Abstract
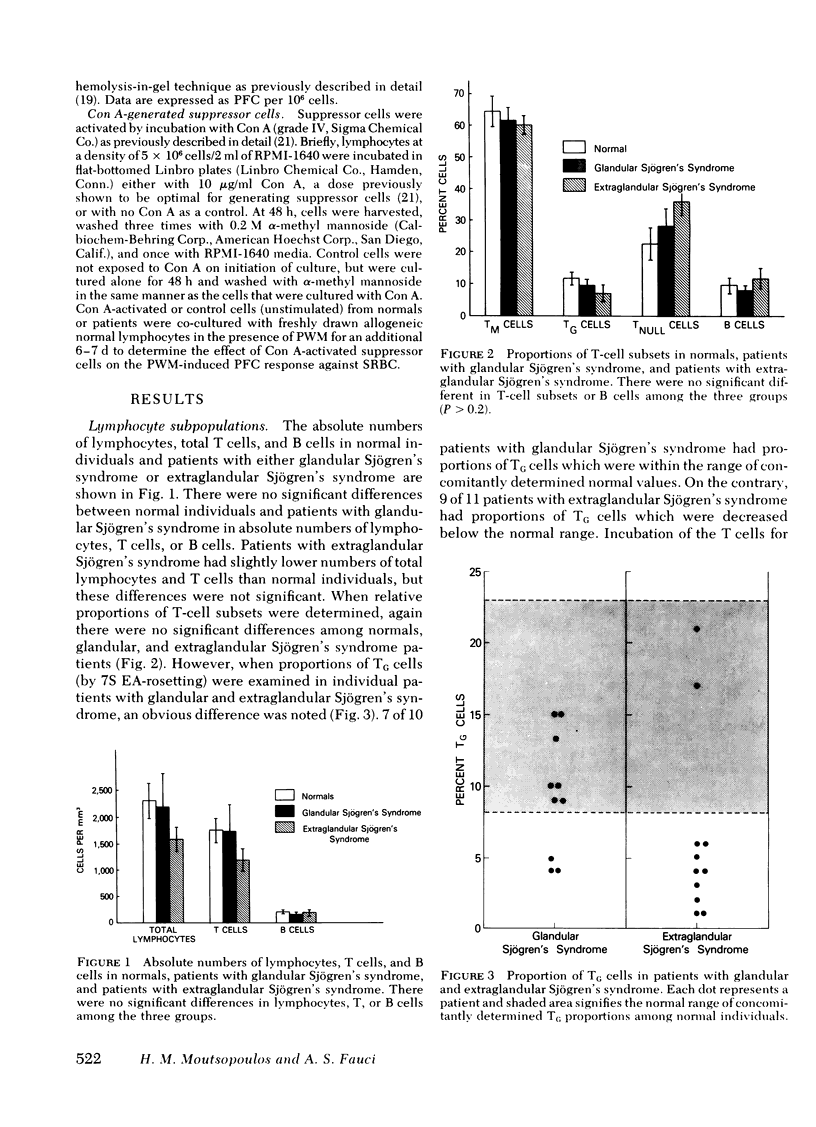
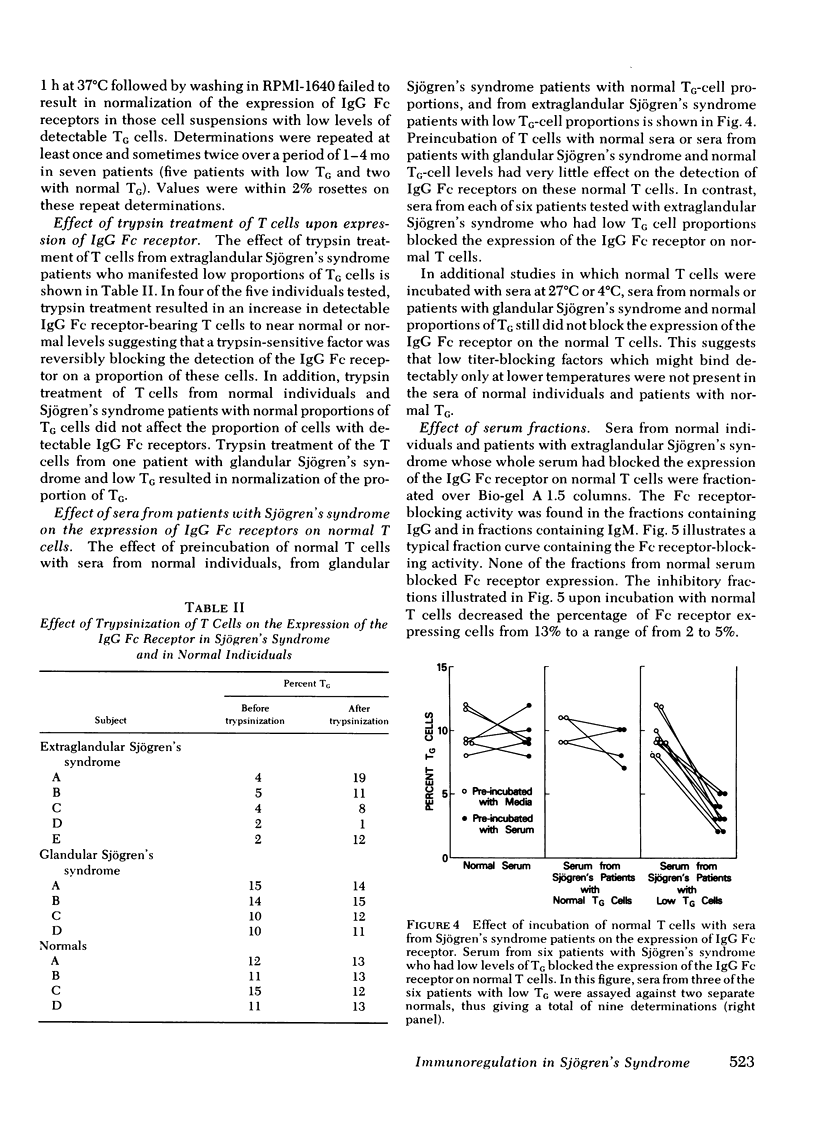
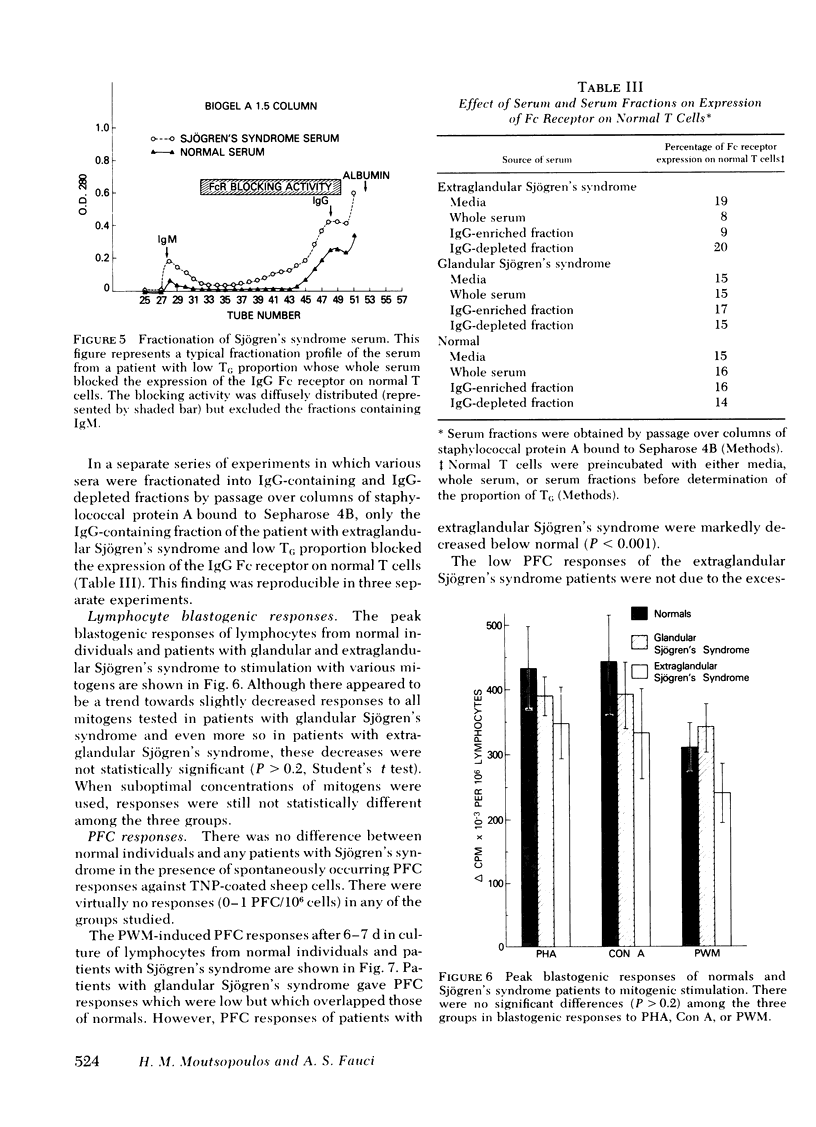
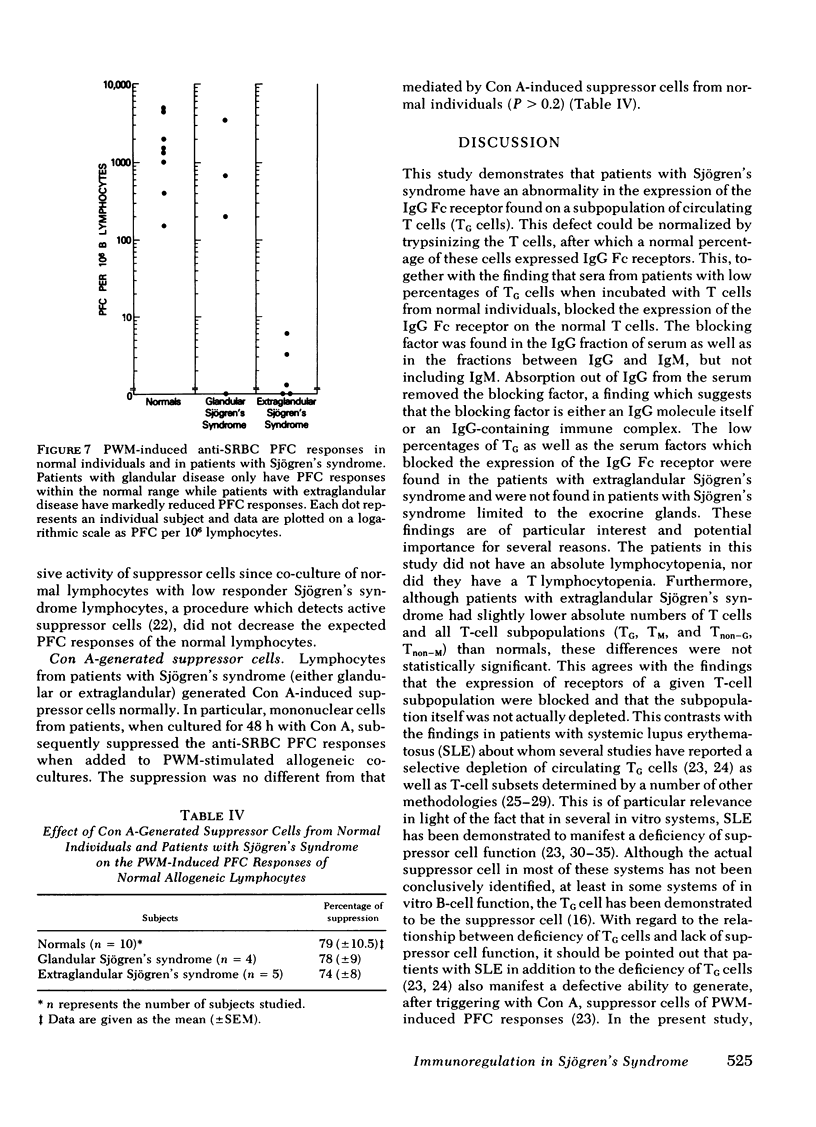
21 patients with Sjögren's syndrome (sicca syndrome) with either glandular or extraglandular involvement, but without other connective tissue diseases, were studied with regard to immunoregulatory T-cell subpopulations, B-cell function, and suppressor cell capabilities. Patients with isolated glandular disease as well as patients with extraglandular disease had normal absolute numbers of total lymphocytes, T cells, and B cells. However, 9 of 11 patients with extraglandular disease and only 3 of 10 patients with glandular disease had decreased relative proportions of T cells bearing receptors for the Fc portion of immunoglobulin (Ig)G (TG) which was explained by a factor that blocked the expression of the IgG Fc receptor on TG cells. This blockage was reversible since the factor could be removed by trypsinizing the T cells before TG determination. Serum from patients with abnormal proportions of TG cells, but not serum from patients with normal proportions of TG cells, blocked the expression of the IgG Fc receptor on normal T cells. The serum factor upon fractionation over Bio-Gel A 1.5 columns as well as over staphylococcal protein A-Sepharose 4B columns was found diffusely within the IgG fraction, and not in the IgM fraction.
Neither patients with glandular nor patients with extraglandular disease manifested increased numbers of in vivo-activated circulating lymphocytes as determined by spontaneous anti-trinitrophenyl (TNP) plaque-forming cells (PFC). However, patients with glandular disease had reduced numbers of pokeweed mitogen-induced anti-sheep erythrocyte PFC (P < 0.01) as compared with normals and patients with glandular disease. Of note was the fact that despite the modulation of TG subpopulation by the serum factor in patients with extra-glandular disease, these patients manifested normal concanavalin A-generated suppressor cells of pokeweed mitogen-induced