Abstract
Cytokine and growth factor signaling mediates essential roles in the differentiation, proliferation, survival and function of a number of cell lineages. This is achieved via specific receptors located on the surface of target cells, with ligand binding activating key intracellular signal transduction cascades to mediate the requisite cellular outcome. Effective resolution of receptor signaling is also essential, with excessive signaling having the potential for pathological consequences. The Suppressor of cytokine signaling (SOCS) family of proteins represent one important mechanism to extinguish cytokine and growth factor receptor signaling. There are 8 SOCS proteins in mammals; SOCS1-7 and the alternatively named Cytokine-inducible SH2-containing protein (CISH). SOCS1-3 and CISH are predominantly associated with the regulation of cytokine receptor signaling, while SOCS4-7 are more commonly involved in the control of Receptor tyrosine kinase (RTK) signaling. Individual SOCS proteins are typically induced by specific cytokines and growth factors, thereby generating a negative feedback loop. As a consequence of their regulatory properties, SOCS proteins have important functions in development and homeostasis, with increasing recognition of their role in disease, particularly their tumor suppressor and anti-inflammatory functions. This review provides a synthesis of our current understanding of the SOCS family, with an emphasis on their immune and hematopoietic roles.
Keywords: SOCS, development, immunity, disease, JAK-STAT, cytokine, growth factor, signalling, receptor tyrosine
Introduction
Overview
Cytokines and growth factors are important mediators of cell-cell communication. These glycoproteins are secreted by cells in response to normal developmental cues or environmental stimuli to relay information to specific target cells expressing the appropriate receptor on their surface. Receptor binding initiates a range of intracellular signaling cascades that lead to appropriate cellular responses, such as proliferation, differentiation, survival and functional activation [1,2]. Subsequent dissipation of receptor signaling is essential to ensure the response of the cell does not become pathogenic. The Suppressor of cytokine signaling (SOCS) proteins represent one key mechanism by which this level of control is achieved [3,4].
SOCS structure and function
There are eight mammalian SOCS family members; SOCS1-7 and the alternatively named Cytokine-inducible SH2-containing protein (CISH) [5] (Figure 1). While SOCS proteins are able to regulate signaling downstream of a range of receptors, current evidence indicates that CISH and SOCS1-3 are most often associated with regulation of cytokine receptor signaling through the JAK-STAT pathway, while SOCS4-7 predominantly regulate growth factor receptor signaling [6-8]. This difference reflects their evolutionary history, with the precursors to the SOCS4-7 sub-family existing prior to the emergence of a functional cytokine receptor pathway, while the sub-family comprising of SOCS1-3 and CISH emerged later, co-incident with the cytokine receptor family [9,10]. Within each sub-family, pairs of SOCS proteins have similar structure and function: CISH/SOCS2, SOCS1/SOCS3, SOCS4/SOCS5 and SOCS6/SOCS7, again reflecting their evolutionary history [9].
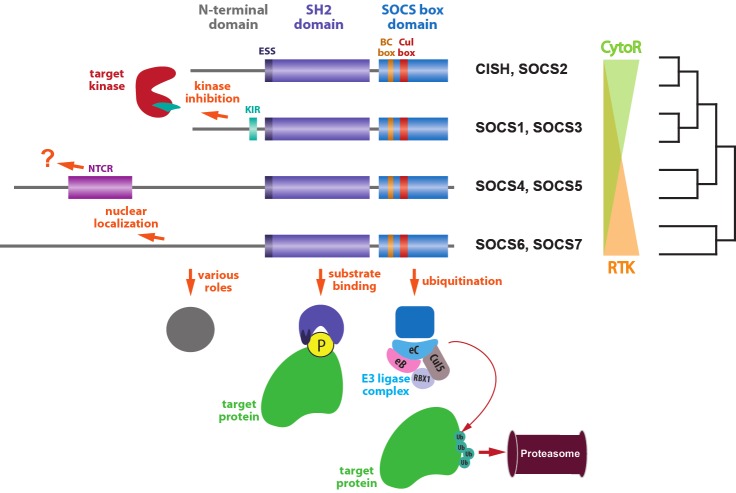
Figure 1.
