Abstract
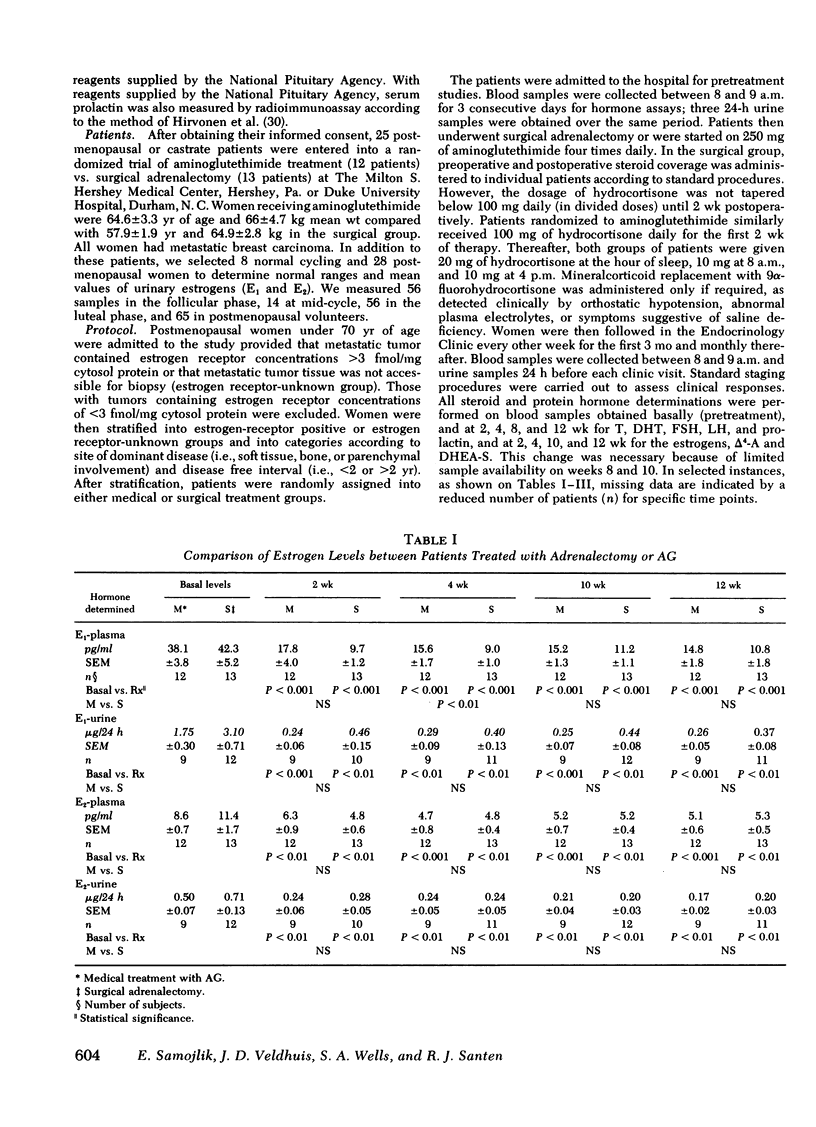
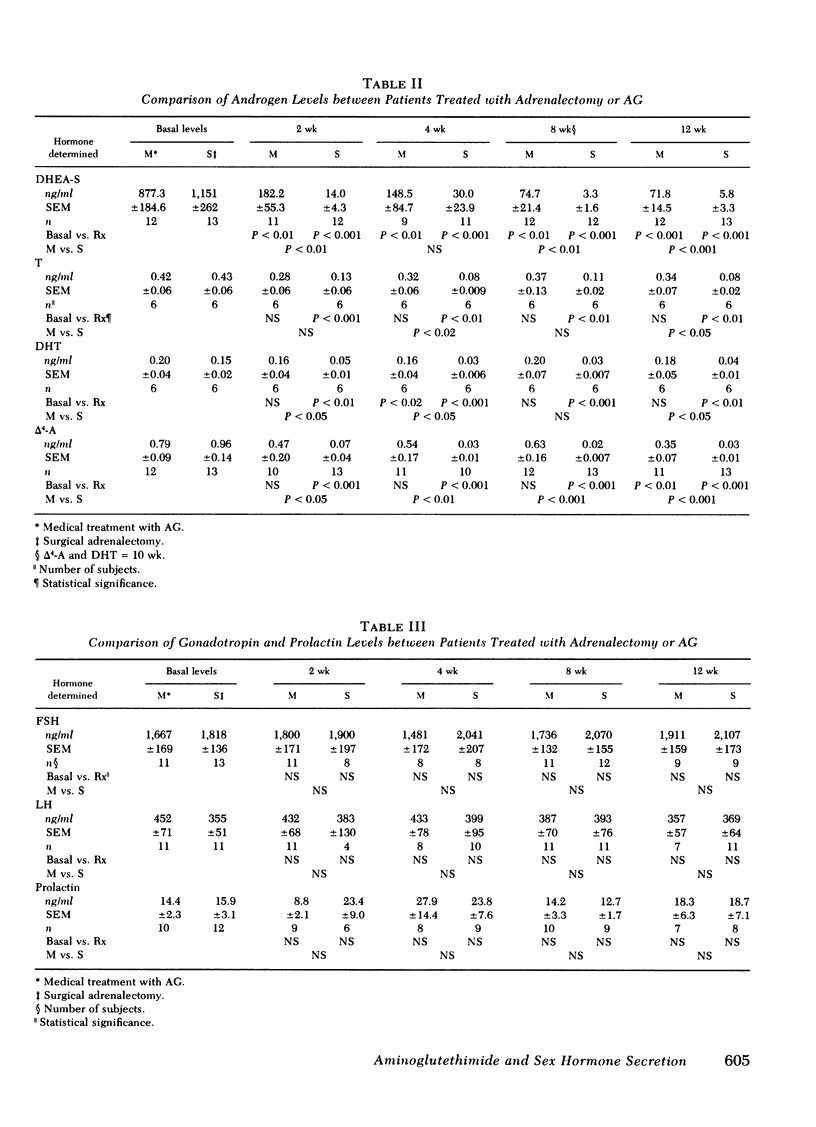
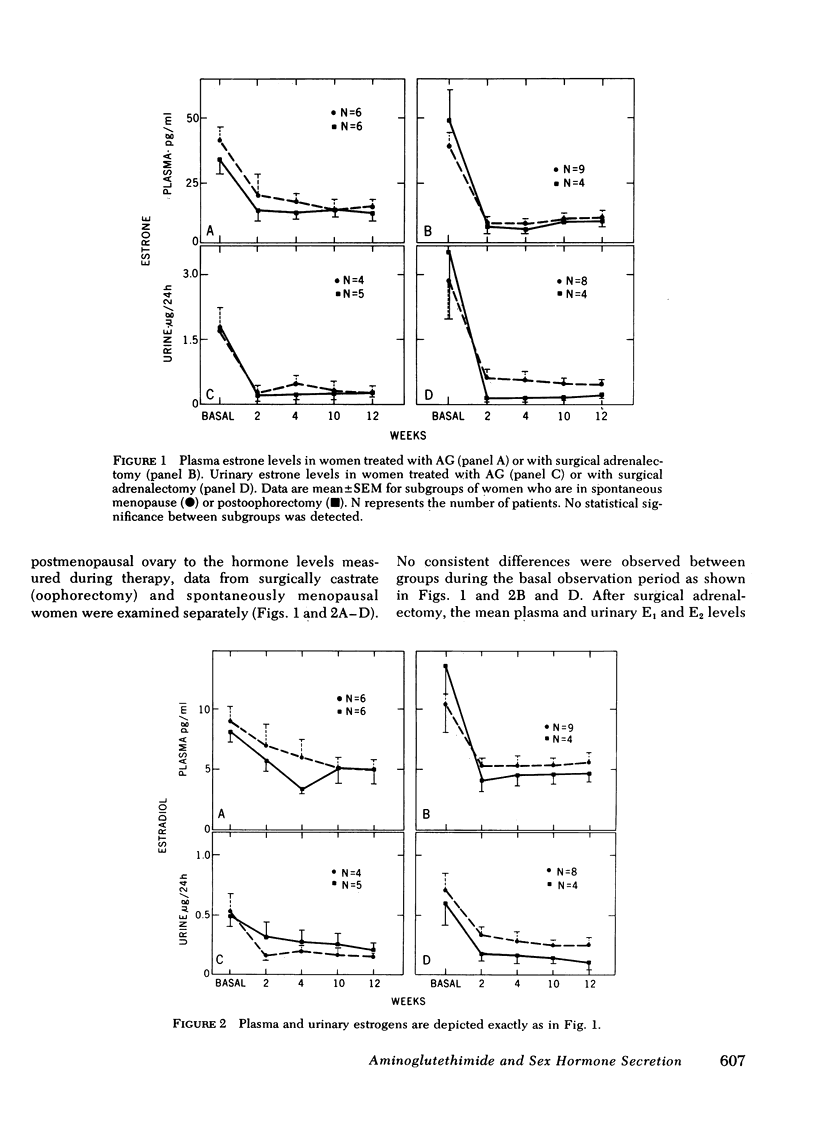
We evaluated the comparative effects of aminoglutethimide (AG) on androgen and estrogen levels estrone ([E1], estradiol [E2], plasma dehydroepiandrosterone-sulfate [DHEA-S], testosterone [T], dihydrotestosterone [DHT], delta 4-androstenedione [delta 4-A]), follicle-stimulating hormone (FSH), luteinizing hormone (LH), and prolactin in postmenopausal patients with breast cancer randomly allocated to either AG treatment or bilateral surgical adrenalectomy as a control group. In response to either treatment, the plasma levels of E1 fell 62-75% (P less than 0.001) and urine E1 85.7-88.7% (P less than 0.001) in all study days over a 12-wk period. Similarly, the concentrations of E2 in plasma and urine fell 40-72% without statistically significant differences between the two treatment modalities. The relatively weak androgen, DHEA-S, was reduced by 92% (877.3 +/- 184.6 to 71.8 +/- 14.5 ng/ml) at 12 wk in women treated with AG, but suppressed nearly 99% (1,151 +/- 262 to 5.8 +/- 3.3 ng/ml) in adrenalectomized women. At all time points after treatment, the DHEA-S levels were significantly higher in patients receiving AG. Plasma concentrations of the potent androgens, T and DHT, were also relatively preserved during AG treatment. T levels were never significantly reduced by AG, and DHT concentrations were decreased only at the 4th wk to a maximum of 20%. delta 4-A levels fell 56% in response to this drug only on the 12th wk of therapy (basal, 0.79 +/- 0.09 ng/ml; 12 wk, 0.35 +/- 0.07 ng/ml). In marked contrast, all androgens fell significantly at each time period in response to surgical adrenalectomy, with an 81% maximum suppression of T, 73% of DHT, and 97% of delta 4-A. In response to estrogen suppression, plasma levels of FSH, LH, and prolactin did not change significantly throughout the treatment period in either therapy group. To examine possible contributions of the postmenopausal ovary to hormone levels during therapy, data from surgically castrate and spontaneously menopausal women were evaluated separately. No significant differences between the two groups were observed for E1, E2, T, DHT, DHEA-S, delta 4-A, LH, FSH, and prolactin. We conclude that