp., and Sph. quercicola (syn. S. querciola).
At the base of the main Septoria clade, a well-supported clade 3 (88 %, 16 strains) includes several species associated with hosts in the Apiaceae, viz., S. oenanthis (CBS 128667) and S. oenanthicola (CBS 128649; a new species proposed by Quaedvlieg et al. (2013), S. sii (CBS 118.96, 102369, 102370), and S. aegopodii (CBS 123740, 123741), and associated with other plant families, S. dearnessii (CBS 128624), a cluster of two strains of S. lactucae (CBS 352.58, 108943) and S. sonchi (CBS 128757), S. campanulae (CBS 128589, 128604), S. mazi (CBS 128656, 128755), and S. gentianae (CBS 128633). In clade 4 (100 %, 183 strains) S. bupleuricola (CBS 128601, 128603) and S. scabiosicola (100 %, 12 strains) occupy a basal position and subclades 4a-d can be distinguished. Subclade 4a (100 %, 46 strains) comprises of a group of 13 strains of miscellaneous host plants, mostly with smaller conidia, viz., two Solanum pathogens S. lycopersici (CBS 354.49, 128654) and S. malagutii (CBS 106.80), S. apiicola (4 strains), S. cucurbitacearum (CBS 178.77), and S. aridis (4 strains), and a second strain identified as S.posonniensis (CBS 128658). Subclade 4b (100 %, 33 strains) harbours several taxa infecting Asteraceae, among others S. obesa (four strains), S. senecionis (three strains), S. putrida (CBS 109087, 109088), S. leucanthemi (6 strains), S.cirsii (CBS 128621), six strains of the S. chrysanthemella complex, S. exotica (CBS 163.78), and S. posoniensis (CBS 128645). Furthermore this group of 33 comprises taxa with relatively large conidia capable of infecting Ranunculaceae, viz. S.lycoctoni, S. napelli (CBS 109104-109106) from Austria and S. pseudonapelli (CBS 128664; a new species proposed by Quaedvlieg et al. 2013) from Korea. It also includes S. lycopicola (128651), CBS 128662 identified as S. stachydicola (probably misidentified), and two strains of S. astericola (CBS 128587, 128593). Subclade 4c (99 %, 15 strains) contains S. matricariae (CBS 109000, 109001), S. lamiicola (8 strains), S. anthrisci (CBS 109019, 109020), and S. petroselini (CBS 182.44, 109521), and subclade 4d (100 %, 103 strains) shows four subgroups, 4d-1-4. Basic to these are found S. dolichospora (CBS 129152) and S. helianthi (CBS 123.81). Subclade 4d-1 (100 %, 45 strains) contains S. cf. stachydicola (CBS 128668; see Quaedvlieg et al. 2013), and many other species infecting herbaceous plants, among others S. stachydis (nine strains), S. phlogis (three strains), S. epambrosiae (CBS 128629, 128636), S. cerastii (five strains), S. galeopsidis (seven strains), S. stachydis (9 strains), S. epilobii (CBS 109084, 109085) and S. digitalis (CBS 391.63, 328.67). Subclade 4d-2 (100 %, 35 strains) comprises among others S. polygonorum (six strains), S. urticae and S convolvuli (three strains each), S.villarsiae, S. crepidis, and S. codonopsidis. Subclade 4d-3 (99 %, 11 strains) containing S. erigerontis (five strains), S. lysimachii (five strains), and S. saccardoi (CBS 128756). Subclade 4d-4 (100 %, 9 strains) contains S. bothriospermi (CBS 128592, 128599), S. tinctoriae (CBS 129154), four strains identified as S. rubi that need to be re-named, and S.agrimoniicola (CBS 128585, 128602).
In clade 5 (92 %, 63 strains) of the main Septoria clade two main clusters are found. At the base of the subclade 5a (77 %, 52 strains), two strains of S. clematidis (CBS 108983-4) and Septoria sp. (CPC 19716) originating from Searsia laevigatum in South Africa. This cluster furthermore comprises three strains isolated from Hibiscus spp., viz., S. hibiscicola (CBS 128611, 128615) and S. abei (CBS 128598), and two main groups, one with S. anthurii (CBS 148.41, 346.58), S. sisyrinchii (CBS 112096), the Chromolaena fungi S. chromolaenae (CBS 113373) and S. ekmanniana (CBS 113385, 113612), and S. passiflorae (CBS 102701) and S. passifloricola (CBS 129431), and a second group comprising at the base S. eucalyptorum (CBS 118505; Crous et al. 2006b), and furthermore S. justiciae (CBS 128610, 128625, and CPC 12509), S. linicola (CBS 316.37), S. cucubali (3 strains, including CBS 124874, an endophytic isolate from Fagus leaf litter), S. lepidiicola (CBS 128635) and and a partially unresolved cluster of 23 strains comprising the plurivorous S. protearum and S. citri complex. A small well-supported cluster (100 %) contains S. verbenae (CBS 113438, 113481), two unidentified species of Septoria (CPC 19304, from Vigna unguiculata subsp. sesquipedalis and CPC 19793, from Syzygium cordatum), and M. coacervata (CBS 113391). Subclade 5b (100 %, 11 strains) comprises S. helianthicola (CBS 122.81), three strains of S. stellariae, CBS 503.76 identified as S. acetosae, three strains of S. astragali, “Cercospora sp.” (CBS 112737), and furthermore S. hippocastani (CBS 411.61 and MP11).
Examining the distribution of host families throughout the tree, an interesting disjunct pattern is found for the families that are represented by more than a few specimens (see legend in Fig. 2). For example, the 28 species infecting Asteraceae are found in all clades and most subclades of the tree, including Sphaerulina; nine species infecting Apiaceae are found in clade 3 and subclades 4a-d of Septoria; 10 species of Rosaceae in Septoria clades 4, 5 and Sphaerulina (clades 1 and 2); six species infecting Lamiaceae are dispersed in subclades 4b, c, and d-1.
Fig. 2.
Consensus phylogram (50 % majority rule) of 17 222 trees resulting from a Bayesian analysis of the combined seven loci sequence alignment using MrBayes v. 3.2.1. Bayesian posterior probabilities values are indicated on their respective branches and the scale bar indicates 0.2 expected changes per site. The tree was rooted to Readeriella mirabilis (Teratosphaeriaceae) (CBS 125000). The family of the host plant from which the strain was isolated is indicated for 12 most prevalently occurring host families in our dataset (colour bar according to the legend).
TAXONOMY
Caryophylloseptoria Verkley, Quaedvlieg & Crous, gen. nov. MycoBank MB804469.
Etymology: Named after the plant family on which these taxa occur, Caryophyllaceae.
Conidiomata pycnidial, epiphyllous or predominantly epiphyllous, globose to subglobose, or slightly depressed, with a central ostiolum. Conidiomatal wall composed of textura angularis or globulosa-angularis. Conidiogenous cells hyaline, holoblastic, proliferating percurrently 1-many times with indistinct annellations, or (in addition) proliferating sympodially. Conidia cylindrical, straight, curved or flexuous, multiseptate, not or somewhat constricted around the septa, hyaline, contents with several oil-droplets and granular material in each cell.
Type species: Caryophylloseptoria lychnidis (Desm.) Verkley, Quaedvlieg & Crous.
Caryophylloseptoria lychnidis (Desm.) Verkley, Quaedvlieg & Crous, comb. nov. MycoBank MB804470. Fig. 3.
Fig. 3.
Caryophylloseptoria lychnidis. A. CBS 109098, colony on OA. B. Ibid., on CMA. C. Conidia and conidiogenous cells in planta (CBS 109098). D. Conidia on OA (CBS 109098). Scale bars = 10 μm.
Basionym: Septoria lychnidis Desm., Annls Sci. Nat., sér. 3, Bot. 11: 347. 1849.
For extended synonymy see Shin & Sameva (2004).
Description in planta: Symptoms leaf spots circular, whitish to pale yellow, surrounded by a brown border; Conidiomata pycnidial, epiphyllous, several in each leaf spot, globose to subglobose, dark brown, semi-immersed, 50-100(-120) μm diam; ostiolum central, initially circular, 25-45 μm wide, later more irregular and up to 100 μm wide, surrounding cells concolorous or somewhat darker; conidiomatal wall 10-20μm thick, composed of textura angularis without distinctly differentiated layers, the cells 3-5 μm diam, the outer cells with brown, somewhat thickened walls, the inner cells with hyaline and thinner walls; Conidiogenous cells hyaline, cylindrical and tapering gradually towards the apex, or narrowly ampulliform with a relatively wide and long neck, holoblastic, proliferating percurrently 1-many times with indistinct annellations, rarely also proliferating sympodially, 6-17.5(-22) × 3-4(-5) μm. Conidia cylindrical, straight, more often slightly curved or flexuous, with a narrowly to broadly rounded, sometimes more distinctly pointed apex, towards the broadly truncate base barely attenuated, (0-)3-5(-7)-septate, not constricted around the septa, hyaline, contents with several oil-droplets and minute granular material in each cell in the living state, with inconspicuous oil-droplets and granular contents in the rehydrated state, (22-)39-75(-85) × 2-3 μm (rehydrated). Sexual morph unknown.
Description in vitro: Colonies on OA (3-)4-6 mm diam in 12 d (7-11 mm in 3 wk), with an even, pure yellow to straw, glabrous margin, the pigment diffusing into the surrounding medium; colonies spreading, but in the centre quite distinctly elevated, immersed mycelium pure yellow to straw, later locally citrine-green or citrine; after 10-15 d darkened by numerous immersed or superficial pycnidia arranged in random patterns, the outer wall of the superficial pycinidia entirely covered by white to glaucous hyphae, tardily releasing initially buff to straw, later salmon conidial slime; reverse pure yellow, but centre olivaceous and citrine to greenish olivaceous after 3 wk. After incubation over about 7 wk olivaceous-black sectors become visible in the colony consisting mostly of immersed strands of dark-walled hyphae, alternating with yellow sectors; some colonies develop wider sectors that remain yellow above, but more ochreous on reverse. Colonies on CMA 4-6 mm diam in 12 d (9-12 mm in 3 wk), as on OA, but sporulating earlier. Colonies on MEA 2-4 mm diam in 12 d (5-7(-9) mm in 3 wk; 17-24 mm in 7 wk), with an even to ruffled, colourless to buff, glabrous margin; no diffusing pigment seen; colonies restricted, irregularly pustulate up to 3 mm high, the surface dark, blackish or chestnut, covered by a short, dense mat of white to glaucous-grey, after 7 wk straw to pale yellow, aerial mycelium; conidiomata releasing droplets, later larger masses of first whitish, then salmon conidial slime; reverse brown-vinaceous in the centre, surrounded by hazel or cinnamon areas. Colonies on CHA 4.5 mm diam in 3 wk (24 mm in 7 wk); colony as on MEA, but the surface almost entirely hidden under a dense mat of woolly, white aerial mycelium, locally with a pure yellow to straw haze which later becomes more intense, and a yellowish pigment diffusing into the surrounding medium; reverse umber to sienna; densely aggregated superficial conidiomata in the centre releasing masses of amber to pale salmon conidial slime. Conidiomata pycnidial, as in planta, but somewhat larger, 70-145 μm diam, mostly single, sometimes merged into complexes, without differentiated ostiolum; conidiogenous cells as in planta, proliferating percurrently with distinct annellations or sympodially, 8.5-25 × 3.5-6 μm; conidia cylindrical, straight, slightly curved or flexuous, with a rounded apex, lower part barely attenuated into a broad truncate base, (0-)1-5-septate, not constricted around the septa, hyaline, with several oil-droplets and minute granular material in each cell, (44-)77-94.5 × (2-)2.5-3 μm.
Hosts: Lychnis spp. and Silene spp. (incl. Melandrium).
Material examined: Austria, Tirol, Inntal, near Telfs, on living leaves of Silene pratensis (syn. M. album), 4 Aug. 2000, G. Verkley 1047, CBS H-21161, living culture CBS 109098, 109102; same loc., host, date, G. Verkley 1048, CBS H-21162, living culture CBS 109099, 109101; Netherlands, Hilversum, on living leaves of Silene dioica (syn. Melandrium rubrum), 22 June 1985, H.A. van der Aa 9524, CBS H-18112.
Notes: This fungus has been reported from several species of Lychnis and Silene (including Melandrium), and the size ranges of conidia given by various authors differ considerably. In the original description by Desmazières, the fungus was characterised as having 5-7-septate conidia, measuring 50-70 ×2.5-3 μm, in widely opening pycnidia. Diedicke (1915) gave the same spore measurements, but Grove (1935) reported 30-50 × 2-3 μm, while Jørstad (1965) gave different ranges on different hosts (overall extremes 27-72 × 2-3 μm). Radulescu et al. (1973) reported 30-76 × 2.2-3.3 μm, and Vanev et al. (1997) 26-93.5 × 1.5-3.2 μm. The characters of the Austrian material studied here generally agree well with previous records, and the range of conidial sizes agrees best with that given by Vanev et al. (1997). The authors cited above have listed various names as synonyms of S. lychnidis, including S. lychnidis var. pusilla (= S. pusilla). Two strains isolated from Lychnis cognata in South Korea (CBS 128614, 128630) first also identitifed as S. lychnidis, were shown by sequence analyses to belong to a distinct species, for which the name C. pseudolychnidis is introduced by Quaedvlieg et al. (2013).
Caryophylloseptoria silenes (Westend.) Verkley, Quaedvlieg & Crous, comb. nov. MycoBank MB804471. Fig. 4.
Fig. 4.
Caryophylloseptoria silenes. A-C. Colonies CBS 109100. A. On OA. B. On CHA. C. On MEA. D. Conidia and conidiogenous cells in planta (CBS H-21160). E. Ibid., on OA (CBS 109100). F-G. Conidia on OA (CBS 109100). Scale bars = 10 μm.
Basionym: Septoria silenes Westend., in Westendorp & Wallays, Herb. crypt. Belge, Fasc. 19, no 955. 1854; Bull. Acad. R. Belg. Cl. Sci., Sér. 2, 2: 575. 1857.
Description in planta: Symptoms leaf spots circular or elliptical, pale yellow to pale brown, surrounded by a dark purplish border; Conidiomata pycnidial, amphigenous but predominately epiphyllous, numerous in each leaf spot, globose to subglobose, immersed, 50-80(-100) μm diam; ostiolum central, initially circular, 20-45 μm wide, later more irregular and up to 50 μm wide, surrounding cells somewhat darker; conidiomatal wall only 10-15 μm thick, composed of textura angularis without distinctly differentiated layers, the outer cells with brown, somewhat thickened walls and 4-7.5 μm diam, the inner cells hyaline and thin-walled and 3.5-5 μm diam; Conidiogenous cells hyaline, ampuliform, or cylindrical and widest near the apex, hyaline, holoblastic, proliferating percurrently 1-several times with distinct scars (annellations), sympodial proliferation not observed, 4-10 × 3-5 μm. Conidia cylindrical, straight or slightly curved, with a rounded apex, lower part attenuated more or less abruptly into a broad truncate base, (0-)1(-4)-septate, somewhat constricted around the septa, hyaline, contents with several oil-droplets in each cell in the living state, with conspicuous oil-droplets and granular contents in the rehydrated state, 21-37 × 2-3.5(-4) μm (rehydrated; in turgescent state up to 4.5 μm wide). Sexual morph unknown.
Description in vitro: Colonies on OA 7-9 mm diam in 12 d (7-10 mm in 3 wk; 21-23 mm in 7 wk), with an even, later undulating, pure yellow to luteous, glabrous margin, the pigment diffusing into the medium around the colony; colonies spreading, but in the centre quite distinctly elevated, immersed mycelium luteous to ochreous-orange, darkened by numerous simple, first brownish, then black pycnidia arranged in concentric patterns, releasing droplets of initally milky white, later pale pure yellow conidial slime; immersed mycelium later mostly luteous to sienna, much darker after 7 wk; most of the colony covered by a high, woolly-floccose mat of pale grey, later straw to pure yellow aerial mycelium; reverse luteous, in the centre umber, ultimately becoming almost black. Colonies on CMA 5-7 mm diam in 12 d (7-9 mm in 3 wk; 18-19 mm in 7 wk), as on OA, but immersed mycelium and (more scarce) aerial mycelium more intensely pigmented, immersed mycelium appearing rust to sienna after 3, but mostly black after 7 wk, and conidial slime earlier pure yellow. Colonies on MEA 3-5 mm diam in 12 d (5-9 mm in 3 wk; 17-20 mm in 7 wk), with a ruffled, yellowish, glabrous margin; diffusing yellow pigment distinct around the colony; colonies restricted, irregularly pustulate up to 3 mm high, the surface dark, blackish or chestnut, covered by a short, dense, almost pruinose mat of grey to pure yellow aerial mycelium; conidiomata releasing droplets of initially pale pure yellow, later almost amber conidial slime; reverse chestnut or blood. Colonies on CHA 3.5-5 mm diam in 12 d (7-8 mm in 3 wk; 12-17 mm in 7 wk), with an even or irregular margin, mostly hidden underneath white aerial hyphae; yellow pigment very clear diffusing beyond the colony margin after 3 wk; colonies restricted, conical or hemispherical, the surface very dark, but mostly covered by a dense mat of woolly, initially white, then pure yellow aerial mycelium; reverse sienna to fulvous. Sporulating scarcely after 3, but more intensely after 7 wk, cirrhi or droplets of pale pure yellow, later amber conidial slime released by superficial conidiomata.
Conidiomata pycnidial, as in planta, but larger, 90-155 μm diam, mostly single, sometimes merged into complexes with several ostioli; conidiogenous cells as in planta, but often with a more elongated neck, proliferating percurrently with distinct annellations or sympodially, 7-17 × 3-5 μm; conidia cylindrical, straight or slightly curved, with a rounded apex, lower part attenuated more or less abruptly into a broad truncate base, (0-)1-3(-4)-septate, somewhat constricted around the septa, hyaline, with several oil-droplets in each cell, (24-)26.5-35(-42) × 3-4(-5) μm.
Hosts: Silene spp.
Material examined: Austria, Tirol, Ötztal, Horlachtal, Mühl near Niederthai, alt. 1500 m, on living leaves of Siline nutans, 3 Aug. 2000, G. Verkley 1041, CBS H-21160, living cultures CBS 109100, 109103.
Notes: Jørstad (1965) examined type material from BR in Westend., Herb. crypt. Belge 955, on Silene armeria. He reported that among numerous immature pycnidia were a few thin-walled pycnidia with 0-septate conidia measuring 21-24 × 2-2.5 μm, but in his opinion there was no doubt that collections from other hosts like Silene cucubalus (= S. inflata), and from Silene rupestris with predominantly 1-septate spores up to 31 μm in length belonged to the same species. In the material collected in Austria, we have observed predominantly 1-septate conidia, but conidial length did vary in different fruitbodies: some pycnidia produced conidia 21-28 μm in length, others conidia measuring 26-37 μm in length. However, isolates from these pycnidia were similar in colony characters and conidia produced did not show such differences in size range.
Priest (2006) noted that there are at least two taxa of Septoria occurring on Silene, a short-spored taxon represented by S. silenes, and a long-spored taxon for which the name S. silenicola applies. This author referred all collections from Australia on this host genus to S. silenicola, for which conidia measure (34-)48-65(-85) × 2-2.5(-3) μm.
As pointed out by Petrak (1925) and Jørstad (1965), several of the Septoria described on Silene spp. (and Melandrium) are likely to be conspecific with S. silenes. Septoria dominii Bubák 1905 was already placed in the synonymy of S. silenes by Jørstad (1965), and the same could be correct for S. dimera from Silene nutans. According to the original diagnosis, the conidia of S. dimera are 1-septate and measure 28-32 × 4 μm. Radulescu et al. (1973) and Markevičius & Treigienė (2003) treated S. dimera as a separate species next to S. silenes, reporting measurements for conidia of S. dimera as 25-40 × 3-4 μm, and 21-35 × 3.2-4.3 μm, respectively. Vanev et al. (1997) also treated S. dimera, reporting conidial measurements 26-65 × 2.5-4 μm, but they included material from Silene spp. and Cucubalus baccifer.
Caryophylloseptoria spergulae (Westend.) Verkley, Quaedvlieg & Crous, comb. nov. MycoBank MB804472. Fig. 5.
Fig. 5.
Caryophylloseptoria spergulae. A, B. Colonies CBS 109010. A. On OA. B. On MEA. C. Conidia and conidiogenous cells in planta (CBS H-21150). D-H. Conidia on OA (CBS 109010). Scale bars = 10 μm.
Basionym: Septoria spergulae Westend., in Westendorp & Wallays, Herb. crypt. Belge, Fasc. 23-24, no. 1155. 1857; Bull. Acad. R. Belg. Cl. Sci., Sér. 2, 2: 576. 1857.
Description in planta: Symptoms absent. Conidiomata pycnidial, black, in dense groups on dead stems and leaves, only partly immersed in the host tissue, globose or slightly depressed, (50-)75-150 μm diam; ostiolum circular, central, 10-12.5 μm wide, without distinctly differentiated cells; pycnidial wall with an outer layer of textura globulosa-angularis containing cells 8-12 μm diam with brown walls, thickened unevenly up to 3μm, and an inner layer of textura globulosa-angularis containing cells 5-8 μm diam with hyaline or pale brown walls. Conidiogenous cells hyaline, ampuliform, or elongated ampulliform with a distinct neck, hyaline or very pale brown near the base, holoblastic, proliferating percurrently 1-many times with indistinct annellations, also sympodially, 5-10(-16) × 3-5 μm. Conidia cylindrical, regularly curved, or abruptly bent in the lower cell, gradually attenuated to the rounded apex, gradually or more abruptly attenuated into a truncate base, 1(-2)-septate, not or indistinctly constricted around the septum, hyaline, contents rich in small guttulae, minutely granular material and large vacuoles in the living state, oil-droplets merged into larger guttules in the rehydrated state, (18-)24-33 (-40) × 2.0-2.5(-3.0) μm (rehydrated). Sexual morph unknown.
Description in vitro: Colonies on OA less than 2 mm diam after 2 wk (6-8 mm in 28 d), restricted, though not much elevated, with an even, colourless, glabrous margin; colony surface covered by a dense continuous or discontinuous mat of grey, finely felted to somewhat woolly, low aerial mycelium, agar around the colony showing a yellow diffusing pigment; immersed mycelium pale luteous to saffron, reverse concolorous, but olivaceous-black under areas with well-developed aerial mycelium or conidiomata. Colony sporulating in the centre after about 2 wk, with spores in large pale salmon droplets oozing from pycnidioid complexes. Colonies on CMA 8-10 mm diam in 28 d, as on OA, but immersed mycelium soon darkening and olivaceous-black, while the aerial mycelium is somewhat more greenish, and the mat denser and more continuous; reverse olivaceous-black. Colonies on MEA 5 mm diam in 2 wk (8-10 mm in 28 d), restricted, with an even, buff, glabrous margin; colony surface black, but with a diffuse mat of greyish white, often with some sulphur yellow (centre), woolly aerial mycelium; fruitbodies developing tardily on the colony surface, sporulating with large, dirty white to pale reddish masses in watery droplets; reverse dark brick to olivaceous-black. Colonies on CHA 7-9 mm diam in 2 wk, as on MEA, but aerial mycelium higher and denser, in the centre also conspicuously yellowish-pale citrine. No sporulation observed.
Conidiomata mostly olivaceous-brown, irregular merged complexes of initially closed, but soon widely opening stromata, only rarely pycnidial and structurally similar to those on the natural substratum. Conidiogenous cells hyaline, ampuliform, or elongated ampulliform with a relatively long neck, hyaline or very pale brown near the base, holoblastic, proliferating percurrently 1-many times with indistinct annellations, also sympodially, mostly after one or more percurrent proliferations, 7-14(-22) × 3-5 μm. Conidia on OA hyaline, pale salmon in mass, cylindrical and regularly curved, or abruptly bent in the lower or upper cell, gradually attenuated to the rounded apex, more abruptly attenuated into a truncate base, contents granular with large vacuoles, 1(-3)-septate, not or indistinctly constricted around the septa, contents rich in minute guttulae and granular material, 25.5-41 × (2.0-)2.5-3.0(-4.5) μm.
Hosts: On dead leaves and stems of Spergula spp.
Material examined: Belgium, Beverloo, on dry leaves and stems of Spergula arvensis, M. Torquinet s.n., isotype BR-MYCO 159328-54, also distributed in Westendorp & Wallay, Herb.crypt.Belg., Fasc. 23-24: no.1155. Germany, Brandenburg, Kreis Nieder-Barnim, near Prenden, on leaves and stems of Spergula vernalis, 24 July 1920, H. & P. Sydow s.n., distributed in Sydow, Mycotheca germanica 1688, CBS H-4765. Netherlands, on Dianthus caryophyllus, Schouten s.n., CBS 397.52 (sub S. dianthi Desm.); Prov. Gelderland, ‘t Harde, Doornspijkse Heide, De Zanden, on decaying leaves of Spergula morisonii, A. Aptroot 48300, 13 June 2000, epitype designated here CBS H-21150 “MBT175350”, living culture ex-epitype CBS 109010.
Notes: This fungus was originally described from dry leaves and stems of Spergula arvensis by Westendorp, who described the conidia as 30 × 2.5 μm. The type from BR is well-preserved and rich in fruitbodies on leaves and stems, where conidia are 1(-2)-septate, 20-38 × 2-2.5(-3) μm. The collection Aptroot 48300 from Spergula morisonii agrees in morphology and can be identified as conspecific, although it contains a larger proportion of 2-septate conidia (that are mostly 30-40 μm long) than in the type. The material on Spergula vernalis that was distributed as Mycotheca germanica 1688 morphologically also agrees with these collections.
Other names were later introduced for Septoria on members of the plant genus Spergularia (= Alsine), which is closely related to Spergula: S. alsines Rostr. 1903 from Spergularia sp., conidia 20-31 × 2-3 μm formed in 55-120 μm wide pycnidia (Teterevnikova-Babayan 1987; conidia 20-25 × 2-3 μm and 3-septate, in the original diagnosis of Rostrup 1903, based on material from Alsine verna non Spergula vernalis), S. spergulariae 1903, on Spergularia rubra (conidia 30-45 × 2.5-3 μm, “multiseptate”), S. vandasii 1906, on Alsine glomerata, and S. spergularina 1945, on Spergularia longipes (no conidial measurements available). Some of these names could be synonymous with S. spergulae or perhaps S. alsines, but in order to corroborate this, new material needs to be collected and compared to the types. According to Teterevnikova-Babayan (1987), S. alsines differs from S. spergulae in conidial shape in that the conidial base is more truncate than in S. spergulae, and in that it is capable of also killing Minuartia glomerata. Rhabdospora alsines Mont. 1892, which was described from dead stems of Alsine tenuifolia, is unlikely to be conspecific with S. spergulae, as its conidia were described as 16-18 × 2 μm and 1-septate.
Muthumary (1999) studied type material of S. dianthi 1849 (PC 344) and by the drawings he made of it the conidia of this fungus and those of S. spergulae appear very similar in shape. Muthumary reported that the conidia of S. dianthi were 32-48 (av. 40) × 3-4 (av. 3) μm, and mostly 1-, rarely 2-septate. Given these measurements, on average, the conidia in the type of S. dianthi are clearly longer than in S. spergulae (on average below or around 30). Moreover, S. dianthi is a fungus causing leaf spots on several Dianthus spp., while S. spergulae is only known from dry and dead host tissues, and is therefore believed to be saprobic (and possibly endophytic). CBS 109010 and the only strain available for S. dianthi (CBS 397.52) show 100 % sequence homology of the LSU, ITS, Btub and Cal, while there are only minor differences in Act (99.25 %), EF (97.54 %), and RPB2 (99.42 %). Further work is required to establish that S. dianthi and S. spergulae are truely distinct taxa.
Septoria Sacc., Syll. Fung. 3: 474. 1884. nom. cons.
Type species: S. cytisi Desm.
A generic description is provided by Quaedvlieg et al. (2013, this volume).
Septoria aegopodii Desm. ex J. Kickx, Pl. Crypt. Fland. 1: 427. 1876 [Annls Sci. Nat., sér. 6, 7: no 616. 1878?]. Fig. 6.
Fig. 6.
Septoria aegopodii. A-E. Conidia in planta. A-C. CBS H-21262. D, E. CBS H-21199. Scale bars = 10 μm.
= Septoria podagrariae Lasch, in Rabenh., Herb. mycol. I, no 458. 1843. nomen nudum.
-
= Sphaeria podagrariae Roth, Catal. Bot. 1: 230. 1797.
≡ Mycosphaerella podagrariae (Roth: Fr.) Petr., Annls mycol. 19 (3/4): 203. 1921.
-
= Cryptosporium aegopodii Preuss, Linnaea 24: 719 (Fungi Hoyersw., no. 322). 1853.
≡ Phloeospora aegopodii (Preuss) Grove, British Stem- and Leaf-fungi (Coelomycetes) 1: 434. 1935.
≡ Septoria aegopodii (Preuss) Sacc., Syll. Fung. 3: 529. 1884 [non Desm. 1878].
?= Septoria podagrariae var. pimpinellae-magnae Kabát & Bubák, in Bubák & Kabát, Ber. naturw.-med. Ver. Innsbruck 30: 19-36 (extr. 11). 1906.
= Mycosphaerella aegopodii Potebnia, Annls mycol. 8(1): 49. 1910.
Description in planta: Symptoms leaf spots numerous but small, angular and delimited by veinlets, visible on both sides of the leaf, white to pale yellow. Conidiomata pycnidial, developing soon after first discolouration of the host tissue, predominantly epiphyllous, mostly also visible from the underside of the lesion, several scattered in each leaf spot, globose to subglobose, pale to dark brown (drying black), immersed, 125-190 μm diam, releasing conidia in white cirrhi; ostiolum central, initially circular and 17-35 μm wide, later becoming more irregular and up to 100 μm wide, surrounding cells dark brown, with thickened cell walls; conidiomatal wall except for the part surrounding the ostiolum poorly developed, about 10-20 μm thick, composed of pale brown to hyaline angular cells 3.5-8 μm diam with thin walls. Conidiogenous cells hyaline, discrete, cylindrical to narrowly or broadly ampulliform, holoblastic, proliferating sympodially, 8-15(-18) × 2.5-4.5 μm. Conidia filiform-cylindrical, straight, curved to somewhat flexuous, attenuated gradually to a relatively broadly rounded apex and broadly truncate base often provided with a collar of gelatinous material, (0-)1-2(-3)-septate (second and later septa very thin and easily overlooked), not constricted around the septa, hyaline, contents with numerous minute oil-droplets and granular material in each cell in the living state, with minute oil-droplets and granular contents in the rehydrated state, (30-)55-95(-115) × 3.5-4 μm (living; 30-72(-80) × 2.5-4 μm, rehydrated).
Description in vitro: All attempts to grow the isolates from conidia failed. Some conidia germinated at the apical cells, but mycelia died within 1-2 d after germination.
Hosts: Aegopodium podagraria and Pimpinella sp.
Material examined: Austria, Tirol, Ötztal, Ötz near Habichen, on living leaves of Pimpinella sp., 24 July 2000, G. Verkley 1001, CBS H-21187. Netherlands, Prov. Overijssel, Losser, in garden at Mollenbergstraat, on living leaves of Aegopodium podagraria, June 1999, G. Verkley 800, CBS H-21192; same substr., Prov. Overijssel, Losser, Arboretum Poort-Bulten, June 1999, G. Verkley 801, CBS H-21193; same substr., Prov. Utrecht, ‘s Graveland, Gooilust, 5 Sep. 1999, G. Verkley 916, CBS H-21199; same substr., Prov. Limburg, St. Jansberg, near Plasmolen, 9 Sep. 1999, G. Verkley 931, CBS H-21211; same substr., Prov. Zeeland, Zuid-Beveland, Community of Borsele, Schouwersweel near Nisse, 27 Aug. 2001, G. Verkley 1116, CBS H-21165; same substr., Prov. Utrecht, Soest, 29 July 2008, G. Verkley 5020, CBS H-21262.
Notes: This species is common on Aegopodium podagraria, especially on plants growing under less favourable conditions. Jørstad (1965) noted that in autumn the pycnidia are commonly accompanied by immature perithecia (or by “sclerotia”) of Mycosphaerella aegopodii in Sweden, but we have not found any in The Netherlands. According to van der Aa (pers. comm.), the sexual morph only matures in montane habitats. Aptroot (2006), who studied herbarium specimens collected at high altitudes in several localities in Europe also did not observe any mature ascomata. Type material of M. podagrariae could not be located (Aptroot 2006). Simon et al. (2009) studied the cellular interactions between M. podagrariae and Aegopodium podagraria based on German material (no cultures preserved).
We have not seen the type of S. podagrariae var. pimpinellae-magnae 1906 described from Pimpinella magna (= P. major?) in Tirol, but since the conidial characters given by Saccardo & Trotter (1913, 45-60 × 2.5-4 μm, 3-septate) are well within the range of S. aegopodii, it is placed here tentatively as a synonym. On Pimpinella, eight other Septoria species or varieties have been described in the literature, but these could not be studied here. The oldest available name would be S. pimpinellae Ellis 1893 (later homonyms Laubert 1920 and Hollós 1926). According to the diagnoses the conidial sizes described for these taxa largely overlap, and range from 15-35 × 1-1.5(-2) μm, thus all considerably smaller than in S. aegopodii.
Septoria aegopodina Sacc., Michelia 1: 185. 1878. Fig. 7.
Fig. 7.
Septoria aegopodina. A, B. Colonies CBS 123740. A. On OA. B. On MEA. C-F. Conidia in planta (CBS H-21249). G. Conidia and conidiogenous cells in planta (CBS H-21249). H, I. Conidia on OA (CBS 123741). J. Conidia on MEA (CBS 123740). Scale bars = 10 μm.
= Septoria aegopodina var. villosa Gonz. Frag., Assoc. españ. Progr. Cienc. Congr. Oporto, 6. Cienc. natur.: 47. 1921.
= Septoria aegopodina var. trailii Grove, British Stem-and Leaf-Fungi (Coelomycetes) 1: 396. 1935.
Description in planta: Symptoms leaf spots numerous, indefinite and soon covering large parts of the leaf lamina, visible on both sides of the leaf, first yellow then pale orange-brown. Conidiomata pycnidial, predominantly hypophyllous, scattered or gregarious, globose to subglobose, pale to dark brown, immersed, 90-160 μm diam, releasing conidia in white cirrhi; ostiolum central, circular and 15-25 μm wide, surrounded by cells with dark brown to almost black, thickened walls; conidiomatal wall 10-28 μm thick, composed of an outer cell layer of pale brown to hyaline isodiametric angular or globose cells, 3.5-8 μm diam with thickened walls, and an inner layer of one or more hyaline cells with not or only slightly thickened walls. Conidiogenous cells hyaline, discrete, mostly broadly ampulliform, holoblastic, rarely proliferating sympodially, possibly also percurrently but no annellations visible, 4-7(-8) × 3-4.5 μm. Conidia filiform to filiform-cylindrical, straight or curved, attenuated gradually to a narrowly rounded to somewhat pointed apex, and attenuated gradually or more abruptly to a narrowly truncate base, (0-)1-3-septate, not constricted around the septa, hyaline, with numerous minute and several larger oil-droplets in each cell in the living state, and minute oil-droplets and granular contents in the rehydrated state, (22-)30-42.5 × 1.5-2(-2.5) μm (rehydrated). Sexual morph unknown.
Description in vitro (20 °C, diffuse daylight): Colonies on OA 7-10 mm diam in 2 wk, with a very narrow, glabrous and rosy-buff margin; colony restricted, somewhat elevated, immersed mycelium colourless to faintly brick, or much darker, brown-vinaceous, but mostly hidden under a dense, woolly mat of pure white to faintly yellow aerial mycelium; reverse olivaceous-black to dark brick; a vinaceous pigment diffusing into the surrounding medium. Colonies on MEA 8-15 mm diam in 2 wk, the margin covered by pure white aerial hyphae; colony restricted, irregularly postulate in the central area, mostly covered by a dense woolly-floccose mat of smoke grey aerial mycelium, but after 2 wk numerous glabrous, black conidiomata appear on the colony surface in the centre, releasing milky white conidial slime. Reverse of colony olivaceous-black.
Conidia on MEA elongated ellipsoidal to cylindrical, straight to distinctly curved, rounded to narrowly pointed at the apex, attenuated gradually to a narrowly truncate base, 0-1-septate, 0-septate 8-12 × 2-2.5(-3), 1-septate 10-21 × 2-2.5 μm; Conidia on OA cylindrical, straight or slightly to distinctly curved, narrowly rounded to slightly pointed at the apex, attenuated gradually to a narrowly truncate base, 1-3-septate, (16-)20-32 × 1.5-2 μm.
Hosts: Aegopodium podagraria and Pimpinella spp.
Material examined: Czech Republic, Moravia, Veltice, Forest of Rendez Vous, on living leaves of Aegopodium podagraria, 16 Sep. 2008, G. Verkley 6013, CBS H-21249, living cultures CBS 123740, 123741.
Notes: Morphologically, the material from the Czech Republic available here agrees well with S. aegopodina as described by Vanev et al. (1997) and Shin & Sameva (2004), although the pycnidia are larger than described by these authors (55-85 μm diam). The species can easily be distinguished from S. aegopodii occurring on the same host plant, as the conidia of that fungus are considerably larger (30-115 × 3.5-4 μm), and appear predominantly 1-septate. The conidia more closely resemble those of S. anthrisci. The diagnoses of S. aegopodina var. trailii based on material on Pimpinella saxifraga, and of S. aegopodina var. villosa on Pimpinella villosa, agree with the description of the type variety. Both varieties are therefore considered synonyms of S. aegopodina. In the multigene phylogeny S. aegopodina groups fairly closely with S. oenanthicola, S. sii and S. oenanthis from the same host family (Apiaceae), but other taxa from that family like S. anthrisci are relative distant and belong elsewhere the Septoria clade (Fig. 2). Other isolates grouping with S. aegopodii include those of S. mazi from Mazus japonicus (Scrophulariaceae), S. campanulae from Campanula takesimana (Campanulaceae), and S. gentianae from Gentiana scabra var. buergeri (Gentianaceae).
Septoria anthrisci Pass. & Brunaud, Rev. Mycol. (Toulouse) 5: 250. 1883 [non P. Karst., Meddn Soc. Fauna Flora fenn. 13: 10. 1884]. Fig. 8.
Fig. 8.
Septoria anthrisci. A. Conidia and conidiogenous cells in planta (CBS H-21185). B. Conidia on OA (CBS 109020). Scale bars = 10 μm.
Description in planta: Symptoms leaf spots numerous but small, circular to elliptical, visible on both sides of the leaf, the centre white to pale ochreous, surrounded by a relatively narrow, somewhat elevated, dark reddish brown to black margin. Conidiomata pycnidial, epiphyllous, sometimes also visible from the underside of the lesion, mostly one, rarely up to three in each leaf spot, subglobose to lenticular, sometimes becoming cupulate, brown to black, immersed, 115-190 μm diam; ostiolum central, initially circular and 30-55 μm wide, later becoming more irregular and up to 100 μm wide, surrounding cells concolorous; conidiomatal wall about 12-20 μm thick, composed of an outer layer of pale brown angular cells 4.5-7 μm diam with somewhat thickened walls, and an inner layer of thin-walled, pale yellow angular to globose cells 2.5-5 μm diam. Conidiogenous cells hyaline, discrete, rarely integrated in 1-septate conidiophores, globose or narrowly or broadly ampulliform, holoblastic, mostly with a relatively narrow elongated neck, proliferating percurrently several times with distinct annellations, often also sympodially after or in between a few percurrent proliferations, 6-14(-18) × 2.5-5(-6) μm. Conidia filiform, straight, curved to flexuous, attenuated gradually to a narrowly pointed apex and narrowly truncate base, (0-)1-3(-4)-septate (septa very thin and easily overlooked), not constricted around the septa, hyaline, contents with several minute oil-droplets and granular material in each cell in the living state, with minute oil-droplets and granular contents in the rehydrated state, (18-)25-59 (-65) × 1-2 μm (living; rehydrated, 1-1.8 μm wide). Sexual morph unknown.
Description in vitro: Colonies on OA 4-6(-9) mm diam in 1 wk (18-22 mm in 22 d), with an even, glabrous, peach, later coral margin, with a concolorous pigment diffusing beyond the colony margin; colonies after 1 wk restricted, distinctly elevated in the centre, immersed mycelium first peach to pale coral, then deep coral, the colony already appearing darker in the centre after 1 wk due to numerous almost black pycnidial conidiomata in part merging into large complexes, releasing pale whitish or rosy-buff droplets of conidial slime from one to several short-papillate or more elongated neck-like openings; reverse in the centre blood colour, surrounded by a first intense peach, later scarlet or coral area. Colonies on CMA 7-8(-9) mm diam in 1 wk (18-21 mm in 22 d), as on OA. Colonies on MEA 6-11 mm diam in 1 wk (24-29 mm in 22 d), with an even, almost glabrous, buff margin, without a diffusing pigment; colonies restricted, irregularly pustulate to hemispherical, already up to 4 mm high after 1 wk, immersed mycelium leaden grey to olivaceous-grey, covered by well-developed white to greyish, appressed, woolly aerial mycelium; conidiomata abundantly developing at the surface in the central area, releasing cirrhi of buff to pale luteous to rosy-buff conidial slime; reverse fuscous black to brown-vinaceous, surrounded by a narrow pale luteous marginal zone. Colonies on CHA 7-12 mm diam in 1 wk (29-31 mm in 22 d), as on MEA, but the surface more glaucous to glaucous blue green, the margin rosy-buff, and the conidial slime pale flesh.
Conidiomata pycnidial, single, brown to black, 100-250 μm diam, conidiogenous cells as in planta; conidia as in planta, 25-55(-69) × 1.2-2 μm.
Hosts: Anthriscus spp., and also Chaerophyllum spp. (Teterevnikova-Babayan 1987; Vanev et al. 1997).
Material examined: Austria, Tirol, Ötztal, Sautens, on living leaves of Anthriscus sp., 30 July 2000, G. Verkley 1022, CBS H-21185, living culture CBS 109019, 109020.
Notes: According to the short and incomplete original diagnosis, the conidia of S. anthrisci are continuous, 40-50 μm long. The type host is Anthriscus vulgaris. The description of the species on the host agrees well with those provided by Vanev et al. (1997) and Teterevnikova-Babayan (1987), although the latter reported conidia up to 75 μm long. The species is close to S. petroselini (CBS 182.44 and CBS 109521), from which it cannot be distinguished by ITS sequence, but the EF and Act sequences proved to differ by 4 and 27 %, respectively.