Structural and functional relationships amongst SOCS proteins. SOCS proteins can be grouped into pairs with similar structure and function, and these further grouped into those mainly acting on Cytokine receptor (CytoR) signaling and those mainly acting on Receptor tyrosine kinase (RTK) signaling, reflecting evolutionary relationships. All SOCS proteins consist of three conserved domains, the N-terminal, SH2 and SOCS box domains. The N-terminal domain is the least conserved and has a variety of roles, with specific sub-domains identified in certain SOCS pairs, including the kinase inhibitory region (KIR) in SOCS1 and SOCS3 and the N-terminal conserved region (NTCR) in SOCS4 and SOCS5. The SH2 domain is lengthened by the addition of an extended SH2 sequence (ESS) and is involved in substrate binding via interaction with specific phosphotyrosine residues on the target protein. The SOCS box consists of BC box and Cul box sub-domains that recruit Elongin B and C, Cullin5, RBX1 and other E3 ligase components to mediate ubiquitination of target proteins and their subsequent proteasomal degradation.
Each SOCS protein contains three distinct domains; an N-terminal domain of low conservation, a conserved central Src-homology 2 (SH2) domain, and a more highly conserved C-terminal domain termed the SOCS box. The N-terminal domain is variable in length between members, with SOCS1-3 and CISH having a shorter N-terminal domain in comparison to SOCS4-7 [11]. Within the N-terminal domains of SOCS1 and SOCS3 is a so-called kinase-inhibitory region (KIR), which is responsible for inhibition of cytokine receptor-associated Janus kinases (JAKs) [12]. SOCS4 and SOCS5 also possess a highly conserved region within their N-terminal domain, termed the N-terminal conserved region (NTCR), although the role of this sequence has not been elucidated [13]. In contrast, the N-terminal domains of SOCS6 and SOCS7 have been shown to be required for their respective nuclear translocation and, in the case of SOCS7, appears to be involved in transporting other proteins into the nucleus [14-16]. The SH2 domains of the SOCS proteins interact in a context-specific manner with phosphotyrosine residues present on their target proteins, including cell surface receptors, imparting on SOCS proteins their target specificity [4]. These are longer than typical SH2 domains due to a so-called extended SH2 sequence (ESS) also found in STAT1 and STAT3, which contributes to their function [12]. Finally, the SOCS box is comprised of two functional sub-domains; a BC box that recruits Elongin B and C, and a Cul box that mediates Cullin5 binding. The resulting complex is able to bind RBX2, leading in turn to recruitment of the remaining components of an E3 ubiquitin ligase complex [17,18].
Control of signalling by SOCS proteins
Certain members of the SOCS family, typically SOCS1-3 and CISH, are induced by cytokine receptors and serve to extinguish signaling from the same receptor, providing a classical negative feedback loop [19] (Figure 2A). This occurs via activation of receptor-associated Janus kinases (JAKs), which phosphorylate tyrosine residues on the receptor complex to recruit signaling molecules, including Signal Transducer and Activator of Transcription (STAT) proteins. These also become tyrosine phosphorylated, leading to dimerization and nuclear translocation, where they stimulate transcription of target genes. They include SOCS genes, the encoded proteins of which are able to inhibit intracellular signaling by a number of different mechanisms that vary between individual family members. All SOCS proteins are able to regulate receptor signaling through the recruitment of proteasomal degradation components to their target proteins, be they specific receptors or associated molecules. This is achieved by binding to these targets through their SH2 domains and recruitment of Elongin B/C heterodimers, Cullin5 and other components of a E3 ubiquitination complex via their SOCS box [18,20]. SOCS-associated molecules are then readily able to be ubiquitinated, which typically targets these proteins to the proteasome. SOCS members can also regulate signaling via alternate methods. This is particularly true of SOCS1 and SOCS3, which bind Cullin5 at a much lower affinity than other SOCS proteins and remain partially active even in the absence of their SOCS box [20,21]. As an alternative mechanism, SOCS1 and SOCS3 are able to directly inhibit JAK kinases, binding via their KIR domain to the JAK activation loop to inhibit kinase activity [12,22-24]. CISH, SOCS2 and SOCS3 can also inhibit signaling via their ability to bind to phosphotyrosine residues typically on receptors, thereby blocking access of other SH2-containing signaling molecules [11,25-28].