equivalent and highly significant estrogen suppression occurs with either AG or surgical adrenalectomy although androgen secretion is preserved during AG treatment but not after surgical adrenalectomy. The combined effects of estrogen deprivation associated with androgen preservation might be significant in the therapeutic action of AG in hormone-responsive neoplasms.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULBROOK R. D., GREENWOOD F. C., HAYWARD J. L. Selection of breast-cancer patients for adrenalectomy or hypophysectomy by determination of urinary 17-hydroxycorticosteroids and aetiocholanolone. Lancet. 1960 May 28;1(7135):1154–1157. doi: 10.1016/s0140-6736(60)91040-0. [DOI] [PubMed] [Google Scholar]
- BULBROOK R. D., HAYWARD J. L., SPICER C. C., THOMAS B. S. Abnormal excretion of urinary steroids by women with early breast cancer. Lancet. 1962 Dec 15;2(7268):1238–1240. doi: 10.1016/s0140-6736(62)92812-x. [DOI] [PubMed] [Google Scholar]
- Cash R., Brough A. J., Cohen M. N., Satoh P. S. Aminoglutethimide (Elipten-Ciba) as an inhibitor of adrenal steroidogenesis: mechanism of action and therapeutic trial. J Clin Endocrinol Metab. 1967 Sep;27(9):1239–1248. doi: 10.1210/jcem-27-9-1239. [DOI] [PubMed] [Google Scholar]
- Cash R., Petrini M. A., Brough A. J. Ovarian dysfunction associated with an anticonvulsant drug. JAMA. 1969 May 19;208(7):1149–1152. [PubMed] [Google Scholar]
- Cohen M. P. Aminoglutethimide inhibition of adrenal desmolase activity. Proc Soc Exp Biol Med. 1968 Apr;127(4):1086–1090. doi: 10.3181/00379727-127-32877. [DOI] [PubMed] [Google Scholar]
- Dexter R. N., Fishman L. M., Ney R. L., Liddle G. W. Inhibition of adrenal corticosteroid synthesis by aminoglutethimide: studies of the mechanism of action. J Clin Endocrinol Metab. 1967 Apr;27(4):473–480. doi: 10.1210/jcem-27-4-473. [DOI] [PubMed] [Google Scholar]
- Fracchia A. A., Farrow J. H., Miller T. R., Tollefsen R. H., Greenberg E. J., Knapper W. H. Hypophysectomy as compared with adrenalectomy in the treatment of advanced carcinoma of the breast. Surg Gynecol Obstet. 1971 Aug;133(2):241–246. [PubMed] [Google Scholar]
