Abstract
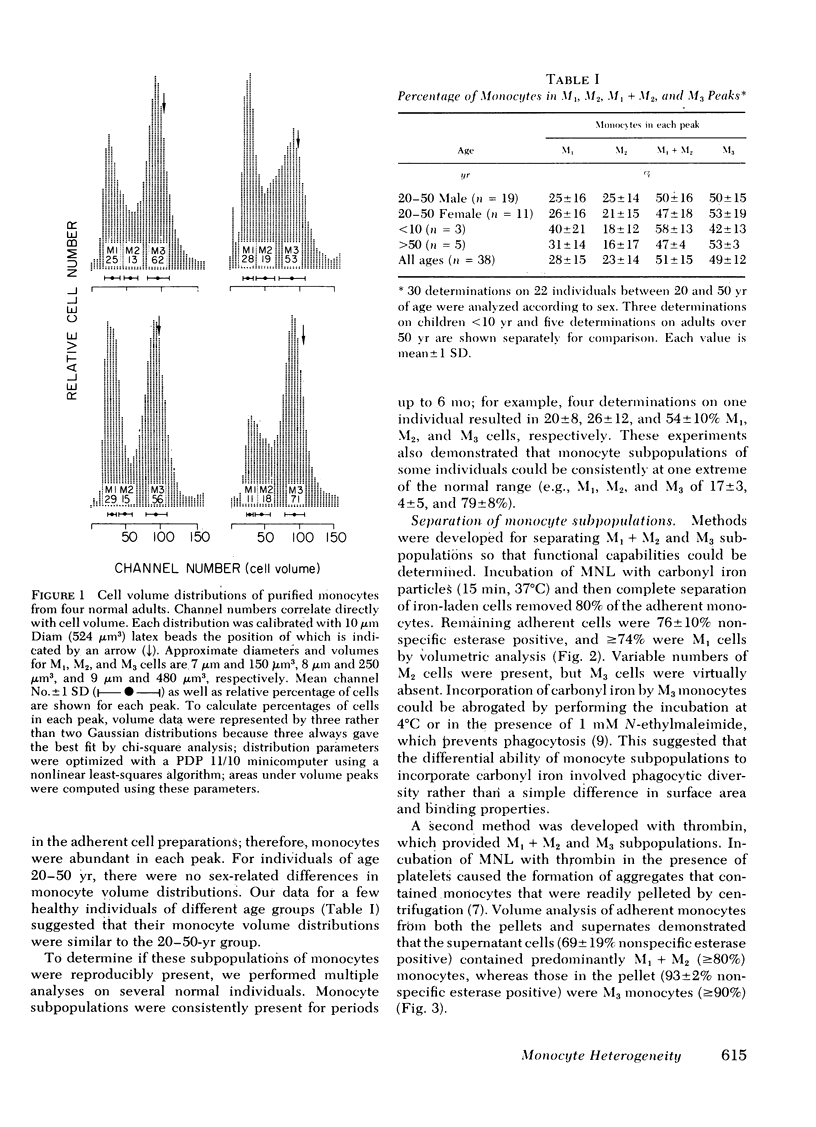
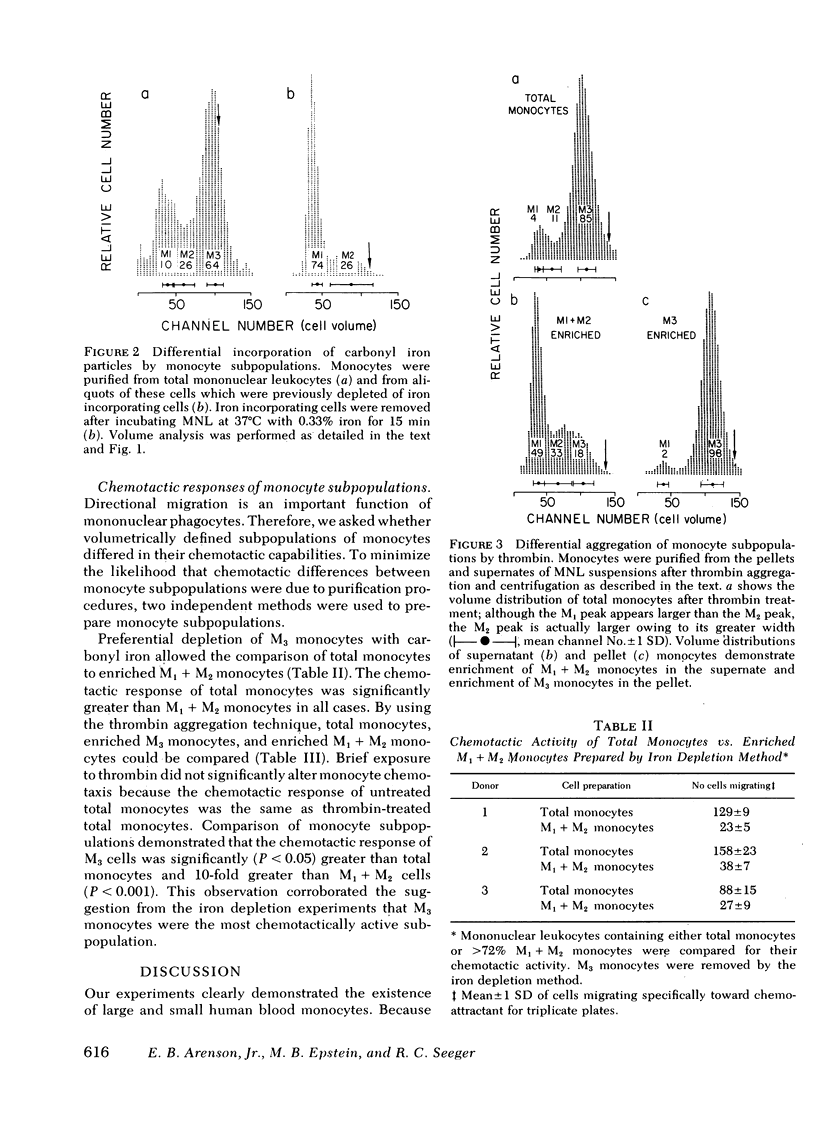
Volume analysis of purified human blood monocytes revealed distinct populations of large and small cells. Computer curve fitting suggested a third, intermediate-sized population. These monocytes were designated M1, M2, and M3 in order of increasing size, and their approximate volumes were 150, 250, and 480 micron3, respectively. The three subpopulations were present in all 30 normal individuals tested. Two new techniques were developed that separate monocytes into M1 + M2 and M3 fractions; one used preferential incorporation of carbonyl iron particles by M3 cells and the other used the selective aggregation of M3 cells by thrombin in the presence of platelets. The chemotactic response to zymosan-activated human serum by total monocytes, M1 + M2 monocytes, and M3 monocytes was determined by the agarose plate method. In all experiments M3 monocytes were 10-fold more responsive than M1 + M2 monocytes and were significantly more so than total monocytes. These findings suggest that M3 cells are the major subpopulation capable of directional migration. This investigation establishes the existence of volumetrically definable subpopulations of human monocytes that are functionally distinct.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böyum A. A one-stage procedure for isolation of granulocytes and lymphocytes from human blood. General sedimentation properties of white blood cells in a 1g gravity field. Scand J Clin Lab Invest Suppl. 1968;97:51–76. [PubMed] [Google Scholar]