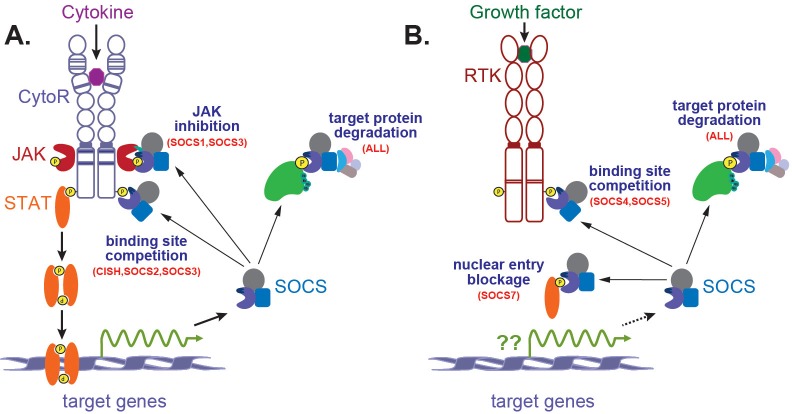
Figure 2.
Signaling via by cytokines and growth factors and its negative regulation by SOCS proteins. Cytokines (A) or growth factors (B) bind to their respective cell surface receptors, Cytokine receptors (CytoRs) and Receptor tyrosine kinases (RTKs), leading to receptor dimerization. For CytoRs this leads to activation of associated intracellular JAKs, which phosphorylate tyrosine residues on the receptor complex, creating docking site for STATs. These are then phosphorylated, enabling dimerization with other STATs, followed by translocation to the nucleus, where they act as transcription factors to induce expression of target genes that mediate a range of biological processes. These targets include SOCS genes capable of regulating receptor signaling, creating a negative feedback loop. RTKs possess intrinsic tyrosine kinase activity and may by-pass JAK and/or STAT activation. Individual SOCS proteins negatively regulate signaling by several mechanisms: degradation of receptors or associated proteins via the proteasomal pathway; inhibition of JAK tyrosine activity; competition for receptor phosphotyrosine residues thereby blocking other signaling molecules; prevention of nuclear translocation of key signaling molecules.
In the case of growth factors, intracellular signalling is mediated by so-called receptor tyrosine kinases (RTKs), with SOCS4-7 more typically involved in their regulation, although the factors regulating their expression are less well characterized (Figure 2B). The SOCS proteins regulate RTKs via target protein degradation, and in the case of SOCS4 and SOCS5 also binding site competition, while SOCS7 has been shown to directly bind signaling proteins to prevent their nuclear translocation, thereby inhibiting their ability to signal [29]. However, the division of SOCS proteins between cytokine receptor and RTKs is not strict. Moreover, SOCS proteins can also participate in cross-talk between receptors and also regulate other pathways, such as Toll-like receptors (TLRs).
Role of SOCS proteins
The function of individual SOCS proteins has been investigated by a range of approaches, with mouse knockout (KO) and transgenic (Tg) models revealing important roles in development (Table 1). Moreover, the SOCS proteins are being increasingly implicated in disease (Table 2).
Table 1.