PFC responses in allogeneic co-cultures. This was unlike the suppressor cell defect previously described in this system with systemic lupus erythematosus patients. The discrepancy was attributed both to the fact that the TG defect was reversible and to the fact that concanavalin A-generated suppressor cells are not limited to the TG subset. Thus, these studies have demonstrated reversible abnormalities in TG cells in patients with extraglandular Sjögren's syndrome which are not associated with suppressor cell defects. The discrepancy between these findings and the immuno-regulatory defects demonstrated in systemic lupus erythematosus may explain the difference in severity of the autoimmune expression in these diseases.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Sagawa A., Pascual E., Hebert J., Sadeghee S. Suppressor T-cell abnormality in idiopathic systemic lupus erythematosus. Clin Immunol Immunopathol. 1976 Sep;6(2):192–199. doi: 10.1016/0090-1229(76)90110-0. [DOI] [PubMed] [Google Scholar]
- Akizuki M., Boehm-Truitt M. J., Kassan S. S., Steinberg A. D., Chused T. M. Purification of an acidic nuclear protein antigen and demonstration of its antibodies in subsets of patients with sicca syndrome. J Immunol. 1977 Sep;119(3):932–938. [PubMed] [Google Scholar]
- Alarcón-Segovia D., Ruíz-Argüelles A. Decreased circulating thymus-derived cells with receptors for the Fc portion of immunoglobulin G in systemic lupus erythematosus. J Clin Invest. 1978 Dec;62(6):1390–1394. doi: 10.1172/JCI109260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspaugh M. A., Talal N., Tan E. M. Differentiation and characterization of autoantibodies and their antigens in Sjögren's syndrome. Arthritis Rheum. 1976 Mar-Apr;19(2):216–222. doi: 10.1002/art.1780190214. [DOI] [PubMed] [Google Scholar]
- Anderson L. G., Talal N. The spectrum of benign to malignant lymphoproliferation in Sjögren's syndrome. Clin Exp Immunol. 1972 Feb;10(2):199–221. [PMC free article] [PubMed] [Google Scholar]