Of other Septoria species found on the family Apiaceae, only S. petroselini is relatively closely related. Septoria petroselini can be distinguished from S. anthrisci by the larger conidia (29-80 × 1.9-2.5 mm) with up to 7 septa on the host plant, usually species of Petroselinum or Coriandrum.
Septoria apiicola Speg., Boln Acad. nac. Cienc. Córdoba 11: 294. 1888. Fig. 9.
Fig. 9.
Septoria apiicola. a. Colony on OA (CBS 400.54). B, C. Conidia in planta (CBS H-21261). D. Conidia on OA (CBS 400.54). E. Conidia and conidiogenous cells on OA (CBS 400.54). F. Ibid., in planta (CBS H-21261). Scale bars = 10 μm.
≡ Rhabdospora apiicola (Speg.) Kuntze, Revisio generum plantarum 3 (2): 509. 1898.
= Septoria apii Chester, Bull. Torrey Bot. Club 18: 371. 1891 [non Rostr., Gartn. Tidende 180. 1893, later homonym].
= Septoria petroselini var. apii Briosi & Cavara, I funghi parassiti delle piante coltivate de utili essicati, delineati e descritti, Fasc. 6, no 144. 1891.
= Septoria apii-graveolentis Dorogin, Mater. Mikol. Fitopat. Ross. 1 (4): 72. 1915.
Description in planta: Symptoms on leaves numerous spots, scattered, separate but not well-delimited, circular to elliptical, or confluent, yellowish or pale brown and in dry conditions also with a white centre, visible on both sides of the leaf. Conidiomata pycnidial, amphigenous, single, numerous in each lesion, scattered, in small clusters or in more or less distinct concentric patterns, globose to subglobose, dark brown to black, immersed, (60-)75-170 μm diam; ostiolum circular, central, somewhat papillate, 15-45(-55) μm wide, surrounded by darker cells with thickened walls; conidiomatal wall composed of textura angularis, 12.5-20 μm thick, with an outer layer of cells, 4-6.5(-8) μm diam with brown, thickened walls, and an inner layer of hyaline and thin-walled cells 3.5-4 μm diam. Conidiogenous cells cylindrical, or broadly to elongated ampulliform mostly without distinct neck, hyaline, holoblastic, proliferating percurrently, annellations indistinct, rarely also sympodially, 4-8(-10) × 3.5-5 μm. Conidia filiform, straight, curved, or flexuous, gradually attenuated to a narrowly rounded to more or less pointed apex, more or less abruptly attenuated into a truncate base, (1-)2-3(-5)-septate, not or only inconspicuously constricted around the septa in the living state, hyaline, containing one to several relatively small oil-droplets in each cell, in the rehydrated state with larger oil-masses, 20-48(-56) × 2-2.5 μm (living; rehydrated, NT 1.5-2 μm wide). Sexual morph unknown.
Description in vitro (based on CBS 400.54): Colonies on OA 12-18 mm diam in 2 wk, with an even to slightly ruffled, glabrous, colourless margin; colonies spreading, remaining almost plane, immersed mycelium dull green to dark herbage green; aerial mycelium moderately to well-developed, woolly-floccose, white; dark brown to black single globose pycnidia developing after 7-10 d scattered over the agar surface, more rarely immersed in the agar, 70-100(-140) μm diam, ostioli often reduced or absent, releasing droplets of milky white conidial slime; reverse dark bluish green to black, diffusing pigment absent. Conidiogenous cells as in planta, but more often proliferating sympodially, 4-12.5 × 3.5-4.5 μm. Conidia as in planta, mostly 30-55(-68) × 2-2.5 μm.
Hosts: Apium australe, A. graveolens var. graveolens (celery), A. graveolens var. rapaceum (celeriac), A. prostratum.
Material examined: Italy, Perugia, culture ex leaf of Apium graveolens, deposited June 1959, M. Ribaldi s.n., CBS 389.59; Netherlands, culture ex Apium sp., deposited Aug. 1952, isolated by G. van den Ende s.n., CBS 395.52; Prov. Utrecht, Baarn, Cantonspark, culture ex living leaves of A.graveolens, 1953, deposited Oct. 1954, J.A. von Arx s.n., CBS 400.54 = IMI 092628; Prov. Limburg, Venray, Vreedepeel, on living leaves of A. graveolens var. graveolens, Aug. 2004, collector unknown (G. Verkley 3046), CBS H-21261; same substr., Noord-Brabant, between Zevenbergen and Zevenbergschen Hoek, 26 Aug. 2004, R. Munning (G. Verkley 3048), CBS H-21163, living culture CBS 116465.
Notes: According to Priest (2006), it is apparent that at least two species of Septoria occur on Apium spp. worldwide. Earlier studies demonstrated considerable variation in the dimensions of conidia in material on Apium spp. especially in conidial width, along with other minor morphological differences, and differences in leaf spot type (Cochran 1932, Sheridan 1968). Gabrielson & Grogan (1964) concluded that there was just one species involved, characterised by pycnidia 55-190 μm diam and conidia 10-72 × 0.9-3.0 μm. They accepted the name S. apiicola, and placed S. apii and S. apii-graveolentis in its synonymy. Jørstad (1965) placed S. apii in the synonymy of S. petroselini, while Sutton & Waterston (1966) followed Gabrielson & Grogan but described the conidia as 22-56 × 2-2.5 μm. As was the case in the material from Australia studied more recently by Priest (2006; conidia 30-48 × 2-2.5 μm), most conidia in the collections available for the present study are 2-2.5 μm wide. These collections proved highly homogenous in DNA sequences of the genes investigated and in most morphological characters. However, morphological and molecular investigations of more material on Apium from various host species and geographical regions is required before conclusions can be drawn about the number of taxa involved on this host genus.
According to Sutton & Waterston (1966) and also Priest (2006), the conidiogenous cells of S. apiicola are phialidic, producing several conidia enteroblastically and seceding at the same level, and these authors did not report sympodial proliferation. In the material we were able to examine however, percurrent proliferation was mostly seen and rarely also sympodial in planta, while sympodially proliferating conidiogenous cells were more common in vitro. The difference may result from the fact that here we studied living material, as we noted that after rehydration of the herbarium vouchers it is indeed very difficult to still see the details, in particular progressive annellations.
Septoria astragali Roberge ex Desm., Annls Sci. Nat., sér. 2, Bot. 19: 345. 1843. Fig. 10.
Fig. 10.
Septoria astragali, CBS 109116. A-C. Colonies (15 C, nUV). A. On OA. B. On CHA. C. On MEA. D. Conidia and conidiogenous cells on OA (CBS 109116); E-G. Conidia in planta (CBS H-21258). H. Conidiogenous cells on OA (CBS 123878). I. Conidia on OA (CBS 123878). Scale bars = 10 μm.
?= Septoria astragali var. brencklei Sacc., Atti Memorie Accad. patavina 33: 171 (as ‘brinklei’). 1917.
Description in planta: Symptoms leaf spots circular or more irregular, often indefinite or delimited by a dark brown border, white, pale ochreous to yellowish brown, usually several on each leaflet. Conidiomata pycnidial, often visible on both sides of the leaf, amphigenous, but either predominantly hypo- (V6023) or epiphyllous (V1036), scattered, globose, immersed to semi-immersed, 125-170 μm diam; ostiolum circular, central, 20-55 μm wide, surrounding cells somewhat darker; conidiomatal wall up to 30 μm thick, composed of an outer layer of isodiametric to irregular cells 3.5-8.5 μm diam with brown walls which are thickened up to 1 μm, and an inner layer of hyaline, thin-walled cells 3-7 μm diam. Conidiogenous cells hyaline, ampuliform, or elongated ampulliform with a distinct neck, hyaline, holoblastic, proliferating sympodially, and sometimes (also) percurrently 1-2 times with indistinct annellations, 10-17 × 5-8 μm. Conidia cylindrical, straight, curved, or flexuous, gradually attenuated to a narrowly rounded to somewhat pointed apex and a truncate base, (5-)7-9(-11)-septate, somewhat constricted around the septa in the living state (“T”), not constricted in the rehydrated state, hyaline, contents granular or with numerous small and a few larger oil-droplets in each cell, (85-) 105-145 × 3.5-4 μm (living; rehydrated, 3-3.5 μm wide). Sexual morph unknown.
Description in vitro: Colonies on OA 2-4 mm diam in 10 d (34-37 mm in 7 wk), with an even or irregular, glabrous, colourless margin; colonies spreading, the surface plane, immersed mycelium mostly colourless to buff with very diffuse, short, whitish aerial mycelium, the centre of the colony darkened by numerous superficial and immersed, separate or confluent pycnidial conidiomata, the outer walls covered with short mycelial outgrowth, with a single opening releasing a stout cirrhus of pale whitish to rosy-buff conidial slime; reverse mostly olivaceous-black due to the conidiomata; after incubation of 5-7 wk, more of the immersed mycelium darkens to olivaceous-black, with traces of a red pigment especially near the margin, and the aerial mycelium becomes more dominant, white or grey. Colonies on CMA 2-3 mm diam in 10 d (27-28 mm in 7 wk), as on OA, but the reddish pigment at the margin more conspicuous in old cultures. Colonies on MEA 1.5-3 mm diam in 10 d ((8-)14-17 mm in 7 wk), with an even to irregular, glabrous, buff margin; colonies first restricted, while later faster growing hyphal strands colonize the medium underneath the surface of the agar, pustulate to hemispherical, the surface first ochreous or amber, later olivaceous-grey or black covered by fairly dense, short, white aerial mycelium; some superficial or immersed pycnidial conidiomata formed, releasing cirrhi of pale buff conidial slime; reverse dark umber to brown-vinaceous. Colonies on CHA 1.5-3 mm diam in 10 d (15-17 mm in 7 wk), with an irregular margin which is hardly visible from above; colonies restricted, irregularly pustulate to hemispherical, the surface dark brick to dark slate blue, covered by a diffuse, very short, felty, white aerial mycelium; abundant superficial conidiomata releasing stout cirrhi of rosy-buff conidial slime; reverse blood colour.
Hosts: Astragalus spp.
Material examined: Austria, Tirol, Ötztal, Ötz, near Habichen W of Ötztaler Aache, 1 Aug. 2000, on living leaves of Astragalus glycyphyllos, G. Verkley 1036, epitype designated here CBS H-21151 “MBT175673”, living cultures ex-epitype CBS 109116, 109117; Carinthia, near Töschling at Wörthersee, on living leaves of A. glycyphyllos, July (year not indicated), Keissler, distributed in Keissler, Kryptogam. exsicc. 1331, PC 0084566. Czech Republic, Moravia, Pavlov, forest around ruin, 18 Sep. 2008, on living leaves of A. glycyphyllos, G. Verkley 6023, CBS H-21258, living culture CBS 123878. France, Lower Normandy, Calvados, Baynes near Forêt de Cerisy, 20-21 Sep. 1842, on leaves of A. glycyphyllos, Roberge, “Col. Desmazieres 1863, no. 8, 59”, isotype PC 0084563; Côte-d’Or, Montagne de Bard, same substr., June 1901, Fautrey, PC 0084565 (herb. Mussat); same substr., Pinsguel, near Toulouse, 30 Aug. 1935, Moesz, PC 0084564. Poland, Puszcza Bialowieska, Aug. 1922, on living leaves of A. glycyphyllos, W. Siemaszko, distributed in W. Siemaszko, Fung. Bialowiezenses exsicc. 73, PC 0084569. Romania, Transsilvania, distr. Istriţa-Năsăud, Arcalia Arboretum, 1 July 1966, on living leaves of A. glycyphyllos, A. Crişan, distributed in Flora Romania exsicc 3127, PC 0084567; same substr., Muntenia, distr. Ilfov, Pantelimon, 18 July 1926, T. Săvulescu & C. Sandhu, distributed in Săvulescu, Herb. Mycol. Romanicum 4, 166, PC 0084568 (sub S. astragali f. santonensis).
Notes: The type specimen in PC of S. astragali contains several mounted leaves and is provided with a hand-written description in French. Conidia observed in this material are mostly 7-9-septate, 85-130 × 2.5-3.5 μm. The type thus agrees well with the original description which indicated conidia 120 × 3 μm, with 9-10 septa. Of the other collections available for this study that generally all agree with the type in morphology and leaf symptoms, 1036 from Tirol is chosen as epitype. Various authors have reported comparable conidial measurements for this large-spored Septoria. Jørstad (1965) reported conidial measurements 48-128 × 3-3.5 μm, Teterevnikova-Babayan (1987) 60-140 × 3-4 μm, Vanev et al. (1997), 58-112 × 2.5-3.5 μm. According to the original diagnosis, S. astragali var. brencklei, described from Lathyrus venosus in North Dakota, has 8-10-septate conidia, 130-150 × 4-5 μm, and Teterevnikova-Babayan (1987) placed it in synonymy with S. astragali. Septoria astragali is one of the first of over 200 Septoria that were described from plants of the family Fabaceae.
Septoria campanulae (Lév.) Sacc., Syll. Fung. 3: 544. 1884. Fig. 11.
Fig. 11.
Septoria campanulae. A. Conidia and conidiogenous cells in planta (CBS H-21178). B. Ibid., on CHA (CBS 109114). Scale bars = 10 μm.
Basionym: Ascochyta campanulae Lév., Annls Sci. Nat., sér. 3, Bot. 5: 277. 1846.
Description in planta: Symptoms definite, circular to irregular, pale to dark brown leaf spots, epigenous, usually delimited by blackened veinlets. Conidiomata pycnidial, predominantly ephiphyllous, rarely hyphyllous, scattered, globose to subglobose, immersed to semi-immersed, 40-125 μm diam; ostiolum circular, central, 10-20 μm wide, surrounding cells darker; conidiomatal wall 10-20 μm diam, composed of an outer layer of brown-walled cells 3.5-10 μm diam, and an inner layer of hyaline cells 3.5-6 μm diam. Conidiogenous cells discrete or integrated in 1-2-septate conidiophores, cylindrical, or ampuliform, sometimes with an elongated neck, hyaline, holoblastic, proliferating sympodially, and often in the same cell also percurrently showing indistinct annellations, 5-15 × 3-5 μm. Conidia filiform, straight or slightly curved, gradually attenuated to a narrowly rounded or somewhat pointed apex, gradually or more abruptly attenuated into a narrowly truncate base, 0-1(-3)-septate, not or indistinctly constricted around the septa, hyaline, contents with small oil-droplets and minutely granular material in the living state and rehydrated state, (12.5-)15-25(-32) × 1.5-2 μm (rehydrated). Sexual morph unknown.
Description in vitro: Colonies on OA 6-9 mm diam in 10 d (28-32 mm in 3 wk; > 65 mm in 7 wk), with an even, somewhat undulating, glabrous, colourless margin; colonies spreading, the surface plane, immersed mycelium pale luteous to ochreous, but radiating greenish or olivaceous hyphal strands soon developing, which later dominate the olivaceous-black colonies, then also a distinct red pigment is produced which diffuses beyond the colony margin; scattered, mostly superficial pycnidial conidiomata, which are first dark olivaceous, then almost black, glabrous, with a single or up to 5 ostioli placed on short papillae or more elongated necks, that release pale whitish conidial slime; aerial mycelium scanty, diffuse, woolly-floccose, white; reverse in the centre most dark slate blue, first surrounded and intermixed with ochreous to rust, later more coral. Colonies on CMA 5-9 mm diam in 10 d (24-28 mm in 3 wk; > 70 mm in 7 wk), with an even, glabrous margin; as on OA but immersed mycelium with a greenish haze throughout, later almost entirely olivaceous-black; aerial mycelium even more scanty, but higher and reverse darker, dark slate blue throughout most of the colony; conidiomata similar as on OA, but necks shorter or absent. Colonies on MEA 7-9 mm diam in 2 wk (24-30 mm in 3 wk; > 70 mm in 7 wk), with an even, undulating to ruffled, glabrous, buff to honey margin; colonies first more restricted, pustulate to almost conical, but later growing faster with a plane submarginal area; immersed mycelium rather dark, near the margin covered by woolly to felty white aerial mycelium; mostly composed of spherical conidiomatal initials, superficial mature conidiomata releasing milky white conidial slime; reverse first dark brick in the centre, near the margin locally grey-olivaceous or cinnamon, later sepia to brown-vinaceous, the margin honey. Colonies on CHA 4-10 mm diam in 10 d (17-32 mm in 3 wk; 45-65 mm in 7 wk), with an irregular or even, buff margin covered by a diffuse, felty white, later grey aerial mycelium; further as on MEA, but the colony surface less elevated and especially near the margin with greyish, felty to tufty aerial mycelium; in the centre numerous conidiomata develop at the surface, after 3 wk releasing milky white to rosy-buff droplets of conidial slime; reverse in the centre blood colour, dark brick to cinnamon at the margin.
Conidiogenous cells as in planta, but often with relatively longer necks due to repetitive percurrent proliferation. Conidia as in planta, but more often 2 and also 3-septate, and mostly 18-34.5 × 1.5-2 μm (OA), 13-32 × 1.5-2 μm (CHA).
Hosts: Campanula glomerata, C. takesimana.
Material examined: Austria, Tirol, Ötztal, Sulztal, Gries, along the river in the village, on living leaves of Campanula glomerata, 1 Aug. 2000, G. Verkley 1034, CBS H-21178, living cultures CBS 109114, 109115. Korea, Taean, on living leaves of C. takesimana, H.D. Shin, living culture SMKC 21949 = KACC 42622 = CBS 128589; Daejeon, same substr., H.D. Shin, living culture SMKC 24476 = KACC 44787 = CBS 128604.
Notes: The first species described on Campanula is S. campanulae, for which Shin & Sameva (2004) provided a detailed description based on material occurring in Korea on C. punctata and C. takeshimana (conidia mostly 1-septate, 13-24 × 1.5-2 μm). Shin & Sameva summerised the history of the Septoria species on the genus Campanula. Of the three species most often accepted, viz., S. campanulae, S. obscura, and S. trachelii, S. campanulae fits the current material best. Septoria arcautei was not mentioned by Shin & Sameva. This species was described from C. glomerata in Spain, and according to the original description by Unanumo, the pycnidia are predominantly epiphyllous, 55.8-74.8 μm diam, and the conidia continuous, 20-25.7 × 0.8 μm. Septoria campanulae is closely related to several species from hosts in Apiaceae, including S. aegopodina, S. oenanthis, and S. sii (Fig. 2). Sequencing results of CBS 109114 and 109115 were puzzling, suggesting possible contamination.
Septoria cerastii Roberge ex Desm., Annls Sci. Nat., sér. 3, Bot. 11: 347. 1849. Fig. 12.
Fig. 12.
Septoria cerastii, CBS 102323. A-C. Colonies (15 °C, nUV). A. On OA. B. On CHA. C. On MEA. D. Conidia and conidiogenous cells in planta (CBS H-21158, epitype). E. Conidia on OA (CBS 102323). Scale bars = 10 μm.
Description in planta: Symptoms indefinite, yellow to brown leaf spots, but more often on withering parts of leaves, stems and bracts. Conidiomata pycnidial, on leaves amphigenous but predominately epiphyllous, scattered or aggregated, globose, semi-immersed, 80-125(-150) μm diam; ostiolum circular, central, 20-45 μm wide, surrounding cells somewhat darker; conidiomatal wall composed of textura angularis without distinctly differentiated layers, the outer cells with brown, somewhat thickened walls and 4-6.5 μm diam, the inner cells hyaline and thin-walled and 3.5-6 μm diam. Conidiogenous cells ampulliform, or elongated ampulliform with a distinct neck, hyaline, holoblastic, proliferating percurrently 1-many times with indistinct annellations, also sympodially, 5-10 × 3-5 μm. Conidia filiform to filiform-cylindrical, straight, curved, or flexuous, gradually attenuated to a rounded or more or less pointed apex, abruptly attenuated into a truncate base, (1-)2-4(-5)-septate, not or indistinctly constricted around the septa, hyaline, contents moderately rich in small guttulae, minutely granular material and large vacuoles in the living state, in the rehydrated state with inconspicuous contents and no oil-droplets, (21-)30-52(-57) × 1.5-2 μm (rehydrated). Sexual morph unknown.
Description in vitro: Colonies on OA 2-4 mm diam in 2 wk (10-13 mm in 6 wk), the margin irregular to ruffled, almost as dark as rest of the colony, covered by diffuse, grey aerial mycelium; the colony spreading, almost plane to somewhat irregularly lifted and pustulate, immersed mycelium olivaceous-black to black, covered with dense, grey, woolly aerial mycelium; conidiomata starting to develop at the surface after 10-15 d; reverse olivaceous-black. Colonies on CMA 2-5 mm diam in 2 wk (13-17 mm in 6 wk), as on OA; conidial slime milky white; reverse greenish grey to almost black. Colonies on MEA 0.5-1.5 mm diam in 2 wk (4-6 mm in 6 wk), as on OA, with equally dense and long, woolly, grey aerial mycelium; colony hemispherical, with scarce pycnidial conidiomata developing tardily; reverse dark slate blue to black. Colonies on CHA 1-3 mm diam in 2 wk (8-12 mm in 6 wk), as on OA, but colonies more distinctly lifted above the agar surface, hemispherical, and aerial mycelium denser but shorter; conidiomata developing scarcely at the surface.
Conidiomata pycnidial and similar as in planta, 100-150 μm diam, or merged into larger complexes especially on the agar surface, dark olivaceous-black to black, up to 250 μm diam; ostiolum as in planta, or absent; Conidiogenous cells hyaline, ampuliform, or elongated ampulliform to cylindrical, with a distinct neck, holoblastic, proliferating percurrently 1-many times with indistinct scars (annellations), also sympodially, 5-12(-15) × 3-5(-6.5) μm. Conidia on OA similar as in planta, 1-3(-5)-septate, indistinctly constricted around the septa, hyaline, contents moderately rich in small guttulae, minutely granular material and large vacuoles in the living state, (26-)35-50(-57) × 1.5-2.5 μm (T), released from superficial conidiomata in whitish cirrhi or slimy masses.
Hosts: In leaf spots and on withering leaves, stems and bracts of Cerastium spp. According to Markevičius & Treigienė (2003), also on Stellaria holostea.
Material examined: Korea, Hoengseong, on C. holosteoides var. hallaisanense, 14 May 2006, H.D. Shin, CBS 128586 =KACC 42367 = SMKC 21781; same loc., substr., H.D. Shin, CBS 128612 =KACC 42831 = SMKC 22609; Jeju, on C. holosteoides, 1 Nov. 2007, H.D. Shin, CBS 128626 =KACC 43220 = SMKC 23137. Netherlands, prov. Utrecht, Baarn, on living leaves of Cerastium sp., 9 Aug. 1968, H.A. van der Aa 731, CBS H-18069; same loc., substratum, 18 Oct. 1962, H.A. van der Aa, CBS H-18070, and 19 Oct. 1963, CBS H-18071; Prov. Noord Holland, Amsterdamse Waterleidingduinen, near Ruigeveld, on withering leaves of Cerastium fontanum subsp. vulgare, 31 Aug. 1999, G. Verkley & A. van Iperen 915, epitype designated here CBS H-21158 “MBT175351”, living culture ex-epitype CBS 102323. Romania, distr. Ilfov, Malu-Spart, on living leaves of C. fontanum subsp. triviale, 20 May 1973, G. Negrean, CBS H-18072, distributed in Herb. Mycol. Romanicum, fasc. 50, no. 2475.
Notes: The material on Cerastium fontanum examined here agrees in morphology with the detailed description of Muthumary (1999), who studied type material of S. cerastii (PC 1324) and also provided excellent illustrations. The type host was identified as C. vulgatum, which is a synonym of C. fontanum subsp. vulgare (and C. holosteoides). According to Muthumary, no definite spots are on the leaves in this collection, but the fungus is nonetheless interpreted as parasitic. We have the impression from our collection that it may be endophytic or a very weak pathogen, but in Korea the fungus causes very characteristic symptoms on C. holosteoides var. hallaisanense (Shin & Sameva 2004).
This species and S. stellariae occur on two very closely related host genera, Cerastium and Stellaria (Smissen et al. 2002), but the two can be distinguished morphologically by conidiogenesis and conidial morphology in planta, and the cultures also differ considerably in pigmentation and growth speed especially on OA. DNA sequence data also support the hypothesis that S. cerastii and S. stellariae are distinct species, as they differ for example by 6 base positions on ITS 1, and the distance in the multilocus tree is considerable. Jørstad (1965) also regarded S. cerastii and S. stellariae as distinct species, indicating that on average the spores in the latter were much longer (22-96 μm) than in the former (20-43 μm). He mentioned that in two collections of S. cerastii from Iceland the conidia reached lengths of 57-60 μm, whereas in collections from Norway attributed to the same species conidia were no longer than 43 μm. In the Dutch collection studied here, conidia also reached 57 μm in length.
Septoria chromolaenae Crous & den Breeÿen, Fungal Diversity 23: 90. 2006.
A detailed description of the species in planta and in vitro was given by Den Breeÿen et al. (2006).
Material examined: Cuba, near Havana, Chromolaena odorata, S. Neser, 28 Oct. 1997, holotype CBS H-19756, culture ex-type CBS 113373.
Notes: This species is closely related to two strains identified as S. ekmanniana (CBS 113385, 113612) originating from Chromolaena odorata (Asteraceae) in Mexico. The two species can readily be distinguished by conidial sizes, particularly in culture (Den Breeÿen et al. 2006). Other species in this clade include S. passiflorae (CBS 102701) and S. passifloricola (CBS 129431), and S. anthurii (CBS 148.41, 346.58) and S. sisyrinchii (CBS 112096).
Septoria chrysanthemella complex
Septoria chrysanthemella Sacc., Syll. Fung. 11: 542. 1895. nom. nov. pro S. chrysanthemi Cavara, Atti Ist. bot. Univ. Lab. crittogam. Pavia, Ser. 2, 2: 266. 1892 [non Allesch., 1891].
A description in planta was provided by Punithalingam (1967a) and Priest (2006). Sexual morph: unknown.
Multilocus sequencing revealed that five of the isolates studied here that were identified as S. chrysanthemella belong to a species complex, showing the presence of two cryptic sister species. The first group includes CBS 354.73, 128616 and 128617, originating from Chrysanthemum morifolium in New Zealand and Korea, respectively. The second group comprises the two European isolates CBS 351.58 and 483.63, and CBS 128622 from Korea, from various Chrysanthemum spp. A description of the isolates is provided below.
Group 1: Description in vitro (CBS 354.73): Colonies on OA 20-23 mm diam in 2 wk, with an even, glabrous margin; colonies spreading, immersed mycelium grey-olivaceous and in the centre with a brown haze, mostly glabrous but locally with some tufts of pure white aerial mycelium; reverse greenish grey to olivaceous-grey. Pycnidia developing immersed and on the agar surface after 10-12 d, releasing pale white conidial slime. Colonies on MEA 17-20 mm diam in 2 wk, with an even, colourless to buff margin; colonies restricted to spreading, in the centre irregularly pustulate, the surface dark, provided with diffuse or more dense mat of grey, appressed aerial mycelium; reverse brown-vinaceous. Conidiomata developing on the agar surface in the centre, releasing milky white masses of conidial slime.
Material examined: New Zealand, Taranaki, Chrysanthemum morifolium, G.F. Laundon, 24 Nov. 1972, LEV 6807, living culture CBS 354.73. South Korea, Hongcheon, Chr. morifolium, H.D. Shin, 10 Sep. 2007, living culture SMKC 22860 = KACC 43086 = CBS 128617.
Group 2: Description in vitro (CBS 351.58): Colonies on OA reaching 32-36 mm diam in 2 wk, with an even, glabrous margin; colonies spreading, immersed mycelium pale luteous to faintly saffron, mostly glabrous but locally with some tufts of pure white aerial mycelium; reverse flesh to saffron. Pycnidia formed immersed or on the agar surface after 10-12 d, releasing pale white conidial slime. Colonies on MEA reaching 36-40 mm diam in 2 wk, with an even, cvolourless to buff margin; colonies spreading, the surface entirely covered by a dense mat of pure white to rose, woolly aerial mycelium; reverse fulvous to ochreous, dark brick in the centre. Pycnidia formed mostly on the agar surface after 10-2 wk, releasing pale white conidial slime.
Material examined: Germany, Berlin, Chrysanthemum indicum, R. Schneider, June 1957, living culture BBA 8432 = CBS 351.58. Netherlands, Baarn, on Chr ysanthemum sp., isol. H.A. van der Aa, dep. J.A. von Arx Nov. 1963, living culture CBS 483.63. South Korea, Hoengseong, on Chr. boreale, H.D. Shin, 16 Oct. 2007, living culture SMKC 23025 = KACC 43191 = CBS 128622.
Notes: Saccardo (1895) did not specify the host species of S. chrysanthemella, but in the original diagnosis of Cavara (for which Saccardo proposed a nomen novum to replace the name S. chrysanthemi because it was antedated by S. chrysanthemi Allesch. 1891), the host was indicated to be Chrysanthemum indicum. The fungus was described to produce conidia 55-65 × 1.5-2 μm, and lacking septa. It will have to be resolved to which group of the complex the name S. chrysanthemella should be applied.
Septoria clematidis Roberge ex Desm., Annls Sci. Nat., sér. 3, Bot. 20: 93. 1853 [non Pandotra & K.S.M. Sastry, nom. illeg., Art. 53]. Fig. 13.
Fig. 13.
Septoria clematidis. A, B. Colonies CBS 108983 (15 °C, nUV). A. On OA. B. On MEA. C. Conidia and conidiogenous cells in planta (CBS H-21182, epitype). D. Ibid., CBS 108983 on OA. Scale bars = 10 μm.
Description in planta: Symptoms leaf spots angular to circular, initially mostly pale yellowish brown, then greyish brown, sometimes surrounded by a darker border, visible on both sides of the leaf. Conidiomata pycnidial, epiphyllous, several in each leaf spot, globose to subglobose, dark brown, immersed, 65-120(-160) μm diam; ostiolum central, circular, 55-80(-100) μm wide, surrounding cells concolorous or somewhat darker; conidiomatal wall 20-35 μm thick, composed of textura angularis without distinctly differentiated layers, the cells 3-10 μm diam, the outer cells with brown, somewhat thickened walls, the inner cells with hyaline and thinner walls. Conidiogenous cells hyaline, narrowly to broadly ampulliform with a relatively wide and sometimes elongated neck, holoblastic, proliferating sympodially and possibly also percurrently in some cells but annellations not observed, 8-12.5 × 4-5(-6) μm. Conidia cylindrical to filiform-cylindrical, straight, more often curved or slightly flexuous, with a relatively broadly rounded, sometimes somewhat pointed apex, barely attenuated towards the broadly truncate base, (1-)4-5(-6)-septate, not or indistinctly constricted around the septa, hyaline, contents with a few oil-droplets and minute granular material in each cell in the living state, with inconspicuous oil-droplets and granular contents in the rehydrated state, (40-)47-67(-80) × (3-)3.5-4 μm (rehydrated). Sexual morph unknown.
Description in vitro: Colonies on OA 3-6(-8) mm diam in 3 wk (12-15 mm in 7 wk), the margin irregular to ruffled, colourless, glabrous; the colony almost plane to somewhat irregularly lifted and pustulate, immersed mycelium initially in the centre pale grey-olivaceous with some long aerial hyphae, darkening entirely in older colonies to olivaceous-black, this darkening starting where pycnicial stromata are formed releasing milky white droplets of conidial slime after about 3 wk; reverse of colony dark slate blue to olivaceous-black. Colonies on CMA 4-7(-9) mm diam in 3 wk (12-17 mm in 7 wk), as on OA, but aerial mycelium denser on sterile parts of the colony. Numerous pycnidial conidiomata developing after 2 wk in the agar, on its surface, and also in the aerial mycelium, but no fertile ones observed. Colonies on MEA 4.5-7 mm diam in 3 wk (11-18(-22) mm in 7 wk), with a barely visible margin; colony restricted, hemispherical, the surface very dark or black, covered by short, diffuse to dense white or grey aerial hyphae; pycnidial conidiomata at the surface releasing clear droplets without conidial slime after 3 wk, and later first buff, then dirty luteous droplets with conidia; reverse dark slate blue to black, margin pale luteous or buff. Colonies on CHA 4.5-7 mm diam in 3 wk (15-18 mm in 7 wk), as on MEA, but aerial mycelium denser with longer hyphae; conidiomatal initials developing scarcely at the surface, still sterile after 3 wk, but later on releasing dirty buff to pale ochreous droplets of conidial slime. In older colonies on MEA and CHA a grey or greyish white, dense mat of aerial hyphae may cover small or larger sectors.
Conidiomata as in vitro, pycnidial, often merged to complex stromata, first brownish, then black, glabrous or the surface covered by short white hyphae; conidiogenous cells as in planta, but larger, 7.5-20 × 3-5(-6) μm, holoblastic, proliferating sympodially, no percurrent proliferation observed; conidia similar in shape as in planta but mostly 3-7-septate, (45-)55-85(-105) × 4-5(-7) μm.
Hosts: Clematis spp.
Material examined: Austria, Tirol, Ötztal, Brunau, on living leaves of Clematis vitalba, 30 July 2000, G. Verkley 1025, epitype designated here CBS H-21182 “MBT175353”, living cultures ex-epitype CBS 108983, 108984; same loc., substr., date, G. Verkley 1026, CBS H-21183; same substr., S. Tirol, Eggenthal, Birchabruck, 23 July 1904, J. Kabát, distributed in Kabát & Bubák, Fungi imperfecti exsicc. 163, PC 0084599. France, Parc de Lébisey, 27 July 1848, Roberge (?), ‘Col. Desmazieres 1863, no. 8, 448’, isotype PC 0084593; same loc., substr., June 1848, Roberge, PC 0084596; same substr., Paris, Parc de St Cloud, Aug. 1908, Ludwig, PC 0084607; same substr., Fontainebleau forest, Aug. 1885, PC 0084604; same substr., Clères, 27 Aug. 1896 (herb. Mussat), PC 0084598; same substr., Seine-et-Oise, Meudon, 15 Nov. 1844, Roussel (Herb. Roussel), PC 0084594, PC 0084595. Romania, distr. Iaşi, Moldova, Bârnova, same substr., 30 Aug. 1934, T. Săvulescu & C. Sandhu, distributed in Săvulescu, Herb. Mycol. Romanicum 24, 1160, PC 0084603, 0084608, 0084597.
Notes: This is one of the large-spored species of Septoria from the genus Clematis. Teterevnikova-Babayan (1987), who studied collections from several species of Clematis observed, 4-6-septate conidia 60-90 × 3-5 μm. Vanev et al. (1997) reported conidia as 39-100 × 2.5-4 μm. The type of S. clematidis in PC showed 4-7-septate conidia 52-78 × 3-3.5 μm, in good agreement with the ones observed in the Austrian material (CBS H-21182), which is designated above as epitype.
The taxonomy of the 15 described species of Septoria on Clematis is still unresolved (Shin & Sameva 2004), and would certainly benefit from study of additional fresh material and cultures which could be compared with type material. Septoria clematidis Roberge is probably distinct from S. clematidis Pandotra & K.S.M. Sastry, a taxon described on Clematis grata in India that should be renamed because it is a later homonym. According to Muthumary (1999), the conidia in the type of S. clematidis Pan. & Sastry are 1-3-septate, 38-66 × 2.5-3 μm, whereas in the original diagnosis the conidia are described as “ septate”, 25.6-44.8 (av. 36.3) × 2.3-3.2 (av. 2.7). Two other large-spored species are S. jackmanii Ellis & Everh. 1892, which was described from Clematis jackmanii in Geneva, New York and, according to the diagnosis, has conidia 40-70 × 2.5-3 μm (number of septa not given), and also S. williamsiae Priest, based on material on C. aristata in Australia, which has (1-)3(-4)-septate conidia 20-45(-55) × (1.5-)2 μm (Priest 2006).
Septoria convolvuli Desm., Annls Sci. Nat., sér. 2, Bot.17: 108. 1842. Fig. 14.
Fig. 14.
Septoria convolvuli. A, B. Conidia in planta (CBS H-21244). C. Conidia and conidiogenous cells on OA (CBS 102325). Scale bars = 10 μm.
Description in planta: Symptoms leaf lesions circular, single or confluent to form irregular extended lesions, pale to dark brown, showing one to several concentric lines and a dark brown, slightly raised line or zone delimiting the lesion, visible on both sides of the leaf. Conidiomata pycnidial, epiphyllous, several in each lesion, immersed, subglobose to globose, brown to black, (65-)90-120(-145) μm diam; ostiolum central, circular to irregular, initially 20-40 μm wide, later becoming more irregular and up to 70 μm wide, surrounding cells somewhat darker; conidiomatal wall 10-15 μm thick, composed of a homogenous tissue of hyaline, angular cells, 2.5-4.5 μm diam, the outermost cells pale brown with slightly thickened walls, the inner cells thin-walled. Conidiogenous cells hyaline, discrete, rarely integrated in 1-septate conidiophores, narrowly to broadly ampulliform, holoblastic, proliferating percurrently several times, with indistinct annellations on a relatively elongated neck, or sympodially, 6-10(-17) × 2.5-3.5(-4) μm. Conidia filiform to filiform-cylindrical, slightly to strongly curved, often elegantly flexuous, attenuated in the upper cell to a narrowly rounded to pointed tip, narrowly truncate at the base, 1-3(-4)-septate, not constricted around the septa, hyaline, contents minute oil-droplets and granular material in the rehydrated state, (15-)23-42(-50) × 1.5-2 μm (rehydrated). Sexual morph unknown.
Description in vitro: Colonies on OA 3-5 mm diam in 1 wk (16-20 mm in 25 d; 40-48 mm in 33 d), with an even, glabrous margin, which is colourless, or faintly salmon due to a diffusable pigment already visible after 1 wk (but fading after 3 wk); colonies first restricted, conical to irregularly pustulate, but later spreading, immersed mycelium in the centre becoming first yellowish or citrine, then herbage green or darker olivaceous, surrounded by a more palid, rosy-buff or pale salmon, later hazel outer zone; pycnidia already developing in clusters or radiating rows at the colony surface, but they remain scarce, later releasing pale rosy-buff or whitish droplets of conidial slime; aerial mycelium remaining scanty, but in the centre it may be well-developed, white, woolly; reverse in the centre olivaceous-black to olivaceous-grey, surrounded by a first salmon or rosy-buff zone where the diffusable pigment is formed, but this becomes hazel. Colonies on CMA 3-5 mm diam in 1 wk [(15-)18-21 mm in 25 d; 38-40 mm in 33 d], as on OA, but salmon pigment only faintly visible after 20 d, the margin becoming rosy-buff; centre much darker earlier on, entirely olivaceous-black, numerous black papillate to rostrate pycnidia developing after 21 d, releasing pale whitish to buff droplets of conidial slime. Colonies on MEA 2-5 mm diam in 1 wk [5-11 mm in 25 d; 16-18(- 23) mm in 33 d], with a ruffled, mostly colourless margin already covered by white aerial hyphae after 1 wk; a halo of a diffusing pigment is visible after 1 wk, which fades later on; colonies restricted, irregularly pustulate and up to 3 mm high after 1 wk, immersed mycelium dark, but mostly invisible from above due to well-developed, white to greyish, dense and short-felted aerial mycelium; black conidiomata already developing after 1 wk, releasing large masses of buff conidial slime; reverse mostly sepia to isabelline. Some colonies may show a more spreading growth after 2 wk in sectors, that are glabrous, immersed mycelium almost black. Colonies on CHA 3-5 mm diam in 1 wk (18-30 mm in 25 d; 30-34 in 33 d), with an even, glabrous, colourless margin; colonies irregularly pustulate, up to 3 mm high after 1 wk, immersed mycelium colourless to pale ochreous, but in the centre the surface may be already almost black, while after 25 d the entire colony attains that colour, the larger part covered by well-developed, low, dense, pure white, later smoke-grey to grey-olivaceous, felty to woolly-floccose, aerial mycelium; conidiomatal initials developing mainly in the centre after 1 wk; reverse mostly fawn, but later almost entirely brown-vinaceous.
Conidiomata single, 60-150 μm diam, or merged to small clusters of up to 350 μm diam, olivaceous to brown, formed mostly on the agar surface; conidiogenous cells as in planta, 6-20 × 2.5-4(-5) μm; conidia as in planta, but often some conidia with cells that are somewhat inflated, and constricted around septa, (22-)30-45(-55) × 1.8-2.5 μm.
Hosts: Calystegia spp. and Convolvulus spp.
Material examined: Germany, Eiffel, Schalkenmehren near Maar, Daun, on living leaves of Convolvulus arvensis, 16 Sep. 1970, H.A. van der Aa 2276, CBS H-18082. Netherlands, Prov. Hoord-Holland, Laren, on living leaves of Calystegia sepium, 18 July 1970, H.A. van der Aa 2198, CBS H-18081; Prov. Flevoland, Erkemeder beach, in edge of marshland bordering the lake, on living leaves of Ca. sepium, 8 Sep. 1999, G. Verkley 927, CBS H-21209, living culture CBS 102325. New Zealand, North Island, Coromandel, Tairua Forest, along roadside of St. Hway 25, near crossing 25A, on living leaves of Ca. sepium, 21 Jan. 2003, G. Verkley 1844, CBS H-21244, living culture CBS 113111; same substr., North Isl., Waikato, Taupiri, Bob Byrne Memorial Park, 27 Jan. 2003, G. Verkley 1896, CBS H-21248; same substr., North Isl., Northland, Russell, 30 Jan. 2003, G. Verkley 2014, CBS H-21245. South Korea, Kangnung, isolated from Ca. soldanella, H.D. Shin, 8 Nov. 2007, KACC 43226 = CBS 128627.
Notes: Morphologically and genetically the collections available proved highly homogeneous. Muthumary (1999) and Priest (2006) both reported sympodial conidiogenesis for this species, but did not observe annellidic conidiogenesis. According to Shin & Sameva (2004), the conidia can be up to 68 μm long and 7-septate. Jørstad (1965) listed several Septoria names that were based on material from Convolvulaceae in the synonymy of S. convolvuli, including S. septulata. Beach (1919) reported physiological differences for the species on Convolvulus arvensis, but whether this correlates with genetic differences still remains to be investigated. Moreover, as already pointed out by Priest (2006), a number of species on Calystegia and Convolvulus still have to be critically re-examined, which would have to include studies in culture.
Septoria coprosmae Cooke, Grevillea 14: 129. 1886.