Function of SOCS proteins revealed by mouse models
| Knockout | Transgenic | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| SOCS protein | Type | Phenotype | Affected pathway | REF | Phenotype | Affected pathway | REF |
| CISH | Not yet reported | [256] | Growth retardation | GH/STAT5B | [54] | ||
| Lactation failure | PRL/STAT5A | [54] | |||||
| Reduced γδ T and NK cells | IL-2/STAT5 | [54] | |||||
| Altered T cell response | IL-2/STAT5 | [54] | |||||
| SOCS1 | Complete | Neonatal death | IFNγ | [90,91] | Spontaneous T cell activation | γc cytokines | [257] |
| Complete | Lymphopenia | IFNγ | [91] | Defective T cell development | γc cytokines | [257] | |
| Complete | Monocytic organ infiltration | IFNγ | [90] | Fewer γδ T cells | γc cytokines | [257] | |
| Complete | Fatty degeneration /liver necrosis | IFNγ/STAT1 & IL-4/STAT6 | [90] | ||||
| Complete+IFNγ-/- | Polycystic kidneys | ? | [93] | ||||
| Complete+IFNγ-/- | Chronic inflammatory lesions | ? | [93] | ||||
| Complete+IFNγ-/- | Perturbed T cell development | γc cytokines | [94,95] | ||||
| Complete+IFNγ-/- | Sensitive to LPS | TLR | [69] | ||||
| T cell | Increased CD8+ differentiation | γc cytokines | [98] | ||||
| Complete but T&B cells | Splenomegaly | IL-4/IFNγ | [52] | ||||
| Complete but T&B cells | Lymphadenopathy | IL-4/IFNγ | [52] | ||||
| Complete but T&B cells | Spontaneous dermatitis | IL-4/IFNγ | [52] | ||||
| SOCS2 | Complete | Gigantism | GH/STAT5B/IGF-1 | [140] | Gigantism | GH/STAT5B/IGF-1 | [143] |
| SOCS3 | Complete | Embryonic lethal | LIF | [190,258] | Embryonic lethal/anaemia | EPO/STAT5 | [258] |
| Complete+LIF+/- | Death by 190 days | ? | [192] | ||||
| Hematopoietic | Neutrophilia/Inflammatory conditions | G-CSF | [181] | ||||
| Neuronal | Resistance to diet induced obesity | Leptin | [259] | ||||
| Complete but T cells | Perturbed CD8+ T cell proliferation | IL-6 & IL-27 | [180] | ||||
| SOCS4 | Not yet reported | Not yet reported | |||||
| SOCS5 | Complete | No reported phenotype | [233] | Reduced Th2 differentiation | IL-4 | [28] | |
| SOCS6 | Complete | Mild growth retardation | IGF-1 | [7] | Increased glucose metabolism | INS/p85 | [241] |
| SOCS7 | Complete (mixed b/g) | Altered glucose homeostasis | INS | [8,252] | Not yet reported | ||
| Complete (mixed b/g) | Large pancreatic islets | ?INS | [8] | ||||
| Complete (C57BL b/g) | Hydrocephalus/growth retardation | ?INS & IGF-1 | [252] | ||||
| Complete (mixed b/g) | Cutaneous disease | [254] | |||||
Table 2.
Association of SOCS proteins with disease
| SOCS protein | Disease association | Mechanism | Reference |
|---|---|---|---|
| CISH | Infectious disease susceptibility | SNP at position -29 (blunted induction by IL-2) | [56] |
| Osteoarthritis | Decreased expression | [60] | |
| SOCS1 | Acute myeloid leukemia | Hypermethylation | [116,117] |
| Glioblastoma multiforme | Hypermethylation | [127] | |
| Barrett’s adenocarcinoma | Hypermethylation | [125] | |
| Chronic myeloid leukemia | Hypermethylation | [118] | |
| Breast cancer | Hypermethylation | [128] | |
| Ovarian cancer | Hypermethylation | [128] | |
| Cervical carcinogenesis | Hypermethylation | [123] | |
| Esophageal squamous cell carcinoma | Hypermethylation | [124] | |
| Hepatocellular carcinoma | Hypermethylation & gene loss | [126] | |
| Chronic myeloid leukemia | Constitutive expression | [121] | |
| Hodgkin lymphoma | SOCS1 mutation, enhanced | [113] | |
| Primary mediastinal B-cell lymphoma | Hypermethylation | [115] | |
| Tuberculosis | Increased expression | [134] | |
| SOCS2 | Ovarian cancer | Hypermethylation | [128] |
| Acromegaly associated colonic polyps | Increased expression | [162] | |
| Osteoarthritis | Decreased expression | [60] | |
| Type 2 diabetes | 5’ SNP | [61] | |
| SOCS3 | Lung cancer | Hypermethylation | [214] |
| Barrett’s adenocarcinoma | Hypermethylation | [125] | |
| Hepatocellular carcinoma | Hypermethylation | [211] | |
| Malignant melanoma | Hypermethylation | [212] | |
| Glioma | Hypermethylation | [219] | |
| Prostate cancer | hypermethylation | [220] | |
| Ulcerative colitis (areas of dysplasia) | Deceased expression | [216] | |
| Breast cancer with lymph node metastasis | Reduced expression | [221] | |
| Atopic asthma/dermatitis (Th2 disease) | Increased SOCS3 expression (peripheral T-cells) | [197] | |