- BLOCH K. J., BUCHANAN W. W., WOHL M. J., BUNIM J. J. SJOEGREN'S SYNDROME. A CLINICAL, PATHOLOGICAL, AND SEROLOGICAL STUDY OF SIXTY-TWO CASES. Medicine (Baltimore) 1965 May;44:187–231. [PubMed] [Google Scholar]
- Bresnihan B., Jasin H. E. Suppressor function of peripheral blood mononuclear cells in normal individuals and in patients with systemic lupus erythematosus. J Clin Invest. 1977 Jan;59(1):106–116. doi: 10.1172/JCI108607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budman D. R., Merchant E. B., Steinberg A. D., Doft B., Gershwin M. E., Lizzio E., Reeves J. P. Increased spontaneous activity of antibody-forming cells in the peripheral blood of patients with active SLE. Arthritis Rheum. 1977 Apr;20(3):829–833. doi: 10.1002/art.1780200312. [DOI] [PubMed] [Google Scholar]
- Cohen P. L., Ziff M. Abnormal polyclonal B cell activation in NZB/NZW F1 mice. J Immunol. 1977 Oct;119(4):1534–1537. [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C. The effect of in vivo hydrocortisone on subpopulations of human lymphocytes. J Clin Invest. 1974 Jan;53(1):240–246. doi: 10.1172/JCI107544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. Human B cell function in a polyclonally induced plaque forming cell system. Cell triggering and immunoregulation. Immunol Rev. 1979;45:93–116. doi: 10.1111/j.1600-065x.1979.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. Human bone marrow lymphocytes. I. Distribution of lymphocyte subpopulations in the bone marrow of normal individuals. J Clin Invest. 1975 Jul;56(1):98–110. doi: 10.1172/JCI108085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R. Polyclonal activation of bone-marrow-derived lymphocytes from human peripheral blood measured by a direct plaque-forming cell assay. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3676–3679. doi: 10.1073/pnas.73.10.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Pratt K. R., Whalen G. Activation of human B lymphocytes. II. Cellular interactions in the PFC response of human tonsillar and peripheral blood B lymphocytes to polyclonal activation by pokeweed mitogen. J Immunol. 1976 Dec;117(6):2100–2104. [PubMed] [Google Scholar]