- Fracchia A. A. Indications for castration and adrenalectomy for advanced breast cancer. Cancer. 1971 Dec;28(6):1699–1701. doi: 10.1002/1097-0142(197112)28:6<1699::aid-cncr2820280660>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Fracchia A. A., Randall H. T., Farrow J. H. The results of adrenalectomy in advanced breast cancer in 500 consecutive patients. Surg Gynecol Obstet. 1967 Oct;125(4):747–756. [PubMed] [Google Scholar]
- Goldenberg I. S., Waters N., Ravdin R. S., Ansfield F. J., Segaloff A. Androgenic therapy for advanced breast cancer in women. A report of the cooperative breast cancer group. JAMA. 1973 Mar 12;223(11):1267–1268. [PubMed] [Google Scholar]
- Gower D. B. Modifiers of steroid-hormone metabolism: a review of their chemistry, biochemistry and clinical applications. J Steroid Biochem. 1974 Aug;5(5):501–523. doi: 10.1016/0022-4731(74)90051-x. [DOI] [PubMed] [Google Scholar]
- Gupta C., Osterman J., Santen R., Bardin C. W. In vivo metabolism of progestins. V. The effect of protocol design on the estimated metabolic clearance rate and volume of distribution of medroxyprogesterone acetate in women. J Clin Endocrinol Metab. 1979 May;48(5):816–820. doi: 10.1210/jcem-48-5-816. [DOI] [PubMed] [Google Scholar]
- Harvey H. A., Santen R. J., Osterman J., Samojlik E., White D. S., Lipton A. A comparative trial of transsphenoidal hypophysectomy and estrogen suppression with aminoglutethimide in advanced breast cancer. Cancer. 1979 Jun;43(6):2207–2214. doi: 10.1002/1097-0142(197906)43:6<2207::aid-cncr2820430608>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Hirvonen E., Ranta T., Seppäla M. Prolactin and thyrotropin responses to thyrotropin-releasing hormone in patients with secondary amenorrhea: the effect of bromocriptine. J Clin Endocrinol Metab. 1976 Jun;42(6):1024–1030. doi: 10.1210/jcem-42-6-1024. [DOI] [PubMed] [Google Scholar]
- Horký K., Küchel O., Gregorvá I., Stárka L. Qualitative alterations in urinary 17-ketosteroid excretion during aminoglutethimide administration. J Clin Endocrinol Metab. 1969 Feb;29(2):297–299. doi: 10.1210/jcem-29-2-297. [DOI] [PubMed] [Google Scholar]
- Krieger D. T., Samojlik E., Bardin C. W. Cortisol and androgen secretion in a case of Nelson's syndrome with paratesticular tumors: response to cyproheptadine therapy. J Clin Endocrinol Metab. 1978 Oct;47(4):837–844. doi: 10.1210/jcem-47-4-837. [DOI] [PubMed] [Google Scholar]