| Breast cancer with lymph node metastasis | Reduced expression | [221] | |
| SOCS4 | Gastric cancer | Hypermethylation | [229] |
| SOCS5 | Uveitis | Increased expression | [236] |
| Thyroid gland cancer | Decreased expression | [239] | |
| SOCS6 | Gastric cancer | Hypermethylation & gene loss | [249] |
| Colorectal cancer | Gene loss | [251] | |
| Primary lung squamous cell carcinoma | Decreased expression | [248] | |
| Liver cancer | Decreased expression | [239] | |
| Thyroid gland cancer | Decreased expression | [239] | |
| SOCS7 | Breast cancer | Decreased expression | [228] |
| Metabolic syndrome | Specific haplotypes | [255] |
CISH
CISH, the founding member of the SOCS family, was identified as an immediate-early gene induced in hematopoietic cells in response to stimulation by a variety of cytokines, including erythropoietin (EPO), interleukin-2 (IL-2), IL-3, and granulocyte-macrophage colony-stimulating factor (GM-CSF), which encoded a protein that was able to bind to activated cytokine receptors [30,31]. Subsequent studies revealed induction by growth hormone (GH), prolactin (PRL) [32-34], IL-9 [35], thrombopoietin (TPO) [36,37] and granulocyte colony-stimulating factor (G-CSF) [38], each of which also activate STAT5. In mice, strong expression of Cish was observed in the kidney, lung and liver [30], with lower expression in heart, stomach, testis, spleen, and thymus [30,36]. Stat5a/b KO mice had no detectable Cish expression [39,40], underpinning the key role of STAT5 in CISH gene regulation. Indeed, CISH has been shown to be a direct STAT5 target gene, with two sets of conserved tandem STAT5 binding sites present in its promoter [31,41]. CISH is also induced by IL-6 [36], IL-10 [42], interferon (IFN)α [43], IFNγ [36], tumor necrosis factor (TNF)α [36], thymic stromal lymphopoietin (TSPL) [44], leptin [45], and ciliary neurotropic factor (CNTF) [46]. Finally, CISH has been found to be an early response gene induced by T cell receptor (TCR) stimulation via an alternative pathway not involving STAT5 [47]. Recent studies suggest that CISH is regulated post-transcriptionally by microRNAs (miRs), with miR-98 or let-7 shown to target the 3’ untranslated region (UTR) of the CISH mRNA, causing translational repression. Stimulation with bacterial lipopolysaccharide (LPS) or Cryptosporidium parvum infection in vitro was able to decrease expression of miR-98 and let-7, thereby relieving the miR-mediated translational suppression of CISH [48]. The CISH 3’UTR also contains ATAA destabilisation motifs, while the CISH protein possesses PEST sequences, which lead to rapid turnover of the mRNA and protein, respectively [30].
CISH is known to negatively regulate signaling induced by EPO [31], GH [26], IL-2 [49], IL-3 [30], and PRL [34,50]. CISH binds via its SH2 domain to phosphorylated tyrosine residues of activated cytokine receptors, where it suppresses signaling via at least two mechanisms [25,26]. Firstly, CISH can bind to the same receptor phosphotyrosine sites as STAT5, thereby physically blocking further STAT5 docking, which has been demonstrated for the EPO receptor (EPOR) and growth hormone receptor (GHR) [25,26,51]. CISH can also negatively regulate signaling at the receptor level by facilitating proteasomal degradation of activated receptor complexes via interactions between its SOCS box, Elongin B/C and Cullin5 [52,53]. Interestingly, CISH has been suggested to be a positive regulator of TCR-mediated MAPK activation in T cells, with increased levels of CISH promoting TCR-mediated T cell proliferation and cytokine secretion [47], although the mechanism remains to be elucidated.
Transgenic mice expressing CISH under the control of the β-actin promoter exhibited growth retardation caused by reduced GHR signaling, defective mammary gland development due to disrupted PRL receptor (PRLR) signaling, and altered T and Natural killer (NK) cell responses as a result of blunted IL-2R signaling in T cells [54]. These phenotypes were consistent with those observed in Stat5a/b-deficient mice [39,40], suggesting a specific in vivo role in the regulation of STAT5. An alternate transgenic mouse expressing CISH in CD4+ T cells showed inhibited TCR signalling, but in a STAT5-independent manner [47].