- Fauci A. S., Steinberg A. D., Haynes B. F., Whalen G. Immunoregulatory aberrations in systemic lupus erythematosus. J Immunol. 1978 Oct;121(4):1473–1479. [PubMed] [Google Scholar]
- Feldmann J. L., Becker M. J., Moutsopoulos H., Fye K., Blackman M., Epstein W. V., Talal N. Antibody-dependent cell-mediated cytotoxicity in selected autoimmune diseases. J Clin Invest. 1976 Jul;58(1):173–179. doi: 10.1172/JCI108447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinski W., Gershwin M. E., Budman D. R., Steinberg A. D. Study of lymphocyte subpopulations in normal humans and patients with systemic lupus erythematosus by fractionation of peripheral blood lymphocytes on a discontinuous Ficoll gradient. Clin Exp Immunol. 1976 Nov;26(2):228–238. [PMC free article] [PubMed] [Google Scholar]
- Glinski W., Gershwin M. E., Steinberg A. D. Fractionation of cells on a discontinuous Ficoll gradient. Study of subpopulations of human T cells using anti-T-cell antibodies from patients with systemic lupus erythematosus. J Clin Invest. 1976 Mar;57(3):604–614. doi: 10.1172/JCI108316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon R., Haire M., Wisdom G. B., Neill D. W. The use of indirect immunofluorescence to evaluate the gel filtration method of fractionating human immunoglobulins. J Immunol Methods. 1975;8(1-2):29–36. doi: 10.1016/0022-1759(75)90078-2. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. III. Concanavalin A-induced generation of suppressor cells of the plaque-forming cell response of normal human B lymphocytes. J Immunol. 1977 Jun;118(6):2281–2287. [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. X. Heterogeneity of concanavalin A-generated suppressor cells of the pokeweed mitogen-induced plaque-forming cell response of human peripheral blood lymphocytes. J Immunol. 1978 Aug;121(2):559–565. [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. The differential effect of in vivo hydrocortisone on the kinetics of subpopulations of human peripheral blood thymus-derived lymphocytes. J Clin Invest. 1978 Mar;61(3):703–707. doi: 10.1172/JCI108982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward A. R., Layward L., Lydyard P. M., Moretta L., Dagg M., Lawton A. R. Fc-receptor heterogeneity of human suppressor T cells. J Immunol. 1978 Jul;121(1):1–5. [PubMed] [Google Scholar]