Description in vitro: Colonies on OA 32 mm diam in 28 d (45 mm in 38 d), with a glabrous, colourless, even margin; colony spreading, the surface glabrous with only a few tufts of pure white aerial mycelium near the centre, immersed mycelium mostly cinnamon, but brick in the centre, reverse concolorous; no diffusing pigments observed. Conidiomata formed after 3-10 d, on the agar surface or submerged, simple or complex, with dark, first reddish-brown, then black walls, preformed opening undifferentiated or lacking, tardily releasing pale salmon to whitish conidial slime (after 30 d or later). Colonies on MEA (Oxoid, 3 %) 35 mm diam in 28 d (45 mm in 38 d), spreading but slightly elevated in the centre, with a colourless to rosy-buff, glabrous, even margin; colony surface leaden-grey to black, but with a fine felt coverage of minute, white aerial hyphae, reverse mostly dark brick to sepia, surrounded by cinnamon near the margin; no diffusing pigments observed. Conidiomata formed from 10 d onwards, mostly superficial, complex, opening by tearing of the upper wall and releasing milky white conidial slime. Spermatogonia of an Asteromella-state also formed.
Conidiomata simple or complex, with several merging cavities, lacking a differentiated ostiolum, opening by tearing of the wall; conidiomatal wall composed of a single layer of isodiametric cells, 6-13 μm diam. Conidiogenous cells discrete, or integrated in short, 1-2-septate conidiophores, hyaline, cylindrical, holoblastic, sympodial; conidia cylindrical, hyaline, smooth-walled, mostly curved, rounded at the tip, attenuated to a truncate base, (0-)1-3-septate, not or only slightly constricted around the septa, with minute oil-droplets near the ends and the septa, 9-31 × 1.8-2.2 μm (MEA), 17-30 × 1.7-2.0(-2.5) μm (OA); Spermatia hyaline, ellipsoid, with rounded ends and minutely granular contents, 3-5 × 0.8-1.2 μm.
Hosts: Coprosma robusta, Coprosma sp.
Material examined: New Zealand, North Island, Bay of Islands area, N. of Russell, mycosphaerella-like sexual morph on living leaves of Coprosma robusta, G. Verkley 2020, CBS H-21246, living single ascospore isolate CBS 113391.
Notes: CBS 113391 was obtained from rehydrated spotted leaves of Coprosma robusta collected in New Zealand that contained a mycosphaerella-like sexual morph. No mature asci were observed in this material, nor a septoria-like morph, but the isolate obtained developed pycnidia agreeing with conidia described for S. coprosmae (30 × 2 μm). In the multilocus phylogeny CBS 113391 groups with CPC 19304, originating from Vigna unguiculata subsp. sesquipedalis in Australia, and CPC 19793, isolated from Syzygium cordatum in Australia, and is also relatively closely related to S. verbenae (CBS 113438, 113481) isolated from Verbena officinalis in New Zealand. Aptroot (2006) investigated an isotype of Mycosphaerella coacervata from BPI and could only find “various coelomycetes”. It is unclear whether it contained a Septoria. Sydow (1924) provided a description of the sexual morph of M. coacervata and an associated spermatial state, but not of a Septoria.
Septoria cruciatae Roberge ex Desm., Annls Sci. Nat., sér. 3, Bot. 8: 20. 1847. Fig. 15.
Fig. 15.
Septoria cruciatae. A, B. Colonies CBS 123747. A. On OA. B. On MEA. C, D. Conidia in planta (CBS H-21250, epitype). E. Conidia on OA (CBS 123748). F. Conidia in planta (CBS H-21250). G. Conidia and conidiogenous cells in planta (CBS H-21250). H. Conidia on OA (CBS 123747). Scale bars = 10 μm.
= Septoria urens Pass., Atti Soc. crittog. ital. 2: 31. 1879.
-
= Septoria aparines Ellis & Kellerm., J. Mycol. 5: 143. 1889.
≡ Rhabdospora aparines (Ellis & Kellerm.) Kuntze, Revisio generum plantarum 3 (2): 509. 1898.
= Septoria asperulae Bäumler, Verh. zool.-bot. Ges. Wien 40: 142. 1890.
= Septoria galii-borealis Henn., Bot. Jahrb. Syst. 37: 163. 1905 [non Bubák & Kabát].
= Septoria galii-borealis Bubák & Kabát, Hedwigia 52: 350. 1912 [non Henn., later homonym].
?= Phleospora bresadolae Allesch., Ber. bot. Ver. Landshut 12: 60. 1892.
?= Septoria relicta Bubák, Annls mycol. 4: 116. 1906.
For more synonyms see Jørstad (1965).
Description in planta. Symptoms leaf lesions indefinite, usually a single one on each leaf expanding to ultimately cover the entire lamina, brown. Conidiomata pycnidial, epiphyllous, numerous, semi-immersed to immersed, subglobose to globose, dark brown to black, 170-240 μm diam; ostiolum central, circular, initially 25-55 μm wide, later becoming more irregular and up to 90 μm wide, surrounding cells concolourous; conidiomatal wall 20-35 μm thick, composed of an inner layer of isodiametric to irregular cells mostly 2.5-4.5 μm diam with hyaline cell walls up to 2 μm thick, and an outer layer of hyphal cells, 8-15 × 5-6.5 μm with orange brown walls thickened up to 2 μm, well developed and up to 15 μm thick in the upper part of the pycnidium wall. Conidiogenous cells hyaline, discrete, rarely integrated in 1-septate conidiophores, cylindrical, or narrowly to broadly ampulliform, holoblastic, proliferating rarely percurrently showing 1-2 indistinct annellations, sometimes (also) proliferating sympodially, 10-15(-22) × 3-5.5 (-6) μm. Conidia filiform, curved to flexuous, rounded to somewhat pointed at the apex, attenuated modestly towards the truncate base, (0-)2-3-septate, not constricted around the septa, hyaline, containing several large oil-droplets and granular material in the living state and rehydrated state, (30-)42-54(-60) × 2.5-3.2 μm (living; rehydrated, 2.0-2.5 μm wide), released in white cirrhi.
Description in vitro (20 °C, diffuse daylight). Colonies on OA 8-12 mm diam in 2 wk, with a glabrous, colourless, even margin; colony restricted, the surface mostly covered by pure white, woolly-floccose aerial mycelium, immersed mycelium mostly bright or darker herbage-green, brick in the centre, reverse dark green to black; a red pigment diffuses into the medium. Conidiomata developing in the centre on the surface of the colony or in the aerial mycelium, releasing pale milky white to rosy-buff conidial slime. Colonies on MEA 5-7 mm diam in 2 wk, with a barely visible, irregularly ruffled margin; colony restricted, hemispherical to irregularly pustulate, the surface entirely covered by a dense felty to woolly mat of pale olivaceous-grey, locally reddish, aerial mycelium, immersed mycelium almost black; reverse olivaceous-black to black; conidiomata developing on the surface in the centre of colonies, releasing milky white to rosy-buff conidial slime. Conidiomata on OA olivaceous-brown to olivaceous, globose, single or aggregated, 200-380 μm diam, on the agar mostly without a well-developed ostiolum, the wall composed of a rather undifferentiated outer layer of loosely interwoven, pale brown hyphae with barely thickened walls, and an inner layer of globose to angular cells with hyaline walls up to 2 μm thick. Conidia as in planta, mostly 3-septate, 35-65 × 2-2.5(-3) μm (OA).
Hosts: Galium spp.
Material examined: Czech Republic, Moravia, Milovice, forest Milovika stran, 15 Sep. 2008, on living or decaying leaves of Galium odoratum, G. Verkley 6007, epitype designated here CBS H-21250 “MBT175354”, living cultures ex-epitype CBS 123747, 123748. France, Libisey near Caen, on living leaves of G. cruciatum, Jul.-Sep. 1844, M. Roberge, “Col. Desmazieres 1863, no. 8, 200”, isotype PC 0084552, with handwritten description in French; Libisey near Caen, on living leaves of G. cruciatum, July 1844, M. Roberge, PC 0084551; Puy-de-Dôme, Ambert, on G. cruciatum, 23 Aug. 1903, L. Brevière, PC 0084553. Germany, Thüringen, Berka a. Ilm, on leaves of G. rotundifolium, 21 July 1912, H. Diedicke, distributed in Sydow, Mycotheca germanica 1132, PC 0084548. Iran, Pass Ghaleh, on G. coronatum, 10 July 1968, Sharif, PC 0084549. Romania, Bucharest, on G. mollugo, 4 Oct. 1974, G. Negrean, distributed in Herb. Mycol. Romanicum 50, 2476, PC 0084550.
Notes: The description given above is based on the collections on Galium odoratum and G. cruciatum, including the well-preserved type specimen from PC and the collection V6007, which agrees well with this type material. Although the latter is from Czech Republic and another host species than the type, it is selected here as epitype as two cultures derived from it are also preserved in CBS. According to Jørstad (1965), on G. boreale conidia are 23-73 × (1-)1.5-2(-2.5) μm (with mostly 3 septa), and on G. aparine 37-88 × 1-1.5 μm (with up to 5 septa). Jørstad placed five names in the synonymy of S. cruciatae, including S. asperulae from G. odoratum. He reported limited differences between material on different species of Galium, and it is not unlikely that there is just one species capable of infecting several species of Galium. In addition to the names he listed as synonyms of S. cruciatae, S. relicta and Phleospora bresadolae, both described from G. odoratum (syn. Asperula odorata) in Czech Republic and Germany, respectively, may also be regarded as synonyms, but we have not studied type material for those (conidia reported 38-60 × 3-3.5 μm and 40-60 × 2.5-3.5 μm for these two respectively). The multigene phylogeny shows that the epitype of S. cruciatae is not part of the main Septoria clade (Fig 1), but basal to a clade of pseudocercosporella-like fungi. A new genus may have to be proposed for it in future.
Septoria cucubali Lebedeva, Materialy po mikol. obsled. Rossii 5, 3: 3. 1921. Fig. 16.
Fig. 16.
Septoria cucubali. A-C. Colonies. A. CBS 102367, on OA. B. Ibid., on CHA. C, D. CBS 102386. C. On MEA. D. On OA. E, F. Conidia on OA (CBS 102386). G. Conidia and spermatia on OA (CBS 102367). H. Conidia and conidiogenous cells in planta (CBS H-21159). I. Conidia on OA (CBS 102386). Scale bars = 10 μm.
Description in planta: Symptoms indefinite colourless to pale yellowish brown lesions, both on the lamina and along the leaf margins. Conidiomata pycnidial, epiphyllous, mostly gregarious, globose, black, semi-immersed, 50-95 μm diam; osiolum central, circular, 20-35 μm wide, provided with slightly darker cells; conidiomatal wall relatively thin, composed of textura angularis, the outer cells 3.5-5 μm diam, with brown, somewhat thickened walls, the inner cells 2.5-4.5 μm diam, with hyaline and thin walls. Conidiogenous cells ampulliform to cylindrical, without a distinct neck, hyaline, holoblastic, appearing to be phialidic, but proliferating percurrently with indistinct and close annellations, rarely also proliferating sympodially, 5-8(-10) × 2-3 μm. Conidia fusiform-cylindrical to cylindrical, weakly curved, gradually attenuated to a rounded or more or less pointed apex, abruptly attenuated into a narrow, truncate base, mostly 0-1(-3)-septate, not or indistinctly constricted around the septa, hyaline, contents minutely granular in the living state, in the rehydrated state with no distinct contents, (9-)15-42(-52) × 2-2.5 μm (rehydrated). Sexual morph unknown.
Description in vitro: Colonies on OA 13-18 mm diam in 2 wk (50-55 mm in 6 wk), with an even, glabrous, first colourless margin; colony spreading, immersed mycelium in the centre pale ochreous to sienna with a distinct citrine to olivaceous tone especially towards the margin, or a faint salmon haze; aerial mycelium scanty to well-developed, woolly-floccose, greyish white, gradually attaining a reddish haze; reverse rust to bay, with olivaceous-black areas. Surface of the colony first plane, but later irregularly lifted, with blackish stromata developing on the surface and immersed in the agar, first spherical, closed, later opening widely to expose a milky white to luteous conidial slime. Colonies on CMA 9-15 mm diam in 2 wk (43-45 mm in 6 wk), with an even, glabrous, colourless to buff margin; further as on OA, but immersed mycelium only in the centre sienna, for the most olivaceous to almost dull green; aerial mycelium similar in colour and texture, but scarcer; reverse olivaceous-black, with distinct rust central areas; conidiomata less developed. Colonies on MEA 9-16 mm diam in 2 wk, with an even, buff or peach to scarlet margin, mostly hidden under tufts of aerial mycelium; colonies hemispherical, sometimes radially striate, immersed mycelium dark ochreous to greyish brown or olivaceous-black, mostly covered by finely felty or floccose-tufty, white, greyish or scarlet aerial mycelium; luteous to reddish diffusable pigment sometimes present; reverse rust to chestnut, margin apricot; stromata scarcely developing, releasing milky white to rosy-buff conidial slime. Colonies on CHA (4-)6-9 mm diam in 2 wk [(30-) 40-46 mm in 6 wk], as on MEA, conidial slime first rosy-buff, later ochreous.
Conidiomata pycnidial, as in planta but often larger, 100-175μm, or merging into larger complexes; conidiogenous cells as in planta, but annellations more distinct. Conidia fusiform-cylindrical to cylindrical, straight or weakly curved, gradually attenuated to a rounded or more or less pointed apex, abruptly attenuated into a narrow, truncate base, (0-)1-3(-4)-septate, not or indistinctly constricted around the septa, hyaline, contents minutely granular with small oil-droplets, (9-)15-29(-52) × 2-2.5 μm.
Both on the plant and in culture spermatogonia of an Asteromella state were produced, in which 0-septate, ellipsoid spermatia were formed 2-3 × 1-1.5 μm. No sexual morph was observed.
Hosts: on living leaves of Cucubalus baccifer and Saponaria officinalis.
Material examined: Germany, isolated from leaf litter of Fagus sylvatica, M. Unterseher, living culture CBS 124874. Netherlands, Prov. Gelderland, Millingen aan de Rijn, Millingerwaard, on living leaves of Cucubalus baccifer, 6 Oct. 1999, G. Verkley 941, CBS H-21159, living cultures CBS 102367, 102368; same loc., date, brown leaf margin on living leaves of Saponaria officinalis, 6 Oct. 1999, G. Verkley 938, CBS H-21218, living culture CBS 102386.
Notes: The material on Cucubalus available for this study showed conidia (9-)15-19(-23) × 2-2.5 μm, thus much shorter and somewhat narrower than reported for S. cucubali in the original diagnosis (34-50 × 1.5-2 μm; based on material collected in July), and by Teterevnikova-Babayan (1987). This Dutch material was collected much later in the season than the type, and under relatively dry conditions. Averages of conidial width and especially lengths seen in specimens collected under adverse conditions such as drought or cold can be lower as compared to material collected under optimal conditions. The isolates obtained from this material were, however, capable of producing conidia up to 52 μm in length. This would be in good agreement with S. cucubali, as are the morphology of the pycnidia, the shape and width of the conidia, as well as the symptoms on the plant described by Teterevnikova-Babayan (1987) for S. cucubali. Markevičius & Treigiene (2003) reported S. dimera on Cucubalus, and that species is characterised by conidia that are wider (21-35 × 3.2-4.3 μm; Vanev et al. 1997 report 26-65 × 2.5-4 μm for that species).
The isolates from Cucubalus were also very similar to those obtained from the material collected in the same area on Saponaria, and the sequences obtained indicate that these isolates all belong to a single species. The material on the plant studied here differs from the description of S. saponariae provided by Teterevnikova-Babayan (1987), who describes conidia as 1-3-septate, 25-59 × 3.3-4.5 μm. That species thus has much wider conidia. Host range of S. cucubali in literature only mentions Cucubalus, but it is clear from the present study that it also includes Saponaria officinalis. The strain isolated from beech leaf litter may be an accidental dweller and originate from a Caryophyllaceae host growing in the vicinity. That the fungus would be capable of infecting Fagus leaves as an endophyte seems unlikely but cannot be excluded.
Septoria cucurbitacearum Sacc., Nuovo G. bot. ital. 8: 205. 1876.
Description in vitro: Colonies on OA 38 mm diam in 5 wk, with an even, or slightly undulating, colourless, glabrous margin; colonies restricted to moderately spreading, almost entirely olivaceous-black, due to brown-walled immersed hyphae, the surface mostly glabrous, yet in the centre and around pycnidia often with greyish white, pruinose aerial hyphae. Conidiomata numerous, scattered or gregarious, black, pycnidial, with a single often quite long ostiolate neck, but fruitbodies often bursting somewhere in the lower wall, conidial slime pale white; reverse concolourous. Conidiogenous cells hyaline, discrete, ampulliform to cylindrical, holoblastic, with 1-3 percurrent proliferations, 8-16 × 3.5-5 μm. Conidia filiform, curved or flexuous, hyaline, 3-5(-7)-septate, not constricted around the septa, narrowly rounded at the top, slighty attenuating to a narrowly truncate base, with minute oil-droplets, (30-)35-55 (-72) × 1.5-2(-2.5) μm.
Hosts: Cucurbita spp., Cucumis spp. and Citrullus vulgaris.
Material examined: New Zealand, culture isolated from living leaves of Cucurbita maxima, date of collection and isolation unknown (deposited in Feb. 1977), H. J. Boesewinkel s.n., CBS 178.77.
Notes: No specimens on plant material were available for this study. A description based on specimens from Cucumis, Cucurbita and Citrullus collected in Australia is provided by Priest (2006), and the sporulating structures observed in CBS 178.77 on OA agree well with that description. Septoria cucurbitacearum is the oldest name on plants of the family Cucurbitaceae, and Punithalingam (1982) discussed the relationship with the other taxa on the host genera Cucurbita and Cucumis. On the basis of the multilocus sequence analysis it can be concluded that S. cucurbitacearum is closely related to S. lycospersici (CBS 354.49 and 128654), S. malagutii (CBS 106.80), and S. apiicola.
Septoria digitalis Pass., Atti Soc. crittog. ital. 2: 36. 1879. Fig. 17.
Fig. 17.
Septoria digitalis. A, B. Colonies CBS 328.67 (15 °C, nUV). A. On OA. B. On MEA. C, D. Colonies CBS 391.63 (15 °C, nUV). C. On OA. D. On MEA. E. Conidia on OA (CBS 328.67). Scale bars = 10 μm.
Description in planta (based on CBS H-18090): Symptoms leaf spots hologenous, scattered, circular to elliptical, pale yellowish brown, definite with a dark brown border, or indefinite, surrounded by a larger area of the leaf which turns reddish purple. Conidiomata pycnidial, epiphyllous, numerous scattered in each leaf spot, subglobose to globose, immersed, brown to black, (70-)85-130 μm diam; ostiolum central, initially circular and 20-45 μm wide, later more irregular and up to 60 μm wide, surrounding cells undifferentiated; conidiomatal wall about 12.5-20 μm thick, composed of an outer layer of isodiametric cells 4.5-8(-10) μm diam or more irregular cells with brown walls 1-2 μm thick, and an inner layer of angular to globose cells 2.5-4(-6) μm diam with relatively thin, hyaline walls. Conidiogenous cells hyaline, discrete, rarely integrated in 1-septate conidiophores, globose, doliiform or ampulliform, holoblastic, proliferating sympodially and often also percurrently, with close indistinct annellations on an elongated neck, 3-8.5(-10) × 2-3.5(-4.5) μm. Conidia filiform-cylindrical to cylindrical, straight to slightly curved, rarely somewhat flexuous, attenuated gradually to a narrowly rounded to pointed apex, and attenuated gradually or more abruptly to a narrowly truncate base, 1-3(-4)-septate, not constricted around the septa, hyaline, contents with minute oil-droplets and granular contents in the rehydrated state, (16.5-)22-44 × 1.5-2(-2.5) μm (rehydrated). Sexual morph unknown.
Description in vitro (18 °C, near UV light) CBS 328.67: Colonies on OA 12-13 mm diam in 2 wk, with an even to slightly ruffled, glabrous margin; colonies restricted to spreading, with some irregular pustulate elevations in the centre, immersed mycelium dark rust to chestnut, mostly covered by a more or less dense mat of low, woolly to woolly-floccose, greyish to somewhat reddish aerial mycelium, with scattered higher tufts, reverse blood colour; producing a red pigment diffusing into the surrounding agar medium. Colonies on MEA 10-13 mm diam in 2 wk, with an even margin which is mostly covered by aerial mycelium; colonies restricted, irregularly pustulate and up to 2 mm high in the centre, immersed mycelium dark, entirely covered by a dense mat of appressed, finely felted, grey to ochreous or rust aerial mycelium, the surface showing numerous sterile black stromata; reverse dark brick or sepia in the centre, surrounded by dark violet slate. No sporulation or diffusing pigment observed. CBS 391.63: Colonies on OA 23-25 mm diam in 2 wk, with an even, glabrous margin; colonies spreading, immersed mycelium fulvous to rust, or some brown-vinaceous, glabrous, or with barely any aerial mycelium, no sporulation observed; reverse blood colour in centre, fading to red or coral towards the margin; producing some red pigment diffusing into the surrounding agar medium. Colonies on MEA 25-30 mm diam in 2 wk, with an even, undulating, glabrous, buff margin; colonies restricted to spreading, radially striate, up to 2 mm high in the centre, immersed mycelium dark, entirely covered by a dense mat of appressed, finely felted, rosy vinaceous to flesh aerial mycelium with greysih or white zones; reverse brown-vinaceous to blood colour. No sporulation or diffusing pigment observed.
Conidia (OA) as in planta, 20-48(-52) × 1.5-2.5 μm.
Hosts: Digitalis spp.
Material examined: Czech Republic, South Bohemia, Písek, on Digitalis lanata, Sep. 1962 V. Holubová-Jechová, living culture CBS 391.63. Netherlands, Doornspijk, herbal garden, in leaf spot on D. lanata, 22 June 1967, H.A. van der Aa 72, CBS H-18090, and dried culture on OA CBS H-18092, living culture CBS 328.67.
Notes: The two strains investigated here showed some notable differences in colony features, and they are therefore described separately above. Nonetheless, these strains showed highly homologous sequences of all loci investigated here. The strains are relatively distant from the closest relatives in the Septoria-clade, viz., among others, S. epilobii (CBS 109084, 109085), S. verbascicola (CBS 102401), and the strains of S. stachydis and S. galeopsidis. According to the original diagnosis, based on material on Digitalis lutea, the conidia of S. digitalis are continuous, 25-30 × 1.5 μm (also in Radulescu et al. 1973, Teterevnikova-Babayan 1983). Although conidia observed in the material on D. lanata studied here are up to 44 μm long and provided with up to 4 septa, it is concluded that the name S. digitalis can be applied to this material.
Septoria epilobii Westend., Bull. Acad. r. Belg., Cl. Sci., Sér. 2, 19: 120. 1852 [non Roberge ex Desm. 1853]. Fig. 18.
Fig. 18.
Septoria epilobii. A-C. Colonies CBS 109084 (15 °C, nUV). A. On OA. B. On CHA. C. On MEA. D. Conidia in planta (CBS H-21171, epitype). E. Conidia and conidiogenous cells on OA (CBS 109094). Scale bars = 10 μm.
= S. epilobii Roberge ex Desm., Annls Sci. Nat., ser. 3, 20: 94. 1853 [Nom. illeg., later homonym].
?= S. epilobii Westend. var. durieui Unamuno, Boln R. Soc. esp. Hist. nat. 34: 250. 1934.
Description in planta: Symptoms leaf lesions sparse to numerous, single, circular to irregular, rarely entended to the margin of the leaf, brown, often with a greyish centre, well-delimited by a dark brown elevated line, visible on both sides of the leaf. Conidiomata pycnidial, epiphyllous, several in each lesion, subglobose to globose, brown to black, 48-75 μm diam; ostiolum central, circular, initially 15-24 μm wide, later becoming more irregular and up to 40 μm wide, surrounding cells dark brown; conidiomatal wall 12-20 μm thick, composed of a homogenous tissue of hyaline, angular cells, 3-6.5 μm diam, the outermost cells pale brown with slightly thickened walls, the inner cells hyaline and thin-walled. Conidiogenous cells hyaline, discrete, rarely also integrated in 1-septate conidiophores, cylindrical, or narrowly to broadly ampulliform, holoblastic, proliferating sympodially, sometimes with a relatively narrow and elongated neck (no annellations seen), 5-14 × 3.5-6 μm. Conidia cylindrical or filiform-cylindrical, straight to slightly curved, narrowly to broadly rounded at the apex, narrowing slightly or more distinctly to a truncate base, (0-)1-3-septate, not or slightly constricted around the septa, hyaline, contents with few minute oil-droplets and granular material in each cell in the rehydrated state, 25-35(-40) × 1.5-2(-2.5) μm (rehydrated). Sexual morph unknown.
Description in vitro: Colonies on OA 12-15(-17) mm diam in 3 wk (45-48 mm in 7 wk), with an even, glabrous, colourless or vaguely buff margin; colonies spreading, plane, in the centre olivaceous-black, surrounded by olivaceous radiating hyphal strands; reverse concolourous; aerial mycelium absent, or a tuft of white or grey woolly aerial mycelium in the centre; abundant olivaceous to brown, then black, pycnidial conidiomata developing after 3 wk, releasing milky white droplets of conidial slime. Colonies on CMA 12-14(-16) mm diam in 3 wk (45-50 mm in 7 wk), as on OA, but centre more homogeneous olivaceous-black after 3 wk; after 7 wk larger outer area saffron to pale ochreous, margin buff; reverse concolourous; sporulation as on OA, but older conidial slime pale saffron. Colonies on MEA (7-)10-16 mm diam in 3 wk (46-50 mm in 7 wk), with an even, glabrous, rosy-buff or buff margin; colonies restricted, conical, in the centre with more irregular pustulate protruberances, after about 4 wk becoming more spreading, the surface brown-vinaceous to almost black, locally ochreous to dirty peach, covered by a diffuse, low, minutely felty whitish to grey aerial mycelium; reverse brown-vinaceous to dark slate blue, locally cinnamon to ochreous; conidiomatal initials developing from 3 wk onwards in most of the colonies, but sporulation occurs sparsely in submarginal pycnidia after 7 wk in dirty white to rosy-buff droplets. Colonies on CHA 7-12(-16) mm diam in 3 wk (34-38 mm in 7 wk), as on MEA, including sporulation.
Conidiomata as in planta, single or merged, with a single or a few papillate openings, which can be positioned on an elongated neck; conidiogenous cells as in planta, proferating sympodially and possibly also percurrently, but the presence of annellations could not be confirmed, 5-18 × 3.5-6 μm; conidia as in planta, 24-41 × 1.8-2.5 μm.
Hosts: Chamaenerion angustifolium and Epilobium spp.
Material examined: Austria, Tirol, Ober Inntal, Samnaun Gruppe, Zanderstal near Spiss, alt. 1800 m, on rocky bank of Zandersbach, on living leaves of Epilobium fleischeri, 11 Aug. 2000, G. Verkley 1068, epitype designated here CBS H-21171 “MBT175355”, living cultures ex-epitype CBS 109084, 109085. Belgium, on the bank of river Wépion, near Namur, on leaves of E. spicatum (= E. angustifolium, Chamaenerion angustifolium), 1829, Bellynck, “Westendorp & Wallay Herb. Crypt. no. 727”, isotype BR-MYCO 158690-95. Netherlands, prov. Utrecht, Baarn, Baarnsche bos, ex leaf spot of E. angustifolium, 17 Sep. 1967, L. Marvanová s.n., living culture CBS 435.67 no longer available (infected with basidiomycete).
Notes: In the type specimen of S. epilobii on Epilobium angustifolium (= Chamaenerion angustifolium), from BR, 1-3-septate conidia, 20-40 × 1-1.5 μm are observed. Although the collection of S. epilobii from Tirol was collected on another host species, E. fleischeri, it agrees morphologically well with the type material, and therefore this Austrian collection is chosen here as epitype. It is considered likely that a single taxon is capable of infecting various members of the genera Epilobium and its sister-genus Chamaenerion. The concept of S. epilobii maintained here concurs with that of most authors (Radulescu et al. 1973, Teterevnikova-Babayan 1987), except Vanev et al. (1997), who gave a much wider length range of conidia, viz., 12-72 ×1-2 μm, but their concept of S. epilobii may erroneously have been based in part on specimens of S. alpicola. Septoria epilobii is very distinct from S. alpicola Sacc. 1897, a species causing systemic infections in Epilobium spp. in alpine and boreal regions (type host E. alpinum), developing pycnidia on symptomless leaves as well as stems that produce conidia, 24-95 × 0.7-1.5(-2) μm, with up to 7 septa (Jørstad 1965).
Septoria epilobii var. durieui Unamuno, which has been described from E. duriaei in Spain, with conidia 30-55 × 1.5 μm, is tentatively placed here in the synonymy of S. epilobi.
As can be seen in the multilocus phylogeny (Fig. 2), the strains of Septoria epilobii are closely related to CBS 102401, which was isolated from Verbascum nigrum, and preliminarily identified as S. verbascicola Berk. & M.A. Curtis. This name is a nomen nudum and the type should be studied. Other closely related species include S. taraxaci (CBS 567.75), S. stachydis, S. galeopsidis, and S. digitalis.
Septoria erigerontis Peck, Rep. N.Y. St. Mus. nat. Hist. 24: 87. 1872 [non Berk. & M.A. Curtis 1874; nec Hollós 1926, later homonyms]. Fig. 19.
Fig. 19.
Septoria erigerontis. A-C. Colonies CBS 109094 (15 °C, nUV). A. On OA. B. On CHA. C. On MEA. D. Conidia in planta (CBS H-21176). E. Conidia and conidiogenous cells on OA (CBS 109094). F, G. Conidia in planta (CBS H-21176). H, I. Conidia on OA (CBS 186.93). Scale bars = 10 μm.
≡ Septoria erigerontea Sacc., Syll. Fung. 3: 547. 1884 [nom. illeg., Art. 52. superfluous nom. nov.].
= Septoria erigeronata Thüm., Bull. Soc. Imp. Nat. Moscou 56: 132. 1881.
-
= Septoria schnabliana (Allesch.) Died., KryptogFl. M. Brandenb. 9: 454. 1914.
≡ Rhabdospora schnabliana Allesch., Hedwigia 34: 273. 1895.
= Septoria chanousii Ferraris, Malpighia 16: 27. 1902.
= Septoria stenactidis Vill, in Sydow, Annls mycol. 8: 493. 1910.
?= Septoria bosniaca Picb., Glasnik Zemal. Muz. Bosn. Herceg. 45: 68. 1933.
Description in planta: Symptoms leaf spots hologenous, scattered, circular to irregular, pale brown, indefinite or surrounded by a slightly darker margin. Conidiomata pycnidial, epiphyllous, numerous scattered in each leaf spot, subglobose to globose, brown to black, semi-immersed, 75-130 μm diam; ostiolum central, initially circular and 15-35 μm wide, later more irregular, up to 55 μm wide, surrounding cells dark brown and with more thickened walls; conidiomatal wall about 8-12.5 μm thick, composed of a homogenous tissue of hyaline, angular cells 2.5-4 μm diam with relatively thin, hyaline walls, surrounded by a layer of pale to dark brown cells, 2-5 μm diam, with somewhat thickened walls. Conidiogenous cells hyaline, discrete, rarely integrated in 1-2-septate conidiophores, cylindrical to doliiform, or narrowly to broadly ampulliform, holoblastic, proliferating mostly sympodially, rarely also percurrently with indistinct annellations, 6-10 × 2.5-4.5 μm. Conidia filiform, straight, slightly curved to flexuous, attenuated gradually to a narrowly rounded to pointed apex and narrowly truncate base, (0-)1-3(-5)-septate, not constricted around the septa, hyaline, contents with several minute oil-droplets and granular material in each cell in the living state, with minute oil-droplets and granular contents in the rehydrated state, (17-)25-50(-62.5) × 1-1.5(-2) μm (rehydrated). Sexual morph unknown.
Description in vitro: Colonies on OA 8-11 mm diam in 12 d (42-44 mm in 7 wk), with an even, glabrous, colourless to pale red or coral margin, the pigment also clearly diffusing beyond the margin; colonies spreading, the surface almost plane, immersed mycelium translucent and red everywhere (12 d), in the centre with densely aggregated superficial pycnidial conidiomata often with distinct papillate to rostrate openings, which later may elongate further, pycnidia elsewhere in radiating rows, later also in concentric rings, releasing milky white to pale buff droplets of conidial slime; aerial mycelium white, felty, scanty, mostly in the centre; reverse concolorous. Colonies on CMA 7-10 mm diam in 12 d (50-59 mm in 7 wk), as on OA, but immersed hyphae darker and olivaceous, but red pigmentation still distinct, especially around the colony margin. Colonies on MEA 4-7 mm diam in 12 d (45-48 mm in 7 wk), with a ruffled, colourless to pale buff, plane marginal zone; colony initially restricted, hemispherical after 12 d, with an irregularly pustulate-worty surface, later for the most plane and spreading, immersed mycelium very dark chestnut to black, aerial mycelium on elevated surface almost absent, but near margin forming short-tufty mat of pure white hyphae; superficial pycnidial conidiomata releasing pale flesh or milky white droplets of conidial slime. Colonies on CHA 6-8 mm diam in 12 d (29-36 mm in 7 wk), as on MEA, but in some sectors with an even, rosy-buff margin; colonies less elevated in the centre than on MEA, covered with diffuse, woolly, greyish aerial mycelium in the centre, and a low, dense mat of reddish hyphae near the margin; pycnidial conidiomata more numerous than on MEA, later in distinct, concentric patterns, producing flesh, later salmon droplets of conidial slime.
Conidiogenous cells (OA) as in planta, but more frequently proliferating percurrently and with distinct annellations. Conidia as in planta, up to 85 μm long and 2.5 μm wide.
Hosts: Conyza spp. and Erigeron spp.
Material examined: Austria, Tirol, Inntal W of Innsbruck, S of Telfs, along road 171, on living leaves of Erigeron annuus, 4 Aug. 2000, G. Verkley 1045, CBS H-21176, living culture CBS 109094, 109095; same substr., country unknown, M. Vurro, living culture CBS 186.93 (sub S. schnabliana). South Korea, Namyangju, same substr., H.D. Shin, 3 May 2006, living culture SMKC 21739 = KACC 42356 = CBS 128606; same country, loc. unknown, same substr., living culture CPC 12340 = CBS 131893.
Notes: The material available for this study agreed generally well with the detailed descriptions given for this species in recent literature (Shin & Sameva 2004, Priest 2006). However, Priest (2006) did not observe sympodial proliferation in the conidiogenous cells. Shin & Sameva (2004) reported conidia up to 70 μm long in material from South Korea. Verkley & Starink-Willemse (2004) already showed that the ITS sequence of CBS 186.93 identified as S. schnabliana is identical to that in S. erigerontis (CBS 109094), and suspected the conspecificity of this material. Strong evidence for this conspecificity is provided here, as the additional genes sequenced were all (almost) identical for the three isolates investigated, and also for CBS 128606 (= KACC 42356) and CBS 131893 (= CPC 12340) from the same host in South Korea.
According to the diagnosis, Septoria stenactidis, described from Stenactis annua (= E. annuum), has continuous (or indistinctly septate) conidia, 35-40 × 1 μm, which agrees well with S. erigerontis on the type host, and it was already placed in the synonymy by Jørstad (1965), and recently also by Priest (2006). Priest also included S. chanousii in the synonymy of S. erigerontis. This fungus was originally decribed on E. uniflora in Italy, with 3-4-septate conidia measuring 45-50 × 1.5 μm. Likewise, S. bosniaca from Erigeron polymorphus described in the diagnosis as a fungus with 0(-3)-septate conidia, 19-42 × 1.3-1.9 μm, is probably also a synonym.
Septoria galeopsidis Westend., Bull. Acad. r. Belg., Cl. Sci., Sér. 2, 2: 577. 1857. Fig. 20.
Fig. 20.
Septoria galeopsidis, CBS 102314. A-C. Colonies (15 °C, nUV). A. On OA. B. On CHA. C. On MEA. D. Conidia on OA. E. Conidia and conidiogenous cells in planta (CBS H-21195). F-H. Conidia on OA (CBS 123744). Scale bars = 10 μm.
= Ascochyta galeopsidis Lasch in Rabenh., Herb. Myc. I, 1058. 1846 [nom. nud.].
= Septoria cotylea Pat. & Har., Bull. Soc. Mycol. France 21: 85. 1905.
Description in planta: Symptoms leaf spots irregular or angular, becoming dark brown, in yellow parts of the leaf lamina. Conidiomata pycnidial, hypophyllous, often numerous in each leaf spot, globose to subglobose, dark brown, almost completely immersed, 75-100(-130) μm diam; ostiolum central, initially circular, 15-25 μm wide, surrounding cells somewhat darker; conidiomatal wall 10-22 μm thick, composed of textura angularis without distinctly differentiated layers, the cells 3-8 μm diam, the outer cells with brown, somewhat thickened walls, the inner cells with hyaline and thinner walls. Conidiogenous cells discrete, sometimes integrated into 1-2-septate conidiophores, hyaline, narrowly or broadly ampulliform with a relatively narrow neck, holoblastic, proliferating percurrently with indistinct annellations, and also sympodially, 6-12(-15) × 3.5-5(-6) μm. Conidia filiform, straight or slightly curved, sometimes flexuous, with a rounded or somewhat pointed apex, attenuated towards the narrowly truncate base, (0-)3(-5)-septate, not constricted around the septa, hyaline, contents with several minute oil-droplets and granular material in each cell in the living state, with inconspicuous oil-droplets and granular contents in the rehydrated state, 20.5-44 × 1.5-2.5 μm (living; rehydrated, 1-2 μm wide). Sexual morph unknown.
Description in vitro: Colonies on OA 7-13 mm diam in 2 wk (35-43 mm in 6 wk), with an even, glabrous, colourless margin; colonies almost plane, immersed mycelium homogeneously olivaceous-black to greenish black (also near the margin); aerial mycelium scanty, woolly-floccose, white or greyish; superficial pycnidial conidiomata scanty, scattered over the central aerea, releasing milky white droplets of conidial slime; reverse dark slate blue to black. Colonies on CMA 7-13 mm diam in 2 wk (33-37 mm in 6 wk), as on OA, but concentration of conidiomatal development in elevated pustules on the elsewhere flat colony. Colonies on MEA 6-11 mm diam in 2 wk (33-39(-46) mm in 6 wk), the margin even, later undulating, buff, narrow and glabrous; colonies hemispherical, often irregularly pustulate or with columnar outgrowths up to 5 mm high, immersed mycelium olivaceous-black to black, mostly covered by a dense mat of finely velted, greyish aerial mycelium; faster growing, glabrous sectors with buff immersed mycelium may appear after several weeks; conidiomata starting to develop on the (dark) colony surface, tardily sporulating with whitish to flesh droplets of conidial slime; reverse brown-vinaceous or olivaceous-black. Colonies on CHA 5-10(-15) mm diam in 2 wk (20-29 mm in 6 wk), with an even, glabrous to nearly so, buff margin; colonies irregularly pustulate, immersed mycelium olivaceous-black, mostly covered by a dense but appressed mat of woolly-floccose, grey aerial mycelium, in some slightly faster growing sectors pure white; scattered but scarce superficial conidiomata releasing pale flesh droplets of conidial slime; reverse blood colour to black.
Conidiomata pycnidial and similar as in planta, 100-150 μm diam, or merged into larger complexes especially on the agar surface, dark brown, up to 200 μm diam; ostiolum as in planta, or absent. Conidiogenous cells hyaline, ampuliform, or elongated ampulliform to cylindrical, with a distinct neck, holoblastic, proliferating percurrently with indistinct scars (annellations), or sympodially, 8-13(-15) × 3-4.5(-5) μm. Conidia cylindrical, straight or slightly curved, tapering to a rounded or somewhat pointed apex, lower part slightly or more clearly attenuated into a broad truncate base, (0-)1-3(-5)-septate, not constricted around the septa, hyaline, with several oil-droplets and minute granular material in each cell, (37-)50-65 (-70) × 2-2.5 μm.
Hosts: Galeopsis angustifolia, G. ladanum, G. pubescens, G. speciosa and G. tetrahit.
Material examined: Belgium, in the vicinity of Mons, on leaves of Galeopsis tetrahit, R. P. Clém. Dumont, distributed in Westendorp & Wallays, Herb. crypt. Belge, Fasc. 23-24, no 1134, isotype BR-MYCO 158116-06. Czech Republic, Moravia, Mikulov, on living leaves of Galeopsis sp., 15 Sep. 2008, G. Verkley 6003, CBS H-21256, living cultures CBS 123744, 123749; same substr., date, Moravia, Milovice, forest Milovika stran, G. Verkley 6006, CBS H-21254, living cultures CBS 123745, 123746. France, Corrèze, Prât Alleyrat, on living leaves of G. tetrahit, 25 July 1976, H.A. van der Aa 5344, CBS H-18099; loc. unknown, isol. C. Killian ex Galeopsis sp., living culture CBS 191.26. Netherlands, prov. Noord-Brabant, Cromvoirt, on living leaves of G. tetrahit, 2 June 1963, H.A. van der Aa s.n., CBS H-18097; prov. Gelderland, Putten, on living leaves of G. tetrahit, 8 Aug. 1984, G. de Hoog s.n., CBS H-18100; prov. Utrecht, Soest, on living leaves of G. tetrahit, 4 Aug. 1999, G. Verkley 902, CBS H-21195, living culture CBS 102314; prov. Limburg, St. Jansberg near Plasmolen, on living leaves of G. tetrahit, 9 Sep. 1999, G. Verkley 934, epitype designated here CBS H-21215 “MBT175356”, living culture ex-epitype CBS 102411. Romania, distr. Satu-Mare, Pir, on living leaves of G. ladanum, 27 Aug. 1973, G. Negrean s.n., CBS H-18098.
Notes: Jørstad (1965) reported comparable conidial size ranges in specimens on different host species, viz. G. speciosa (extreme values 20-64 × 1-2.5 μm) and G. tetrahit (28-60 × 1-2 μm), although in most Norwegian collections on G. tetrahit, the maximum conidial length varied downwards to 48 μm. In the original diagnosis of S. galeopsidis conidia are described as 30-40 × 1-1.5 μm (Saccardo 1884), while Radulescu et al. (1973) reported measurements ranging between 20-45 μm in length in collections on various hosts. In the type material from BR investigated here conidia are mostly 3-5-septate, 19-40 × 1.5-2 μm. In other material available for the present study, maximum length of conidia was only 44 μm in planta, whereas the strains obtained from it were capable of forming conidia with a maximum length of 70 μm on OA. The differences with S. lamiicola are discussed under that species.
Septoria galeopsidis is closely related to only some of the other Septoria species occurring on plants from the family Lamiaceae, especially S. melissae (CBS 109097) and S. stachydis. Septoria lamiicola on Lamium spp., which is morphologically quite similar to S. galeopsidis, proves genetically very distinct, although these taxa can barely be distinguished by their ITS sequence (99.5 %). Several house-keeping genes do allow an easy identification of these species.