No Cish KO mouse has been described. However, a recent study has indicated a role for CISH during the GM-CSF-mediated ex vivo development of mouse bone-marrow-derived dendritic cells (BMDCs). CISH was found to be induced by GM-CSF treatment, and CISH knockdown caused a decline in MHC class I, pro-inflammatory cytokines, and co-stimulatory molecule expression, concurrent with a substantial increase in the production of BMDCs via increased proliferation and reduced apoptosis. This was associated with enhanced STAT5 activation. Therefore, CISH expression appears to regulate progenitor cell proliferation late in development via feedback inhibition of STAT5 activation to allow differentiation to proceed effectively [55]. Functional analysis of a zebrafish CISH homologue, cish.a, has revealed it to be a direct downstream target of the JAK2/STAT5 pathway in vivo, with specific ablation of cish.a leading to a significant enhancement of embryonic erythropoiesis, myelopoiesis and lymphopoiesis, consistent with a role for CISH in the negative regulation of the JAK2/STAT5 pathway in vivo (ACW, unpublished).
CISH has also been implicated in disease. An association has been found between CISH single nucleotide polymorphisms (SNPs) and susceptibility to infectious diseases, including bacteremia, malaria, and tuberculosis. A SNP at position -292 of the CISH promoter was the most highly associated, increasing the overall risk of infectious disease by at least 18% among persons carrying the variant allele. This allele blunted the induction of CISH mRNA following IL-2 stimulation, suggesting enhanced IL-2R signaling might be responsible for the increased susceptibility [56]. The -292 SNP was also found to be associated with hepatitis B virus infection in a Vietnamese population [57]. Other studies have suggested that CISH contributes to expansion of regulatory T cells (Tregs) in response to microbial infection [58]. Levels of CISH mRNA were also found to be higher in peripheral blood mononuclear cells (PBMCs) from systemic lupus erythematous (SLE) patients in acute phase compared to either normal individuals or patients with inactive phase of disease, suggesting that CISH represents a marker of SLE and may be involved in disease pathogenesis [59]. Alterations in CISH levels have also recently been linked to osteoarthritis, although in this case CISH mRNA levels were found to be 10-fold lower in chondrocytes from osteoarthritic patients compared to control samples [60]. This suggests CISH plays a clinically-relevant role that might provide new strategies for controlling infectious agents and inflammatory diseases.
SOCS1
SOCS1 was shown to be highly expressed in both mouse and human thymus and spleen [5,36,61]. It was also expressed in the lung, testis [36], colon and mesenteric lymph nodes [5]. SOCS1 has been shown to be induced by numerous cytokines in vitro and ex vivo, including IL-2 [62], IL-4 [22,63], IL-6 [22], IL-13 [64], IFNα/β [65,66], IFN-γ [36,67], EPO [23,36], G-CSF [22], LIF [22], PRL [34], GH [32], CNTF [46] and TNFα [68]. SOCS1 is also induced by Toll-like receptor (TLR) ligands, such as LPS [69,70] and CpG DNA [71], as well as INS [72] and thyroid stimulating hormone (TSH) [73]. Bioinformatic analysis has identified the 3’UTR region of SOCS1 as a potential target of miR-155. This miR is normally induced by TNF-α through the JNK pathway with knockdown of miR-155 in mouse osteoblastic cells resulting in increased SOCS1 protein expression following TNFα stimulation. In contrast, transfection with miR-155 inhibited wild-type SOCS1 [74]. In T cells, FOXP3 negatively regulates miR-155 thereby contributing to the maintenance of SOCS1 levels in these cells [75].