- Horowitz S., Borcherding W., Moorthy A. V., Chesney R., Schulte-Wisserman H., Hong R. Induction of suppressor T cells in systemic lupus erythematosus by thymosin and cultured thymic epithelium. Science. 1977 Sep 2;197(4307):999–1001. doi: 10.1126/science.302032. [DOI] [PubMed] [Google Scholar]
- Jasin H. E., Ziff M. Immunoglobulin synthesis by peripheral blood cells in systemic lupus erythematosus. Arthritis Rheum. 1975 May-Jun;18(3):219–228. doi: 10.1002/art.1780180305. [DOI] [PubMed] [Google Scholar]
- Kassan S. S., Thomas T. L., Moutsopoulos H. M., Hoover R., Kimberly R. P., Budman D. R., Costa J., Decker J. L., Chused T. M. Increased risk of lymphoma in sicca syndrome. Ann Intern Med. 1978 Dec;89(6):888–892. doi: 10.7326/0003-4819-89-6-888. [DOI] [PubMed] [Google Scholar]
- Koike T., Kobayashi S., Yoshiki T., Itoh T., Shirai T. Differential sensitivity of functional subsets of T cells to the cytotoxicity of natural T-lymphocytotoxic autoantibody of systemic lupus erythematosus. Arthritis Rheum. 1979 Feb;22(2):123–129. doi: 10.1002/art.1780220204. [DOI] [PubMed] [Google Scholar]
- Lawley T. J., Moutsopoulos H. M., Katz S. I., Theofilopoulos A. N., Chused T. M., Frank M. M. Demonstration of circulating immune complexes in Sjögren's syndrome. J Immunol. 1979 Sep;123(3):1382–1387. [PubMed] [Google Scholar]
- Mackenzie M. R., Warner N. L., Mitchell G. F. The binding of murine immunoglobulins to staphylococcal protein A. J Immunol. 1978 May;120(5):1493–1496. [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Mingari M. C., Moretta A., Webb S. R. Subpopulations of human T cells identified by receptors for immunoglobulins and mitogen responsiveness. J Immunol. 1976 Dec;117(6):2171–2174. [PubMed] [Google Scholar]