- Klein R. B., Fischer T. J., Gard S. E., Biberstein M., Rich K. C., Stiehm E. R. Decreased mononuclear and polymorphonuclear chemotaxis in human newborns, infants, and young children. Pediatrics. 1977 Oct;60(4):467–472. [PubMed] [Google Scholar]
- Lee K. C., Berry D. Functional heterogeneity in macrophages activated by Corynebacterium parvum: characterization of subpopulations with different activities in promoting immune responses and suppressing tumor cell growth. J Immunol. 1977 May;118(5):1530–1540. [PubMed] [Google Scholar]
- Norris D. A., Morris R. M., Sanderson R. J., Kohler P. F. Isolation of functional subsets of human peripheral blood monocytes. J Immunol. 1979 Jul;123(1):166–172. [PubMed] [Google Scholar]
- Rotman B., Papermaster B. W. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc Natl Acad Sci U S A. 1966 Jan;55(1):134–141. doi: 10.1073/pnas.55.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkamp J. A., Fulwyler M. J., Coulter J. R., Hiebert R. D., Horney J. L., Mullancy P. F. A new multiparameter separator for microscopic particles and biological cells. Rev Sci Instrum. 1973 Sep;44(9):1301–1310. doi: 10.1063/1.1686375. [DOI] [PubMed] [Google Scholar]
- Terasaki P. I., Bernoco D., Park M. S., Ozturk G., Iwaki Y. Microdroplet testing for HLA-A, -B, -C, and -D antigens. The Phillip Levine Award Lecture. Am J Clin Pathol. 1978 Feb;69(2):103–120. doi: 10.1093/ajcp/69.2.103. [DOI] [PubMed] [Google Scholar]
- Tucker S. B., Pierre R. V., Jordon R. E. Rapid identification of monocytes in a mixed mononuclear cell preparation. J Immunol Methods. 1977;14(3-4):267–269. doi: 10.1016/0022-1759(77)90137-5. [DOI] [PubMed] [Google Scholar]