SOCS1 has also been found to regulate signaling by a raft of receptors in vitro, including those for the cytokines IFNα [67], IFNγ [67], EPO [23], PRL [33,34], GH [32,51], LIF [22], TNF-α [68], IL-2 [23,62], IL-3 [23], IL-4 [63], IL-6 [22], IL-7 [76], IL-12 [77], IL-15 [78], EPO [23], TPO [36], TSLP [44], oncostatin M (OSM) [36], and leptin [79], as well as the receptors for INS [80] and IGF-1 [81], and the TLRs [82]. SOCS1 is known to regulate signaling via two mechanisms. Firstly, it can bind directly to cytokine receptor-associated JAK1, JAK2, and TYK2 to inhibit their tyrosine kinase activity via its ESS/KIR domains, and consequently suppress activation of downstream pathways [12,21-23]. However, like other SOCS family members, SOCS1 can also interact with Elongin B/C and Cullin5 via its SOCS box, facilitating ubiquitination and proteasomal degradation of target substrates [17,83], including JAK1 [83], JAK2 [84], TEL-JAK2 [85,86], GEF, VAV [87], insulin receptor substrate (IRS)-1 and IRS-2 [88], as well the TLR2/4 adaptor protein MAL [82]. Interestingly, the SOCS box has also been shown to protect SOCS1 against proteolytic degradation [89].
SOCS1 has been shown to have several essential roles in immunity. Socs1 KO mice developed a fatal neonatal disease that resulted in death by three weeks of age [90,91]. These animals exhibited severe lymphopenia and T cell-mediated autoimmune inflammatory disease, characterized by monocytic infiltration of major organs along with fatty degeneration and necrosis of the liver [90]. These phenotypes were significantly reduced in Ifnγ/Socs1 double KO mice and in Socs1 KO mice treated with anti-IFNγ antibodies [92,93], indicating that hyper-responsiveness to IFNγ was the chief cause and confirming SOCS1 as a potent in vivo regulator of IFNγ. However, these Ifnγ/Socs1 double KO mice developed additional phenotypes, including polycystic kidneys, chronic infections, and inflammatory lesions, which resulted in survival to only 6 months of age [93]. T cell development was also perturbed, including reduced T cells numbers [94], disrupted Th2 responses [95], a reduced CD4/CD8 ratio [94], as well as abnormal development of Th17 cells [96], resulting from hypersensitivity to cytokines acting via the γc receptor: IL-2, IL-4, IL-7, IL-15 [97] and IL-12 [77].
T cell-specific Socs1 KO mice did not develop the lethal multi-organ inflammation, but rather specific lymphoid deficiencies, including increased differentiation toward CD8(+) T cells and phenotypes correlating with hypersensitivity to γc receptor utilizing cytokines [98]. These mice also showed a 10-fold increase in FOXP3(+) CD4(+) T regulatory (Treg) cells in the thymus, indicating SOCS1 potentially negatively regulates the generation and/or accumulation of these cells [99]. The increase in Treg cells was still apparent when these mice were crossed with those lacking IFNγ or IL-7 indicating other cytokines mediate this effect [99]. Further supporting a Treg role, mice lacking Socs1 expression in all but their T and B cells developed spontaneous dermatitis, splenomegaly, and lymphadenopathy. They showed an accumulation of DCs in their thymi and spleens, which were hyper-responsive to both IFNγ and IL-4, resulting in increased levels of BAFF/BLyS and APRIL, facilitating the generation of autoantibodies. This resulted in the development of systemic autoimmune-like diseases with hypergammaglobulinemia at an early age [52]. Whilst it has been demonstrated that SOCS1 is important in helper T cell (Th) differentiation, there is conflicting data regarding exactly which cell fate SOCS1 drives differentiation towards. Some studies have suggested that SOCS1 favors Th1 differentiation, while others suggest that IL-6 induced SOCS1 blocks Th1 development via the inhibition of IFNγR, leading to accelerated Th2 differentiation [100]. To add to the complexity, SOCS1 has been shown to be necessary for Th17 differentiation via its suppressive effects on IFNγ [101].
SOCS1 has been demonstrated to have several other in vivo regulatory functions. Thus, Socs1 KO mice also showed impaired osteoblast differentiation [102], as well as enhanced insulin signaling [103]. In addition, cells from SOCS1 transgenic mice were unable to respond to LPS, suggesting that SOCS1 inhibited TLR/NF-κB signaling in vivo [69]. Socs1 deficiency also resulted in excessive macrophage and dendritic cell activation [52,69], potentially caused by the combined effects of unrestrained signaling via IFNRAR1 [104] and TLRs [82,105]. Knockdown of zebrafish socs1 resulted in perturbation of specific myeloid populations during embryogenesis prior to the commencement of lymphopoiesis, along with reduced numbers of T cells. Zebrafish SOCS1 was shown to interact with the zebrafish JAK2/STAT5 pathway both in vitro and in vivo. This demonstrated SOCS1 has a conserved role in T cell development, but exerts a T cell-independent function in embryonic myelopoiesis likely mediated via regulation of receptors that utilise the JAK2-STAT5 pathway [106].