- Moretta L., Mingari M. C., Moretta A., Cooper M. D. Human T lymphocyte subpopulations: studies of the mechanism by which T cells bearing Fc receptors for IgG suppress T-dependent B cell differentiation induced by pokeweed mitogen. J Immunol. 1979 Mar;122(3):984–990. [PubMed] [Google Scholar]
- Moretta L., Mingari M. C., Romanzi C. A. Loss of Fc receptors for IgG from human T lymphocytes exposed to IgG immune complexes. Nature. 1978 Apr 13;272(5654):618–620. doi: 10.1038/272618a0. [DOI] [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Abe T., Hara M., Homma M. In vitro TNP-specific antibody formation by peripheral lymphocytes from patients with systemic lupus erythematosus. Scand J Immunol. 1977;6(6-7):575–579. doi: 10.1111/j.1365-3083.1977.tb02135.x. [DOI] [PubMed] [Google Scholar]
- Morimoto C. Loss of suppressor T-lymphocyte function in patients with systemic lupus erythematosus (SLE). Clin Exp Immunol. 1978 Apr;32(1):125–133. [PMC free article] [PubMed] [Google Scholar]
- Moutsopoulos H. M., Boehm-Truitt M., Kassan S. S., Chused T. M. Demonstration of activation of B lymphocytes in New Zealand black mice at birth by an immunoradiometric assay for murine IgM. J Immunol. 1977 Nov;119(5):1639–1644. [PubMed] [Google Scholar]
- Moutsopoulos H. M., Webber B. L., Vlagopoulos T. P., Chused T. M., Decker J. L. Differences in the clinical manifestations of sicca syndrome in the presence and absence of rheumatoid arthritis. Am J Med. 1979 May;66(5):733–736. doi: 10.1016/0002-9343(79)91110-0. [DOI] [PubMed] [Google Scholar]
- Moutsopoulos H., Fye K. H., Sawada S., Becker M. J., Goldstein A., Talal N. In vitro effect of thymosin on T-lymphocyte rosette formation in rheumatic diseases. Clin Exp Immunol. 1976 Dec;26(3):563–573. [PMC free article] [PubMed] [Google Scholar]