- Li K., Foo T., Adams J. B. Products of dehydroepiandrosterone metabolism by human mammary tumors and their influence on estradiol receptor binding. Steroids. 1978 Jan;31(1):113–127. doi: 10.1016/0039-128x(78)90023-5. [DOI] [PubMed] [Google Scholar]
- Lipton A., Santen R. J. Proceedings: Medical adrenalectomy using aminoglutethimide and dexamethasone in advanced breast cancer. Cancer. 1974 Feb;33(2):503–512. doi: 10.1002/1097-0142(197402)33:2<503::aid-cncr2820330227>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Newsome H. H., Brown P. W., Terz J. J., Lawrence W., Jr Medical and surgical adrenalectomy in patients with advanced breast carcinoma. Cancer. 1977 Feb;39(2):542–546. doi: 10.1002/1097-0142(197702)39:2<542::aid-cncr2820390224>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Newsome H. H., Jr, Brown P. W., Terz J. J., Lawrence W., Jr Medical adrenalectomy and plasma steroids in advanced breast carcinoma. Surgery. 1978 Jan;83(1):83–89. [PubMed] [Google Scholar]
- PEARSON O. H., RAY B. S. Results of hypophysectomy in the treatment of metastatic mammary carcinoma. Cancer. 1959 Jan-Feb;12(1):85–92. doi: 10.1002/1097-0142(195901/02)12:1<85::aid-cncr2820120114>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Poortman J., Thijssen J. H., Schwarz F. Androgen production and conversion to estrogens in normal postmenopausal women and in selected breast cancer patients. J Clin Endocrinol Metab. 1973 Jul;37(1):101–109. doi: 10.1210/jcem-37-1-101. [DOI] [PubMed] [Google Scholar]
- Ray B. S. Current cancer concepts. Hypophysectomy as palliative treatment. JAMA. 1967 Jun 12;200(11):974–975. [PubMed] [Google Scholar]
- SEGALOFF A., CARABASI R., HORWITT B. N., SCHLOSSER J. V., MURISON P. J. Hormonal therapy in cancer of the breast. VI. Effect of ACTH and cortisone on clinical course and hormonal excretion. Cancer. 1954 Mar;7(2):331–334. doi: 10.1002/1097-0142(195403)7:2<331::aid-cncr2820070218>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Samojlik E., Santen R. J. Adrenal suppression with aminoglutethimide. III. Comparison of plasma delta 4- and delta 5-steroids in postmenopausal women treated for breast carcinoma. J Clin Endocrinol Metab. 1978 Oct;47(4):717–724. doi: 10.1210/jcem-47-4-717. [DOI] [PubMed] [Google Scholar]