Septoria heraclei (Lib.) Desm., Pl. crypt. Fr., Fasc. 11, no 534. 1831. Fig. 21.
Fig. 21.
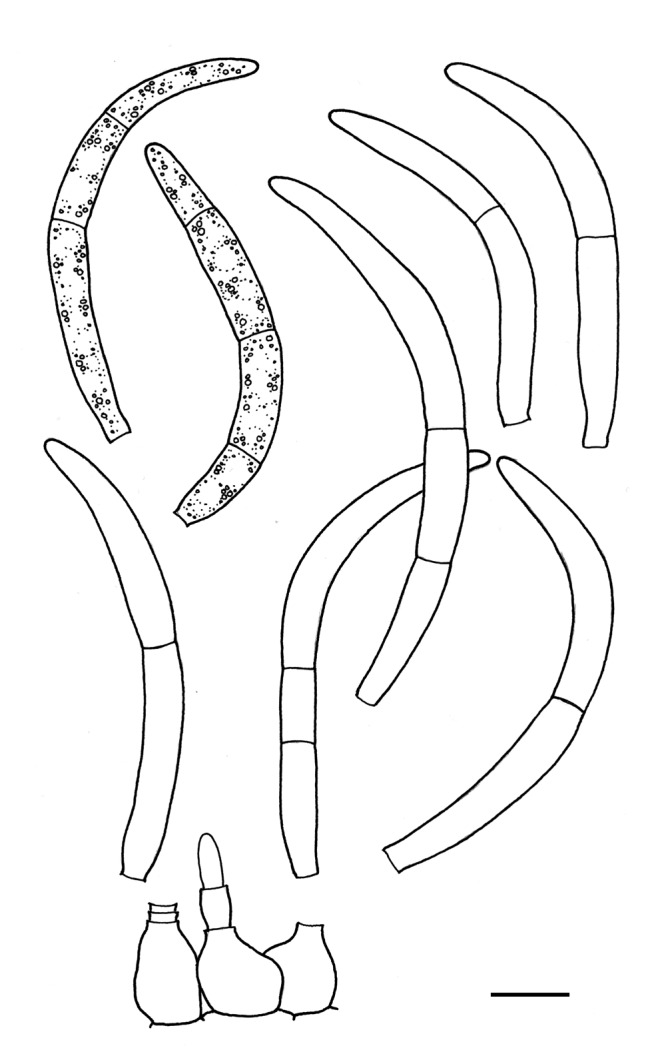
Septoria heraclei, conidia and conidiogenous cells in planta (CBS H-21224). Scale bars = 10 μm.
Basionym: Ascochyta heraclei Lib., Pl. crypt. Ard., Cent. 1: no. 51. 1830.
≡ Cylindrosporium heraclei (Lib.) Höhn., Sber. Akad. Wiss. Wien, Math.-naturw. Kl. 115, I: 378. 1906 [non Oudem. 1873, nec Ellis & Everh. 1888].
≡ Phloeospora heraclei (Lib.) Petr., Annls mycol. 17: 71. 1919 [non (Lib.) Maire, Bull. Soc. Mycol. France 46: 241. 1930].
≡ Cylindrosporium umbelliferarum Wehm., Mycologia 39: 475. 1947. nom. nov.
= Septoria heraclei-palmati Maire, Bull. Soc. Mycol. France 21: 167. 1905.
Description in planta: Symptoms leaf spots numerous but small, irregular in outline, best visible on the upper side of the leaf, initially yellowish or ochreous, later becoming pale to dark brown, in places white due to loosening of the epidermis. Conidiomata pseudopycnidial, hypophyllous, one, rarely up to three in each leaf spot, lenticular, immersed, the upper wall rupturing in an early stage and conidial masses breaking through the leaf epidermis, pale brown, 115-200 μm diam; ostiolum absent; conidiomatal wall about 15-28 μm thick, composed of an outer layer of pale brown angular cells, 5-10 μm diam with somewhat thickened walls, and an inner layer of thin-walled, pale yellow angular to globose cells, 4.5-8 μm diam. Conidiogenous cells hyaline, discrete, rarely integrated in 1-septate conidiophores, cylindrical, or broadly ampulliform, holoblastic, proliferating percurrently one to several times with distinct annellations, sometimes also sympodially, 10-25 × 5-7(-8) μm. Conidia cylindrical, usually strongly curved, attenuated gradually to a blunt to somewhat pointed apex, attenuated gradually, or more abruptly just above the broadly truncate base, (0-)1-2(-4)-septate, not or indistinctly constricted around the septa, hyaline, contents with numerous small oil-droplets and granular material in each cell in the living state, with amorphous granular contents in the rehydrated state, 40-55(-70) × 4-6 μm (living; rehydrated, 3-5 μm wide).
Description in vitro: Several attempts were made to isolate this species but unfortunately no conidia survived after germination.
Hosts: Heracleum spp.
Material examined: Austria, Tirol, Ötztal, Ötz near Habichen, on living leaves of Heracleum sphondylium, 24 July 2000, G. Verkley 1002, CBS H-21186. Netherlands, Prov. Limburg, Gulpen, near Stokhem, on living leaves of H. sphondylium, 28 June 2000, G. Verkley 957, CBS H-21224; same substr., Prov. Limburg, upper edge of Savelsbos, G. Verkley 959, CBS H-21225.
Notes: The conidia of this fungus are much wider than in most other Septoria species on Apiaceae. Jørstad (1965) reported conidia 35-57 × 3-5 μm, usually with 1 septum. Vanev et al. (1997) observed conidia up to 85 μm long, and 1.8-3.5 μm wide. Septoria heraclei-palmati was originally described from Heracleum palmatum in Greece, with 1-septate conidia, 50-70 × 3 μm. Jørstad (1965) already considered this name as a synonym of S. heraclei. Other authors have mostly accepted S. heracleicola as a further Septoria species on Heracleum, describing the conidia as continuous and ranging roughly in size 20-40 × 1-2 μm (Radulescu et al. 1973, Teterevnikova-Babayan 1987, Vanev et al. 1997). Four further Septoria and two Rhabdospora species have been described in the literature based on material found on various members of the genus Heracleum, all of which according to their original descriptions have conidia more or less within this range, so with much narrower conidia than S. heraclei.
Septoria hypochoeridis Petrov, Materialy po mikol. i fitopat. Rossii (Leningrad) 6 (1): 55. 1927. Fig. 22.
Fig. 22.
Septoria hypochoeridis, CBS H-21234. A, B. Conidia in planta. Scale bars = 10 μm.
Description in planta: Symptoms leaf spots scattered, 2-5 mm diam, definite, circular, hologenous, grey to white in the centre, surrounded by a slightly elevated, dark reddish purple or black zone. Conidiomata pycnidial, hypophyllous, one to a few in each leaf spot, (sub)globose, immersed, dark brown, 60-95 μm diam; ostiolum central, circular, 15-28 μm diam, surrounded by darker cells; conidiomatal wall about 10-20 μm thick, composed of an outer layer isodiametric or more irregular cells, 5-10 μm diam, with somewhat thickened, pale brown walls, and an inner layer of thin-walled, hyaline angular to globose cells, 4.5-7 μm diam. Conidiogenous cells hyaline, discrete, cylindrical, or broadly ampulliform, holoblastic, proliferating sympodially, percurrent proliferation not observed, 8-15 × 3.5-4(-5.5) μm. Conidia filiform, straight to slightly curved, attenuated gradually to a somewhat pointed apex, or more abruptly just above the broadly truncate base, 0-1(-2)-septate, not constricted at the septum, hyaline, contents with granular material in the rehydrated state, 15-24 × 1-1.5 μm (rehydrated). Sexual morph unknown.
Description in vitro: No cultures could be obtained. Conidia placed on MEA and OA died shortly after germination.
Hosts: Hypochoeris radicata and other Hypochoeris spp.
Material examined: New Zealand, North Island, Taupo distr., Tongariro Nat. Park, Taurewa, along road 47, on decaying leaf base of Hypochoeris radicata, 25 Jan. 2003, G. Verkley 1871, CBS H-21234.
Additional material examined: New Zealand, North Island, Taupo distr., Lake Taupo, shoreline E of Motutaiko Island, on living leaves of Crepis capillaris, 25 Jan. 2003, G. Verkley 1870, CBS H-21235.
Notes: The material on Hypochoeris radicata from New Zealand agrees well with the original description and drawing of Septoria hypochoeridis; conidia are reported as continuous to 1-septate, 19-22 × 1.5 μm. According to Teterevnikova-Babayan (1987), the conidia of this species can be somewhat larger, 20-25 × 1.5-2 μm, and Hypochoeris grandiflora is also infected. Rhabdospora hypochoeridis was described from dead stems of H. radicata in Germany, with curved conidia, 16-30 × 0.6-1 μm, which, according to Priest (2006), is suggestive of a Phomopsis with β-conidia rather than a Septoria. Another species ocurring on this host and other Asteraceae is Septoria lagenophorae, which occurs in association with Puccinia spp. and other fungi (Priest 2006). This fungus can be distinguished from S. hypochoeridis by 1-2-septate conidia, 15-32 μm long, and conidiogenous cells which are not proliferating sympodially but produce successive conidia enteroblastically at the same level through a narrow opening (Priest 2006), so appearing phialidic.
The collection on Crepis capillaris studied here may also belong to S. hypochoeridis, but no earlier reports from the host genus Crepis have been documented. This material agrees in all morphological characters with the collection on Hypochoeris, but the conidia lack septa. It is certainly morphologically different from Septoria crepidis, which produces much larger, mostly 3-septate conidia [22-55 × 1.5-2(-2.5) cf. Shin & Sameva 2004]. The S. crepidis strains CBS 128608 (= KACC 42396), 128619 (= KACC 43092) and 131895 (= CPC 12539) isolated from Crepis japonica (syn. Youngia japonica) in South Korea, group with CBS 128650, Septoria sp. (originally identified as S. taraxaci), but by lack of cultures and molecular data for S. hypochoeridis the phylogenetic relationship with S. crepidis and allied Septoria remains to be resolved.
Septoria lactucae Pass., Atti Soc. crittog. ital. 2: 34. 1879 [non Peck, Bot. Gaz. 4: 170. 1879. Later homonym, nom. illeg. Art. 53]. Fig. 23.
Fig. 23.
Septoria lactucae. A-D. Colonies CBS 108943 (15 °C, nUV). A. On OA. B. On MEA. C. Colony margin on OA. D. Colony margin on MEA. E-I. Conidia on OA (CBS 108943). Scale bars = 10 μm.
Description in vitro: Colonies on OA 8-9 mm diam in 2 wk, with an even to undulating, colourless margin; colonies spreading to restricted, immersed mycelium pale luteous, without aerial mycelium, conidiomata developing immersed and on the agar surface, mostly in the centre and in radiating rows, conidiomata releasing milky white to rosy-buff conidial masses; reverse hazel with a tinge of ochreous. Colonies on MEA 4.5-6 mm diam in 2 wk, with a minutely ruffled, buff margin; colonies restricted, irregularly pustulate, the surface almost black, with low and weakly developed, finely felted, white to grey aerial mycelium but also glabrous areas occur; reverse chestnut to brown-vinaceous. No sporulation observed.
Conidia (OA) filiform to cylindrical, weakly to strongly curved, attenuated gradually towards the relatively broadly, more rarely narrowly rounded apex, attenuated gradually or more abruptly to a truncate base, hyaline, (0-)1-3-septate, contents granular and sometimes also with minute oil-droplets, (22-)28-38.5(-46) × 2-2.5 μm (living). Sexual morph unknown.
Hosts: Lactuca sativa and L. serriola.
Material examined: Germany, Potsdam, on leaf of Lactuca sativa, 20 Nov. 1958, G. Sörgel 628, living culture CBS 352.58. Netherlands, on seed of L. sativa, Sep. 2000, P. Grooteman s.n., living culture CBS 108943.
Notes: Septoria lactucae is the oldest name described in Septoria from the host Lactuca sativa. Three others have been described from lettuce (including two later homonyms), and another eight from other species of the genus Lactuca. Symptoms of the minor leaf spot disease of lettuce were described by Punithalingam & Holiday (1972). They describe the conidia as 1-2(-3)-septate, 25-40 × 1.5-2 μm. Muthumary (1999) examined the type and described the conidia as fusiform, straight to slightly curved, narrowed at the tip, truncate at the base, 1-3 septate, 32-52 (av. 35) × 2-2.5 μm. According to Jørstad (1965), conidia of S. lactucae are 19-48 × 1.5-2 μm with up to 2 septa, while Priest (2006) describes them as 1-3-septate, 22-33(-36) × 2-2.5(-3) μm. CBS 128757 (KACC 43221) isolated from Sonchus asper in South Korea, and identified as Septoria sonchi, is very closely related and groups in a cluster with 100 % bootstrap support with the strains of S. lactucae (Fig. 2).
Septoria lamiicola Sacc., Syll. Fung. 3: 358. 1884. nom. nov. pro S. lamii Sacc., Michelia 1: 180. 1878. Fig. 24.
Fig. 24.
Septoria lamiicola, CBS 102329. A-C. Colonies (15 °C, nUV). A. On OA. B. On CHA. C. On MEA. D. Conidia in planta (CBS H-21181). E. Ibid. (CBS H-21216). F. Conidia and conidiogenous cells on OA (CBS 102380). Scale bars = 10 μm.
≡ Septoria heterochroa Roberge ex Desm. f. lamii Desm., Annls Sci. Nat., sér. 3, Bot. 8: 22. 1847.
= Septoria lamii Westend., in Bellynck, Bull. Acad. Roy. Sci. Belgique 19: 63. 1852.
= Septoria lamii Pass., in Thüm., Mycoth. univ., Cent. 12, no 1183. 1878; Atti Soc. crittog. ital. 2: 37. 1879.
Description in planta: Symptoms leaf spots circular to angular, white to pale brown, surrounded by a dark brown border. Conidiomata pycnidial, epiphyllous, several in each leaf spot, globose to subglobose, dark brown, immersed to semi-immersed, 65-100 μm diam; ostiolum central, initially circular, 20-35 μm wide, later up to 50 μm wide, surrounding cells concolorous or somewhat darker; conidiomatal wall 12-25 μm thick, composed of textura angularis without distinctly differentiated layers, the cells 3.5-8 μm diam, the outer cells with brown, somewhat thickened walls, the inner cells with hyaline and thinner walls. Conidiogenous cells hyaline, narrowly or broadly ampulliform with a relatively narrow neck, holoblastic, proliferating sympodially, and towards the apex often also percurrently 1-many times with indistinct annellations, 5-10(-12) × 3.5-4(-5) μm. Conidia filiform to filiform-cylindrical, straight or slightly curved, rarely flexuous, with a rounded or somewhat pointed apex, attenuated towards the narrowly truncate base, (0-)3(-5)-septate, not constricted around the septa, hyaline, contents with several minute oil-droplets and granular material in each cell in the living state, with inconspicuous oil-droplets and granular contents in the rehydrated state, (26-)35-50(-54) × 1.5-2.5(-3) μm (living; rehydrated, 1-2 μm wide; V1032, rehydrated, 33-52 × 1.5-2). Sexual morph unknown.
Description in vitro: Colonies on OA 8-14 mm diam in 2 wk (40-45 mm in 6 wk), with an even, glabrous, colourless margin; colonies plane, immersed mycelium colourless to pale primrose or buff, later becoming homogeneously dark herbage green, soon appearing darker by numerous immersed and superficial pycnidial conidiomata, that release dirty white to rosy-buff conidial slime; aerial mycelium absent, only developing in the centre after several wk as a sharply delimited, dense, white, woolly floccose mat; reverse buff at the margin, inwards dark olivaceous-grey. Colonies on CMA 4-8 mm diam in 2 wk (20-27 mm in 6 wk), with an even, glabrous margin; as on OA but immersed mycelium more honey to pale luteous throughout, later becoming more greenish, the pycnidial conidiomata as on OA, but in more regular concentric rings, releasing rosy-buff, later salmon conidial slime. Colonies on MEA 7-9 mm diam in 2 wk (28-33 mm in 6 wk), with an even (later undulating), glabrous, buff to honey margin; colonies pustulate to almost hemispherical, immersed mycelium rather dark, locally covered by woolly to felty white aerial mycelium; mostly composed of spherical conidiomatal initials, superficial mature conidiomata releasing a dirty white, later buff conidial slime; reverse dark brick in the centre, near the margin cinnamon to honey. Colonies on CHA 8-14 mm diam in 2 wk (35-42 mm in 6 wk), with an even, but later irregular, buff margin covered by a diffuse, felty white aerial mycelium; further as on MEA, but the colony surface less elevated, and more homogeneously covered by diffuse, felty, white aerial mycelium; conidial slime abundantly produced, first milky white, later dirty honey; reverse in the centre blood colour, dark brick at the margin.
Conidiomata pycnidial, first olivaceous, then almost black, glabrous, 150-450 μm diam, with 1-5 ostioli placed on short papillae or more elongated necks up to 350 μm long; conidiogenous cells as in planta, proliferating sympodially and mostly also percurrently with distinct annellations, 8-16 × 3-8 μm; conidia cylindrical, straight or slightly curved, tapering to a rounded apex, lower part slightly attenuated into a broad truncate base, (0-)1-5-septate, not constricted around the septa, hyaline, with several oil-droplets and minute granular material in each cell, (34-)50-65(-70) × 2-3 μm.
Hosts: Lamium album, L. maculatum, L. purpureum and several other Lamium spp.
Material examined: Austria, Tirol, Ötztal, Sulztal, Gries, alt. 1570 m, on living leaves of Lamium album, 1 Aug. 2000, G. Verkley 1032, CBS H-21181, living cultures CBS 109112, 109113. Czech Republic, Moravia, Pavlov, forest around ruin, on living leaves of Lamium sp., 18 Sep. 2008, G. Verkley 6020, CBS H-21251, living cultures CBS 123882, 123883, and 6021, CBS H-21252, living culture CBS 123884. Netherlands, prov. Limburg, St. Jansberg near Plasmolen, on living leaves of L. album, 9 Sep. 1999, G. Verkley 925, CBS H-21207, living cultures CBS 102328, 102329; prov. Gelderland, Millingen aan den Rijn, Millingerwaard, on living leaves of L. album, 6 Oct. 1999, G. Verkley 936, CBS H-21216, living cultures CBS 102379, 102380.
Notes: According to Jørstad (1965), conidia of S. lamiicola are 3-septate, 24-60 × 1-2 μm, while Teterevnikova-Babayan (1987) reported 35-50 × 0.75-1.5 μm from seven Lamium species. For the current study, only fresh material on Lamium album was available. Jørstad (1965) mentioned the resemblance of the conidia with those in S. galeopsidis, but also noted a difference in the wall thickness of the pycnidia, which we did not observe. A much more profound difference is seen between cultures of the two species, with colonies of S. galeopsidis on OA being opaque and dark olivaceous-black even at the margin, while colonies of S. lamiicola are more translucent yellowish to ochreous, becoming darker only due to the formation of pycnidia. Priest (2006) pointed towards differences in conidial width and conidiogenesis between S. lamiicola and S. galeopsidis, but having compared both species morphologically in planta and in vitro, we conclude that these species cannot be distinguished using these criteria. These two species are, however, readily distinguished by DNA sequence data, and the multilocus phylogeny provides evidence for a close relationship with S. matricariae (CBS 109000, CBS 109001), while other Septoria occurring on the same plant family as S. lamiicola (Lamiaceae) are all much more distant (Fig. 2). The Austrian and Dutch collections of S. lamiicola on L. album are sufficiently homogenous to consider them conspecific.
Septoria leucanthemi Sacc. & Speg., in Saccardo, Michelia 1: 191. 1878. Fig. 25.
Fig. 25.
Septoria leucanthemi. A-C. Colonies CBS 109090 (15 °C, nUV). A. On OA. B. On MEA. C. On CHA. D. Conidia and conidiogenous cells on OA (CBS 109090). E, F. Conidia in planta (CBS H-21243). G. Conidia on MEA (CBS 109090). Scale bars = 10 μm.
≡ Rhabdospora leucanthemi (Sacc. & Speg.) Petr., Sydowia 11: 351. 1957.
For addditional synonyms see Punithalingam (1967b).
Description in planta: Symptoms leaf spots hologenous or epigenous, scattered, circular to irregular, pale to dull brown throughout or with whitish central area, indefinite with concentric zones or delimited by a slightly darker margin. Conidiomata pycnidial, predominantly epiphyllous, numerous scattered in each leaf spot, subglobose to globose, brown to black, semi-immersed, 130-220(-240) μm diam; ostiolum central, circular, 35-100 μm wide, surrounding cells dark brown and with more thickened walls; conidiomatal wall about 8-12.5 μm thick, composed of a homogenous tissue of hyaline, angular cells, 2.5-5 μm diam with relatively thin, hyaline walls, surrounded by a layer of pale to dark brown angular to more irregular cells, 3-6.5 μm diam with slightly thickened walls. Conidiogenous cells hyaline, discrete, rarely integrated in 1-2-septate conidiophores, cylindrical to doliiform, or ampulliform, holoblastic, proliferating percurrently with indistinct annellations, or sympodially, 6-18 × 4-6.5(-7.5) μm. Conidia filiform to filiform-cylindrical, straight, curved, sometimes slightly flexuous, attenuated gradually to a narrowly rounded to pointed apex, widest near the base, where attenuating abruptly or more gradually into a narrowly truncate base, (5-)6-13-septate (later secondary septa are developed in some cells), not constricted around the septa, hyaline, contents with several minute oil-droplets and granular material in each cell in the living state, with minute oil-droplets and granular contents in the rehydrated state, (67-)80-100(-125) × 2.5-3.0(-3.5) μm (rehydrated). Sexual morph unknown.
Description in vitro: Colonies on OA 6-8(-11) mm diam in 2 wk (11-14(-17) mm in 3 wk), with an even, glabrous, colourless margin; colonies spreading, the surface plane, immersed mycelium pale buff, later more or less rosy-buff; in the centre complexes of pycnidial conidiomata with pale brown or olivaceous walls release masses of pale whitish to buff conidial slime; reverse concolorous, but honey in the centre. Colonies on CMA 9-11(-13) mm diam in 2 wk (15-18 mm in 3 wk), as on OA, but with some white diffuse and high aerial hyphae in the centre, and later more elevated in the centre; reverse in the centre hazel to fawn after 3 wk; conidiomata much more numerous and larger than on OA, developing in concentric or random patterns as discrete, large acervuloid (later almost discoid to cupulate) stromata with olivaceous sterile tissues, releasing large masses of pale white to pale buff conidial slime. Colonies on MEA 7-10 mm diam in 2 wk (14-17 mm in 3 wk), with an even, colourless, glabrous margin; colonies restricted, irregularly pustulate to hemispherical, the bumpy surface consisting of numerous protruding conidiomatal initials, appearing dark, with sepia, dark brick and cinnamon tinges, aerial mycelium mostly absent, locally dense, pure white and woolly; reverse mostly sepia to fawn or vinaceous buff. Sporulation only observed after about 7 wk. Colonies on CHA 7-13 mm diam in 2 wk (15-20 mm in 3 wk), with an even, glabrous, pale vinaceous buff margin; colonies restricted, irregularly pustulate to conical, the surface bumpy, immersed mycelium honey to hazel, covered by dense to diffuse, pure white, woolly aerial mycelium; conidiomata sparsely developing at the surface after 2 wk, the wall slightly darker than the surrounding hyphae, releasing pale white conidial slime (even after 3 wk); reverse cinnamon in the centre, vinaceous buff or pale ochreous at the margin.
Conidiomata and conidiogenous cells as in planta. Conidia as in planta, 5-13(-17)-septate, 70-125(-175) × 3-4 μm (OA).
Hosts: Various species of the genera Chrysanthemum, Tagetes, Achillea, Centaurea and Helianthus (Waddell & Weber 1963, Punithalingam 1967b, c).
Material examined: Austria, Tirol, Ober Inntal, Samnaun Gruppe, Böderweg on Lazidalm, on living leaves of Chrysanthemum leucanthemum, 8 Aug. 2000, G. Verkley 1055, CBS H-21173, living cultures CBS 109090, 109091; Same substr., Tirol, Zanderstal near Spiss, 11 Aug. 2000, G. Verkley 1069, CBS H-21170, living cultures CBS 109083, 109086. Germany, Hamburg, on living leaves of Chr. maximum, Sep. 1958, R. Schneider s.n. CBS H-18111, living culture CBS 353.58 = BBA 8504 = IMI 91322. New Zealand, Coromandel distr., Coromandel peninsula, Waikawau, coast along St. Hwy 25, on living leaves of Chr. leucanthemum, 22 Jan. 2003, G. Verkley 1826, CBS H-21247; same substr., North Island, Coromandel, Tairua Forest, along roadside of St. Hway 25, near crossing 25A, 23 Jan. 2003, G. Verkley 1842b, CBS H-21243, living culture CBS 113112.
Notes: The six strains studied here showed minor differences in morphological characters and DNA sequences, which show highest similarity to sequences of CBS 128621, an isolate originating from Cirsium setidens in South Korea, and identified as S. cirsii (Fig. 2). Septoria leucanthemi is also closely related to a number of other Septoria species from Asteraceae, such as S. senecionis and S. putrida (Senecio spp.), S. obesa (Chrysanthemum spp., Artemisia) and S. astericola (Aster sp.). It is confirmed here that Septoria obesa, which has been regarded as a synonym of S. leucanthemi by Jørstad (1965), should be treated as a separate species (see also the note on S. obesa).
Septoria lycoctoni Speg. ex Sacc., Michelia 2: 167. 1880. Fig. 26.
Fig. 26.
Septoria lycoctoni. A-C. Colonies CBS 109089 (15 °C, nUV). A. On OA. B. On CHA. C. On MEA. D. Conidia and conidiogenous cells in planta (CBS H-21155). E. Conidia on OA (CBS 109089). Scale bars = 10 μm.
Description in planta: Symptoms leaf spots epigenous, numerous, circular to irregular, single or confluent, white to pale greyish, surrounded by an initially red, then dark brown to black and thickened border. Conidiomata pycnidial, epiphyllous, inconspicuous, up to a few in each leaf spot, globose to subglobose, brown, immersed, 90-145(-220) μm diam; ostiolum central, circular, more or less papillate, 25-55 μm wide; conidiomatal wall 17-35 μm thick, composed of textura angularis, differentiated layers absent, the cells mostly 3.5-5(-11) μm diam, the outer cells with brown, somewhat thickened walls, the inner cells with thinner, hyaline walls. Conidiogenous cells hyaline, cylindrical, or elongated ampulliform with a relatively narrow neck which widens at the top, hyaline, holoblastic, proliferating sympodially, 7-18 × 3.5-6 μm. Conidia filiform, straight, more often curved, sometimes flexuous, gradually attenuated to the pointed apex, more or less attenuated towards the broadly truncate base, (0-)2-5(-6)-septate, not or indistinctly constricted around the septa, hyaline, with several oil-droplets and granular contents in each cell in the rehydrated state, 26-47 × 1.5-2 μm (rehydrated; up to 2.5 μm wide in the living state). Sexual morph unknown.
Description in vitro: Colonies on OA 9-11 mm diam in 2 wk (18-20 mm in 3 wk), with an even, glabrous, colourless margin; immersed mycelium mostly coral to scarlet, the pigment diffusing into the surrounding medium; in the centre black and slightly elevated with mostly superficial, glabrous pycnidia, surrounded by an area with more scattered pycnidia, releasing pale white to pale flesh droplets of conidial slime; aerial mycelium only present in the centre, but well-developed, dense, appressed, woolly, white or greyish, locally with a flesh haze; reverse scarlet to coral, the centre darker, blood colour. Colonies on CMA 9-12 mm diam in 2 wk (17-19 mm in 3 wk), as on OA, but pycnidia more numerous, usually only formed in the centre of the colony. Colonies on MEA (3-)5-9 mm diam in 2 wk (13-18 mm in 3 wk), with an irregular margin; colonies restricted, the surface cerebriform to irregularly pustulate, up to 3 mm high, the surface pale brown, later black, at first almost glabrous, or (especially in brighter-coloured faster growing sectors/colonies) already covered by dense mat of pure white to flesh, woolly aerial mycelium, that later covers most of the colony surface; large masses of honey or pale amber conidial slime locally emerging from immersed conidiomata; reverse of the colony either dark brick or luteous to ochreous, paler towards the margin. Colonies on CHA 8-13 mm diam in 2 wk (15-19(-22) mm in 3 wk), with an even or undulating, colourless margin mostly hidden under aerial hyphae; immersed mycelium greenish grey, grey-olivaceous to olivaceous-black, throughout covered by well-developed, tufty whitish grey aerial mycelium that later shows a reddish haze; reverse blood colour, but margin paler; in the central part of the colony numerous pycnidia develop, releasing pale whitish to rosy-buff conidial slime; in older colonies the central surface becomes cerebriform and about 3 mm high, much like on MEA.
Conidiomata as in planta, pycnidial with barely protruding ostioli, which later often grow out to elongated necks up 50-150 μm long; on CMA less differentiated and fairly large, opening by tearing of the upper wall; conidiogenous cells as in planta, but larger, 9-25 × 3.5-7.5 μm, proliferating sympodially and also percurrently, but annellations on the necks are inconspicuous; conidia similar in shape as in planta but longer, 3-5(-6)-septate, 30-75 × 1.5-2.5 μm.
Hosts: Aconitum vulparia (= A. lycoctoni), A. anthora, A. conversiflorum and several other Aconitum spp.
Material examined: Austria, Ober Inntal, Samnaun Gruppe, Lawenalm near Serfaus, alt. 2000 m., on living leaves of Aconitum vulparia (syn. A. lycoctonum), 8 Aug. 2000, G. Verkley 1053, CBS H-21155, living culture CBS 109089.
Notes: In the diagnosis of S. lycoctoni, the conidia were described as “indistinctly multiseptate”, measuring 25-35 × 1.5-2 (Saccardo 1884). This fungus was found on A. lycoctonum in Italy. Teterevnikova-Babayan (1987) gave conidial size ranges of 25-70 × 1-2 μm for this species, and she included several of the varieties which were described after 1880, viz., var. sibirica 1896, var. macrospora 1909, var. anthorae 1928. Petrak (1957) observed conidia 20-60 (rarely 70 to 80) × 1.5-2 μm in his collection on Aconitum moldavicum.
The colonies of Septoria lycoctoni and S. napelli look very similar on all media tested, although in S. napelli more red pigment seems to be produced than in S. lycoctoni, and the conidial slime is salmon rather than flesh. The two species can more readily be distinguished from each other by the shape of their conidia. In S. lycoctoni, the mature conidia only attenuate towards the apex above the uppermost septum, while in S. napelli, the tapering of the conidium walls is visible below the second septum from the top. The difference between the conidia of these species is also clear on the plant. Because the conidia of S. napelli are wider, the septa and the attenuations are easier to observe. In the case of S. lycoctoni the apical attenuation of conidia is not so clear, which may explain why Petrak (1957), who compared this species also to collections identified as S. napelli (but for reasons explained below probably misidentified), circumscribed the conidia of S. lycoctoni as not-attenuated.
The strains of S. napelli (CBS 109104-109106) originating from Aconitum napellus and CBS 109089 of S. lycoctoni are very closely related and form a monophyletic group in the multilocus phylogeny (Fig. 2).
Septoria lysimachiae (Lib.) Westend., Bull. Acad. r. Belg., Cl. Sci., Sér. 2, 19: 120. 1852. Fig. 27.
Fig. 27.
Septoria lysimachiae. A-C. Colonies (15 °C, nUV). A. CBS 123794 on OA. B. CBS 108998 on MEA. C. Ibid., colony margin on MEA. D. Conidia in planta (CBS H-21196). E. Conidia on OA (CBS 108998). F. Conidia in planta (CBS H-21196). G. Conidia on OA (CBS 108998). H. Conidia on OA (CBS 108999). Scale bars = 10 μm.
Basionym: Ascochyta lysimachiae Lib., Pl. Crypt. Ard. Fasc. 3, 252. 1834.
Description in planta: Symptoms leaf lesions indefinite, usually only a few scattered over the leaf lamina, or a single one, most often developing from the tip to the petiole, greyish to reddish brown. Conidiomata pycnidial, epiphyllous, immersed, subglobose to globose, black, 95-120(-165) μm diam; ostiolum central, circular, initially 25-35 μm wide, later becoming more irregular and up to 90 μm wide, surrounding cells concolourous; conidiomatal wall 10-20 μm thick, composed of an outer layer of angular to irregular cells mostly 4.5-10 μm diam with pale to orange brown walls, and an inner layer of isodiametric, hyaline cells 3-6 μm diam. Conidiogenous cells hyaline, discrete, rarely integrated in 1-septate conidiophores, cylindrical, or narrowly to broadly ampulliform, holoblastic, proliferating sympodially, and often also percurrently showing 1-3 indistinct annellations on a neck-like protrusion, 8-15 × 3-5(-6) μm. Conidia cylindrical to filiform-cylindrical, slightly to strongly curved, rarely somewhat flexuous, narrowly rounded to pointed at the apex, attenuated gradually or more abruptly towards a narrowly truncate base, (0-)3-5, later with secondary septa dividing the cells, conidia sometimes breaking up into smaller fragments in the cirrhus, not or slightly constricted around the septa, hyaline, containing several large oil-droplets and granular material in the living and rehydrated state, (28-)35-70(-88) × 2.5-3.5 (-4) μm (living; rehydrated, 2.0-3 μm wide). Sexual morph unknown.
Description in vitro: Colonies on OA rather variable in growth speed and pigmentation, 3.5-7 mm diam in 1 wk (20-26 mm in 2 wk), with an even, glabrous, colourless margin; colonies spreading, flat,immersed mycelium first mostly buff, then either rosy-buff to pale salmon turning olivaceous or hazel, or long colourless and later becoming olivaceous-black to greenish black; aerial mycelium woolly-floccose, white or greyish, mostly developing only in the centre; reverse olivaceous-black to greenish grey or dark slate blue to black. Conidiomata developing scarcely immersed in the agar, producing small amounts of conidia that are released as rosy-buff droplet. Colonies on CMA 2-4 mm diam in 1 wk (15-20 mm in 3 wk), as on OA, but centre of the colony somewhat elevated, and colourlous marginal zone narrow, immersed mycelium becoming more rapidly pigmented with a vinaceous buff tint, in the centre becoming brown-vinaceous; reverse hazel, in the centre almost black. Colonies on MEA 3.5-6 mm diam in 1 wk (8-17(-19) mm in 3 wk), with an even to slightly ruffled, buff to rosy-buff, glabrous margin; some strains with a more uneven outline, strongly fimbriate, with faster growing deeply immersed mycelium often extending well beyond the colony margin at the level of the agar surface; colonies spreading, but often distinctly elevated or irregularly pustulate in the centre; immersed mycelium variable in colour, buff, ochreous or brownish, and in the faster growing sectors often with a glaucous haze; aerial mycelium diffuse to dense, pure white, (vinaceous) greyish or brownish, finely felted to woolly; reverse versicoloured, margin and parts of faster growing sectors buff to honey, in other parts darker, hazel to brown-vinaceous, sometimes mostly olivaceous-black. Some strains show a conspicuous halo of diffusing reddish pigment (CBS 108996, 108997). Scarce dark conidiomata beginning to develop in the centre after 1 wk, releasing pale white droplets of conidial slime after about 3 wk. Colonies on CHA 2-4(-6) mm diam in 1 wk [18-24(-26) mm in 21 d], with an even or slightly ruffled, glabrous, colourless to buff margin; colonies irregularly pustulate, immersed mycelium olivaceous-black; aerial mycelium soon covering most of the colony, woolly-floccose, smoke grey with an olivaceous haze, locally grey-olivaceous, in slightly faster growing sectors sometimes pure white; reverse mostly brown-vinaceous. Superficial, blackish conidiomata in the centre releasing pale rosy-buff to white masses of conidial slime after 1 wk; reverse mostly blood colour, or fawn and brown-vinaceous in the centre.
Conidia (OA) cylindrical, slightly to strongly curved to flexuous, narrowly rounded to somewhat pointed at the apex, attenuated gradually or more abruptly towards a truncate base, mostly 3-7(-11)-septate, including the soon formed secondary septa, cells soon loosing their turgesence and often separating into smaller fragments, in the turgescent state constricted around the septa, hyaline, with many vacuuoles and also containing several large oil-droplets and granular material in the living state and rehydrated state, (30-)40-80-(90) × 2.5-3.5 (-4) μm (living; rehydrated NT 2.0-3 μm wide).
Hosts: Lysimachia spp.
Material examined: Belgium, near Namur, on leaves of Lysimachia vulgaris, Bellynck, isotype BR-MYCO 145978-90, also distributed in M. A. Libert, Pl. Crypt. Ard. Fasc. 3, no. 253. Czech Republic, Mikulov, on living leaves of Lysimachia sp., 15 Sep. 2008, G. Verkley 6004, CBS H-21255, living cultures CBS 123794, 123795. Netherlands, Prov. Utrecht, Baarn, De Hooge Vuursche, in the forest, on L. vulgaris, 22 June 2000, G. Verkley 955, epitype designated here CBS H-21227 “MBT175357”, living cultures ex-epitype CBS 108998, 108999; Prov. Utrecht, Soest, Stadhouderslaan near monument “De Naald”, on living leaves of L.vulgaris, 4 Aug. 1999, G. Verkley 903, CBS H-21196, living culture CBS 102315; Prov. Gelderland, Amerongen, Park Kasteel Amerongen, on living leaves of L. vulgaris, 11 July 2000, G. Verkley 971, CBS H-21230, living culture CBS 108996, 108997.
Notes: Shin & Sameva (2003) provided a detailed description of S. lysimachiae (conidia 35-80 × 1.5-2.5 μm, 3-7-septate). In the type material from BR the conidia are mostly 3-5-septate, 25-72 × 2.5-3.5 μm, and very similar in shape to those observed in the material that was collected from the field for the present study. The isolates show more variation in colony characters than observed in most other species of Septoria, but this phenotypic heterogeneity is neither reflected in the sporulating structures nor in the sequence data obtained. The EF, Btub and RPB2 gene sequences proved 100 % identical among strains originating from the Netherlands (CBS 102315, 108998 and 108999) and Czech Republic (CBS 123794, 123795), while differences found between the Dutch and Czech isolates for Cal and Act were only 3 (99.3 % similarity) and 1 bp (99.6 %), respectively. It is concluded therefore that the material studied belongs to a single species. Septoria saccardoi, based on material from Lysimachia vulgaris in Italy, is characterised by cylindrical, curved, 3-septate conidia, 38-40 × 3.5 μm (Saccardo 1906). Quaedvlieg et al. (2013) describe this species in detail based on an isolate originating from of Lysimachia vulgaris var. davuricai in Korea (CBS 128665 =KACC 43962) and because it is distant to other septoria-like fungi, they propose a new genus name to accommodate it, Xenoseptoria. CBS 128758, isolated from L. clethoroides in Korea was identified as S. lysimachiae, but based on sequence analyses it is a distant fungus belonging in the genus Sphaerulina.
Septoria matricariae Hollós, Annls Mus. nat. Hung. 8: 5. 1910 [non Syd. 1921; nec Cejp, Fassatiova & Zavrel, Zpravy 153: 13. 1971; later homonyms]. Fig. 28.
Fig. 28.
Septoria matricariae. A. Conidia and conidiogenous cells in planta (CBS H-21228). B. Conidia on OA (CBS 109001). Scale bars = 10 μm.
= S. chamomillae Andrian., Mikol. i Fitopat. 30: 10. 1996. Nom. nov. pro S. matricariae Syd., Annls mycol. 19: 143. 1921; nom. illeg. Art. 53 [non Marchal & Sternon, 1923].
?= S. chamomillae Marchal & Sternon, Bull. Soc. r. Bot. Belg. 55: 50. 1922.
Description in planta: Symptoms lesions indefinite, leaves becoming affected from the top towards base, discolouring to yellow and brown. Conidiomata pycnidial, amphigenous, numerous, more or less evenly dispersed over the affected area, globose to subglobose, dark brown to black, immersed, 75-125(-150) μm diam; ostiolum central, circular, often papillate, breaking through the leaf epidermis, 25-43(-50) μm wide, surrounding cells concolorous or somewhat darker; conidiomatal wall 10-20 μm thick, composed of textura angularis without distinctly differentiated layers, the cells 2-6 μm diam, the outer cells with yellowish brown, thickened walls, the inner cells with hyaline, also relatively thick walls; Conidiogenous cells hyaline, discrete or integrated in 1-2-septate conidiophores up to 17.5 μm long, doliiform, narrowly to broadly ampulliform, holoblastic, proliferating sympodially and/or also percurrently with one or two indistinct annellations, 3.5-10 × 3-4.5(-5.5) μm. Conidia filiform, straight, curved or slightly flexuous, attenuated gradually towards a relatively narrowly rounded to pointed apex, barely attenuated towards the broadly truncate base, indistinctly (1-)2-3(-6)-septate, not or indistinctly constricted around septa, hyaline, contents with a few minute oil-droplets and granular material in each cell in the living state, with inconspicuous oil-droplets and granular contents in the rehydrated state, 41-58 × 2-3 μm (living; rehydrated, 1.5-2.4 μm wide). Sexual morph unknown.
Description in vitro: Colonies on OA 19-24 mm diam in 3 wk (44-48 mm in 6 wk), with an even, glabrous, colourless margin; colonies spreading, the surface plane, immersed mycelium olivaceous-black to very dark dull green, with numerous dark, radiating hyphae, almost entirely glabrous, few tufts of greyish aerial mycelium in the centre; numerous scattered single or complex pycnidial conidiomata developed already after 1 wk, with a single ostiole or several papillate or rostrate openings, from which pale rosy-buff droplets of conidial slime are released; reverse concolourous. Colonies on CMA 16-18(-20) mm diam in 3 wk (38-50 mm in 6 wk), as on OA. Colonies on MEA 9-12(-14) mm diam in 3 wk (27-39 mm in 6 wk), with an even to slightly ruffled buff margin; colonies restricted, conical and up to 3 mm high after 3 wk, immersed mycelium near the margin grey-olivaceous, but most of the colony surface iron grey to greenish black, the outer areas mostly covered by a low but dense, finely felted, grey aerial mycelium, the centre almost glabrous; superficial semi-immersed conidiomata releasing pale whitish droplets of conidial slime after 2-3 wk; reverse mostly dark slate blue with olivaceous areas. Colonies on CHA 16-22 mm diam in 3 wk (39-46 mm in 6 wk), as on MEA, but conidiomata more numerous, releasing pale whitish to pale rosy-buff droplets or cirrhi of conidial slime, and reverse with a brown-vinaceous tinge.