- Pichler W. J., Lum L., Broder S. Fc-receptors on human T lymphocytes. I. Transition of Tgamma to Tmu cells. J Immunol. 1978 Oct;121(4):1540–1548. [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Sagawa A., Abdou N. I. Suppressor-cell dysfunction in systemic lupus erythematosus. Cells involved and in vitro correction. J Clin Invest. 1978 Oct;62(4):789–796. doi: 10.1172/JCI109190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Green I. Studies of immune functions of patients with systemic lupus erythematosus. I. Dysfunction of suppressor T-cell activity related to impaired generation of, rather than response to, suppressor cells. Arthritis Rheum. 1978 Jul-Aug;21(6):657–664. doi: 10.1002/art.1780210608. [DOI] [PubMed] [Google Scholar]
- Sjögren's syndrome--newer aspects of research, diagnosis, and therapy. Ann Intern Med. 1971 Dec;75(6):937–950. [PubMed] [Google Scholar]
- Steinberg A. D., Klassen L. W., Budman D. R., Williams G. W. Immunofluorescence studies of anti-T cell antibodies and T cells in systemic lupus erythematosus. Selective loss of brightly staining T cells in active disease. Arthritis Rheum. 1979 Feb;22(2):114–122. doi: 10.1002/art.1780220203. [DOI] [PubMed] [Google Scholar]
- TALAL N., BUNIM J. J. THE DEVELOPMENT OF MALIGNANT LYMPHOMA IN THE COURSE OF SJOEGREN'S SYNDROME. Am J Med. 1964 Apr;36:529–540. doi: 10.1016/0002-9343(64)90101-9. [DOI] [PubMed] [Google Scholar]
- Talal N. Disordered immunologic regulation and autoimmunity. Transplant Rev. 1976;31:240–263. doi: 10.1111/j.1600-065x.1976.tb01456.x. [DOI] [PubMed] [Google Scholar]
- Talal N., Sokoloff L., Barth W. F. Extrasalivary lymphoid abnormalities in Sjögren's syndrome (reticulum cell sarcoma, "pseudolymphoma," macroglobulinemia). Am J Med. 1967 Jul;43(1):50–65. doi: 10.1016/0002-9343(67)90148-9. [DOI] [PubMed] [Google Scholar]
- Talal N., Sylvester R. A., Daniels T. E., Greenspan J. S., Williams R. C., Jr T and B lymphocytes in peripheral blood and tissue lesions in Sjögren's syndrome. J Clin Invest. 1974 Jan;53(1):180–189. doi: 10.1172/JCI107536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey J. J., Laughter A. H., Steinberg A. D. A serum inhibitor of immune regulation in patients with systemic lupus erythematosus. J Clin Invest. 1978 Sep;62(3):713–715. doi: 10.1172/JCI109180. [DOI] [PMC free article] [PubMed] [Google Scholar]