- Samojlik E., Santen R. J., Wells S. A. Adrenal suppression with aminoglutethimide. II. Differential effects of aminoglutethimide on plasma androstenedione and estrogen levels. J Clin Endocrinol Metab. 1977 Sep;45(3):480–487. doi: 10.1210/jcem-45-3-480. [DOI] [PubMed] [Google Scholar]
- Santen R. J., Leonard J. M., Sherins R. J., Gandy H. M., Paulsen C. A. Short- and long-term effects of clomiphene citrate on the pituitary-testicular axis. J Clin Endocrinol Metab. 1971 Dec;33(6):970–979. doi: 10.1210/jcem-33-6-970. [DOI] [PubMed] [Google Scholar]
- Santen R. J., Santner S., Davis B., Veldhuis J., Samojlik E., Ruby E. Aminoglutethimide inhibits extraglandular estrogen production in postmenopausal women with breast carcinoma. J Clin Endocrinol Metab. 1978 Dec;47(6):1257–1265. doi: 10.1210/jcem-47-6-1257. [DOI] [PubMed] [Google Scholar]
- Santen R. J., Wells S. A., Cohn N., Demers L. M., Misbin R. I., Foltz E. L. Compensatory increase in TSH secretion without effect on prolactin secretion in patients treated with aminoglutethimide. J Clin Endocrinol Metab. 1977 Oct;45(4):739–746. doi: 10.1210/jcem-45-4-739. [DOI] [PubMed] [Google Scholar]
- Santen R. J., Wells S. A., Runić S., Gupta C., Kendall J., Rudy E. B., Samojlik E. Adrenal suppression with aminoglutethimide. I. Differential e-fects of aminoglutethimide on glucocorticoid metabolism as a rationale for use of hydrocortisone. J Clin Endocrinol Metab. 1977 Sep;45(3):469–479. doi: 10.1210/jcem-45-3-469. [DOI] [PubMed] [Google Scholar]
- Schwarzel W. C., Kruggel W. G., Brodie H. J. Studies on the mechanism of estrogen biosynthesis. 8. The development of inhibitors of the enzyme system in human placenta. Endocrinology. 1973 Mar;92(3):866–880. doi: 10.1210/endo-92-3-866. [DOI] [PubMed] [Google Scholar]
- Siiteri P. K., Thompson E. A. Studies of human placental aromatase. J Steroid Biochem. 1975 Mar-Apr;6(3-4):317–322. doi: 10.1016/0022-4731(75)90149-1. [DOI] [PubMed] [Google Scholar]
- Siiteri P. K., Williams J. E., Takaki N. K. Steroid abnormalities in endometrial and breast carcinoma: a unifying hypothesis. J Steroid Biochem. 1976 Nov-Dec;7(11-12):897–903. doi: 10.1016/0022-4731(76)90008-x. [DOI] [PubMed] [Google Scholar]
- Silverstein M. J., Byron R. L., Jr, Yonemoto R. H., Riihimaki D. U., Schuster G. Bilateral adrenalectomy for advanced breast cancer: a 21 year experience. Surgery. 1975 Jun;77(6):825–832. [PubMed] [Google Scholar]