Conidiomata as in planta, pycnidial with a single ostiolum, dark brown to black, rarely merged into complex fruitbodies; conidiogenous cells as in planta, but larger and more often integrated in 1-3-septate conidiophores, 10-15(-23) × 3-6(-7) μm; conidia as in planta, but longer, 36-78(-90) × (1.6-)1.7-2.2 μm, contents several oil-droplets in each cell.
Hosts: Matricaria spp.
Material examined: Netherlands, prov. Limburg, Zuid-Limburg, along roadside near Savelsbos, on living leaves of Matricaria discoidea (= M. matricarioides), 28 June 2000, G. Verkley 960, CBS H-21228, living cultures CBS 109000, 109001. Romania, Suceava, Siret, on leaves of M. discoidea, 7 July 1969, distributed in Contantinescu & Negrean, Herb. mycol. Romanicum Fasc. 40, no. 199, CBS H-18115.
Notes: The phylogenetic analyses indicate that S. matricariae is closest to S. lamiicola, yet rather distant from other Septoria occurring on Asteraceae. The indefinite lesions caused by this species are reminiscent of those developed by S. stellariae on Stellaria media. The leaves seem to whither more rapidly and pycnidia develop soon after discolouration of the leaf tissues starts. Stems are not affected. In the original diagnosis of S. matricariae, based on material from Matricaria discoidea in Hungary, the conidia are described as continuous and 40-60 × 2-2.5 μm. The Dutch and also the Romanian material studied here contain conidia with mostly 1-3 septa, but otherwise agree well with Hollós’ description of the type. According to Radulescu et al. (1973) the conidia in material from the same host plant are also continuous, measuring 25-50 × 1.5-2 μm. As in several other Septoria spp., the septa in S. matricariae are not easy to observe, and Hollós and others may have overlooked them.
Sydow described a Septoria under the same name from Matricaria chamomilla in Germany with multiseptate conidia 30-60 × 1-1.5 μm. The name he proposed was illegitimate because it is a later homonym of S. matricariae Hollós, as is also S. matricariae Cejp et al.. Septoria chamomillae was also described from M. chamomillae in Belgium and has 3-5-septate conidia 35-52 × 1-2 μm. Although we have not seen the types of either of these names, we consider them tentatively as synonyms of S. matricariae.
Septoria melissae Desm., Annls Sci. Nat., sér. 3, Bot., 20: 87. 1853. Fig. 29.
Fig. 29.
Septoria melissae, CBS 109097. A-C. Colonies (15 °C, nUV). A. On OA. B. On MEA. C. On MEA, detail of colony margin. D. Conidia and conidiogenous cells on OA. E-F. Conidia on OA. Scale bars = 10 μm.
≡ Phloeospora melissae (Desm.) Parisi, Bull. Bot. R. Univ. Napoli 6: 292. 1921.
Description in vitro: Colonies on OA 12-13 mm diam in 2 wk, with an even to slightly ruffled, mostly colourless margin; colonies restricted to spreading, somewhat elevated in the centre, immersed mycelium greenish black, with greenish hyphal strands radiating into or even beyond the colourless margin, the surface mostly glabrous or provided with very diffuse, finely felted, grey aerial hyphae, the elevations in the centre bearing tufts of more well-developed, grey aerial mycelium; conidiomata developing mostly in the centre immersed or on the agar surface, releasing pale rosy to rosy-buff conidial slime. No diffusing pigment observed. Colonies on MEA 5-7(-9) mm diam in 2 wk, with a slightly ruffled margin; colonies restricted, pustulate with cerebriform elevations in the centre, the surface black, covered by a diffuse to dense mat of finely felted, mostly grey aerial mycelium; reverse very dark brown-vinaceous. Conidiomata sparsely developing on the colony surface, releasing dirty reddish brown conidial slime. A very faint pigment is visible around the colony.
Conidiogenous cells (OA) globose to ampulliform, holoblastic, hyaline, discrete or integrated in 1(-2)-septate conidiophores, proliferating sympodially, percurrent proliferation not observed, 4-10 × 3-5 μm. Conidia filiform, straight to flexuous, weakly to more strongly curved, attenuated gradually to a narrowly rounded, typically pointed apex, attenuated gradually to a narrowly truncate to somewhat rounded base, hyaline, with fine granular material and minute oil-droplets, (0-)3(-5)-septate, (22-)30-50(-61) × 1.5-2 μm. Sexual morph unknown.
Host: Melissa officinalis.
Material examined: Netherlands, Baarn, garden Eemnesserweg, on living leaves of Melissa officinalis, 11 Sep. 2000, H.A. van der Aa s.n. (G. Verkley 1073), CBS H-21169, living cultures CBS 109096, 109097.
Notes: This species is the only Septoria described from the genus Melissa. The type material originates from Melissa officinalis in France (not seen). According to the short original diagnosis, S. melissae produces conidia 30 × 1.6 μm, and no septa were reported. Radulescu et al. (1973) described the conidia as continuous or with 1-3 septa, 25-38 × 1.6 μm. These measurements agree quite well with those given by Teterevnikova-Babayan (1987; 28-38 × 1.5 μm), but Vanev et al. (1997) gave a much wider range of measurements, 20.5-58 × 1.5-2.2 μm (septa 2-5). Genetically CBS 109097 is very closely related to S. galeopsidis, but a 5 bp insertion found in the Btub gene is absent in all sequenced strains of S. galeopsidis. Septoria melissae can furthermore be distinguished in culture from S. galeopsidis by the narrower conidia on OA (1.5-2 μm, in S. galeopsidis 2-2.5 μm), and the conidiogenous cells, which only proliferate sympodially and not percurrently.
Septoria napelli Speg, Decades mycologicae italicae I-XII: no. 117. 1879; Atti Soc. crittog. ital., Ser. 2, 3: 69. 1880. Fig. 30.
Fig. 30.
Septoria napelli. A-C. Colonies CBS 109104 (15 °C, nUV). A. On OA. B. On CHA. C. On MEA. D. Conidia and conidiogenous cells on OA (CBS 109104). E. Conidia in planta (CBS H-21153). Scale bars = 10 μm.
≡ Rhabdospora napelli (Speg.) Petr., Sydowia 11: 376. 1957 [misapplication].
Description in planta: Symptoms leaf spots hologenous, circular to irregular, single, white to pale greyish, surrounded by a first red, then black, relatively wide border, often completely blackening the narrow leaflets. Conidiomata pycnidial, epiphyllous, rarely also hypohyllous, conspicuous, one to many in each leaf spot, globose to subglobose, black, semi-immersed, 100-150(-200) μm diam; ostiolum central, circular, initially 15-25 μm wide, later opening more widely; conidiomatal wall 15-28 μm thick, composed of textura angularis, differentiated layers absent, the cells mostly 4-10 μm diam, the outer cells with brown, somewhat thickened walls, the inner cells with hyaline and thinner walls. Conidiogenous cells hyaline, cylindrical, broadly to narrowly ampulliform, with a distinct neck of variable length, hyaline, holoblastic, with several distinct percurrent proliferations, more rarely also sympodial after a sequence of percurrent proliferations of the same cell, 10-22 × 3.5-8 μm. Conidia filiform, straight, more often irregularly curved, gradually attenuated to the pointed apex, weakly or more distinctly attenuated towards the broadly truncate base, (3-)4-5(-7)-septate, not constricted around the septa, hyaline, with several relatively large oil-droplets and also minute granular contents in each cell in the rehydrated state, 59-80 × (1.5-)2-3.5 μm (rehydrated; up to 4 μm wide in the living state). Sexual morph unknown.
Description in vitro: Colonies on OA 9-15 mm diam in 2 wk (45-53 mm in 49 d), with an even, glabrous, colourless margin; immersed mycelium coral to scarlet, with pigment diffusing beyond the colony margin; colony becoming black in the centre and somewhat elevated due to superficial pycnidia, surrounded by an area with more scattered pycnidia, releasing flesh to salmon droplets of conidial slime; aerial mycelium well-developed and dense in the centre, appressed, woolly, white to pale grey; reverse scarlet to coral, in the centre blood colour. Colonies on CMA 8-12 mm diam in 2 wk (62-65 mm in 49 d), as on OA. Colonies on MEA 5-9 mm diam in 2 wk (38-44 mm in 49 d), the margin irregular; colonies restricted, with a cerebriform surface, becoming about 5 mm high, the surface soon black, first almost glabrous, later mostly covered by a dense mat of white to flesh, woolly aerial mycelium; honey or amber conidial slime masses are released from immersed pycnidia; reverse of the colony dark brick or luteous, paler towards the margin. Colonies on CHA 8-13 mm diam in 2 wk (55-58 mm in 49 d), with an even or undulating, colourless margin, partly hidden under aerial hyphae; immersed mycelium grey-olivaceous or olivaceous-black, covered with well-developed, grey and partly greenish glaucous, later reddish, aerial mycelium; reverse blood colour, the margin paler; in the central part of the colony numerous pycnidia develop, releasing rosy-buff conidial slime.
Conidiomata as in vitro pycnidial, ostioli initially barely protruding, but later often growing out to form elongated necks up to 100 μm long; on CMA conidiomata less differentiated, sometimes without ostiolum and opening by tearing of the upper wall; conidiogenous cells as in planta, but larger, 10-32 × 3.5-8.5(-10) μm, proliferating sympodially and also percurrently, with distinct annellations on the elonated necks. Conidia similar in shape as in planta but longer, 5-7(-11)-septate, 64-95(-118) × 2-3.5(-4) μm.
Hosts: Aconitum spp.
Material examined: Austria, Ober Inntal, Samnaun Gruppe, Zanderstal near Spiss, alt. 1800 m., on living leaves of Aconitum napellus, 11 Aug. 2000, G. Verkley 1070, CBS H-21153, living cultures CBS 109104, 109105; same loc., host, date, G. Verkley 1071, CBS H-21154, living culture CBS 109106. Romania, reg. Mureş-Autonomă Maghiară, on living leaves of A. degenii, 25 Aug. 1953, C. Sandu-Ville s.n., CBS H-18117, distributed in Herb. Mycol. Romanicum, fasc. 35, no. 1742.
Notes: According to the brief original diagnosis, S. napelli is characterised by 120-130 μm wide hypophyllous pycnidia, and indistinctly septate conidia measuring 50-100 × 2-4 μm. Teterevnikova-Babayan (1987) reported up to 9-septate conidia measuring 40-100 × 3-4 μm, and Shin & Sameva (2004) 3-9-septate conidia, 40-105 × (2.5-)3-5 μm in Korean material. It is doubtful whether the description by Petrak (1957) of S. napelli was based on correctly identified material. Pycnidia of that fungus were mostly hypophyllous, with 3-7, rarely 8-9-septate conidia measuring 40-70 (rarely up to ca. 100) × 3-4 μm, arising from septate and branched conidiophores. The pycnidial wall was composed of globose to angular cells 5-8(-10) μm diam, with walls thickened to an extent which would avoid any compression. Petrak (1957) also observed young fruitbodies of a sexual morph on dead leaves in between old and empty conidiomata. Although this sexual morph was immature, in his opinion it was “undoubtedly Pleosporaceae, perhaps a species of Leptosphaeria, but certainly not Mycosphaerella”. Certain similarities in the walls of the asexual and sexual morph, made him suspect that they were produced in different stages in the life-cycle of a single fungus. Because of the large size of the pycnidia of Petrak’s S. napelli, the structure of the pycnidial wall and conidial ontogeny, which were unlike typical Septoria, he proposed the combination Rhabdospora napelli. Petrak’s observations of S. napelli probably pertained to a different septoria-like fungus (Stagonospora?), probably with pleosporalean affinities, but of which the exact identity remains unclear.
The fungus studied in the present study, which is a member of the Septoria clade, generally agrees with the original description of S. napelli. It is unknown whether S. napelli has a sexual morph. Two Mycosphaerella names have been published from Aconitum, M. antonovii on Aconitum excelsum in Siberia, and M. aconitorum, on Aconitum sp. in Austria. Both names were introduced by Petrak, who did not observe associated asexual morphs for these Mycosphaerella spp. A comparison with S. lycoctoni, including the molecular results, is provide above in the notes on S. lycoctoni.
CBS 128664 isolated from Aconitum pseudolaeve var. erectum in Korea, is genetically distinct from both Septoria spp. on Aconitum in Europe. The new name S. pseudonapelli is proposed for this fungus by Quaedvlieg et al. (2013, this volume).
Septoria obesa Syd., in Syd. & P. Syd., Annls mycol. 12: 163. 1914.
= S. artemisiae Unamuno, Assoc. españ. Progr. Cienc. Congr. Salamanca: 46. 1923 [nom. illeg., later homonym, non Passerini, 1879].
Descriptions in planta are provided by Punithalingam (1967c) and Priest (2006). Sexual morph unknown.
Hosts: Artemisia lavandulaefolia and Chrysanthemum spp.
Material examined: Germany, Weihenstephan, on Chrysanthemum indicum, R. Schneider Sep. 1957, living culture CBS 354.58 = BBA 8554 = IMI 091324. South Korea, Hongcheon, on Artemisia lavandulaefolia, H.D. Shin, 28 June 2006, living culture SMKC 21934 = KACC 42453 = CBS 128588; Bonghwa, on Chr. indicum, H.D. Shin, 18 Oct. 2007, living culture SMKC 23048 = KACC 43193 = CBS 128623; Jeju, on Chr. morifolium, 5 July 2008, living culture KACC 43858 = CBS 128759.
Notes: Jørstad (1965) regarded S. obesa as a synonym of S. leucanthemi, as both have similar conidial morphologies and occur on several Chrysanthemum spp. Punithalingam (1967b, c), however, recognised S. obesa and S. leucanthemi as separate species, noting that the conidia of S. obesa are consistently wider than those of S. leucanthemi. Verkley & Starink-Willemse (2004) found additional, molecular support for the treatment.as separate species in eight polymorphisms found on the ITS sequences of strains representing these species. Further evidence is now provided here based on sequences of six other loci. The host ranges of the two species are also different: S. leucanthemi is capable of infecting various species of a wide range plant genera, viz. Chrysanthemum, Tagetes, Achillea, Centaurea and Helianthus (Waddell & Weber 1963, Punithalingam 1967b). Septoria obesa seems to mainly infect Chrysanthemum spp., but it does also infect Artemisia lavendulaefolia, as could be demonstrated in this study with CBS 128588, a strain originally identified as S. artemisiae. The strain is genetically very close to the other strains of S. obesa studied here and therefore regarded as conspecific. The conidia produced by CBS 128588 are in good agreement with S. obesa as well, being much larger than in S. artemisiae (30-33 × 1.5 μm, according to the original diagnosis of S. artemisiae Passerini). The later homonym S. artemisiae described by Unamuno based on material on Artemisia vulgaris in Spain with 4-septate conidia 35.5-52.5 × 2.5-3 μm, is placed here in the synonymy of S. obesa.
The conidia of the sunflower pathogen S. helianthi (50-85 × 2-3 μm) are similar to those of S. obesa (50-90 × 2.5-3.5 μm, cf. Priest 2006), but they can be distinguished by the number of septa formed, viz., seldom more than 5 in S. helianthi and 5-11 septa in S. obesa. Verkley & Starink already showed that ITS sequences of these species differ by more than 20 base positions, which is also supported by the results found in the present study for other genes (Fig. 2).
Septoria paridis Pass., Atti Soc. crittog. ital. 2: 41. 1879. Fig. 31.
Fig. 31.
Septoria paridis. A-C. Colonies (15 °C, nUV). A. On OA (CBS 109110). B. On CHA (CBS 109110). C. On MEA (CBS 109108). D. Conidia and conidiogenous cells in planta (CBS H-21177, Paris quadrifolia). E. Ibid. (CBS H-21152, Viola palustris). F. Conidia on OA (CBS 109108). Scale bars = 10 μm.
Description in planta: Symptoms leaf spots single, scarce, circular to irregular, white to pale ochreous, surrounded by a vague orange to reddish brown zone, visible on both sides of the leaf, decaying to shot-holes. Conidiomata pycnidial, epiphyllous, one to a few in each leaf spot, globose, black, immersed, 60-100 μm diam; ostiolum central, circular and 35-40 μm wide, surrounding cells concolorous to slightly darker; conidiomatal wall up to 15 μm thick, composed throughout of hyaline, angular cells, 2.5-5 μm diam, the outermost cells brown with somewhat thickened walls, the inner cells hyaline and thin-walled. Conidiogenous cells hyaline, discrete, globose, doliiform, or broadly ampulliform, holoblastic, proliferating percurrently several times with distinct annellations thus forming a relatively narrow neck, rarely also sympodially, 5-8(-11) × 2.5-5 μm. Conidia filiform, straight, or slightly curved, attenuated gradually to a narrowly pointed apex and a narrowly truncate base, 0-3-septate (septa very thin and easily overlooked), not constricted around the septa, contents with several minute oil-droplets and granular material in each cell in the living state, with minute oil-droplets and granular contents in the rehydrated state, (18-)20-28.5(-34) × 1-1.5(-2) μm (living; rehydrated, 1 μm wide). Sexual morph unknown.
Description in vitro: Colonies on OA 8-11 mm diam in 10 d (30-35 mm in 3 wk; more than 75 mm in 7 wk), with an even, glabrous, colourless margin; immersed mycelium mostly homogeneously pale coral to pale red, some pigment diffusing beyond the colony margin, olivaceous to greenish hyphal radial strands also weakly or more strongly developing in some sectors or entire colonies (especially after 7 wk, when most of the red pigment is no longer visible); in the centre olivaceous-black and slightly elevated due to superficial and immersed pycnidia, surrounded by an area with more scattered pycnidia, releasing pale whitish droplets of conidial slime; aerial mycelium very scanty, few minute white tufts; reverse olivaceous-black to greenish grey, surrounded by coral to sienna areas. Colonies on CMA 7-10 mm diam in 10 d (28-33 mm in 3 wk; more than 75 mm in 7 wk), as on OA, but the colonies sooner pigmented, dark green, dark blueish green or olivaceous, and a red pigment tardily formed, but more persistent and still well visible after 7 wk. Sporulation as on OA. Colonies on MEA 6-11 mm diam in 10 d (23-30 mm in 3 wk; 64-75 mm in 7 wk), the margin even, glabrous, buff; colonies spreading, but the centre elevated, irregularly pustulate, up to 2 mm high, the surface dark greyish brown, later black, covered by short felty white aerial mycelium, or higher tufts; reverse of the colony brown-vinaceous or sepia, paler towards the margin. Pycnidia mostly superficial, in dense groups. Colonies on CHA 5-8 mm diam in 10 d (28-35 mm in 3 wk; 45-55 mm in 7 wk), with an even to ruffled, glabrous, colourless to buff margin; immersed mycelium in areas where first sporulation occurs becoming dark, greenish grey to dark slate blue, later more throughout colony, covered by well-developed, tufty whitish grey aerial mycelium that later shows a reddish haze; reverse olivaceous-black to sepia, but margin paler; in the central part of the colony numerous pycnidia develop; in older colonies the centre becomes up to 3 mm high.
Conidiomata (OA) as in planta, immersed or developing on the agar surface, single or merged into complexes 100-220 μm diam, superficial pycnidia mostly forming one to several elongated necks, initially pale brown, then almost black, releasing pale whitish conidial slime, later becoming rosy-buff. Conidiogenous cells as in planta, 7-12(-14) × 2.5-5 μm. Conidia as in planta but some considerably longer, 22-38(-45) × 1-1.5 μm.
Hosts: Paris quadrifolia, P. incompleta and Viola palustris.
Material examined: Austria, Tirol, Leutaschtal Weidach, on river bank, on living leaves of Paris quadrifolia, 2 Aug. 2000, G. Verkley 1038, CBS H-21177, living cultures CBS 109110, 109111; Tirol, Ötztal, Sölden, near Hoch-Sölden, on living leaves of Viola palustris, 31 July 2000, G. Verkley 1037, CBS H-21152, living cultures CBS 109108, 109109.
Notes: According to the original description, conidia of S. paridis are 20 × 1 μm and aseptate. Vanev et al. (1997) describe the conidia as 18-25 × 1-1.3 μm, Teterevnikova-Babayan (1983), 20-25 × 1 μm. As is seen in several other Septoria, the conidia can reach considerably greater length in culture than on the natural host plant. In shape of the conidia the species strongly resembles S. galeopsidis and S. scabiosicola, as do the cultures, although S. galeopsidis does not produce a red pigment on OA. The material on Viola palustris (Violaceae) collected in Tirol was initially identified as S. violae-palustris, but based on the DNA sequence analyses of seven loci (Fig. 2) and the agreeing phenotype it is concluded that the material is conspecific with S. paridis. This is the first report of this fungus on another host genus than Paris, and also outside the Liliaceae. A second Septoria occurring on Paris quadrifolia is S. umbrosa. That species differs from S. paridis by much larger conidia, 30-85 × 3-4.5 μm, which are 5-7-septate.
Septoria passifloricola Punith., CMI Descr. Pathogenic Fungi & Bacteria no. 670. 1980.
≡ S. passiflorae Louw, Sci. Bull. Dept. Agric. For. Un. S. Africa 229: 34. 1941. Nom. illeg. Art 53 [non Syd., Annls mycol. 37: 408. 1939].
Description in vitro: Colonies on OA 12-15 mm diam in 2 wk, with an even, glabrous, buff margin; colonies spreading, immersed mycelium mostly homogeneous orange, but no diffusion of pigments beyond the margin observed; the surface covered by appressed, greyish white to grey aerial mycelium developing in concentric areas, beneath which mostly superficial, dark brown to almost black pycnidia or more complex conidiomata develop, releasing pale whitish to dirty greyish droplets of conidial slime; reverse orange to sienna. Colonies on CMA 10-14 mm diam in 2 wk, as on OA. Colonies on MEA 5-7(-10) mm diam in 2 wk, with an even, weakly lobed, black margin, which may be covered by short fluffy, pure white aerial mycelium; colonies spreading but elevated at the centre, the surface almost black, with immersed conidiomatal complexes soon covered by masses of first pale white, buff, and then brick conidial slime; the central area later entirely covered by cerebriform, brick masses of slime; reverse brick to almost vinaceous, and fawn. Colonies on CHA 8-10(-14) mm diam in 2 wk, with an even, buff margin covered by a diffuse, felty aerial mycelium; further as on MEA, but surface less elevated, and largely covered by diffuse, felty, grey-white aerial mycelium; conidial slime as on MEA abundantly produced from similar conidiomatal complexes, but more intensely pigmented, deep scarlet; reverse blood colour.
Conidiogenous cells (OA) hyaline, discrete, broadly ampulliform to cylindrical, holoblastic, with one or two indistinct percurrent proliferations (sympodial proliferation not observed), 8-14 × 3-6 μm; conidia filiform, hyaline, narrowly rounded at the top, attenuated to a truncate base, straight to somewhat curved, 1-2(-3)-septate, not constricted around the septa, mostly 10-30(-35) × 1.5-2(-2.5) μm.
Host: Passiflora edulis.
Material examined: Australia, Victoria, Wonthaggi, on Passiflora edulis, Mar. 2011, C. Murdoch, living culture CBS 129431. New Zealand, Auckland, Mt Albert, on living leaves of P. edulis, 21 Feb. 2000, C. F. Hill MAF LYN-118a, living culture CBS 102701.
Notes: Priest (2006) provided a description of the fungus on the host, and discussed the nomenclature. He also mentioned the anonymous reporting of a Septoria state observed in ascospore isolates from a Mycosphaerella sp. found on fruits lesions, but whether this truly is the sexual morph of S. passifloricola remains to be corroborated. The multilocus phylogeny (Fig. 2) provides evidence of a close relationship with S. ekmanniana (CBS 113385, 113612) and S. chromolaenae (CBS 113373), and also S. sisyrinchii (CBS 112096) and S. anthurii (CBS 148.41, 346.58).
Septoria petroselini (Lib.) Desm., Mem. Soc. Roy. Sci. Lille 1843: 97. 1843. Fig. 32.
Fig. 32.
Septoria petroselini. A, B. Colonies CBS 109521 (15 °C, nUV). A. On OA. B. On MEA. C, D. Conidia on OA (CBS 109521). E. Conidia and conidiogenous cells in planta (CBS H-21166). F. Conidia on OA (CBS 182.44). G. Conidia on OA (CBS 109521). Scale bars = 10 μm.
Basionym: Ascochyta petroselini Lib., Pl. Crypt. Arduenna 3: 252. 1834.
≡ Phleospora petroselini (Lib.) Westend., Bull. Acad. r. Bruxelles 12 (9): 252. 1845.
Description in planta: Symptoms leaf spots indefinite, without a distinct border, pale brown, visible on both sides in green parts of leaves or barely discoloured petioles. Conidiomata pycnidial, numerous, mostly epiphyllous, semi-immersed, black, mostly 80-200 mm diam, with a central, first narrow, later wider opening, releasing pale white cirrhi of conidia; conidiomatal wall composed of one or two layers of brown-walled, angular cells, lined by a layer of hyaline cells. Conidiogenous cells hyaline, discrete, holoblastic, sympodially or percurrently proliferating, ampulliform, 6-10 × 3-6 mm. Conidia hyaline, filiform, straight to somewhat flexuous, the upper cell tapered into the obtuse apex, relatively widely truncate at the base, (1-)3-5(-7) septate, not or only indistinctly constricted at the septa, contents granular or with minute oil-droplets around the septa and at the ends, 29-80 × 1.9-2.5 mm (living; rehydrated, 1.2-1.5 mm wide). Sexual morph unknown.
Description in vitro (18 °C, near UV) CBS 109521: Colonies on OA 13-16 mm diam in 2 wk, with an even, colourless margin; colonies spreading, immersed mycelium mostly pale ochreous, soon appearing dull green due to the development of dark green hyphal strands, particularly in a discontinuous submarginal zone; reverse in the centre ochreous to fulvous, surrounded by olivaceous-grey. Conidiomata developing after 5-7 d immersed in the agar or on its surface, most numerous in the centre of the colony, releasing milky white to rosy-buff conidial slime. Conidia also produced directly from mycelium near the centre of the colony. Colonies on MEA 17-20 mm diam in 2 wk, with an even to somewhat ruffled, buff margin; colonies spreading to restricted, somewhat elevated towards the centre, the surface black with many stromata developing and releasing milky white droplets of conidial slime, aerial mycelium diffuse to more dense and low, grey; reverse mostly greenish grey to iron-grey, in the centre with fawn to dark brick haze.
Conidiomata and conidiogenous cells as in planta. Conidia (OA) filiform to filiform-cylindrical, straight, flexuous or curved, attenuated gradually to the narrowly rounded to pointed apex, attenuated gradually or more abruptly to the narrowly truncate base, (0-)3-5(-7)-septate, 30-54(-65) × 2-2.5(-3) μm.
Hosts: Petroselinum crispum (syn. Apium petroselinum), other Petroselinum spp. and Coriandrum sativum (Priest 2006).
Material examined: Netherlands, Prov. Utrecht, Baarn, garden Eemnesserweg 90, on living leaves of Petroselinum crispum, 29 Mar. 2001, H.A. van der Aa 12642, CBS H-21166, living culture CBS 109521; Laren, on living leaves of P. sativum, June 1944, S. Dudok de Wit s.n., living culture CBS 182.44 = IMI 100279, dried specimen of culture on CMA, CBS H-18128.
Notes: CBS 182.44, isolated from Petroselinum sativum, produces conidia 29-49 × 1-2 μm, and this range of sizes agrees with those given for S. petroselini by most authors [26-45(-52) × (1-)1.5-2 μm cf. Priest 2006; 16-46 × 1-2 mm cf. Jørstad 1965 on Petroselinum]. In contrast, the conidia in the collection on P. crispum (CBS H-21166), as well as in the isolate CBS 109521 derived from it, were up to 80 μm long and 2.5 μm wide, and the pycnidia were also larger than described for S. petroselini, for which this material was initially identified as S. apiicola, but the molecular data provide evidence that it also belongs to S. petroselini. The material is 100 % homologous on ITS, Act, RPB2 and EF, and 99.7 % on Cal with CBS 182.44. The range of conidial sizes for S. petroselini is therefore expanded here, although it should be noted that the conidia formed in vitro are not over 65 μm in length in the material available. The ITS sequence of S. anthrisci is distinct from that of S. apiicola, but identical to that of S. petroselini and other species. Septoria anthrisci can be distinguished from S. petroselini by the Act, EF and RPB2 sequences.
Septoria phlogis Sacc. & Speg., in Sacc., Michelia 1: 184. 1878 [as “phlocis”; non Ellis & Everh., in G. Martin, J. Mycol. 3: 85. 1887; nec P. Syd., Mycoth. March., Cent. 18, no 1757; Cent. 23, no 2278. 1887; later homonyms]. Fig. 33.
Fig. 33.
Septoria phlogis. Conidia and conidiogenous cells in planta (CBS H-21198). Scale bars = 10 μm.
Description in planta: Symptoms leaf lesions developing in areas of the leaf lamina that first turn yellow, indefinite or delimited by darkening veinlets, hologenous, pale to dark brown. Conidiomata pycnidial, epiphyllous, numerous, semi-immersed to immersed, subglobose to globose, dark brown to black, 100-160 μm diam; ostiolum central, circular, initially 25-35 μm wide, later becoming more irregular and up to 70 μm wide, surrounding cells concolourous; conidiomatal wall 15-28 μm thick, composed of an outer layer of isodiametric to irregular cells mostly 5-9 μm diam with pale brown cell walls up to 2 μm thick, and an inner layer of hyphal to isodiametric cells 3-5 μm diam with thin, hyaline walls. Conidiogenous cells hyaline, discrete or integrated in 1-2-septate conidiophores up to 22 μm long, cylindrical, or narrowly to broadly ampulliform, holoblastic, often proliferating percurrently with indistinct annellations as well as sympodially, 5-7.5(-8) × 2.5-4(-5) μm. Conidia cylindrical, filiform, straight to slightly curved, narrowly rounded to somewhat pointed at the apex, attenuated gradually or more abruptly towards the narrowly truncate base, (0-)1-3(-4)-septate, not constricted around the septa, hyaline, containing minute oil-droplets and granular material in the living and rehydrated state, (22-)32-50(-60) × 1.5-2 μm (rehydrated; living, 2-2.5 μm wide). Sexual morph unknown.
Description in vitro: Colonies on OA 15-18 mm diam in 19 d, with an even, glabrous, buff to rosy-buff margin; colonies spreading, plane; immersed mycelium variably pigmented over sectors, usually either brownish olivaceous, or cinnamon to saffron (honey with a reddish haze); aerial mycelium scanty, white, locally forming a diffuse woolly-floccose mat; reverse olivaceous-black and cinnamon or saffron. Colonies on CMA 13-18 mm diam in 19 d, as on OA. Colonies on MEA 12-17 mm diam in 19 d, with an even, glabrous, buff margin; colonies spreading, the surface mostly plane, only somewhat elevated or folded towards the centre; immersed mycelium mostly dark salmon to olivaceous-black, covered by a dense, appressed mat of woolly, mostly white to faintly rosy-buff aerial mycelium; an ochreous pigment diffuses into the surrounding medium; reverse mostly sienna or blood colour, with an ochreous to saffron margin. Colonies on CHA 12-18 mm diam in 19 d, as on MEA.
Hosts: Phlox spp.
Material examined: Netherlands, Prov. Noord-Holland, Enkhuizen, on living leaves of Phlox sp., 6 Sep. 1949, J.A. von Arx s.n., CBS H-4862; Prov. Utrecht, Baarn, Cantonspark, on living leaves of Phlox sp., 27 Aug. 1999, G. Verkley 911, CBS H-21198, living culture CBS 102317; same substr., Jan. 1932, D. Moll s.n., living culture CBS 312.32; Garden in Baarn, same substr., 16 Oct. 1990, H.A. van der Aa 10919, CBS H-18130, living culture CBS 577.90; same substr., loc., 27 Aug. 1997, H.A. van der Aa 12302, CBS H-18131.
Notes: Priest (2006) described the conidia of S. phlogis as filiform, 1-4-septate, straight to curved, (35-)50-73 × (1-)1.5-2 μm, hyaline, with a truncate base and obtuse apex. He accepted S. divaricatae as a separate species, with Phlox drummondi (syn. P. divaricata) as the only known host plant, and S. drummondi as a synonym. Septoria divaricatae has similarly shaped but smaller conidia than S. phlogis, 1-3-septate, (13-)25-40(-45) × 1-1.5 μm. The overlap in length of the conidia of the two is minimal, at least on the host plant, indicating that they might be truly separate taxa. Several other authors have also accepted S. divaricatae as a distinct entity (Teterevnikova-Babayan 1987, Muthumary 1999). However, Jørstad (1965) considered S. divaricatae a synonym of S. phlogis, and also S. phlogina. Both S. phlogis and S. divaricatae occur on P. drummondi and this may have contributed to the confusion. Investigations based on fresh material on different Phlox species, and studies of cultures derived thereof, as well as type material of the names mentioned above, will be required in order to settle the complicated taxonomy of Septoria on Phlox.
Molecular identification of S. phlogis is straight-forward, as all protein-coding genes investigated here, particularly Btub, Cal and RPB2, show unique diagnostic sequences. Septoria epambrosiae (CBS 128629, 128636) is a sister species to S. phlogis. Septoria epambrosiae is a pathogen of Ambrosia artemisiifolia (Asteraceae), which today is the prime cause of hay fever in many areas where this weed occurs.
Septoria polygonorum Desm., Annls Sci. Nat., sér. 2, Bot.17: 108. 1842. Fig. 34.
Fig. 34.
Septoria polygonorum. A-C. Colonies CBS 102331 (15 °C, nUV). A. On OA. B. On CHA. C. On MEA. D. Conidia and conidiogenous cells in planta (CBS H-21212). E. Ibid., on OA (CBS 108982). F, G. Conidia on OA (CBS 347.67). Scale bars = 10 μm.
≡ Spilosphaeria polygonorum (Desm.) Rabenh., Herb. Mycol. II, no. 442a. 1856.
Description in planta: Symptoms leaf spots small, circular, hologenous, ochreous to brown, sharply delimited by a dark red-brown zone. Conidiomata pycnidial, mainly epiphyllous, several to many developed in each leaf spot after some time, subglobose to lenticular, not protruding strongly, brown to almost black, 50-120 μm diam; ostiolum central, initially circular and 25-45 μm wide, surrounding cells concolorous to somewhat darker brown; conidiomatal wall about 10-25 μm thick, composed of angular cells 2.0-6.5 μm diam, the outermost cells pale yellowish brown with somewhat thickened walls, the inner cells thin-walled. Conidiogenous cells hyaline, discrete, narrowly or broadly ampulliform with a relatively wide neck, holoblastic, often first proliferating sympodially, and later also percurrently 1-several times with distinct annellations, 5-10(-14) × 3.-5.5(-6.5) μm. Conidia filiform to filiform-cylindrical, straight or slightly curved, or flexuous, attenuated gradually to a narrowly rounded to pointed apex, attenuated more abruptly towards the truncate base, 1-4-septate, not or only inconspicuously constricted around the septa, hyaline, contents with several minute oil-droplets and granular material in each cell in the living state, with inconspicuous oil-droplets and granular contents in the rehydrated state, (17-)22-45(-53) × 1.5-2 μm (living; rehydrated, 1.2-1.8 μm wide). Sexual morph unknown.
Description in vitro: Colonies normally slow-growing, but sometimes with fast-growing sectors (diam including these between brackets) on all media except MEA. On OA 3-5 [6-7] mm diam in 2 wk [6-7 (22-30) mm in 6 wk], the margin regular, glabrous, colourless; colonies spreading, plane, immersed mycelium olivaceous-black, but grey-olivaceous to greenish grey in faster growing sectors that sometimes develop from typically slow-growing colonies; aerial mycelium generally absent or very scanty, but woolly-floccose appressed on the above mentioned sectors; white conidial slime produced from numerous, scattered pycnidial or stromatic conidiomata; reverse dark slate blue to olivaceous-black. Colonies on CMA 4-5 (6-7) mm diam in 2 wk [5-7 (22-27) mm in 6 wk], as on OA, with similar fast-growing sectors. Colonies on MEA 3-4 mm diam in 2 wk (6-8 mm in 6 wk), the margin regular, glabrous, barely visible; colonies irregularly pustulate to hemispherical, immersed mycelium olivaceous-black to black, glabrous, the surface bearing numerous droplets of milky white to dirty buff conidial slime emerging from scattered pycnidial conidiomata; reverse olivaceous-black to black. Colonies on CHA 3-5 mm diam in 2 wk [7-10 (22-26) mm in 6 wk], the margin distinctly ruffled, glabrous, ochreous to greyish; colonies irregularly pustulate, immersed mycelium olivaceous-black, lacking aerial mycelium; milky white to dirty buff conidial slime emerging from scattered pycnidial conidiomata; reverse blood colour.
Conidiomata (OA) as in planta, single and pycnidial, brown to black, glabrous, 85-150 μm diam, with a single ostiolum up to 50 μm wide, rarely also merged into multilocular stromata up to 300 μm diam which may have several openings; conidiogenous cells as in planta, proliferating sympodially and/or percurrently, 9-20 × 4-7 μm; conidia as in planta but longer, 30-65(-72) × 1.5-2(-2.2) μm.
Hosts: Polygonum spp.
Material examined: Austria, Tirol, Ötztal, Sautens, on living leaves of Polygonum persicaria, 30 July 2000, G. Verkley 1024, CBS H-21213, living culture CBS 108982. Netherlands, prov. Utrecht, Baarn, Zandvoordtweg, same substr., 9 July 1967, H.A. van der Aa 98, CBS H-18695, living culture CBS 347.67; same substr., prov. Limburg, St. Jansberg, near Plasmolen, 9 Sep. 1999, G. Verkley 926, CBS H-21208, living cultures CBS 102330, 102331; same substr., prov. Limburg, Savelsbos, 28 June 2000, G. Verkley 967, CBS H-21212, living cultures CBS 109007, 109008; Prov. Zeeland, Zuid-Beveland, community of Borsele, Valdijk near Nisse, 27 Aug. 2001, G. Verkley 1110, CBS H-21164, living culture CBS 109834. New Zealand, North Island, Coromandel, Tairua Forest, along roadside of St. Hway 25, near crossing 25A, 23 Jan. 2003, G. Verkley 1843, CBS H-21242, living culture CBS 113110.
Notes: More than ten Septoria species have been described from the host genus Polygonum, of which S. polygonorum is the oldest one. The material available for the present study agrees generally well in morphology with the description of S. polygonorum provided by other authors. Priest (2006) described the conidiogenous cells as holoblastic (first conidium), producing subsequent conidia enterobastically, seceding at the same level (mode “Event 13: enteroblastic non-progressive”). Muthumary (1999), who studied type material of S. polygonorum from PC, observed sympodially proliferated cells. Priest may have overlooked the sympodial conidiogenesis, as in the present study sympodially proliferating cells were also observed in field specimens of S. polygonorum. The strains available from distant geographical origins showed highly similar sequences for seven loci. The multilocus phylogeny indicates a rather isolated position of S. polygonorum (Fig. 2).
Septoria protearum Viljoen & Crous, S. Afr. J. Bot. 64: 144. 1998.
Description in planta: Symptoms leaf spots varied according to the host. Conidiomata pycnidial, epiphyllous or amphigenous, semi-immersed or becoming erumpent, subglobose to globose, dark brown to black, 65-200 μm diam; ostiolum central, circular, slightly papillate, 18-30(-60) μm wide, surrounding cells concolourous, releasing white cirrhi of conidial slime; conidiomatal wall 10-22 μm thick, composed of 3-4 layers of brown, isodiametric to irregular cells mostly 5-10 μm diam with dark brown cell walls up to 2 μm thick, sometimes with an an inner layer of hyphal to isodiametric cells 3.5-5 μm diam with thin, hyaline walls. Conidiogenous cells hyaline, discrete and globose or doliiform often with an elongated neck, or integrated in 1-5-septate conidiophores up to 30 μm long and narrowly to broadly ampulliform, holoblastic, proliferating percurrently with indistinct annellations as well as sympodially, 4-12 × 1.5-3.5(-5) μm. Conidia hyaline, cylindrical, subcylindrical to obclavate, straight to curved, rounded to somewhat pointed at the apex, attenuated gradually or more abruptly towards the truncate base, (0-)1-3(-4)-septate, not constricted around the septa, containing minute oil-droplets and granular material in rehydrated state, (6-)12-22(-30) × 1.5-2 μm (rehydrated). Sexual morph unknown.
Description in vitro (18 °C, near UV): Colonies on OA 11-16 mm diam in 1 wk, 23-30 mm in 2 wk, with an even, slightly undulating, colourless margin; colonies plane, spreading, immersed mycelium ochreous to pale luteous or rosy-buff and rarely also with greenish tinges, aerial mycelium absent or scarce with few grey to rosy-buff tufts; conidiomata developing mostly immersed in the agar, scattered or in concentric zones, olivaceous-black, releasing droplets of milky white to pale salmon conidial slime. Reverse cinnamon to hazel or fawn, or rosy-buff. Colonies on MEA 32-36 mm diam in 2 wk, with an even, (vinaceous) buff to colourless undulating margin; colonies restricted with a cerebriform elevated central area or lower and more spreading, radially striate, the entire surface covered by a dense mat of finely felted, somewhat woolly, white to greysh, or salmon to flesh aerial mycelium; reverse dark, fawn to brown-vinaceous, or olivaceous-black mixed with bright rust to coral. Conidiomata developing after 1 wk, mostly immersed and releasing whitish conidial slime. Colonies on CHA 17-19 mm diam in 1 wk, 25-31 mm in 2 wk, with an even, saffron margin with some diffuse white aerial mycelium; colonies spreading but slightly elevated in the centre, entirely covered by a dense mat of pure white, locally weakly salmon, woolly and somewhat sticky aerial mycelium, in the marginal area later with a glaucous haze; reverse in the centre chestnut, surrounded by rust and apricot zones, margin saffron. Sporulation as on MEA.
Conidiomata (OA) pycnidial, globose, single or merging into complexes up to 220 μm diam, brown to black, the wall composed of pale brown textura angularis with cells up to 10 μm diam, inner cells smaller and hyaline. Conidiogenous cells hyaline, discrete or integrated in simple, 1(-2)-septate conidiophores, cylindrical or narrowly to broadly ampulliform, holoblastic, proliferating sympodially, and/or percurrently with indistinct annellations, and then often showing a narrow neck of variable length, 5-10(-13.5) × 2.5-3(-3.5) μm. Conidia filiform to cylindrical, straight, more often curved or flexuous, or bent irregularly, rounded to somewhat pointed at the apex, attenuated gradually or more abruptly towards the narrowly truncate base, (0-)1-3-septate, not constricted at the septa, hyaline, contents as in planta, (8-)12-22(-25) × 1.5-2 μm (CBS 119942), (12-)15-23.5(-31) × 1-1.5 μm (CBS 179.77), 17-35 × 1-1.5(-2) μm (CBS 658.77).