- Smith J. A., King R. J. Effects of steroids on growth of an androgen-dependent mouse mammary carcinoma in cell culture. Exp Cell Res. 1972 Aug;73(2):351–359. doi: 10.1016/0014-4827(72)90058-4. [DOI] [PubMed] [Google Scholar]
- Thijssen J. H., Poortman J., Schwarz F. Androgens in postmenopausal breast cancer: excretion, production and interaction with estrogens. J Steroid Biochem. 1975 May;6(5):729–734. doi: 10.1016/0022-4731(75)90060-6. [DOI] [PubMed] [Google Scholar]
- Thompson E. A., Jr, Siiteri P. K. The involvement of human placental microsomal cytochrome P-450 in aromatization. J Biol Chem. 1974 Sep 10;249(17):5373–5378. [PubMed] [Google Scholar]
- Tormey D. C., Simon R. M., Lippman M. E., Bull J. M., Myers C. E. Evaluation of tamoxifen dose in advanced breast cancer: a progress report. Cancer Treat Rep. 1976 Oct;60(10):1451–1459. [PubMed] [Google Scholar]
- Uzgiris V. I., Whipple C. A., Salhanick H. A. Stereoselective inhibition of cholesterol side chain cleavage by enantiomers of aminoglutethimide. Endocrinology. 1977 Jul;101(1):89–92. doi: 10.1210/endo-101-1-89. [DOI] [PubMed] [Google Scholar]
- Wang C. F., Lasley B. L., Yen S. S. Gonadotropin secretion in response to low and high doses of LRF in normal and hypogonadal women (functional disparity of the gonadotrophs). J Clin Endocrinol Metab. 1976 Mar;42(3):427–431. doi: 10.1210/jcem-42-3-427. [DOI] [PubMed] [Google Scholar]
- Wells S. A., Jr, Santen R. J., Lipton A., Haagensen D. E., Jr, Ruby E. J., Harvey H., Dilley W. G. Medical adrenalectomy with aminoglutethimide: clinical studies in postmenopausal patients with metastatic breast carcinoma. Ann Surg. 1978 May;187(5):475–484. doi: 10.1097/00000658-197805000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis K. J., London D. R., Ward H. W., Butt W. R., Lynch S. S., Rudd B. T. Recurrent breast cancer treated with the antioestrogen tamoxifen: correlation between hormonal changes and clinical course. Br Med J. 1977 Feb 12;1(6058):425–428. doi: 10.1136/bmj.1.6058.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachmann M., Gitzelmann R. P., Zagalak M., Prader A. Effect of aminoglutethimide on urinary cortisol and cortisol metabolites in adolescents with Cushing's syndrome. Clin Endocrinol (Oxf) 1977 Jul;7(1):63–71. doi: 10.1111/j.1365-2265.1977.tb02940.x. [DOI] [PubMed] [Google Scholar]