Hosts: Asplenium ruta-muraria, Boronia denticulata, Geum sp., Ligustrum vulgare, Myosotis sp., Nephrolepis sp., Pistacia vera, Protea cynaroides, Protea sp., Skimmia sp. and Zanthedeschia aethiopica.
Material examined: Germany, Potsdam, Maulbeerallee beneath the Orangerie, on living leaves of Asplenium ruta-muraria, 17 Nov. 2005, V. Kummer 0045/3, CBS H-19729, living culture CBS 119942. Italy, details of loc. unknown, on Pistacia vera, June 1951, deposited by G. Goidánich, living culture CBS 420.51; on Ligustrum vulgare, June 1959, M. Ribaldi, living culture CBS 390.59. Netherlands, Reeuwijk, in leaf spot of Skimmia sp., commercially cultivated under plastic ‘tunnels’, 1996, J. de Gruyter, CBS H-21190, PD 96/11330 = CBS 364.97. New Zealand, Auckland, on Myosotis sp., Dec. 1976, H.J. Boesewinkel, CBS H=18209, living culture CBS 179.77; same area, on Nephrolepis sp., Sep. 1977, H.J. Boesewinkel, CBS H-18211, living culture CBS 164.78; same area, on leaves and stems of Boronia denticulata, 5 Apr. 1977, H. J. Boesewinkel, CBS H-18120, living culture isolated, CBS 658.77; same area, Albert Park, on leaves of Geum sp., 21 Jan. 2003, G. Verkley V1821, CBS H-21233, living culture CBS 113114. South Africa, Gauteng Province, on leaves of Protea cynaroides, Sep. 1996, L. Viljoen, living ex-type culture of Septoria protearum STE-U 1470 = CBS 778.97; Pilgrims Rest, on Zanthedeschia aethiopica, 15 July 2011, P.W. Crous, living culture CPC 19675.
Notes: The description of S. protearum given by Crous et al. (2004) has been emended here using observations on material isolated from other hosts than Protea. These fungi are, despite minor differences in colony characteristics, genetically very similar, and therefore regarded as conspecific. The name S. protearum is adopted as it is based on well-decribed type material and ex-type cultures. The distinction with a number of strains isolated from Citrus spp., Fragaria sp., Gerbera jamesonii, Gevuina avellana, Hedera helix, Lobelia erinus, and Masdevallia sp. is doubtful but, based on the morphological differences in combination with a limited number of polymorphisms on the house-keeping genes, they are treated here as part of Septoria citri (which clusters in the S. protearum complex), which is a species complex that needs to be further resolved. Material studied and some cultural characters of CBS 113392 are provided below.
Additional material of the Septoria citri complex examined: Country and host unknown, May 1937, L.L. Huiller, living culture CBS 315.37 (sub Septoria citri). Argentina, in leaf spot of Lobelia erinus, S. Wolcon s.n., ‘V1466’, living culture CBS 113392. Italy, Sicilia, on Gerbera jamesonii, Nov. 1961, W. Gerlach, living cultures CBS 410.61 = BBA 9588 (sub S. gerberae). Netherlands, Paterwolde, in glasshouse, in leaf spots of Masdevallia sp., Feb. 1998, W. Veenbaas-Rijks (CBS H-18124), living culture CBS 101013 (sub S. orchidacearum). New Zealand, leaf of Gevuina avellana, Nov. 1998, S. Ganev, living culture CBS 101354; Waitakere, culture isolated from leaf of Fragaria sp., Nov. 1975, H. J.Boesewinkel, living culture CBS 177.77 (sub Septoria aciculosa). Portugal, Algarve, Monchique, in leaf spot on Hedera helix, 14 June 1988, H.A. van der Aa 10494, living culture CBS 566.88 (sub S. hederae Desm.).
Description in vitro (18 °C, near UV, CBS 113392): Colonies 23-26 mm diam in 2 wk, with an even, glabrous colourless margin; colonies spreading, immersed mycelium orange, lacking aerial mycelium; reverse bay to scarlet. Conidiomata developing in concentric patterns, immersed and on the agar surface, releasing milky white masses of conidial slime. Colonies on MEA 17-23 mm diam in 2 wk, with an even colourless margin mostly covered by white aerial hyphae; colonies spreading but developing cerebriform elevations in the centre, immersed mycelium livid vinaceous to vinaceous buff, with diffuse to dense, appressed, whitish to vinaceous buff aerial mycelium.
Conidiogenous cells (OA) varied in shape, globose, doliiform to ampulliform or cylindrical, discrete, rarely integrated in 1-septate conidiophores, holoblastic, proliferating sympodially, and also percurrently with several close and indisctinct annellations, hyaline, 4.5-8(-10) × 3-5 μm. Conidia filiform to cylindrical, straight to flexuous, often weakly curved, attenuated gradually to a narrowly rounded to somewhat pointed apex, attenuated gradually or more abruptly to a narrowly truncate to almost rounded base, contents granular with few minute oil-droplets in the living state, (0-)1-3-septate, (12-)15-28 × 1.5-2 μm (living); CBS 177.77 (OA) 17-35.5 × 1-2 μm (living).
Septoria putrida Strasser, Verh. zool.-bot. Ges. Wien 65: 180. 1915. Fig. 35F-J.
Fig. 35.
A-E. Septoria senecionis. A-C. Colonies CBS 102381 (15 °C, nUV). A. On OA. B. On CHA. C. On MEA. D. Conidia and conidiogenous cells in planta (CBS H-21219, epitype). E. Conidia in planta (CBS H-21219). F-J. Septoria putrida. F, G. Conidia in planta (CBS H-21174). H. Conidiogenous cells in planta (CBS H-21174). I. Conidia and conidiogenous cells in planta (CBS H-21174). J. Ibid., on OA (CBS 109088). Scale bars = 10 μm.
Description in planta: Symptoms definite leaf spots, hologenous or epigenous, scattered or in clusters, initially pale yellowish, later grey to white, surrounded by a black elevated zone or merely delimited by leaf veins. Conidiomata pycnidial, one to several in each leaf spot, scattered, semi-immersed, predominantly epiphyllous, pale brown, lenticular to globose, 80-180 μm diam; ostiolum circular, central, initially 25-50 μm wide, later opening to 80 μm diam, lacking distinctly differentiated cells; conidiomatal wall composed of textura angularis without distinctly differentiated layers, mostly 10-20 μm thick, the outer cells with brown, somewhat thickened walls and 4.5-10 μm diam, the inner cells hyaline, thin-walled, 4-9 μm diam. Conidiogenous cells hyaline, discrete or integrated in short, 1-septate conidiophores, cylindrical, or ampuliform with a mostly relatively short, but sometimes strongly elongated neck (8-10 μm long), hyaline, holoblastic, proliferating percurrently with distinct annellations, sometimes also sympodially, 6.5-12(-19.5) × 3.5-5 μm. Conidia cylindrical, usually strongly curved or flexuous, gradually attenuated to a rounded apex, gradually attenuated into a broadly truncate base, (0-)3-5-septate, not or indistinctly constricted around the septa, hyaline, contents with several small guttulae and numerous granules in each cell in the living state, oil-droplets rarely merged into larger guttules in the rehydrated state, (32-)40-70(-85) × 2-2.5(-3.0) μm (rehydrated). Sexual morph unknown.
Description in vitro: Colonies on OA 5.5-8.5 mm diam in 12 d (13-15 mm in 3 wk; 50-55 mm in 7 wk), with an even, somewhat undulating, glabrous, colourless margin; colonies plane, immersed mycelium buff to primrose, in some sectors also with dark herbage green to dull green radiating hyphal stands, after 7 wk mostly dark greenish; pycnidial conidiomata scattered immersed and superficial, which are first dark olivaceous, then almost black, glabrous or beset with short hyphal protrusions, 150-450 μm diam, mostly with a single ostiolum placed on short papillae, that releases pale whitish or buff conidial slime; aerial mycelium diffuse, woolly-floccose, white to grey; reverse dull green to olivaceous-black in the centre. Colonies on CMA 4-7 mm diam in 12 d (11-14 mm in 3 wk; 50-55 mm in 7 wk), with an even, glabrous, colourless margin; immersed mycelium apart from margin olivaceous-black, at the margin with some local production of a coral pigment after 7 wk; aerial mycelium higher, diffuse woolly, greyish; reverse darker as on OA; conidiomata similar as on OA. Colonies on MEA 2.5-5 mm diam in 12 d (11-13 mm in 3 wk; 42-46 mm in 7 wk), with an even to ruffled, glabrous, colourless to buff margin, which may be irregularly lobate after 7 wk; colonies restricted, pustulate to almost hemispherical, immersed mycelium rather dark, aerial mycelium diffuse, short, felty white, behind the margin denser and higher; superficial mature conidiomata releasing first milky white, later pale luteous to saffron, then salmon conidial slime; reverse olivaceous-black in the centre, near the margin honey. Colonies on CHA 5-7 mm diam in 12 d (8-11 mm in 3 wk), with an irregular, ruffled, colourless margin, older colonies distinctly lobate; the surface mostly covered by a low, dense to diffuse, felty white, later grey aerial mycelium, near the margin pure white felty to tufty; further as on MEA; conidial slime abundantly produced, first milky white, later salmon or saffron; reverse in the centre blood colour, dark brick to cinnamon at the margin.
Conidia as in planta, (0-)3-5(-6)-septate, 40-85(-97) × 2-2.5(-3) μm.
Host: Senecio nemorensis.
Material examined: Austria, Tirol, Ober Inntal, Samnaun Gruppe, Lawenalm, on living leaves of Senecio nemorensis subsp. fuchsii, 8 Aug. 2000, G. Verkley 1052a,
CBS H-21174, living cultures CBS 109087, 109088.
Notes: Septoria putrida was originally described from Senecio nemorensis found in Austria (Sonntagberg), reportedly with 0(-9-11?)-septate conidia, 70-80 × 2 μm. The multilocus sequence analysis indicates that S. putrida and S. senecionis are closely related but genetically distinct species (Fig. 2). Morphologically these sister taxa can best be distinguished based on conidial length; conidia in S. putrida can be up to 85 μm long in planta and even longer (up to 97 μm) in culture, whereas those of S. senecionis are rarely longer than 65 in planta and not over 70 μm long in culture.
Thirteen more taxa have been described in Septoria on Senecio, of which S. anaxaea Sacc. is another distinctive, long-spored species described from Senecio grandidentatus (?= S. praealtus), and recently also from several other Senecio spp. in Australia. According to Priest (2006), conidia are 3(-6)-septate, 28-75 × 2.5-3 μm (50-130 × 3.5-5 μm, Teterevnikova-Babayan 1987). Most other Septoria spp. on Senecio may be synonyms of S. senecionis, and this needs to be confirmed by study of the type material.
Septoria rumicum Sacc. & Paol., in Saccardo, Bull. Soc. r. Bot. Belg. 28: 23. 1889.
Description in vitro: Colonies on OA 3-5 mm diam in 3 wk, with an even colourless margin; colonies restricted, irregularly pustulate, immersed mycelium olivaceous-black mostly hidden under a low, dense mat of felty grey to white aerial mycelium; reverse olivaceous-grey. Colonies on MEA 6-10(-12) mm diam in 3 wk, with an even or lobed, colourless margin; colonies restricted, irregularly pustulate, immersed mycelium appearing olivaceous-grey under a dense mat of woolly-floccose, white to grayish aerial mycelium; reverse olivaceous-black. No sporulation observed.
Conidia (OA) cylindrical, filiform, straight or slightly curved, attenuated gradually towards a narrowly rounded to almost pointed apex, attenuated gradually or more abruptly towards the narrowly truncate base, 3-5(-7)-septate, mostly 60-82 × 2-3 μm.
Hosts: Rumex spp. (R. acetosa, R. alpinum).
Material examined: France, Corrèze, Roumignac, on leaves of Rumex acetosa, H.A. van der Aa 5338, CBS H-18050, living culture CBS 503.76; Haute-Savoie, Mt. Beaudin, on stem of R. alpinus, July 1978, H.A. van der Aa 9594c, CBS H-18163, living culture CBS 522.78.
Notes: Jørstad (1965) noted that S. rumicis Trail, which was published in the same year as S. rumicum, may be conspecific. Septoria acetosae Oud. was also regarded as a synonym. According to Saccardo (1892, Syll. Fung. 10: 380), S. rumicum produces mostly epiphyllous pycnidia 100-125 μm diam, and continuous (?) conidia 50-68 × 3 μm. Septoria rumicis produces chiefly epiphyllous pycnidia 90-100 μm diam and conidia 24-40 × 2-2.5 μm (Teterevnikova-Babayan 1987), according to Jørstad (1965), 20-50 × 2.5-3.5, with 2-3(-5) septa. Septoria acetosae was treated as a separate species by Teterevnikova-Babayan (1987). According to the latter author, it is characterised by 1-3-septate conidia, 28-50 × 3-5 μm. As the conidial sizes of the material available here agree best with the original description of S. rumicum, this name is adopted here. Several other species of Septoria have been described from Rumex, most of which need to be restudied to assess their status.
Septoria scabiosicola (Desm.) Desm., Annls Sci. Nat., sér. 3, Bot. 20: 96. 1853. Fig. 36.
Fig. 36.
Septoria scabiosicola, CBS 102333. A-C. Colonies (15 °C, nUV). A. On OA. B. On CHA. C. On MEA. D. Conidia and conidiogenous cells in planta (CBS H-21180). E. Conidia on OA (CBS 109021). Scale bars = 10 μm.
Basionym: Depazea scabiosicola Desm., Annls Sci. Nat., sér. 2, Bot. 6: 247. 1836.
Description in planta: Symptoms leaf spots numerous but small, circular, some merging to irregular patterns, centre white, surrounded by a relatively broad, dark margin with a distinct red or purple periphery. Conidiomata pycnidial, epiphyllous but sometimes also visible from the underside of the lesion, one to a few in each leaf spot, subglobose to globose, brown to black, usually fully immersed, 65-130 μm diam; ostiolum central, initially circular and 35-60 μm wide, later becoming more irregular and up to 80 μm wide, surrounding cells concolorous to pale brown; conidiomatal wall about 10-15 μm thick, composed of a homogenous tissue of hyaline, angular cells 2.5-6.5 μm diam, the outermost cells pale brown with somewhat thickened walls, the inner cells thin-walled. Conidiogenous cells hyaline, discrete, doliiform, or narrowly to broadly ampulliform, holoblastic, with a relatively narrow elongated neck, proliferating percurrently several times with distinct annellations, often also sympodially after a few percurrent proliferations, 6-9(-12) × 2.5-3(-5) μm. Conidia filiform to filiform-cylindrical, straight, slightly curved to flexuous, attenuated gradually to a narrowly pointed apex and narrowly truncate base, (0-)3-5(-6)-septate (septa very thin and easily overlooked), not constricted around the septa, hyaline, contents with several minute oil-droplets and granular material in each cell in the living state, with minute oil-droplets and granular contents in the rehydrated state, (17-)30-55 (-79) × 1-2 μm (living; rehydrated, 1-1.8 μm wide). Sexual morph unknown.
Description in vitro: Colonies on OA 9-13 mm diam in 2 wk, with an even, glabrous, colourless margin; immersed mycelium mostly homogeneously coral to scarlet, the pigment diffusing beyond the colony margin; in the centre black and slightly elevated due to immersed and more frequently superficial pycnidia, surrounded by an area with more scattered pycnidia, releasing pale flesh droplets of conidial slime; aerial mycelium scanty, consisting of minute white tufts; reverse scarlet to coral, the centre darker, blood colour. Colonies may develop sectors that are unpigmented and glabrous. Colonies on CMA 8-11 mm diam in 2 wk; similar as on OA, but generally less strongly pigmented. Colonies on MEA 6-9 mm diam in 2 wk, the margin irregular; colonies restricted, the centre elevated and cerebriform to irregularly pustulate, up to 2 mm high, the surface pale brown, later black, with scanty white areal mycelium; reverse of the colony dark brick, paler towards the margin. Colonies on CHA 6-11 mm diam in 2 wk, with an even, glabrous, colourless margin; immersed mycelium greenish grey to dark slate blue, throughout covered by well-developed, tufty whitish grey arial mycelium that later attains a reddish haze; reverse blood colour, but margin paler; in the central part of the colony numerous pycnidia develop, releasing pale vinaceous to rosy-buff conidial slime; in older colonies the centre becomes cerebriform and up to 3mm high, much as on MEA.
Conidiomata (OA) as in planta, pycnidial, sometimes merged into larger complex stromata dark brown, glabrous, 80-180 μm diam, with a single ostiolum, or without preformed opening and simply bursting open; conidiogenous cells as in planta, but more often integrated in 1-2-septate conidiophores, often only proliferating percurrently and/or sympodially, 6-15 × 3-7.5 μm; conidia as in planta, 1-6(-7)-septate, not constricted around the septa, hyaline, with several minute oil-droplets and numerous granules in each cell, (30-)40-80(-100) ×1.5-2(-2.5) μm.
Hosts: Knautia spp., Succisa spp. and Scabiosa spp.
Material examined: Austria, Tirol, Ötztal, Brunau, along roadside, on living leaves of Knautia arvensis, 30 July 2000, G. Verkley 1023, CBS H-21184, living cultures CBS 108981, 109021; Tirol, Ötztal, Sautens, in meadow, 30 July 2000, G. Verkley 1030, CBS H-21180, living cultures CBS 108985, 108986; Tirol, Ötztal, Ötz, near Piburger See along forest road, on living leaves of K. dipsacifolia, 1 Aug. 2000, G. Verkley 1033, CBS H-21179, living cultures CBS 109092, 109093; Tirol, Ober Inntal, Samnaun Gruppe, Serfaus, on living leaves of K. dipsacifolia, 9 Aug. 2000, G. Verkley 1062, CBS H-21172, living cultures CBS 109128, 109129. France, on living leaves of Succissa pratensis, H.A. van der Aa 11375, living culture CBS 182.93. Germany, on living leaves of Scabiosa lucida, R. Schneider, living culture CBS 356.58. Netherlands, prov. Gelderland, near Winssen, along Waalbanddijk, on living leaves of K. arvensis, 9 Sep. 1999, G. Verkley 919, CBS H-21201, living cultures CBS 102333, 102334; same loc., host, date, G. Verkley 920, CBS H-21203, living cultures CBS 102335, 102336; same loc., host, date, G. Verkley 921, CBS H-21202; unknown host, July 1937, living culture CBS 317.37.
Notes: Jørstad (1965) and Radulescu et al. (1973) reported variability in the maximum length of conidia on the host plant. This is confirmed in the present study, where the highest and lowest maximum lengths observed in specimens were 79 and 42 μm, in specimens CBS H-21184 and CBS H-21180, respectively. Both specimens were collected from the same host at comparable altitudes (ca. 700 m), from localities in Tirol, Austria less than three kilometers apart. Isolates obtained from these two collections proved equally capable of producing conidia up 100 μm long under standard conditions of incubation.
These isolates as well as other from Knautia arvensis, and strains originating from Scabiosa and Succissa showed no correlation between conidial sizes and host, and although some variation in gene sequences was observed, especially in Act and EF, the data firmly support the hypothesis that they belong to a single taxon. Several formae have been described in S. scabiosicola, but evidence to support these as separate entities is wanting. Septoria scabiosicola is relatively distantly related from other members of the Septoria clade (Fig. 2).
Septoria senecionis Westend., Bull. Acad. r. Belg., Cl. Sci., Sér. 2, 19: 121. 1851. Fig. 35A-E.
Description in planta: Symptoms indefinite, hologenous leaf lesions, often eventually affecting large parts of the leaf lamina, initially pale yellowish, later pale to dark brown. Conidiomata pycnidial, numerous, scattered, immersed, mostly epiphyllous, pale brown, lenticular to globose, (45-)65-120(-160) μm diam; ostiolum circular, central, initially 20-35 μm wide, later opening to 60 μm diam, lacking distinctly differentiated cells; conidiomatal wall composed of textura angularis without distinctly differentiated layers, mostly 15-20 μm thick, the outer cells with brown, somewhat thickened walls and 4.5-10 μm diam, the inner cells hyaline and thin-walled and of comparable diam. Conidiogenous cells hyaline, discrete or integrated in short, 1-2-septate conidiophores, cylindrical, or ampuliform with a relatively short neck, hyaline, holoblastic, proliferating sympodially, and sometimes also percurrently with indistinct annellations, 6.5-10(-12.5) × 2.5-4.5 μm. Conidia cylindrical, weakly to strongly curved, or flexuous, gradually attenuated to a rounded apex, gradually or more abruptly attenuated into a broadly truncate base, (0-)2-5(-6)-septate, not or indistinctly constricted around the septa, hyaline, contents with several small guttules and numerous granules in each cell in the living state, oil-droplets rarely merged into larger guttules in the rehydrated state, (20-)40-65 × 2-2.5(-3) μm (rehydrated). Sexual morph unknown.
Description in vitro: Colonies on OA 7-10 mm diam in 2 wk (22-26 mm in 6 wk), with an even, somewhat undulating, glabrous, colourless margin; colonies spreading, the surface plane, immersed mycelium pale luteous or buff, with scattered immersed and superficial pycnidial conidiomata, which are first dark olivaceous, then almost black, glabrous, 150-450 μm diam, with a single or several (up to 5!) ostioli placed on short papillae or more elongated necks (up to 350 μm), that release buff to rosy-buff later salmon conidial slime; aerial mycelium diffuse, woolly-floccose, white; reverse honey, but isabelline to hazel in the centre. Colonies on CMA 6-8 mm diam in 2 wk (18-23 mm in 6 wk), with an even, glabrous margin; as on OA but immersed mycelium with a greenish haze; aerial mycelium higher and reverse darker, later hazel with olivaceous and yellow tinges; conidiomata similar as on OA. Colonies on MEA 7-9 mm diam in 2 wk (18-21 mm in 6 wk), with an even or somewhat undulating, glabrous, buff to honey margin; colonies pustulate to almost hemispherical, immersed mycelium rather dark, near the margin covered by woolly to felty white aerial mycelium; mostly composed of spherical conidiomatal initials, superficial mature conidiomata releasing rosy-buff to salmon, later honey conidial slime; reverse dark brick in the centre, near the margin cinnamon to honey. Colonies on CHA 7-14 mm diam in 2 wk (20-28 mm in 6 wk), with an irregular, buff margin covered by a diffuse, felty white, later grey aerial mycelium; further as on MEA, but the colony surface less elevated and especially near the margin with greyish felty to tufty aerial mycelium; conidial slime abundantly produced, first rosy-buff, later salmon to ochreous; reverse in the centre blood colour, dark brick to cinnamon at the margin.
Conidiomata on OA see above. Conidia as in planta, mostly (0-)3-5(-6)-septate, 44-63(-70) × 2.5-3 μm.
Hosts: Senecio fluviatilis and S. nemorensis.
Material examined: Belgium, Château de Namur, on leaves of Senecio sarracenica, 1829, A. Bellynck, isotype BR-MYCO 155500-09. Netherlands, Prov. Gelderland, Millingen a/d Rijn, Millingerwaard, on living leaves of S. fluviatilis, 6 Oct. 1999, G. Verkley 939, epitype designated here CBS H-21219 “MBT175358”, living cultures ex-epitype CBS 102366, 102381.
Notes: The first Septoria that was described on the genus Senecio was S. senecionis. The type host is Senecio sarracenica (= Senecio fluviatilis), and in later literature it has also been reported from several other species of Senecio (Radulescu et al. 1973). According to the diagnosis by Westendorp, the conidia are 40 × 1.5 μm and 3-4-septate. Vanev et al. (1997) described the conidia of S. senecionis as 2-6-septate, 29-68 × 2-2.5 μm, Radulescu et al. (1973) as 3-4-septate, 33-57 × 1.2-2 μm. By examining the type specimen from BR it is here confirmed that conidia are in fact wider than described by Westendorp. It contains a single leaf with a few lesions, and conidia observed are 30-55 × 1.5-2.5 μm, and mostly 3-5-septate. The fresh material that was collected in the Netherlands from the same host species, Senecio fluviatilis, and from which CBS 102366 and 102381 were isolated, is in sufficient agreement with the type and is therefore designated here as epitype of S. senecionis. Differences with Septoria putrida are discussed under that species.
Septoria sii Roberge ex Desm., Pl. crypt. Fr., Fasc. 44, no 2185; Annls Sci. Nat., sér. 3, Bot. 20: 92. 1853. Fig. 37.
Fig. 37.
Septoria sii. A-C. Colonies CBS 102370 (15 °C, nUV). A. On OA. B. On CHA. C. On MEA. D. Conidia and conidiogenous cells in planta (CBS H-21223). E. Ibid., on OA (CBS 102369). Scale bars = 10 μm.
Description in planta: Symptoms leaf spots, yellow to brown, initially vaguely delimited but later well-delimited by veinlets, scattered, later often confluent over large areas, visible on both sides of the leaf. Conidiomata pycnidial, epiphyllous, rarely also hypophyllous, single, scattered or in small clusters, globose to subglobose, immersed, (60-)80-110 μm diam; ostiolum circular, central, 12.5-25(-35) μm wide, surrounding cells concolorous; conidiomatal wall composed of textura angularis 5-10 μm thick, with an outer layer of cells 3-4.5 μm diam with brown, thickened walls, and an inner layer of hyaline and thin-walled cells, 2.5-4 μm diam. Conidiogenous cells hyaline, broadly or elongated ampulliform, normally with a distinct neck, hyaline, holoblastic, proliferating percurrently, annellations indistinct, 5-8.5 × 3-5 μm. Conidia cylindrical, straight, curved, or flexuous, gradually attenuated to a relatively broadly rounded apex, more or less abruptly attenuated into a truncate base, 1-3(-4)-septate, slightly to distinctly constricted around the septa in the fresh, fully hydrated state, hyaline, containing one to several relatively large oil-droplets in each cell, in the rehydrated state with irregular oil-masses (20-)29-35(-42) × 2-2.5(-3) μm (living; rehydrated, 1.5-2 μm wide). Sexual morph unknown.
Description in vitro: Colonies on OA 4-9 mm diam in 2 wk [(15-) 19-23 mm in 6 wk], with an even, glabrous, colourless margin; colonies remaining almost plane, immersed mycelium olivaceous-black, locally however peach is dominant, which becomes scarlet after several wk; aerial mycelium mostly well-developed, woolly-floccose, white; scattered, mostly immersed pycnidial to stromatic conidiomata developing in the centre, releasing droplets of milky white to rosy-buff conidial slime; reverse dark slate blue to olivaceous-black, and locally peach, the pigment not diffusing into the medium. Colonies on CMA up to 1.5 mm diam in 2 wk [7-10 (-25) mm in 6 wk], as on OA, but peach pigment diffusing into the medium, while the colony itself is predominantly olivaceous-black. Colonies frequently develop faster growing sectors that first are buff and sporulate directly from the mycelium, later become pale luteous with a distinct scarlet pigmentation and forming numerous mostly superficial pycnidia. Colonies on MEA 3-6 mm diam in 2 wk [12-14(-26) mm in 6 wk], the margin ruffled, olivaceous-black; colony concolorous, irregularly pustulate-worty, covered by diffuse to dense felty white or greyish aerial mycelium; numerous conidiomatal initials developing at the surface, mature ones releasing cirrhi of conidia that first are milky white, later salmon, sometimes merging to form slimy masses covering areas of the colony surface; the agar surrounding the colony slightly discoloured by diffusing pigment(s). Colonies on CHA 5-6 mm diam in 2 wk [8-13(-15) mm in 6 wk], as on MEA; some parts of the colonies pale ochreous, tardily sporulating, releasing pale flesh to salmon droplets of conidial slime from superficial pycnidial conidiomata.
Cultures sporulating with conidiogenous cells developing in (superficial) mycelial hyphae, solitary or in sequences, in addition to conidiomata. Conidiomata on OA pycnidial, single, dark brown to black, 80-185 μm diam, ostiolum single 30-60 μm diam, or stromatic without a differentiated opening and up to 220 μm diam; conidiogenous cells inside pycnidia as in planta but often with more elongated neck, holoblastic, percurrently proliferating one to several times with indistinct annellations, 7-12.5 × 3-6 μm. Conidia as in planta, 22-43 × 2.2-2.5 μm.
Hosts: Sium latifolium, other Sium spp. and Berula erecta (syn. Sium erectum).
Material examined: Netherlands, Prov. Friesland, Terschelling, ditch in polder S of Hoorn, on living leaves of Berula erecta, 19 Aug. 1995, H.A. van der Aa 12029, CBS H-18173, living culture CBS 118.96; same substr., Prov. Utrecht, ‘s Graveland, Kortenhoefse plassen, “Oppad”, 14 Oct. 1999, G. Verkley & H.A. van der Aa 945, CBS H-21223, living culture CBS 102369; same loc., substr., date, G. Verkley & H.A. van der Aa 946, CBS H-21222, living culture CBS 102370.
Notes: The stout conidia with blunt apices and distinct constrictions around the septa (at least in the living, turgescent state) and the absence of sympodial proliferation in conidiogenesis distinguish this species from most other Septoria on Apiaceae here investigated, including S. apiicola. According to the original diagnosis, based on material from Sium latifolium in France, the conidia are 30-40 × 2.5 μm. Most later authors have reported somewhat different size ranges; for example Teterevnikova-Babayan (1985) observed conidia 20-60 × 1-1.5 μm, Vanev et al. (1997) 20-41 × 1.5-2.2 μm, and Radulescu et al. (1973) reported 30-40 × 2-3 μm. The material available for this study proved homogeneous in morphology and genotype. The phylogenetic data indicate that this species is very closely related to S. mazi, a fungus occurring on Mazus japonica (Scrophulariaceae), but also to S. aegopodina on Aegopodium sp. (Apiaceae). The conidia of S. mazi morphologically resemble those of S. sii, but they are narrower and the septa normally indistinct [15-42 × 1.5-2(-2.5) μm, Shin & Sameva 2004].
Septoria sisyrinchii Speg., An. Mus. nac. Hist. nat. B. Aires, 6: 324. 1899. Fig. 38.
Fig. 38.
Septoria sisyrinchii, CBS 112096. A-D. Colonies (15 °C, nUV). A. On OA. B. Ibid., reverse. C. On MEA. D. Ibid., detail of colony margin. E-G. Conidia on OA. Scale bars = 10 μm.
Description in planta: Symptoms leaf lesions developing in large areas of the leaf lamina that first turn yellow, indefinite, hologenous, pale to dark brown, appearing black due to numerous conidiomata. Conidiomata pycnidial, amphigenous, numerous, semi-immersed to immersed, subglobose to globose, black, 70-100(-120) μm diam; ostiolum central, circular, 15-35 μm wide, sometimes opening more widely, releasing white to pale yellowish cirrhi of conidial slime, surrounding cells concolourous or somewhat darker; conidiomatal wall 15-20 μm thick, composed of an outer layer of isodiametric cells 5-8 μm diam with brown, slightly thickened cell walls up to 1 μm thick, and an inner layer of globose to isodiametric cells 3-6 μm diam with thin, hyaline walls. Conidiogenous cells hyaline, discrete or integrated in 1-septate conidiophores up 15 μm long, cylindrical, or ampulliform, holoblastic, proliferating sympodially, percurrent proliferations not observed, 5-10 × 2.5-3.5 μm. Conidia cylindrical to cylindrical-filiform, slightly to strongly curved, sometimes flexuous, narrowly rounded to somewhat pointed at the apex, attenuated gradually or more abruptly towards the truncate base, (0-)1-3-septate, not constricted around the septa, hyaline, containing minute oil-droplets and granular material in the rehydrated state, (15.5-)20-30 × 1.5-2(-2.5) μm (rehydrated). Sexual morph unknown.
Description in vitro (18 °C, near UV): Colonies on OA 11-15 mm diam in 2 wk, with an even, buff margin; colonies restricted to spreading, immersed mycelium a mixture of luteous and saffron, the surface provided with a very diffuse, white fluffy to woolly aerial mycelium, which is denser in zones; reverse sienna; numerous conidiomata developing after 5-7 d especially in the centre, releasing milky white rosy-buff conidial slime. Colonies on MEA 10-14 mm diam in 2 wk, with a buff, minutely ruffled margin; colonies restricted, radially striate and somewhat elevated in the centre, the surface dirty greyish brown, soon covered by large masses ochreous to pale brown masses of conidia. Reverse chestnut to blood color, or brown-vinaceous.
Conidiomata and conidiogenous cells as in planta. Conidia as in planta, mostly 18-35× 1.5-2.5 μm.
Hosts: Sisyrinchum spp.
Material examined: New Zealand, Auckland, Manurewa, Auckland Botanical Gardens, on leaf of Sisyrinchum sp., 28 Dec. 2002, C. F. Hill LYN 755, CBS H-21259, living culture CBS 112096.
Notes: The material from Auckland agrees well with the original diagnosis of S. sisyrinchii, which was based on material from Sisyrinchium bonariense in Argentina. Conidia were described as 0-3-septate, 15-24 × 2.5 μm. The multilocus phylogeny indicates that S. anthurii of the genus Anthurium (Araceae) is a closely related species (Fig. 2).
Septoria stachydis Roberge ex Desm., Annls Sci. Nat., sér. 3, Bot. 8: 19. 1847. Fig. 39.
Fig. 39.
Septoria stachydis. A-C. Colonies CBS 102337 (15 °C, nUV). A. On OA. B. On CHA. C. On MEA. D. Conidia in planta (CBS H-21226). E. Conidia in planta (CBS H-21175). F-I. Conidia on OA (CBS 123750). Scale bars = 10 μm.
Description in planta: Symptoms leaf spots angular or irregular, greyish to yellowish brown, with a somewhat darker to black border. Conidiomata pycnidial, epiphyllous, rarely also hypophyllous, mostly 1-5 in each leaf spot, globose to subglobose, dark brown, semi-immersed, 65-100(-125) μm diam; ostiolum central, circular, 12-20 μm wide, later opening more widely up to 50 μm, surrounding cells somewhat darker; conidiomatal wall 12-18 μm thick, composed of angular and irregular cells 2.5-6 μm diam, the outer cells with brown, somewhat thickened walls, the inner cells with hyaline and thinner walls. Conidiogenous cells discrete, sometimes integrated into 1-septate conidiophores, hyaline, broadly ampulliform with a relatively narrow neck, holoblastic, proliferating percurrently with indistinct annellations, rarely also sympodially, 5-8(-10) × 2.5-3.5(-5) μm. Conidia filiform to filiform-cylindrical, curved or irregularly bent, rarely straight or flexuous, with a narrowly rounded or somewhat pointed apex, with a truncate base, (0-)1-3(-5)-septate, not constricted around the septa, hyaline, contents with several minute oil-droplets and granular material in each cell in the living state, with inconspicuous oil-droplets and granular contents in the rehydrated state, (17-)20-42 × 1-2 μm (living; rehydrated, 1-1.5 μm wide). Sexual morph unknown.
Description in vitro: Colonies on OA 13-16 mm diam in 2 wk (V1049: 8-10 mm in 12 d, 16-18 mm in 3 wk; > 50 mm in 7 wk), with an even, glabrous, colourless margin; immersed mycelium mostly homogeneously coral after 2 wk, the centre of the colony already appearing almost black by numerous superficial and immersed pycnidia; olivaceous-black sectors with dark pigmented radiating sterile hyphae also present, later becoming more dominant, or sectors covered by salmon masses of conidia formed directly from mycelial hyphae; aerial mycelium absent; reverse concolorous, but blood colour in the centre, later mainly olivaceous-black or dark slate blue. Surface of the colony smooth. Pycnidia numerous after 2 wk, superficial or immersed, releasing salmon or rosy-buff droplets of conidial slime. Colonies on CMA 8-12 mm diam in 2 wk (11-14 mm in 12 d, 14-24 mm in 3 wk), as on OA, but olivaceous-black sectors more dominant, sometimes colony almost entirely so. Colonies on MEA 8-10 (slow growing sectors) to 12-16 (fast growing sectors) in 2 wk (18-21 mm in 3 wk; 43-58 mm in 7 wk), with an even, glabrous, honey to buff margin; immersed mycelium very dark blood colour; centre of the colony rising high above the agar surface, cerebriform, covered by dirty ochreous conidial slime formed from separate or fused pycnidial conidiomata. Aerial mycelium in slow-growing sectors scanty, scattered minute tufts of white aerial mycelium, in faster growing sectors well-developed, dense, woolly-cottony, first white, later olivaceous-grey to glaucous grey, locally with a reddish discoloration; some colonies with a more homogeneous, olivaceous-black felty surface, sporulating after 3 wk in the centre, with superficial black pycnidial conidiomata releasing milky white masses of conidial slime. Colonies on CHA 12-18 mm in 2 wk (15-18 mm in 3 wk; 34-38 mm in 7 wk), with an even, glabrous, colourless margin; immersed mycelium greenish grey to dark slate blue, the outer zone covered by well-developed, tufty whitish grey aerial mycelium; reverse blood colour, but margin paler; in the central part of the colony numerous pycnidia develop, releasing pale vinaceous to rosy-buff conidial slime; in older colonies the centre becomes cerebriform, much as on MEA.
Conidiomata (OA) immersed in the agar or on the agar surface, black, single, globose, 100-175 μm diam, or irregular, and merged into large complexes 190-350 μm diam, with relatively thick walls; ostiolum as in planta, or absent; Conidiogenous cells as in planta, but more often integrated in 1-3-septate conidiophores. Conidia as in planta, 22-47(-54.5) × 1-2 μm.
Hosts: Stachys spp.
Material examined: Austria, Tirol, Ober Inntal, Lawenwald near Serfaus, on living leaves of Stachys sylvatica, 8 Aug. 2000, G. Verkley 1049, CBS H-21175, living cultures CBS 109126, 109127. Czech Republic, Moravia, Veltice, Forest of Rendez Vous, on living leaves of Stachys sp., 16 Sep. 2008, G. Verkley 6008, CBS H-21253, living cultures CBS 123750, 123879. Netherlands, prov. Utrecht, Baarn, Kasteel Groeneveld, on living leaves of St. sylvatica, 7 July 1968, H.A. van der Aa 685, CBS H-18175, living culture CBS 449.68; prov. Gelderland, Wageningen, Binnenveld, on living leaves of Stachys sp., 23 July 1981, H.A. van der Aa 7952, CBS H-18176; prov. Gelderland, Winssen, Kasteel Doddendael, on living leaves of St. sylvatica, 9 Sep. 1999, G. Verkley 922, CBS H-21204, living cultures CBS 102326, 102337; prov. Limburg, Gulpen, near Stokhem, on living leaves of St. sylvatica, 28 June 2000, G. Verkley 965, CBS H-21226, living cultures CBS 109005, 109006. Romania, distr. Ilfov, pădurea Malu Spart, on living leaves of St. sylvatica, 27 June 1971, G. Negrean & A. Voicu s.n., CBS H-18178, distributed in Herb. Mycol. Romanicum, fasc. 41, no. 2001; distr. Prahova, Sinaia, Valea Peleşului, on living leaves of St. sylvatica, 4 Sep. 1971, G. Negrean s.n., CBS H-18177, distributed in Herb. Mycol. Romanicum, fasc. 41, no. 2002.
Additional material examined - Germany, loc. unknown, isol. Ziekler, living culture CBS 307.31, preserved as S. stachydis, identity uncertain.
Notes: According to Jørstad (1965), the conidia of S. stachydis on Stachys sylvatica are 16-57 × 1-1.5(-2) μm, with a lowest maximum length for any collection of 32 μm. In the collections available for the present study, conidia are up to 42 μm in length in planta, and 54.5 μm long in vitro. The species differs morphologically from S. stachydicola (Bubák. ex Serebrian.) Jacz., which occurs on the same host genus. Shin & Sameva (2004) gave a description of S. stachydicola, based on two collections of Stachys riederi var. japonica from Korea. According to these authors, the conidia of that species are 38-72 × 2-3 μm (3-7-septate), so longer and wider than those of S. stachydis. Also, the pycnidia are smaller in diam (40-80 μm) and ostioli much wider (20-36 μm) than in S. stachydis. CBS 128668 (= KACC 44796) is described by Quaedvlieg et al. (2013) as Septoria cf. stachydicola. This isolate, and also CBS 128662 (=KACC 43871) are both distant from European isolates of S. stachydis.
Septoria stellariae Roberge ex Desm., Annls Sci. Nat., sér. 3, Bot. 8: 22. 1847. Fig. 40.
Fig. 40.
Septoria stellariae. A-D. Colonies CBS 102364. A, B. On OA. C. On CHA. D. On MEA. E. Conidia and conidiogenous cells on OA (CBS 102364). Scale bars = 10 μm.
-
? = Sphaeria isariphora Desm., Annls Sci. Nat., sér. 2, Bot. 19: 358. 1843.
≡ Mycosphaerella isariphora (Desm.) Johanson, Öfvers. K. Svensk. Vetensk.-Akad. Förhandl. 41 (no. 9): 165. 1884.
Description in planta: Symptoms indefinite white or pale yellow to pale brown leaf lesions on lower leaves of plants, often starting at the leaf margin, extending rapidly over the lamina and leading to complete withering of leaves and their petioles. Conidiomata pycnidial, brown, in dense groups on withering petioles and leaves, where mostly epiphyllous, only partly immersed in the host tissue, globose or lenticular, (85-)120-160(-210) μm diam; ostiolum circular, central, initially 20-35 μm wide, later opening to 80 μm diam, without distinctly differentiated cells; conidiomatal wall composed of textura angularis without distinctly differentiated layers, mostly 15-25 μm thick, the outer cells with brown, somewhat thickened walls and 4.5-8 μm diam, the inner cells hyaline and thin-walled and 3.5-6.5 μm diam; conidiogenous cells lining the whole inner surface of the pycnidium. Conidiogenous cells hyaline, discrete or integrated in short simple, 1-2-septate conidiophores, cylindrical, or ampuliform to elongated ampulliform with a relatively short neck, hyaline, holoblastic, proliferating sympodially, 5-12(-15) × 2.5-4 μm. Conidia cylindrical to filiform, weakly curved or abruptly bent in the lower cell, sometimes flexuous, gradually attenuated to the rounded apex, gradually or more abruptly attenuated into a broadly truncate base, (0-)1-3(-5)-septate, not or indistinctly constricted around the septa, hyaline, contents with several small guttulae and numerous granules in each cell in the living state, oil-droplets rarely merged into larger guttules in the rehydrated state, (21-)30-64 (-70) × 1.5-2.5(-3) μm (living; rehydrated, 1-2 μm wide).
Description in vitro: Colonies on OA 3-5 mm diam in 2 wk, with an even, glabrous, colourless margin; a yellow pigment diffusing into the agar beyond the margin; immersed mycelium mostly colourless to buff or saffron with scanty, whitish aerial mycelium, the centre of the colony darkened by numerous superficial and immersed, separate or confluent pycnidial conidiomata, releasing rosy-buff to salmon conidial slime; reverse pale luteous to saffron, but olivaceous-black in areas with numerous conidiomata. Colonies on CMA 3-6 mm diam in 2 wk, as on OA. Colonies on MEA 2-5 mm diam in 2 wk, with an even, glabrous, colourless margin, locally with rapidly outgrowing hyphae forming superficial pycnidial conidiomata; colonies pustulate to hemispherical, the surface greenish grey to olivaceous-black covered by fairly dense greyish to saffron, woolly aerial mycelium; some superficial or immersed pycnidial conidiomata formed; reverse dark umber to blood colour. Colonies on CHA 4-8 mm diam in 2 wk, remaining almost plane, with an irregular margin; immersed mycelium greenish grey to dark slate-blue in the centre, buff near the margin; aerial mycelium well-developed, greyish to white, with a distinct flesh discoloration especially at the margin; reverse blood colour; abundant immersed and superficial pycnidial conidiomata formed, releasing a buff to saffron conidial slime.
Conidiomata (OA) pycnidial and similar as in planta, single, 100-250 μm diam, but more often merged into larger complexes, brown to olivaceous brown, and up to 350 μm diam; ostiolum as in planta, or absent. Conidiogenous cells hyaline, as in planta but predominantly cylindrical, holoblastic, proliferating sympodially, rarely percurrently with indistinct annellations, 5-15(-22) × 2.5-4.5 μm. Conidia similar as in planta, (0-)3-5-septate, not or indistinctly constricted around the septa, hyaline, contents with several small guttules and numerous granules in each cell, (20-)30-75(-84) × 2-2.5(-3.0) μm.
Hosts: Stellaria spp. and Myosoton spp.
Material examined: Germany, Eifel, Gunderath, near Heilbachsee, on living leaves of Stellaria media, 22 June 1992, H.A. van der Aa 11341, CBS H-5333. Netherlands, Prov. Utrecht, Baarn, on leaves of S. media, 18 May 1985, H.A. van der Aa 9492, CBS H-18179; Prov. Noord-Holland, Laren, on leaves of S. media, 18 Feb. 1967, H.A. van der Aa s.n., CBS H-18180; prov. Noord-Brabant, Valkenswaard, on withering leaves and stems of St. media, 1 May 1967, H.A. van der Aa s.n., CBS H-18179; Ameland, Nes, on leaves of St. media, 27 May 1967, H.A. van der Aa s.n., CBS H-18182; Prov. Gelderland, Landgoed Staverden, on withering leaves and petioles of St. media, 1 Aug. 1999, G. Verkley 901, CBS H-21156, living cultures CBS 102364, 102410; Prov. Limburg, Mook en Middelaar, St. Jansberg, near Plasmolen, on withering leaves and petioles of St. media, 9 Sept 1999, G. Verkley 933, CBS H-21157, living culture CBS 102378; Prov. Flevoland, Erkemeder strand, on withering leaves and petioles of St. media, 8 Sept 1999, G. Verkley 929, CBS H-21217, living culture CBS 102376; Prov. Flevoland, Ketelmeer, IJsseloog, on withering leaves and petioles of St. media, 22 May 2002, G. Verkley 1141, CBS H-21260. Romania, distr. Vîlcea, Muntele Cozia, Stîna Foarfeca, on living leaves of S. media, 14 Oct. 1976, G. Negrean s.n., CBS H-18183, distributed in Herb. Mycol. Romanicum, fasc. 60, no. 2990.
Notes: This fungus is a weak pathogen of Stellaria media in the Netherlands, on which it is only seen under very humid conditions. Especially the lower parts of plants that are sheltered by the surrounding vegetation are affected. Jørstad (1965) observed conidia up to 82 μm in length on Stellaria crassifolia, and up to 96 μm long on Stellaria media, the type host. It has also been reported from other Stellaria spp., and Myosoton (Radulescu et al. 1973, Vanev et al. 1997, Markevičius & Treigienė 2003). Septoria stellariae var. macrospora was originally described from the same host as S. stellariae, Stellaria media. According to Teterevnikova-Babayan (1987), conidia of this variety measure 50-120 × 2.5-4 μm. On fresh plant material studied here conidia longer than 70 μm were not observed, but the isolates obtained thereof did produce conidia up to 84 μm long. Sequence analyses of CBS 102376, 102378, and 102410 originating from three different localities showed no significant polymorphisms in the seven loci, indicating that material belongs to a single taxon. Whether the variety macrospora is tenable, is unclear at this point. We agree with Jørstad (1965), that the connection with the sexual morph Mycosphaerella isariphora suggested in the literature, requires confirmation. It is therefore listed as a tentative synonym of S. stellariae.
Septoria urticae Roberge ex Desm., Annls Sci. Nat., sér. 3, Bot. 8: 24. 1847. Fig. 41.
Fig. 41.
Septoria urticae, epitype. A. Conidia and conidiogenous cells in planta (CBS H-21221). B. Ibid., on OA (CBS 102371). Scale bars = 10 μm.
Description in planta: Symptoms leaf spots small, angular, often merging to irregular patterns, initially pale yellowish brown, partly becoming dark greyish brown later, with a dark border. Conidiomata pycnidial, epiphyllous, several in each leaf spot, subglobose to lenticular, pale brown, usually fully immersed, 70-120 μm diam; ostiolum central, initially circular and 30-45 μm wide, later becoming more irregular and up to 80 μm wide, surrounding cells concolorous to pale brown; conidiomatal wall about 10-17 μm thick, composed of a homogenous tissue of hyaline, angular cells 2.5-6.5 μm diam, the outermost cells pale yellowish brown with somewhat thickened walls, the inner cells thin-walled. Conidiogenous cells hyaline, mostly discrete, narrowly or broadly ampulliform with a relatively narrow neck, holoblastic, often first proliferating sympodially, and later also percurrently 1-several times with distinct annellations, 6-12(-16) × 4-5.5(-7) μm. Conidia cylindrical, straight or slightly curved, flexuous, or irregularly bent, with a narrowly rounded apex, attenuated towards the narrowly truncate base, (0-)1-5(-7)-septate, not constricted around the septa, hyaline, contents with several oil-droplets and granular material in each cell in the living state, with inconspicuous oil-droplets and granular contents in the rehydrated state, (18-) 30-57(-75) × 2-3 μm (living; rehydrated, 2-2.5 μm wide). Sexual morph unknown.
Description in vitro: Colonies on OA 6-7 mm diam in 2 wk (19-22 mm in 6 wk), with an even, glabrous, red to coral margin, the pigment also clearly diffusing beyond the margin; colonies almost plane, immersed mycelium near the margin red, in the centre very dark, blood colour to black, also due to mostly superficial pycnidial conidiomata releasing pale flesh droplets of conidial slime; white, felty aerial mycelium scanty, mostly only just behind the margin; reverse concolorous. Colonies on CMA 4-6 mm diam in 2 wk (16-17 mm in 6 wk), as on OA. Colonies on MEA 6-7(-9) mm diam in 2 wk [20-22(-28) mm in 6 wk], with an even, buff to very pale flesh plane marginal zone; the pigment diffusing into the medium; colony often hemispherical with an irregularly pustulate-worty surface, immersed mycelium very dark chestnut to black, aerial mycelium absent, except in faster growing sectors, which are entirely covered by a dense, felty mat of reddish aerial mycelium; superficial pycnidial conidiomata releasing dirty white to flesh droplets of conidial slime. Colonies on CHA 4-6 mm diam in 2 wk (17-22 mm in 6 wk), as on MEA, but with an initially ruffled (later more even), rather dark margin and more numerous conidiomata producing flesh droplets of conidial slime.
Conidiomata (OA) pycnidial, pale brown to dark brown, glabrous, 100-230 μm diam, with a single ostiolum as in planta, or ostioli barely differentiated; conidiogenous cells as in planta, but more often integrated in 1-2-septate conidiophores, often only proliferating percurrently with distinct annellations on an elongated neck, 6-14 × 3-7.5 μm; conidia cylindrical, straight or slightly curved, tapering to a rounded apex, lower part attenuated into a broad truncate base, 1-7(-9)-septate, not constricted around the septa, hyaline, with several minute oil-droplets and numerous granulae in each cell, (34-)40-70(-90) ×2.5-3(-3.5) μm.
Hosts: Urtica spp. and Glechoma hederacea.
Material examined: Netherlands, Prov. Utrecht, Soest, Overhees, on living leaves of Glechoma hederacea, in leaf spots associated with Puccinia glechomatis, 8 Aug. 1999, G. Verkley 904, CBS H-21197, living culture CBS 102316; Prov. Utrecht, ‘s Graveland, Kortenhoefse plassen, “Oppad”, on living leaves of Urtica dioica, 14 Oct. 1999, H.A. van der Aa & G. Verkley 947, epitype designated here CBS H-21221 “MBT175359”, living cultures ex-epitype CBS 102371, 102375.
Notes: Muthumary (1999) provided a description and illustration of type material of S. urticae (PC 1309). Because there are only insignificant differences between his observations of the type and those observed here in the Dutch collection on the same host, Urtica dioica, the latter is selected as epitype. Muthumary reported ostioli 20-40 μm wide, while in the Dutch material the ostioli eventually open up further to about 80 μm wide. Muthumary observed conidia 35-50 × 2-2.5 μm with 3-4 septa, but other authors have found that conidia in planta can be much longer and have more septa. Jørstad (1965) found that conidia in Norwegean material on U. dioica were 22-81 × 1-1.5 μm, with up to 6 septa. Priest (2006), who studied material on U. insidia and U. urens in Australia reported conidia (26-)35-50(-70) × 1.5-2 μm, 3-5-septate. The present study shows that in vitro conidia can even be up to 90 μm long in this species. The material from Glechoma hederaceae sporulating in association with the rust Puccinia glechomatis, proved morphologically in good agreement with that on Urtica dioica, and since it is also genetically similar to the material from that host, it is regarded conspecific. Other Septoria species have also occasionally been found in association with rust sori, viz., S. lagenophorae, which is regarded to be a hyperparasite of rusts, and occasionally also other leaf-spotting fungi (Priest 2006).
According to Muthumary, the conidiogenous cells of S. urticae each produce a solitary terminal conidium and often also proliferate sympodially. It is established here that S. urticae is also capable of proliferating percurrently, and that this mode of proliferation is more frequent in pure culture. In contrast, Priest (2006) observed conidiogenous cells that first produced a conidium holoblastically, and subsequent conidia enteroblastically at the same level from a narrow conidiogenous locus, viz. like in phialidic conidiogenesis. It is unclear whether this is truly phialidic conidiogenesis, or just cryptic percurrent proliferation as observed in S. chrysanthemella, where the scars of the subsequent secessions are indistinguishable due to the limitations in the resolution of the light microscope (Verkley 1998a).
Septoria verbenae Roberge ex Desm., Annls Sci. Nat., sér. 3, Bot., 8: 19. 1847. Fig. 42.
Fig. 42.
Septoria verbenae. A. Conidia and conidiogenous cells in planta (CBS H-21241). B. Conidia on OA (CBS 113438). Scale bars = 10 μm.
Description in planta: Symptoms stem lesions and leaf spots small, angular to irregular, and merging to elongated areas, initially red to purplish red, then becoming pale in the centre with a darker border. Conidiomata pycnidial, epiphyllous, one to a few in each lesion, globose, dark brown, immersed, 70-140 μm diam; ostiolum central, circular, 25-40 μm wide, surrounding cells dark; conidiomatal wall about 12.5-20 μm thick, composed of a homogenous tissue of textura angularis with hyaline cells 2.5-7.5 μm diam, the outermost cells mid brown with somewhat thickened walls, the inner cells thin-walled and pale yellowish brown. Conidiogenous cells hyaline, discrete, or integrated in 1-2-septate conidiophores, narrowly ampulliform to almost cylincrical, often with a relatively narrow neck, holoblastic, first proliferating sympodially, and in some cells later also percurrently 1-several times with indistinct annellations, 12-18(-20) × 2.5-6 μm. Conidia cylindrical, straight or slightly curved, flexuous, with a narrowly rounded to somewhat pointed apex, attenuated towards the narrowly truncate base, (1-)3(-5)-septate, not constricted around the septa, hyaline, contents with several oil-droplets and granular material in each cell in the living state, with inconspicuous oil-droplets and granular contents in the rehydrated state, (22-)16-48 × (1-)1.5-2 μm (rehydrated). Sexual morph unknown.
Description in vitro: Colonies on OA 10-13 mm diam in 2 wk, with an even, colourless margin; colonies restricted to spreading, immersed mycelium citrine to grey-olivaceous, locally soon darker radiating strands occur, glabrous but in the centre of colonies, where irregular elevations are formed, covered by well-developed, grey to white finely felted aerial mycelium; reverse greenish grey to olivaceous-black. Conidiomata developing immersed or on the agar surface after 10-2 wk. Colonies on MEA 10-13 mm diam in 2 wk, with a slighlty ruffled, buff to amber margin; colonies restricted, irregularly pustulate, the surface entirely covered by a low, dense mat of whitish to grey finely felted aerial mycelium; reverse dark brown to almost black, locally fulvous to sienna. No sporulation observed.
Conidia (OA) filiform to cylindrical, typically weakly to strongly curved, sometimes straight or flexuous, attenuated gradually to a somewhat pointed apex, attenuated gradually or more abruptly to the narrowly truncate to almost rounded base, hyaline, with granular contents and minute oil droplets, (1-)3-5(-7)-septate, (22-)28-46(-54) × 1.5-2(-2.5) μm.
Host: Verbena officinalis.
Material examined: New Zealand, North Isl., Northland, Bay of Islands area, Manawaora along roadside, on living leaves of Verbena officinalis, 30 Jan. 2003, G. Verkley 2017, CBS H-21240; same loc., date, on stems of V. officinalis, G. Verkley 2023, CBS H-21241, living culture CBS 113438, 113481.
Notes: Priest (2006) gave a detailed description based on a collection from New South Wales, Australia [conidia (1-)3-septate, 26-48 × 1.5(-2) μm]. The two strains available proved morphologically similar. These New Zealand strains proved to have identical Act, Btub, Cal, EF, and RPB2 sequences, distinct from other Septoria.
Sphaerulina
Type species: Sphaerulina myriadea (DC.) Sacc., Michelia 1: 399. 1878.
Quaedvlieg et al. (2013, this volume) provide a description based on the sexual morph and treat several additional species with septoria-like asexual morphs.
Sphaerulina aceris (Lib.) Verkley, Quaedvlieg & Crous, comb. nov. MycoBank MB804473. Fig. 43.
Fig. 43.
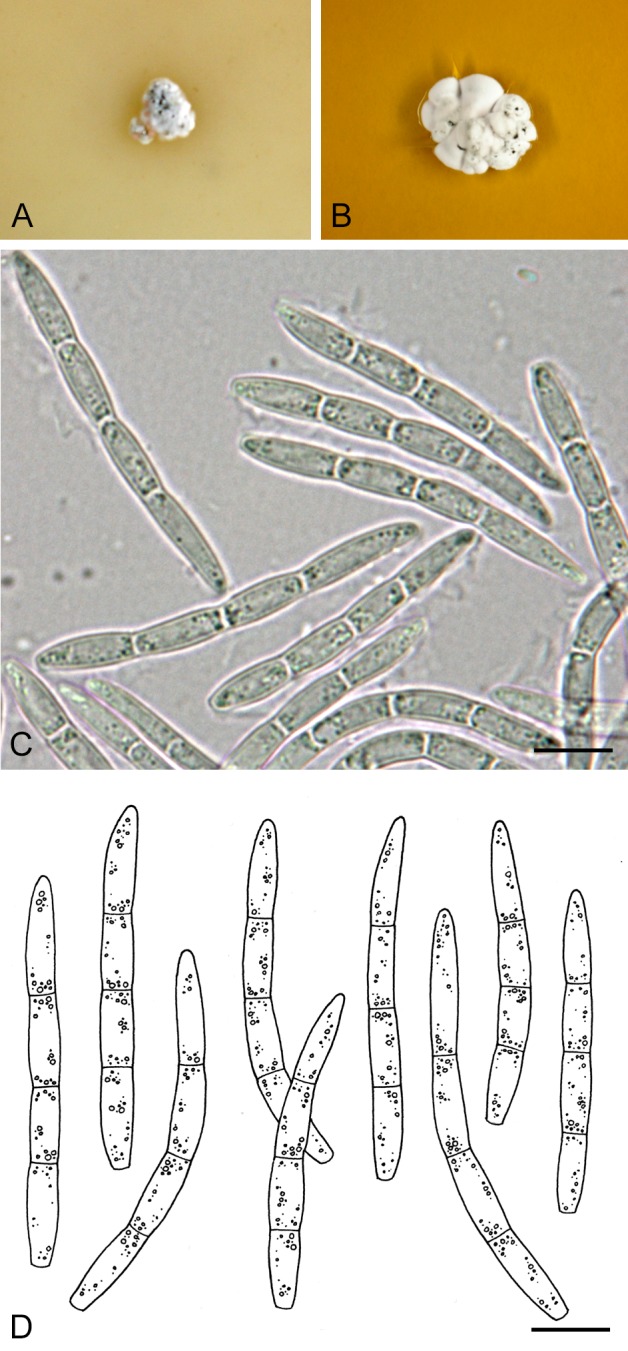
Sphaerulina aceris. A, B. Colonies CBS 183.97. A. On OA. B. On MEA. C, D. Conidia in planta (CBS H-21239). Scale bars = 10 μm.
Basionym: Ascochyta aceris Lib., Pl. crypt. Ard., Cent. 1: no. 54. 1830.
≡ Septoria aceris (Lib.) Berk. & Broome, Ann. Mag. Nat. Hist. Ser. 2, 5: 379. 1850.
≡ Phloeospora aceris (Lib.) Sacc., Syll. Fung. 3: 577. 1884.
-
= Septoria pseudoplatani Roberge ex Desm., Annls Sci. Nat., sér. 3, Bot. 8: 21. 1847.
≡ Cylindrosporium pseudoplatani (Roberge ex Desm.) Died., Annls mycol. 10: 486. 1912.
-
= Sphaerella latebrosa Cooke, Handb. Brit. Fungi 2: no. 2754. 1871.
≡ Mycosphaerella latebrosa (Cooke) J. Schröt., in Cohn, Krypt.-Fl. Schlesien (Breslau) 3.2(3): 334. 1894 [1908].
≡ Carlia latebrosa (Cooke) Höhn., Hedwigia 62: 73. 1920.
-
= Septoria seminalis var. platanoidis Allesch., Hedwigia 35: 34. 1896.
≡ Cylindrosporium platanoidis (Allesch.) Died., Annls mycol. 10(5): 486. 1912.
= Septoria epicotylea Sacc., Malpighia 11: 314. 1897.
= Phloeospora pseudoplatani Bubák & Kabát in Bubák, Sber. K. böhm. Ges. Wiss., Math.-naturw, Kl., 7: 16. 1903.
Description in planta: Symptoms small (0.2-0.5 mm diam), circular to angular, hologenous reddish brown leaf spots. Conidiomata acervular, epi- or hypophyllous, one to a few in each leaf spot, pale brown (drying dark brown), 105-180(-220) μm diam, releasing conidia in white columnar masses; conidiomatal wall mainly consisting of a basal 15-25(-35) μm thick layer of angular to subglobose, subhyaline to pale brown cells 5-10 μm diam, lateral wall absent or very poorly developed, composed of similar, somewhat darker cells. Conidiogenous cells hyaline, discrete or integrated in 1(-2)-septate conidiophores, subglobose, doliiform or ampulliform, holoblastic, proliferating percurrently with one to several disctinct annellations, or sympodially, sometimes both types of proliferation occur in a single conidiogenous cell, 8-15 (-20) × 2.5-4 μm. Conidia cylindrical, straight or more or less curved, attenuated gradually to a broadly rounded apex, attenuated more or less abruptly to a truncate base, (1-)3-septate, conspicuously constricted around the septa in fresh and rehydrated state, hyaline, contents with numerous minute oil-droplets and granular material in each cell in the living state, with minute oil-droplets and granular contents in the rehydrated state, (32-)37-47(-50) × 3-4 μm (living; rehydrated, 2-3 μm wide).
Description in vitro. Colonies on OA 3-4 mm diam in 2 wk, with a undulating even margin; colonies restricted, irregularly pustulate, the surface buff or much darker grey to brown, locally glabrous but mostly covered by a dense mat of finely felted white aerial mycelium, conidiomata developing on the surface releasing conidia in clear droplets, or in milky white to rosy-buff masses; reverse dark greyish or brown-vinaceous. Colonies on MEA 3-4(-8) mm diam in 2 wk, with a undulating even margin; colonies restricted, irregularly pustulate, the surface almost black provided with low and finely felted, diffuse, grey to white aerial mycelium, conidiomata developing just beneath the colony surface, releasing white cirrhi of conidia; reverse a palet of brown-vinaceous, cinnamon and olivaceous-grey.
Conidia (OA) as in planta, (31-)34-50(-58) × 3.5-5 μm. Microconidia (spermatia of the Asteromella state) ellipsoid, hyaline, 0-septate, 3-4 × 1.5 μm.
Hosts: Acer campestre, A. circinatum, A. hyrcanum (Vanev et al. 1997) and A. pseudoplatanus.
Material examined: France, locality unknown, on leaves of Acer campestre, distributed in Libert, Pl. Cryptog. Ard. Fasc. 1 (1830): no. 54, isotype BR-MYCO 153858-16, type of Ascochyta aceris Lib. Netherlands, prov. Utrecht, Baarn, on Acer pseudoplatanus, July 1969, I. Blok, living culture CBS 514.69; Baarn, garden WCS, on living leaves of Acer pseudoplatanus, 23 July 1985, H.A. van der Aa 9537, CBS H-14666, living culture CBS 652.85; same substr., prov. Zuid-Holland, Wassenaar, Hollandsch Duin, 14 Aug. 1994, G. Verkley 227, CBS H-18040, living culture CBS 687.94; same substr., prov. Zuid-Holland, Wassenaar, Ganzenhoek, 8 Aug. 1995, G. Verkley 307, CBS H-21239, living culture CBS 187.96; same substr., prov. Utrecht, Baarn, Eemnesserweg, 7 May 1996, H.A. van der Aa 12120, CBS H-14665, living culture CBS 183.97; USA, Oregon, Lane Co., Proxy Falls Trail, on living leaves of Acer circinatum, 11 Oct. 1996, J. K. Stone & G. Verkley 480, CBS H-21236, living culture CBS 655.97.
Notes: This is the oldest septoria-like species described from members of the family Aceraceae. It occurs on several species of the genus Acer. In the original diagnosis of Libert, three host species were mentioned, viz., A. campestre, A. pseudoplatanus and A. platanoides. Jørstad (1965) treated forms on A. platanoides with conidia 26-60 × 2-2.5 μm as S. apatela All. (synonyms S. seminalis var. platanoidis All., Phleospora platanoidis Kabát & Bubák, Phloeospora samarigena Bubák & Krieg.), while those on A. campestre remained unsettled. According to Jørstad (1965) conidia of S. aceris are 24-43 × 2-3 μm, with 3 septa, which agrees well with the sizes observed in the type specimen available in the present study. This material also showed a small proportion of 4-septate conidia in one of the fruitbodies. More species with conidia longer than 60 μm have been described from A. platanoides, and these need to be critically assessed in a comprehensive study including isolates of all Septoria occurring on the genus Acer. No isolates from the the type host A. campestre that would be most suitable as epitype, were available, hence no epitypification is proposed here. The ultrastructure of conidiogenesis and conidia of S. aceris was studied by Verkley (1998b), who showed that in a single cell percurrent as well as sympodial proliferation can occur.
A description of the sexual morph known as Mycosphaerella latebrosa was provided by Kuijpers & Aptroot (2002), but their species concept included several discrete entities that are distinguishable by their conidial states and occur on distantly related host plants. It is unlikely that these entities can be distinguished at all by the morphology of the sexual state (Verkley & Starink-Willemse 2004).
Sphaerulina cornicola (DC.: Fr.) Verkley, Quaedvlieg & Crous, comb. nov. MycoBank MB804474. Fig. 44.
Fig. 44.

Sphaerulina cornicola. A-C. Colonies CBS 102324. A. On OA. B. On CHA. C. On MEA.
Basionym: Depazea cornicola DC.: Fr., in De Candolle & Lamarck, Flore Française VI: 146. 1815.
≡ Septoria cornicola (DC.: Fr.) Desm., Pl. crypt. Fr., Fasc. 7, no 342. 1828; Index Pl. crypt. Fr.: 24. 1851.
= S. cornicola var. ampla H. C. Greene, Amer. Midl. Nat. 41: 755. 1949 (fide Farr 1991).
For extended synonymy see Farr (1991). Neotype on Cornus sanguinea, France (BPI, designated by Farr 1991), not seen.
Description in planta: Symptoms starting as red discolorations of the leaf lamina and margin, which develop to scattered, circular to irregular, hologenous leaf spots, that later become pale brown, and surrounded by a dark brown to black bordering zone and a distinct red or purple periphery. Conidiomata pycnidial, epiphyllous, numerous scattered in each leaf spot, subglobose to globose, brown to black, immersed or semi-immersed, 55-100(-120) μm diam; ostiolum central, initially circular and 25-40 μm wide, later becoming more irregular and up to 60 μm wide, surrounding cells concolorous to pale brown. Conidiomatal wall about 10-15 μm thick, composed of a outer layer of hyphal to irregular cells 3.0-8 μm diam with brown walls, and an inner layer of hyaline cells 3-5 μm diam; Conidiogenous cells hyaline, discrete, doliiform, or narrowly to broadly ampulliform, holoblastic, proliferating sympodially, sometimes also percurrently with indistinct annellations, 5-12.5(-15) × 3-4(-8) μm. Conidia cylindrical, regularly curved, attenuated gradually to a rounded or somewhat pointed apex and a narrowly truncate base, (0-)1-3(-5)-septate, distinctly constricted around the septa only in the fresh state, hyaline, contents with several minute oil-droplets and granular material in each cell in the living state, with amorphous material and granular contents in the rehydrated state, (20-)24-40 × 3-4 μm (living; rehydrated, 2-3 μm wide). Sexual morph unknown.
Description in vitro: Colonies on OA 4-7mm diam in 2 wk (12-16 mm in 6 wk), with an even, glabrous, buff margin; colonies spreading, the surface first plane, then somewhat pustulate, immersed mycelium a mixture of fawn and rosy-buff tinges, locally darker olivaceous, the surface largely covered by a rosy-buff to vinaceous buff masses or a film of conidial slime produced directly by the mycelium; reverse rosy-buff with isabelline to hazel areas, later darker in the centre. Colonies on CMA 3-4 mm diam in 2 wk (8-12 mm in 6 wk), as on OA. Colonies on MEA 4.5-7 mm diam in 2 wk (9-14(-16) mm in 6 wk), restricted, the entire surface of the colony regularly cerebriform with large masses of conidial slime (also covering the margin), first salmon, later darkening to ochreous or umber, eventually even chestnut; reverse sienna to bay. Colonies on CHA 4-6 mm diam in 2 wk (11-14 mm in 6 wk), as on MEA.
Conidia (OA) as in planta, but showing secondary conidiation, 1-8(-16)-septate, conidia germinating from intermediate cells (laterally) or the basal cells (axially) to form new conidial fragments of variable length, or branched complexes, rendering a heterogeneous mixture.
Host: Cornus sanguinea.
Material examined: Germany, Baden-Württemberg, Kussa-Rheinheim, 3 Sep. 1999, A. Aptroot 46371, CBS H-21191. Netherlands, Prov. Noord Brabant, Eindhoven, Milieu- & Educatiecentrum Eindhoven, on living leaves of Cornus sanguinea, 4 Sep. 1999, A. van Iperen (G. Verkley 918), CBS H-21237, living cultures CBS 102324, 102332; same substr., prov. Limburg, Gulpen, near Stokhem, 28 June 2000, G. Verkley 963, CBS H-21238. USA, Maryland, Prince Georges Co., on C. sanguinea, 14 Sep. 2004, A. Y. Rossman 4089 (BPI), living culture CBS 116778.
Notes: The material examined has the typical conidia of Sphaerulina cornicola, agreeing with those described by Farr (1991). Septoria cornina can be distinguished from Sphaer. cornicola by more variously curved, most commonly hooked, falcate or lunate conidia (23-)32-90(-110) × 2-4(-5) μm with rounded apex (Farr 1991, Shin & Sameva 2004). The phylogenetic relationship with S. cornina remains to be clarified.
Sphaerulina frondicola (Fr.) Verkley, Quaedvlieg & Crous, comb. nov. MycoBank MB804477.
Basionym: Septoria populi Desm, Annls Sci. Nat., sér 2, Bot., 19: 345. 1843. nom. nov. pro Depazea frondicola Fr., Observationes mycologicae, 2: 365, t. 5: 6-7. 1818.
≡ Sphaeria frondicola (Fr.) Fr., Syst. Mycol. 2: 529. 1822.
-
= Sphaerella populi Auersw., in Gonnermann & Rabenhorst, Mycol. eur. Abbild. Sämmtl. Pilze Eur. 5-6: 11 .1869.
≡ Mycosphaerella populi (Auerw.) J. Schroet., in Cohn, Krypt.-Fl. Schlesien (Breslau) 3.2 (3): 336. 1894.
Description in vitro (CBS 391.59): Colonies on OA 3-5 mm diam in 2 wk, with an even or slightly ruffled, colourless, glabrous margin; colonies restricted and up to 2 mm high after 2 wk, immersed mycelium mostly olivaceous to dark herbage green, with moderately developed, greyish white, woolly-floccose aerial mycelium; numerous large, simple or complex, olivaceous to reddish brown stromatic conidiomata formed that open widely to release masses of rosy-buff conidial slime; reverse mostly olivaceous-black. Colonies on MEA 2-3(-4) mm diam in 2 wk, with a ruffled, buff, glabrous margin; colonies restricted, up to 2 mm high, irregularly pustulate, the surface appearing dark brown to black, but with numerous hemispherical stromata at the surface which are fawn to vinaceous brown, some of which start sporulating directly from the surface forming masses of rosy-buff conidial slime after 2 wk; aerial mycelium scarce, locally denser, white; reverse almost black. Colonies on CHA 4-6 mm diam in 2 wk, with an even, rosy-buff margin covered by pure white, woolly aerial mycelium; colonies restricted, up to 2 mm high, immersed mycelium entirely hidden under a dense mat of pure white, high, woolly aerial mycelium; reverse brown-vinaceous in the centre, surrounded by a rosy-buff to buff marginal zone.Conidiomata not well-developed. Conidiogenous cells observed holoblastic, some cells with a single percurrent proliferation. Conidia showing signs of degeneration. In addition, cylindrical to dumpbell-shaped spermatia or microconidia, (5.5-)7.5-13.5(-14.5) × 1.2-1.7 mm, are formed from phialides in the same fruitbodies.
Host: Populus pyramidalis.
Material examined: Germany, Berlin-Kladow, on living leaves of Populus pyramidalis, Dec. 1959, R. Schneider s.n., BBA 8987, CBS H-18150, living culture CBS 391.59
Notes: CBS 391.59 groups in a subclade of the Sphaerulina-clade (Fig. 2), that was named after the type species Sphaerulina myriadea that resides in it (Quaedvlieg et al. 2013). Closest relatives are the other poplar pathogens Sphaer. populicola (syns Septoria populicola Peck, Mycosphaerella populicola, CBS 100042) and several isolates of Sphaer. musiva (synonyms Septoria musiva, Mycosphaerella populorum). CBS 391.59 now only develops atypical sporulating structures not described in detail here.
Sphaerulina gei (Roberge ex Desm.) Verkley, Quaedvlieg & Crous, comb. nov. MycoBank MB804475. Fig. 45E-G.
Fig. 45.
A-D. Sphaerulina hyperici. A-C. Colonies CBS 102313. A. On OA. B. On CHA. C. On MEA. D. Conidia and conidiogenous cells in planta (CBS H-21194, epitype). E-G. Sphaerulina gei. E. Colony on OA (KACC 44051 = CBS 128632). F. Conidia and conidiogenous cells in planta (CBS H-21194, epitype). G. Ibid., on OA (CBS 102318). Scale bars = 10 μm.
Basionym: Septoria gei Roberge ex Desm., Annls Sci. Nat., sér. 2, Bot. 19: 343. 1843.
Description in planta: Symptoms leaf lesions irregular, greyish brown, well-delimited by a dark brown line, surrounding leaf tissue often yellowish; Conidiomata pycnidial, amphigenous though predominantly epiphyllous, numerous in each lesion, subglobose to cupulate, brown to black, 35-80 μm diam; ostiolum central, circular, initially 35-60 μm wide, later becoming more irregular and up to 80 μm wide, surrounding cells dark brown; conidiomatal wall 10-15 μm thick, composed of a homogenous tissue of hyaline, angular cells 2.5-6.5 μm diam, the outermost cells pale brown with slightly thickened walls, the inner cells thin-walled. Conidiogenous cells hyaline, discrete, rarely also integrated in 1-2-septate conidiophores, cylindrical or narrowly to broadly ampulliform, holoblastic, often with a relatively narrow and elongated neck, proliferating percurrently several times with distinct annellations, rarely also sympodially, 6-10(-15) × 3.5-5(-6) μm. Conidia filiform, slightly curved to flexuous, rarely straight, narrowly rounded at the apex, narrowly truncate at the base, (0-)2-5(-8)-septate (septa very thin and easily overlooked), not constricted around the septa, hyaline, contents with several minute oil-droplets and granular material in each cell in the living state, with minute oil-droplets and granular contents in the rehydrated state, 33-65(-75) × 2-2.8(-3) μm (living; rehydrated, 1.8-2.5 μm wide). Sexual morph unknown.
Description in vitro: Colonies on OA 6-8(-15) mm diam in 3 wk, with an even, glabrous, colourless to buff margin; colonies spreading, immersed mycelium at first buff to rosy-buff, tardily becoming olivaceous to olivaceous-black, occassionally some sectors remaining buff; aerial mycelium mostly wanting, but sometimes with a few greyish tufts, the surface of the colony centre soon covered by rosy-buff masses of conidial slime, produced from conidiogenous cells directly on the mycelium or in pycnidial conidiomata; reverse olivaceous-black, margin buff. Colonies on CMA 7-9 mm diam in 3 wk, as on OA, but green pigmentation developing more rapidly. Colonies on MEA 7-9(-11) mm diam in 3 wk, with an irregular, glabrous, rosy-buff margin; a reddish pigment diffusing into the agar; colony spreading to restricted, the surface cerebriform to irregularly lobed, up to 2 mm high, very dark, but locally covered either by grey, felted aerial mycelium or masses of salmon conidial slime, produced directly from hyphae or in superficial stromatal conidiomata; reverse rust to chestnut. Colonies on CHA 6-7(-10) mm diam in 3 wk, colony features and sporulation as on MEA, but the margin covered by whitish aerial mycelium; diffusing pigment also present. Sporulating structures on OA very similar to those in planta, but conidia up to 85 μm long.
Hosts: Geum spp.
Material examined: Czech Republic, Bohemia, near Tábor, on living leaves of Geum urbanum, 20 July 1903, F. Bubák, distributed in Kabát & Bubák, Fungi imperfecti exsicc. 114, PC 0084558. France, Caen, on living leaves of G. urbanum, “Col. Desmazieres 1863, no. 8, 58”, “Jun-Sep. 1842”, isotype PC 0084556; forest near Caen, on living leaves of G. urbanum, 1841, Roberge, PC 0084555. Germany, Brandenburg, Buchmühle near Lagow, on living leaves of G. urbanum, 10 Sep. 1909, P. Sydow, PC 0084559. Korea, Hoengseong, on living leaves of G. japonicum, H.D. Shin, living culture CBS 128616 =KACC 43029 = SMKC 22748; same substr., Pyeongchang, H.D. Shin, living culture CBS 128632 =KACC 44051 = SMKC 23686. Latvia, prov. Vidzeme, Kr. Riga, Ogre, on living leaves of G. urbanum, 19 July 1936, J. Smarods, PC 0084557. Netherlands, Prov. Limburg, Schimperbosch, SW of Vaals, on the same substr., 29 Aug. 1999. H.A. van der Aa s.n., CBS H-21168; Prov. Noord Holland, Amsterdamse Waterleidingduinen, Panneland, on living leaves of G. urbanum, 31 Aug. 1999, G. Verkley & A. van Iperen 914, epitype designated here CBS H-21167 “MBT175360”, living culture ex-epitype CBS 102318. Romania, distr. Prahova, Muntenia, Cheia, on living leaves of G. rivale, T. Săvulescu & C. Sandhu, distributed in Săvulescu, Herb. Mycol. Romanicum 8, 377, PC 0084560. Sweden, Gotland, Endre parish, Hulte, on living leaves of G. urbanum, 16 July 1898, T. Vestergren, PC 0084561.
Notes: The type material from PC studied contains one leaf showing the typical symptoms, and although only old empty fruitbodies were observed in it, it is almost certain that these are the product of this well-known and common “Septoria” species. The other material studied here was in much better condition and proved highly homogeneous in both symptoms and morphology of the sporulating structures, including the collection from Geum rivale, with most conidia observed below 70 μm long. Some authors found conidia up to about 75 μm long in various European collections (Jørstad 1965, Vanev et al. 1997). In the fresh material from The Netherlands, conidia were no longer than 65 μm on the host plant, but the isolates obtained from it produced conidia up to 85 μm long. This material is chosen here to epitypify Sphaer. gei because it is geographically the closest one for which also a culture is available.
Several authors have recognised Septoria gei f. immarginata for material on Geum urbanum with smaller conidia, viz. Radulescu et al. (1973), reporting conidia as continuous, 33-56 × 1.1-1.5 μm (in majority 40-46 × 1.5 μm), and Teterevnikova-Babayan (1983), reporting 20-33 × 1.5 μm. Shin & Sameva (2004) considered this forma a synonym of S. gei, for which they noted the wide range of conidial sizes. In Asian collections identified as S. gei the conidia appear to be longer than in material from elsewhere (Shin & Sameva 2004), but the Korean isolates included here are genetically very close to the ex-epitype strain CBS 102318, and regarded as conspecific. Sequence analyses of the cultures of Sphaer. gei indicate a close relationship with species such as Sphaer. patriniae (CBS 128653, 129153), from Patrinia scabiosaefolia and P. villosa (Valerianaceae) and Sphaer. cercidis (Quaedvlieg et al. 2013).
Sphaerulina hyperici (Roberge ex Desm.) Verkley, Quaedvlieg & Crous, comb. nov. MycoBank MB804476. Fig. 45A-D.
Basionym: Septoria hyperici Roberge ex Desm., Annls Sci. Nat., sér. 2, Bot.17: 110. 1842.
≡ Phleospora hyperici (Roberge ex Desm.) Westend., Bull. Acad. r. Bruxelles 12 (9): 251. 1845.
Description in planta: Symptoms leaf lesions indefinite, usually starting to develop from the tip of leaf lamina and progressing towards the basis, irregular, reddish brown, surrounding leaf tissue often yellowish; Conidiomata pycnidial, amphigenous, densly dispersed in each lesion, only partly immersed, subglobose to globose or flask-shaped, brown to black, 55-90(-130) μm diam; ostiolum central, circular, often lifted above the leaf surface, 25-35(-50) μm wide, surrounded by concolorous or somewhat darker cells; conidiomatal wall 10-22 μm thick, composed of a homogenous tissue of hyaline, angular cells 2-5.5 μm diam, the outermost cells pale brown with slightly thickened walls, the inner cells thin-walled. Conidiogenous cells hyaline, discrete or integrated in 1-2-septate conidiophores, terminal ones narrowly to broadly ampulliform, holoblastic, producing a single conidium or proliferating sympodially, 6-8(-10) × 3.5-5 μm. Conidia cylindrical, straight, more often slightly curved or flexuous, broadly rounded at the apex, narrowing slightly to the truncate base, 1-3(-5)-septate, not or slightly constricted around the septa, hyaline, contents with a few oil-droplets and minute granular material in each cell in the living state, with oil-droplets and granular contents in the rehydrated state, 24-55(-63) × 2.5-3.5 μm (living; rehydrated, 1.8-2.8 μm wide). Sexual morph unknown (see notes).
Description in vitro: Colonies on OA 4-7 mm diam in 2 wk, with an even, glabrous, colourless margin; centre and some outgrowing sectors entirely pale luteous to buff, where conidia are formed directly on the immersed and superficial mycelium; submarginal area blackish, due to dark pigmented hyphae and superficial pycnidia, covered by diffuse, white tufty to woolly aerial mycelium; reverse concolourous. Colonies on CMA as on OA. Colonies on MEA 3-7 mm diam in 2 wk (32-40 mm in 6 wk), with an irregular, glabrous margin; a reddish pigment diffusing into the agar; colony restricted, the surface cerebriform to irregularly lobed, up to 2 mm high, immersed mycelium dark, mostly covered by dense, pure white, woolly aerial mycelium, or salmon to saffron by masses of conidia; reverse cinnamon to brick. Colonies on CHA 3-5 mm diam in 2 wk, with an irregular, glabrous margin; colony restricted, the surface cerebriform to irregularly lobed, up to 2 mm high, dark but mostly covered by salmon to saffron conidial masses, and some areas with a dense, pure white, woolly-floccose aerial mycelium; reverse dark brick.
Hosts: Hypericum spp.
Material examined: Bulgaria, Camkorije, on leaves of Hypericum quadrangulum, 31 Aug. 1907, Fr. Bubák, distributed in Kabát & Bubák, Fungi imperfecti exsicc. 469 (PC 0084544). Czech Republic, Bohemia, Bukovina, on leaves of H. perforatum, 9 June 1906, J. Kabát, distributed in Kabát & Bubák, Fungi imperfecti exsicc. 421 (PC 0084542); same substr., E. Moravia, M. Weisskirchen, Aug. 1941, F. Petrak (PC 0084545). France, loc. unknown, on leaves of H. perforatum, isotype PC 0084532; Lighhouse of Libisey near Caen, same substr., June 1841, M. Roberge, PC 0084531; same substr., Bois de Plaisir, 16 July 1935 (Herb. G. Viennot-Bourgin), PC 0084533; same substr., Allier, Gennetines, 5 Apr. 1959, A. Lachmann, PC 0084535; Landes, Etang near Seignosse, on H. helodes, 5 Aug. 1964, G. Durrieu, PC 0084536; Seine-et-Marne, Fontainebleau forest, on leaves of H. hirsutum, July 1888, Feuilleaubols, PC 0084537, 0084540. Germany, Hessen-Nassau, Dillkreis, Langenaubach, on leaves of H. quadrangulum, 12 July 1931, A. Ludwig, distributed in Sydow, Mycotheca germanica 2570, PC 0084538; Brandenburg, Sadowa, on leaves of H. perforatum, 4 Aug. 1907, P. Sydow, distributed in Sydow, Mycotheca germanica 625, PC 0084543. Netherlands, Prov. Utrecht, Soest, along railroad between Lange Duinen and De Zoom, on living leaves of Hypericum sp., 28 July 1999, G. Verkley 900, epitype designated here CBS H-21194 “MBT175361”, living culture ex-epitype CBS 102313. Romania, Moldova, distr. Iaşi, Poeni, on leaves of H. hirsutum, 1 Aug. 1948, C. Sandu-Ville & I. Rădulescu, distributed in Tr. Săvulescu, Herb. Mycol. Romanicum, fasc. 29, no. 1445, PC 0084534, 0084546. Sweden, E. Götland, Gryt parish, ca. 300 m E.-S.E. of Strömmen, on leaves of H. maculatum, 18 July 1947, J.A. Nannfeldt 9386, distributed in S. Lundell & J.A. Nannfeldt, Fungi exsicc. Suecici, praes. Upsal. 1910, PC 0084547.
Notes: According to Jørstad (1965), the pycnidia of Sphaer. hyperici are immersed hypophylously, but in most collections investigated here they protrude with their ostioli from either side of the leaf in about equal numbers. Jørstad (1965) further noted that the conidial sizes varied considerably between collections, with extreme values ranging between 15 and 57 μm for length and 1.5-2.5 μm for width of conidia. Vanev et al. (1997) reported conidia 21.5-54 × 2-3.2 μm. In the type specimen, which is rich in conidiomata with protruding dry spore-masses, conidia are mostly 1-3-septate, 25-50 × 2-2.5 μm, thus in good agreement with the collection V900, which is designated as epitype.
Four varieties of Septoria hyperici and a few more Septoria species have been described on species of the genus Hypericum. Most of these taxa have conidia in the size range given here for Sphaer. hyperici, indicating that these might be conspecific. However, more strains should be isolated from the different species of Hypericum and compared with type material of these taxa, before firm conclusions about their status can be drawn. Septoria hypericorum, which was described from H. perforatum with conidia reported 15-35 × 4-6 μm, is likely to belong in Stagonospora or another related asexual morph. The ex-epitype strain of Sphaer. hyperici CBS 102313 is closely related to strains identified as S. menispermi (CBS 128666, 128761), and somewhat more distant from species such as Sphaer. gei, and Sphaer. cercidis (CBS 501.50).
Petrak (1925) stated that Mycosphaerella hyperici is the sexual morph of Septoria hyperici, but this has not been confirmed by culture studies. The only culture available of M. hyperici for comparison, CBS 280.49, was sequenced by Zalar et al. (2007) and shown to group with isolates of Cladosporium halotolerans, so it may be a culture contaminant. No strain is available for M. hypericina, a species originally described from Hypericum prolificum in the US. No asexual morph is known for this taxon which, according to Aptroot (2006), is morphologically indistinguishable from M. punctiformis (anam. Ramularia endophylla; Verkley et al. 2004c).
Sphaerulina socia (Pass.) Quaedvlieg, Verkley & Crous, comb. nov. MycoBank MB804478.
Basionym: Septoria socia Pass., Funghi Parm. Septor.: no. 74; Atti Soc. crittog. ital. 2: 33. 1879.
Description in planta: Symptoms leaf lesions circular to irregular, single or confluent to form irregular extended lesions, pale to dark brown, usually surrounded by a red or purple zone, mostly visible on both sides of the leaf. Conidiomata pycnidial, mostly epiphyllous, a few to many in each lesion, immersed, globose, brown to black, 80-100(-110) μm diam; ostiolum central, circular, 15-25 μm wide, surrounding cells darker; conidiomatal wall 10-17 μm thick, composed 2-3 layers of isodiametric cells, 2-3.5(-5) μm diam, the cells in the outermost layer(s) pale brown with slightly thickened walls, the inner cells thin-walled. Conidiogenous cells hyaline, discrete, rarely integrated in 1(-2)-septate conidiophores, globose, or narrowly to broadly ampulliform, holoblastic, proliferating percurrently and/or sympodially, sometimes with indistinct annellations on an elongated neck, 4-8.5(-12) × 2-3(-3.5) μm. Conidia cylindrical, straight to slightly curved, rarely flexuous, attenuated in the upper cell to a pointed to narrowly rounded tip, attenuated gradually or more abruptly towards a sub-truncate base, 1-3(-5)-septate, not constricted around the septa, hyaline, contents minute oil-droplets and granular material in the rehydrated state, (19-)22-34 × 1-1.5(-2) μm (rehydrated). Sexual morph unknown.
Hosts: Chrysanthemum leucanthemum and other wild or cultivated Chrysanthemum spp.
Material examined: Germany, Torstedt near Harburg, Sep. 1957, R. Schneider s.n., BBA 8514, living culture CBS 357.58. New Zealand, North Island, Coromandel, Tairua Forest, along roadside of St. Hway 25, near crossing 25A, on living leaves of Chrysanthemum leucanthemum, 23 Jan. 2003, G. Verkley 1842a, CBS H-21243.
Additional material examined: Netherlands, on leaf of Rosa sp., isolated June 1958 by Plant Protection Service, Wageningen, CBS 355.58 (preserved as S. rosae; possibly infection of a fungus originally identified as S. rosae).
Notes: Punithalingam (1967d) described the conidiogenous cells as obpyriform, undifferentiated cells producing blastospores, while Muthumary (1999) also observed sympodially proliferating cells in a collection from India; the present material from New Zealand clearly showed both percurrent and sympodial conidiogenesis, even in a single conidiogenous cell. In this respect, S. socia is similar to S. chrysanthemella, for which both these proliferations were observed with transmission electron microscopy (Verkley 1998a).
According to Teterevnikova-Babayan (1987) conidia are 21-35 × 1-1.5 μm, so with these measurements the present observations are in good agreement. Verkley & Starink-Willemse (2004) noted that the ITS sequence of CBS 357.58 identified as S. socia suggested a relatively distant relationship with other Septoria species on the family Asteraceae, and that it was more closely related to species such as the maple pathogen Sphaerulina aceris (syn. Septoria aceris, Mycosphaerella latebrosa) and poplar pathogen Sphaerulina populicola. Multilocus sequencing performed here confirms that CBS 357.58 groups in the Sphaerulina-clade, and that CBS 355.58 originally identified as S. rosae likely got infected with S. socia. Septoria rosae is a large spored species (70-90 × 3.5-4 μm) for which the name of the presumed sexual morph Sphaerulina rehmiana would be accepted (Quaedvlieg et al. 2013). Based on the huge difference in conidial size it seems very unlikely that it was confused with S. socia. The material from New Zealand studied here failed to grow in culture, so a genetic comparison was not possible. More isolates will be required to determine the affinities of Sphaerulina rehmiana.
Sphaerulina tirolensis Verkley, Quaedvlieg & Crous, sp. nov. MycoBank MB804479. Fig. 46.
Fig. 46.
Sphaerulina tirolensis. A. Conidia in planta (CBS H-21232, holotype). B. Conidia on OA (CBS 109017). Scale bars = 10 μm.
Etymology: named after the region in Austria where the type material was collected, Tirol.
Description in planta: Symptoms leaf lesions numerous, circular to irregular, mostly single, or confluent, dull brown, amphigenous but on the lower surface barely visible due to the white hairs of the host; Conidiomata pycnidial, epiphyllous, many in each lesion, immersed, subglobose to globose, brown to black, 55-100 μm diam; ostiolum central, circular, initially 15-30 μm wide, later up to 50 μm wide, surrounding cells somewhat darker; conidiomatal wall 15-22 μm thick, composed of an outer layer of pale brown angular to irregular cells, 8-12 μm wide with walls thickened to 1.5 μm, and an inner layer of hyaline, angular to globose, thin-walled cells. Conidiogenous cells hyaline, discrete, rarely integrated in 1-septate conidiophores, cylindrical or narrowly to broadly ampulliform, holoblastic, some proliferating percurrently 1-several times with indistinct annellations and forming an elongated neck, rarely proliferating sympodially, 5-12.5(-15) × 3.5-4(-5) μm. Conidia cylindrical, straight, slightly curved to flexuous, narrowly to broadly rounded at the apex, truncate or slightly narrowed at the base, (1-)3-7(-9)-septate, not constricted around the septa, hyaline, with granular contents and minute oil-droplets, 40-70(-78) × 2.5-3(-3.5) μm (rehydrated). Sexual morph not observed.
Description in vitro: Colonies on OA 2.5-4(-5) mm diam in 2 wk; 16-20 mm in 7 wk), with an even, glabrous, colourless or buff to rosy-buff margin; immersed mycelium dark green or dull green, showing some salmon or rosy-buff colours only after more than 6 wk of incubation; colonies restricted, but with irregular elevations in the centre on which complexes of stromatic conidiomata and single pycnidia are formed, releasing whitish conidial slime; aerial mycelium variable, almost wanting, to well developed as a dense, white, woolly-floccose mat; reverse mostly olivaceous-black, locally buff to rosy-buff. Colonies on CMA 3-4.5(-5) mm daim in 2 wk, 6-8 mm in 3 wk (22-25 mm in 7 wk), as on OA, but with a narrower colourless margin. Conidial slime also milky white, as on OA. Colonies on MEA 2-4(-6) mm diam in 2 wk, 6-9 mm in 3 wk (16-22 mm in 7 wk), with an even, glabrous colourless to buff margin; colonies restricted, irregularly pustulate to hemispherical, sometimes with rather high, subglobose outgrowths; immersed mycelium buff to honey usually only near the margin, olivaceous-black in the centre; almost entirely covered by a dense, appressed mat of white or grey aerial mycelium; a diffusable pigment staining the surrounding agar more or less ochreous; reverse usually dark umber or olivaceous-black in the centre, surrounded by ochreous, which later becomes fulvous to apricot. Colonies on CHA 3-4 mm diam in 2 wk, 5-6 mm in 3 wk (12-16 mm in 7 wk), with an even but later more irregular, glabrous, buff, rosy-buff or flesh margin; colonies pustulate to almost hemispherical, the surface olivaceous-black to dark slate blue, glabrous, or covered by diffuse, greyish or flesh aerial mycelium, some colonies later covered by a pure white, dense mat of aerial mycelium; diffusable pigment not observed; reverse blood colour to umber. Cultures produce large masses of pale flesh conidial slime, aggregating around the colony margin.
Conidiomata pycnidial or merged into stromatic complexes. Conidiogenous cells as in planta. Conidia straight to curved or flexuous, narrowly to broadly rounded at the apex, narrowly truncate at the base, 3-7(-9)-septate, not constricted around the septa, hyaline, contents granular with minute oil-droplets, 54-96(-108) × 2.5-3 μm.
Host: Rubus idaeus.
Material examined: Austria, Tirol, Pitztal, Arzl, on living leaves of Rubus idaeus, 30 July 2000, G. Verkley 1021, holotype CBS H-21232, living cultures ex-type CBS 109017, 109018.
Notes: Sphaerulina tirolensis differs from another septoria-like fungus described on R. idaeus, viz. Rhabdospora rubi var. rubi-idaei described from stems of R. idaeus in Romania, with conidia (36-)40-50(-60) × 2(-2.5) μm. Demaree & Wilcox (1943) studied Septoria leaf-spot diseases of raspberry (R. idaeus) in North America. Cylindrosporium rubi, of which the sexual morph is Sphaerulina rubi cf. Demaree & Wilcox (1943), is also different. The sequences of the various protein-coding genes fully support Sphaer. tirolensis as a separate species from the next taxon, Sphaer. westendorpii. The latter can be distinguished from Sphaer. tirolensis by the smaller conidia in planta [24-45(-50) × 1.8-2.2 μm] and also in culture [30-68(-80) × 1.5-2(-2.5) μm].
Sphaerulina westendorpii Verkley, Quaedvlieg & Crous, comb. et nom. nov. MycoBank MB804480. Fig. 47.
Fig. 47.
Sphaerulina westendorpii. A. conidia in planta (CBS H-21229, epitype); B. conidia on OA (CBS 102327). Scale bars = 10 μm.
Basionym: Septoria rubi Westend., in Westend. & Wallay, Herb. crypt. Belge, Fasc. 19, no. 938. 1854; Kickx, Fl. crypt. Flandr. 1: 432. 1867.
= Mycosphaerella rubi Roark, Phytopathology 11: 329. 1921.
Description in planta: Symptoms leaf lesions numerous, circular to irregular, single or confluent, pale yellowish brown to greyish brown, partly well-delimited by a dark red brown line or zone. Conidiomata pycnidial, epiphyllous, several in each lesion, immersed, subglobose to globose, brown to black, 55-90 μm diam; ostiolum central, circular, initially 20-40 μm wide, later becoming more irregular and up to 70 μm wide, surrounding cells somewhat darker; conidiomatal wall 10-15 μm thick, composed of a homogenous tissue of hyaline, angular cells 2.5-3.5 μm diam, the outermost cells pale brown with slightly thickened walls, the inner cells thin-walled. Conidiogenous cells hyaline, discrete, rarely integrated in 1-septate conidiophores, narrowly to broadly ampulliform, holoblastic, proliferating percurrently several times with indistinct annellations thus forming a relatively elongated neck, rarely also sympodially, 5-10(-15) × 2.5-3.5(-4) μm. Conidia filiform-cylindrical, straight, slightly curved to flexuous, narrowly to broadly rounded at the apex, narrowly truncate at the base, (0-)2-3(-5)-septate, not constricted around the septa, hyaline, contents granular material, sometimes with minute oil-droplets both in the living and rehydrated state, 24-45(-50) × 1.8-2.2 μm (living; rehydrated, 1.5-2.0 μm wide).
Description in vitro: Colonies on OA 8-10 mm diam in 19 d, with an even, glabrous, colourless or buff to rosy-buff margin; immersed mycelium dark green or dull green, but sectors or other parts of colonies may be only olivaceous-buff or rosy-buff to salmon; colonies spreading, with irregular elevations in the centre on which conidiomata are formed, releasing a whitish conidial slime; aerial mycelium almost absent to well developed and forming a dense, white, woolly-floccose mat; reverse olivaceous-black, locally buff to rosy-buff. Colonies on CMA 5-7(-10) mm diam in 19 d, as on OA, but more distinctly elevated and restricted. In faster growing sectors salmon to ochreous pigmentation (due to weak production of red pigment?) in a peripheral zone preceedes the formation a dominant greens. Conidial slime also milky white, as on OA. Colonies on MEA 9-12 mm diam in 19 d, with an even, glabrous colourless to buff margin; colonies restricted, irregularly pustulate to hemispherical; immersed mycelium buff to honey near the margin, olivaceous-black in the centre, sometimes mostly honey; almost entirely covered by a dense, appressed mat of white or grey aerial mycelium; a diffusable pigment staining the surrounding agar more or less ochreous; reverse usually dark umber or olivaceous-black in the centre, surrounded by ochreous, which later becomes fulvous to apricot. Colonies on CHA 7-9 mm diam in 19 d, with an even but later more irregular, glabrous, buff, rosy-buff or flesh margin; colonies pustulate to almost hemispherical, the surface ochreous to sienna, glabrous, or covered by diffuse, greyish or flesh aerial mycelium; diffusable pigment not observed; reverse blood colour to umber.
Conidiomata pycnidial or merged into stromatic complexes, as in planta. Conidiogenous cells as in planta, mostly cylindrical and proliferating percurrently, rarely also sympodially, 7-15(-18) × 2.5-3.5(-4) μm; Conidia as in planta but mostly 3-5-septate and considerably longer, 30-68(-80) × 1.5-2(-2.5) μm.
Hosts: Rubus spp.
Material examined: Belgium, Oostacker, near Gand, on leaves of Rubus sp., isotype BR-MYCO 159265-88, also distributed in Westend. & Wallay, Herb. crypt. Belge, Fasc. 19, no. 938. Czech Republic, Mikulov, on living leaves of Rubus sp., 15 Sep. 2008, G. Verkley 6002, CBS H-21257. Netherlands, prov. Limburg, Gerendal, on living leaves of R. fruticosus s.l., 28 June 2000, G. Verkley 964, epitype designated here CBS H-21229 “MBT175362”, living cultures ex-epitype CBS 109002, 109003; Prov. Limburg, Mookerheide, in mixed forest, on living leaves of R. fruticosus s.l., 9 Sep. 1999, G. Verkley 923, CBS H-21205, living culture CBS 102327; same loc. and substr., 23 Aug. 2004, G. Verkley & M. Starink 3036, CBS H-21263, living culture CBS 117478; same substr., Prov. Limburg, St. Jansberg near Plasmolen, in mixed forest, G. Verkley 924, CBS H-21206; Prov. Flevoland, Erkemeder strand, in sandy dunes, on living leaves of R. fruticosus s.l., 8 Sep. 1999, G. Verkley 930, CBS H-21210.
Notes: Jørstad (1965) discussed the problems regarding the taxonomy of Septoria species described from Rubus. Some of the later described taxa have been placed in synonymy with Septoria rubi, but most still need to be reevaluated based on fresh material, culture studies, and molecular characterisation. The type material in BR contains several well-preserved leaves of the R. fruticosus complex, showing typical symptoms. Fruitbodies investigated contained mostly 1-3-septate conidia, 17.5-40 × 1-1.5 μm, and with the typical shape of this common fungus on Rubus spp. The specimen CBS H-21229 from R. fruticosus in the south of the Netherlands, is chosen as epitype. This species is nested within the Sphaerulina-clade, and a new name in Sphaerulina should therefore be proposed for it. Sphaerulina rubi Demaree & Wilcox is already in use for another fungus with a Cylindrosporium sexual state (C. rubi Ellis & Morgan, conidia 40-55 × 2.5 μm cf. Saccardo), so Sphaer. westendorpii is proposed here as nomen novum. Sphaerulina rehmiana has been associated with Septoria rosae CBS 355.58, which has been identified as S. rosae, is genetically distinct from Sphaer. westendorpii (Quaedvlieg et al. 2013).
Insufficiently known species
For the following species no host material was available and these have only been studied in culture, mostly based on older isolates, for which details are not described when the strain is regarded as degenerate.
Septoria hippocastani Berk. & Broome, Ann. Mag. nat. Hist., Ser. 2, 5: 379. 1850.
Material examined: Germany, Pfälzer Wald, on Aesculus hippocastanum, Sep 1961, deposited Nov 1961, W. Gerlach, living culture CBS 411.61 (= BBA 9619).
Note: CBS 411.61 is degenerated and sterile, but based on multilocus sequence analysis it can be concluded that it is a Septoria s. str. (Fig. 2).
Septoria limonum Pass., Atti Soc. crittog. ital., 2: 23. 1879.
Description in vitro (18 °C, near UV): Colonies on OA 20-29 mm diam in 3 wk, with an even, colourless margin; colonies plane, spreading, immersed mycelium in the centre flesh, surrounded by a broad zone of dark vinaceous to brown-vinaceous, aerial mycelium absent, or scarce, with few tufts of pure white aerial hyphae; reverse concolorous. No sporulation observed. Colonies on MEA 25-32 mm diam in 3 wk, with an even to somewhat ruffled, buff to colourless margin; colonies spreading, somewhat elevated in the centre, immersed mycelium appearing grayish, the colony surface almost entirely covered by a dense mat of white to grey, woolly-floccose aerial mycelium; reverse in the centre rust, surrounded by a broad zone of olivaceous-grey to greenish grey, which is sharply bordered by the narrow buff to luteous margin. No sporulation observed.
Material examined: Italy, Citrus limonium, isolated Mar. 1951, deposited by G. Goidanich, living culture CBS 419.51.
Notes: In the multilocus sequence analysis (Fig. 2) this strain groups with CBS 356.36 (S. citricola) and few other strains in a weakly supported clade close to the plurivorous Septoria protearum and isolates of Septoria citri. Due to the lack of morphological information linked to this strain, its identity remains uncertain.
DISCUSSION
The type species of the genus Septoria, S. cytisi, could not be included in the multilocus analysis due to the fact that only LSU and ITS sequences were available for this species. However, as shown by Quaedvlieg et al. (2011), the position of this taxon is beyond doubt central to the clade indicated here as the main Septoria clade. Several “typical” Septoria species infecting herbaceous plants proved genetically distant from S. cytisi and its relatives, and can best be classified in separate genera, Sphaerulina (Quaedvlieg et al. 2013) and Caryophylloseptoria.
The identification of Septoria has thus far mainly relied on host taxonomy and morphological characters of the shape, size, and septation of conidia (Jørstad 1965, Teterevnikova-Babayan 1987, Andrianova 1987, Vanev et al. 1997, Muthumary 1999, Shin & Sameva 2004, Priest 2006). Taxonomists have noted that conidial width is generally a more reliable character for species identification than conidial length, which is more variable. Some also noticed that Septoria material collected from the same location and host species, but under different environmental conditions or at different times in the same season, can differ considerably in average conidial sizes, particularly length (Jørstad 1965). These findings are also confirmed in our study. Reliable identification based on morphological comparison alone is not possible for many Septoria species, and reference sequences will have to be produced for many more taxa in future. This will require critical studies of type specimens and also require the recollection of fresh material. It is crucial that the types of the oldest names available for Septoria on certain hosts will need to be studied as part of such work, and where necessary epitypes designated to fix the genetic application of these names. Although hardly practised thus far by taxonomists, isolation and study in culture is a valuable and indispensable tool for Septoria species delimitation and identification. We noted that the shape of conidia on OA generally agree best with those in the source material on the natural substrate. Under standardised incubation conditions on standard media cultures originating from deviant voucher material, for example because it developed under adverse conditions, show again their “normal” phenotypes which is better for comparison purposes. Extracting DNA from axenic cultures is straight-forward and less prone to errors caused by contaminants, a problem often encountered when extracting DNA from plant tissue.
The K2P results show that the five protein coding genes used during this research should all theoretically be able to distinquish every species in this dataset as their average inter- to intraspecific distance ration is over 10:1. The problem is that these are average numbers, not absolute numbers. For example, the Btub K2P graph in Fig. 1 starts at 0 and not at at 0.29, meaning that there actually are a few species in our dataset that are not distinguishable by Btub alone (although obviously by far most species in fact are). To avoid this, we recommend using at least two of the protein coding loci used in this study for identification of Septoria and allied genera. Because EF and Btub both have very high PCR success rates and have the highest species resolution percentage of all the loci used in this study, we recommend using these two loci for species identification purposes. It is advisable, however, to first sequence the ITS and LSU for a preliminary genus identification by blasting in GenBank and other useful databases.
The multilocus sequence dataset generally provided good resolution, with maximum to high bootstrap support for almost all terminal and most of the deeper nodes of the phylogenetic tree. The intraspecific variation in the genes investigated is limited for most taxa, even if specimens originate from such distant geographic origins as New Zealand, Korea and Europe (S. convolvuli, S. leucanthemi, S. polygonorum). Strains assigned to Septoria citri possibly represent a species complex, one of few groups within the main Septoria clade that was not resolved. One case of cryptic speciation is revealed in the S. chrysanthemella complex, where at least two genetically discrete entities can be found that are phenotypically difficult to distinguish.
Our results confirm that most species of Septoria have narrow host ranges, being limited to a single genus or a few genera of the same plant family. There were a few notable exceptions, however. We demonstrated that the supposed single-family host ranges of Septoria paridis (Liliaceae) and S. urticae (Urticaceae), each actually included one additional family (Violaceae and Lamiaceae, respectively). More surprisingly Septoria protearum, previously only associated with Proteaceae (Protea) (Crous et al. 2004), was now found to be also associated with Araceae (Zanthedeschia), Aspleniaceae (Asplenium), Rutaceae (Boronia), Boraginaceae (Myosotis), Oleandraceae (Nephrolepis), and Rosaceae (Geum). To our knowledge this is the first study to provide DNA-based evidence confirming that multiple family-associations occur for a single species in Septoria. It is to be expected that collecting and sequencing of more material will show more taxa to be plurivorous, and perhaps S. paridis and S. urticae will be among those.
Coevolution of plant pathogenic fungi and their hosts has been documented for several groups. Other possible patterns of evolution have already been suggested for septoria-like fungi in previous studies but the data available were not sufficient to fully understand the evolution of these fungi (Feau et al. 2006). The robust phylogeny we inferred revealed polyphyletic distribution patterns over the entire range of the Septoria clade for no less than 10 (singletons excluded) of the host families represented. These results clearly reject the coevolution hypothesis for Septoria, as species do not seem to consistently coevolve with hosts from a single host family but frequently jump successfully to hosts in new families. Caryophylloseptoria seems an exceptional genus in that it only comprises species infecting Caryophyllaceae, but it should be noted that it now only contains four species, as three other species infecting this family cluster distant within the Septoria clade (S. cucubali, S. cerastii, and S. stellariae). In the other clades some single-host family clusters can be found, but they do not comprise more than six fungal species (S. chrysanthemella and close relatives of Asteraceae within subclade 4b).
We conclude that trans-family host jumping must be a major force driving the evolution of Septoria and Sphaerulina. Species like S. paridis and S. urticae infecting (at least) two plant families may in fact be cases in point, as they could be in a transitional period of gradually changing from one principal host family to another, unrelated one. The genetic basis for successful host jumping is unclear. It may involve horizontal gene transfer, transient phases of endophytic infections in “non-hosts” as a first step in a process of genetic adaptation to new optimal hosts, or perhaps a combination of both. Plant pathological research may shed more light on the mechanisms driving Septoria evolution which would be important, as it may in future allow accurate assessment of risks involved with the introduction of new crops in areas where Septoria species occur on the local flora.
HOST FAMILY INDEX
The taxa fully described in the Taxonomy section of this study are listed below according to the host family.
Aceraceae
Sphaerulina aceris
Apiaceae
Septoria aegopodii
S. aegopodina
S. anthrisci
S. apiicola
S. heraclei
S. petroselini
S. sii
Araceae
Septoria protearum
Aspleniaceae
Septoria protearum
Asteraceae
Septoria chromolaenae
S. chrysanthemella
S. ekmanniana
S. erigerontis
S. hypochoeridis
S. lactucae
S. leucanthemi
S. matricariae
S. putrida
S. senecionis
Sphaerulina socia
Betulaceae
Sphaerulina betulae
Boraginaceae
Septoria protearum
Campanulaceae
Septoria campanulae
S. citri complex
Caryophyllaceae
Caryophylloseptoria lychnidis
C. silenes
C. spergulae
Septoria cerastii
S. cucubali
S. stellariae
Convolvulaceae
Septoria convolvuli
Cornaceae
Sphaerulina cornicola
Cucurbitaceae
Septoria cucurbitacearum
Dipsacaceae
Septoria scabiosicola
Fabaceae
Septoria astragali
Hypericaceae
Septoria hyperici
Iridaceae
Septoria sisyrinchii
Lamiaceae
Septoria galeopsidis
S. lamiicola
S. melissae
S. stachydis
Liliaceae
Septoria paridis
Oleandraceae
Septoria protearum
Onagraceae
Septoria epilobii
Passifloraceae
Septoria passifloricola
Polemoniaceae
Septoria phlogis
Polygonaceae
Septoria polygonorum
S. rumicum
Primulaceae
Septoria lysimachiae
Ranunculaceae
Septoria clematidis
S. lycoctoni
S. napelli
Rosaceae
Septoria citri complex
Sphaerulina gei
Sphaer. tirolensis
Sphaer. westendorpii
Rubiaceae
Septoria cruciatae
S. coprosmae
Rutaceae
Septoria protearum
Salicaceae
Sphaerulina frondicola
Scrophulariaceae
Septoria digitalis
Urticaceae
Septoria urticae
Verbenaceae
Septoria verbenae
Violaceae
Septoria paridis
Acknowledgments
We thank the technical staff of the CBS Collection for their support during this project. Arien van Iperen (cultures) and Marjan Vermaas (photographic plates) are acknowledged for their invaluable assistance. Huub van der Aa is thanked for his help during collecting and identifying material. Simeon Vanev is gratefully acknowledged for his support with collecting bibliographic data and translating Russian diagnoses. Part of the DNA barcode sequencing results used in this study was financially supported by the Fonds Economische Structuurversterking (FES, Dutch Ministery of Education, Culture and Science grant BEK/BPR-2009/137964-U, “Making the Tree of Life Work”).
REFERENCES
- Andrianova TV. (1987). Problems of classification and phylogeny of Septoria species. Mikologiya i Fitopatologiya 21: 393–399 [Google Scholar]
- Aptroot A. (2006). Mycosphaerella and its anamorphs 2. Conspectus of Mycosphaerella. CBS Biodiversity Series 5 CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands: [Google Scholar]
- Arx JA von. (1983). Mycosphaerella and its anamorphs. Proceedings Koninklijke Nederlandse Akademie van Wetenschappen, Series C - Biological and Medical Sciences 86 (1): 15–54 [Google Scholar]
- Beach WS. (1919). Biological spezialization in the genus Septoria. American Journal of Botany 6: 1–33 [Google Scholar]
- Braun U. (1995). A monograph of Cercosporella, Ramularia and allied genera (Phytopathogenic Hyphomycetes). Vol. 1 IHW-Verlag, Eching: [Google Scholar]
- Breeÿen A den, Groenewald JZ, Verkley GJM, Crous PW. (2006). Morphological and molecular characterisation of Mycosphaerellaceae associated with the invasive weed, Chromolaena odorata. Fungal Diversity 23: 89–110 [Google Scholar]
- Cheewangkoon R, Crous PW, Hyde KD, Groenewald JZ, To-anan C. (2008). Species of Mycosphaerella and related anamorphs on Eucalyptus leaves from Thailand. Persoonia 21: 77–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran LC. (1932). A study of two Septoria leaf spots of celery. Phytopathology 22: 791–812 [Google Scholar]
- Constantinescu O. (1984). Taxonomic revision of Septoria-like fungi parasitic on Betulaceae. Transactions of the British Mycological Society 83: 383–398 [Google Scholar]
- Crous PW, Aptroot A, Kang J-C, Braun U, Wingfield MJ. (2000). The genus Mycosphaerella and its anamorphs. Studies in Mycology 45: 107–121 [Google Scholar]
- Crous PW, Groenewald JZ. (2005). Hosts, species and genotypes: opinions versus data. Australasian Plant Pathology 34: 463–470 [Google Scholar]
- Crous PW, Denman S, Taylor JE, Swart L, Palm ME. (2004a).Cultivation and diseases of Proteaceae: Leucadendron, Leucospermum and Protea. CBS Biodiversity Series 2: 1–228 [Google Scholar]
- Crous PW, Groenewald JZ, Pongpanich K, Himaman W, Arzanlou M, Wingfield MJ. (2004b). Cryptic speciation and host specificity among Mycosphaerella spp. occurring on Australian Acacia species grown as exotics in the tropics. Studies in Mycology 50: 457–469 [Google Scholar]
- Crous PW, Kang J-C, Braun U. (2001). A phylogenetic redefinition of anamorph genera in Mycosphaerella based on ITS rDNA sequences and morphology. Mycologia 93: 1081–1101 [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, et al. (2006a). Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, Samson RA. (2009). CBS Laboratory Manual Series 1 CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands: [Google Scholar]
- Crous PW, Wingfield MJ, Mansilla JP, Alfenas AC, Groenewald JZ. (2006b). Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. II. Studies in Mycology 55: 99–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaree JB, Wilcox MS. (1943). The fungus causing the so-called “Septoria leaf-spot disease” of raspberry. Phytopathology 33: 986–1003 [Google Scholar]
- Diedicke H. (1915). Pilze VII, Sphaeropsidaea, Melanconiae,. Kryptogamenfl. Mark Brandenburg 9: 1–962 [Google Scholar]
- Farr DF. (1991). Septoria species on Cornus. Mycologia 83: 611–623 [Google Scholar]
- Farr DF. (1992). Species of Septoria on the Fabaceae, subfamily Faboidae, tribe Genistae. Sydowia 44: 13–31 [Google Scholar]
- Feau N, Hamelin RC, Bernier L. (2006). Attributes and congruence of three molecular data sets: inferring phylogenies among Septoria-related species from woody perennial plants. Molecular Phylogenetics and Evolution 40: 808–829 [DOI] [PubMed] [Google Scholar]
- Gabrielson RL, Grogan RG. (1964). The late blight organism Septoria apiicola. Phytopathology 54: 1251–1257 [Google Scholar]
- Goodwin SB, Dunkle LD, Zismann VL. (2001). Phylogenetic analysis of Cercospora and Mycosphaerella based on the internal transcribed spacer region of ribosomal DNA. Phytopathology 91: 648–658 [DOI] [PubMed] [Google Scholar]
- Grove WB. (1935). British stem and leaf fungi (Coelomycetes). Vol. I: Sphaeropsidales, Sphaerioideae with hyaline conidia: 1–488 [Google Scholar]
- Hall TA. (1999). BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98 [Google Scholar]
- Hebert PDN, Cywinska A, Ball SL, DeWaard JR. (2003). Biological identifications through DNA barcodes. Proceedings of the Royal Society of London Series B 270: 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. (2001). MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755 [DOI] [PubMed] [Google Scholar]
- Jørstad I. (1965). Septoria and septoroid fungi on dicotyledones in Norway. Skrifter utgitt av Det Norske Videnskaps-Akademi i Oslo, I: Mat-Naturv. Klasse 22:1–110 [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers AFA, Aptroot A. (2004). A revision of Mycosphaerella section Longispora (Ascomycetes). Nova Hedwigia 75: 451–468 [Google Scholar]
- Lombard L, Crous PW, Wingfield BD, Wingfield MJ. (2010). Species concepts in Calonectria (Cylindrocladium). Studies in Mycology 66: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markevičius V, Treigienė A. (2003). Sphaeropsidales. Genus Septoria. Mycota Lithuaniae 10, 3: 1–199 [Google Scholar]
- Mason-Gamer RJ, Kellogg EA. (1996). Testing for phylogenetic conflict among molecular data sets in the tribe Triticeae (Gramineae). Systematic Biology 45: 524–545 [Google Scholar]
- Muthumary J. (1999). First contribution to a monograph of Septoria species in India. Madras: Centre for advanced studies in Botany; [Google Scholar]
- Nylander JAA. (2004). MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre Uppsala University. [Google Scholar]
- Petrak F. (1925). Beiträge zur Pilzflora Südost-Galiziens und der Zentralkarpathen. Hedwigia 65: 179–330 [Google Scholar]
- Petrak F. (1957). Die auf Aconiten vorkommenden Arten der Gattung Septoria. Sydowia 11: 375–379 [Google Scholar]
- Priest MJ. (2006). Fungi of Australia. Septoria. ABRS, Canberra: CSIRO Publishing, Melbourne; [Google Scholar]
- Punithalingam E. (1967a). Septoria chrysanthemella. CMI Descriptions of Pathogenic Fungi and Bacteria 137 Commonwealth Mycological Institute, Kew: [Google Scholar]
- Punithalingam E. (1967b). Septoria leucanthemi. CMI Descriptions of Pathogenic Fungi and Bacteria 138 Commonwealth Mycological Institute, Kew: [Google Scholar]
- Punithalingam E. (1967c). Septoria obesa. CMI Descriptions of Pathogenic Fungi and Bacteria 139 Commonwealth Mycological Institute, Kew: [Google Scholar]
- Punithalingam E. (1967d). Septoria socia. CMI Descriptions of Pathogenic Fungi and Bacteria 140 Commonwealth Mycological Institute, Kew: [Google Scholar]
- Punithalingam E. (1982). Septoria cucurbitacearum. CMI Descriptions of Pathogenic Fungi and Bacteria 740 Commonwealth Mycological Institute, Kew: [Google Scholar]
- Punithalingam E, Holiday P. (1972). Septoria lactucae. CMI Descriptions of Pathogenic Fungi and Bacteria 335 Commonwealth Mycological Institute, Kew: [Google Scholar]
- Punithalingam E, Wheeler BEJ. (1965). Septoria spp. occurring on species of Chrysanthemum. Transactions of the British Mycological Society 48: 423–439 [Google Scholar]
- Quaedvlieg W, Kema GHJ, Groenewald JZ, Verkley GJM, Seifbarghi S, Razavi M, Gohari A, Mirzadi, Mehrabi R, Crous PW. (2011). Zymoseptoria gen. nov.: a new genus to accommodate Septoria-like species occurring on graminicolous hosts. Persoonia 26: 57–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W, Groenewald JZ, Jesús Yáñez-Morales M, Crous PW. (2012). DNA barcoding of Mycosphaerella species of quarantine importance to Europe. Persoonia 29: 101–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W, Verkley GJM, Shin H-D, Barreto RW, Algenas AC, Swart WJ, Groenewald JZ, Crous PW. (2013). Sizing up Septoria. Studies in Mycology 75: 307–390 (this volume). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulescu E, Negru A, Docea E. (1973). Septoriozele din România. Bucureşti: Editura Academiei Republicii Socialiste România; [Google Scholar]
- Rayner RW. (1970). A mycological colour chart. CMI and British Mycological Society; Kew, UK: [Google Scholar]
- Saccardo PA. (1884). Sylloge Fungorum: Sylloge Sphaeropsidearum et Melanconiearum. 3: 542 Padova, Italy: [Google Scholar]
- Saccardo PA. (1895). Sylloge fungorum: Supplemental Universale, Pars III 11: 1–753 Padova, Italy: [Google Scholar]
- Saccardo PA. (1906). Sylloge fungorum: Supplemental Universale, Pars VII 18: 1–838 Padova, Italy: [Google Scholar]
- Saccardo PA, Sydow P. (1899). Sylloge Fungorum: Supplemental Universale, Pars IV 14: 1–1316 Padova, Italy: [Google Scholar]
- Sheridan JE. (1968). Conditions for infection of celery by Septoria apiicola. Plant Disease Report 52: 142–145 [Google Scholar]
- Shin HD, Sameva EF. (2004). Septoria in Korea (Plant Pathogens of Korea 11). National Institute of Agricultural Science and Technology, Republic of Korea: [Google Scholar]
- Simon UK, Groenewald JZ, Stierhof Y-D, Crous PW, Bauer R. (2009). A necrotrophic phytopathogen forming a special cellular interaction with its host Aegopodium podagraria. Mycological Progress 9: 49–56 [Google Scholar]
- Smissen RD, Clement JC, Garnock-Jones PJ, Chambers JK. (2002). Subfamilial relationships within Caryophyllaceae as inferred from 5’ ndhF sequences. American Journal of Botany 89: 1336–1341 [DOI] [PubMed] [Google Scholar]
- Stewart EL, Liu Z, Crous PW, Szabo LJ. (1999). Phylogenetic relationships among some cercosporoid anamorphs of Mycosphaerella based on rDNA sequence analysis. Mycological Research 103: 1491–1499 [Google Scholar]
- Sutton BC. (1980). The Coelomycetes. Fungi imperfecti with Pycnidia, Acervuli and Stromata. Commonwealth Mycological Institute, Kew, Surrey, England: [Google Scholar]
- Sutton BC, Pascoe IG. (1987). Septoria species on Acacia. Transactions of the British Mycological Society 89: 521–532 [Google Scholar]
- Sutton BC, Pascoe IG. (1989). Some Septoria species on native Australian plants. Studies in Mycology 31: 177–186 [Google Scholar]
- Sutton BC, Hennebert GL. (1994). Interconnections amongst anamorphs and their possible contribution to ascomycete systematics. In Ascomycete systematics: problems and perspectives in the nineties (Hawksworth DL, ed.), pp. 77–98 Plenum Press, New York, USA: [Google Scholar]
- Sutton BC, Waterston JM. (1966) Septoria apiicola. CMI Descriptions of Pathogenic Fungi and Bacteria 88 Commonwealth Mycological Institute, Kew: [Google Scholar]
- Sydow H von. (1924). Beiträge zur Kenntnis der Pilzflora Neu-Seelands. I. Annales Mycologici 22: 299–317 [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. (2007). MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24: 1596–1599 [DOI] [PubMed] [Google Scholar]
- Teterevnikova-Babayan DN. (1987). Fungi of the genus Septoria in the U.S.S.R. Yerevan: Akademia Nauk Armyanskoi SSR; [Google Scholar]
- Vanev SG, Sameva EF, Bakalova GG. (1997). Fungi Bulgaricae 3. Ordo Sphaeropsidales. Sofia: Editio Academica Prof. Marin Drinov; [Google Scholar]
- Verkley GJM. (1998a). Ultrastructural evidence for two types of proliferation in a single conidiogenous cell of Septoria chrysanthemella. Mycological Research 102: 368–372 [Google Scholar]
- Verkley GJM. (1998b). Ultrastructure of conidiogenesis and conidia in two species of Septoria sensu lato. Mycologia 90: 189–198 [Google Scholar]
- Verkley GJM, Priest MJ. (2000). Septoria and similar coelomycetous anamorphs of Mycosphaerella. Studies in Mycology 45: 123–128 [Google Scholar]
- Verkley GJM, Starink-Willemse M. (2004a). A phylogenetic study of some Septoria species pathogenic to Asteraceae based on ITS ribosomal DNA sequences. Mycological Progress 3: 315–322 [Google Scholar]
- Verkley GJM, Starink-Willemse M, Iperen A van, Abeln ECA. (2004b). Phylogenetic analyses of Septoria species based on the ITS and LSU-D2 regions of nuclear ribosomal DNA. Mycologia 96: 558–571 [PubMed] [Google Scholar]
- Verkley GJM, Crous PW, Groenewald JZ, Braun U, Aptroot A. (2004c). Mycosphaerella punctiformis revisited: morphology, phylogeny, and epitypification of the type species of the genus Mycosphaerella (Dothideales, Ascomycota). Mycological Research 108: 1271–1282 [DOI] [PubMed] [Google Scholar]
- Waddell HT, Weber GF. (1963). Physiology and pathology of Septoria species on Chrysanthemum. Mycologia 55: 442–452 [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor JW. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications (Innis MA, Gelfland DH, Sininsky JJ, White TJ, eds). Academic Press, San Diego, USA: 315–322 [Google Scholar]
- Zalar P, Hoog GS de, Schroers H-J. (2007). Phylogeny and ecology of the ubiquitous saprobe Cladosporium sphaerospermum, with descriptions of seven new species from hypersaline environments. Studies in Mycology 58: 157–183 [DOI] [PMC free article] [PubMed] [Google Scholar]