Defective SOCS1 signaling has been associated with a range of inflammatory disorders. In a murine arthritis model, SOCS1 was expressed in multiple cell types in the arthritic joint, with the extent of joint destruction and synovial inflammation exacerbated in Socs1 KO mice [107]. SOCS1 expression has been shown to correlate inversely with the severity of disease in idiopathic pulmonary fibrosis patients, with adenovirally-delivered SOCS1 decreasing fibrosis, inflammation and mortality in a murine model of pulmonary inflammation [108]. However, SOCS1 does not always confer protection against inflammatory/immune diseases. Thus, SOCS1 Tg mice spontaneously develop colitis, with severe intestinal inflammation [109]. Moreover, a SOCS1 promoter SNP that increases SOCS1 expression was associated with adult-onset asthma [110]. SOCS1 was also shown to protect β-cells from cytotoxic T cells in a murine type 1 diabetes model [111]. Further, increased expression of SOCS1 was observed in insulin-resistant mice, with down-modulation of SOCS1 leading to increased insulin-sensitivity in these mice [112].
SOCS1 has also been suggested to have a tumor suppressor role, particularly in hematological malignancies and proliferative disorders. Thus, the SOCS1 gene has been found to be frequently mutated in both classical Hodgkin lymphoma [113,114] and primary mediastinal B-cell lymphoma [115], leading to augmented signaling by STAT5 [113,115] and STAT6 [114]. The SOCS1 gene was commonly silenced by hypermethylation (and occasionally mutation) in acute myeloid leukemia [116,117], with the reintroduction of SOCS1 causing growth suppression in affected cells [117]. Chronic myeloid leukemia patients also demonstrated hypermethylation of SOCS1 that reverted to an unmethylated state during remission [118]. Some Philadelphia chromosome (Ph)-negative myeloproliferative disorders (MPDs) exhibit SOCS1 hypermethylation, which may complement other mutations, such as the hyperactive JAK2V617F mutation [119]. Others have alternatively found that SOCS1 is overexpressed in Ph-negative MPDs, potentially acting as a compensatory feedback mechanism [120]. Indeed, constitutive expression of SOCS1 has been observed in chronic myeloid leukemia (CML) [121], in line with hypomethylation of this gene [122]. SOCS1 expression in CML also correlated with a poor response to interferon α, treatment, likely due to a direct effect on receptor signaling [121]. Hypermethylation of SOCS1 has been commonly reported in solid tumors, including 61% of cervical cancer samples [123], and 45% of esophageal squamous cell carcinoma samples [124], as well as occasionally in Barrett’s adenocarcinoma [125], with combined hypermethylation/gene loss observed in hepatocellular carcinoma [126]. Hypermethylation-mediated silencing has also been seen in glioblastoma multiforme, with concomitant enhancement of radio-resistance, indicative of a pro-apoptotic function [127]. Hypermethy-lation of the SOCS gene has also been observed in breast and ovarian cancer, where SOCS1 reintroduction was again able to suppress cell growth [128]. Spontaneous colorectal cancer was also seen in Socs1 KO mice in an IFNγ-dependent manner [129]. Finally, SOCS1 has also been shown to suppress oncogenic forms of VAV [87], c-MET [130], ABL and c-KIT [131], as well as TEL-JAK2 and BCR-ABL fusions [131].
SOCS1 also has roles in the response to infectious agents. For example, SOCS1 has been shown to protect against lethal inflammation induced by Chlamydia pneumoniae, although it hampered bacterial clearance due to its effects on IFNα/β-induced STAT1 [132]. SOCS1 also inhibited the antiviral response to influenza [133], and increased SOCS1 levels were associated with enhanced disease severity in tuberculosis [134]. In fact, many infectious agents specifically target SOCS1 to augment the infection process. Thus, Toxoplasma gondii induces SOCS1 expression leading to inhibition of cytokine signaling and suppression of immune responses [135]. Similarly, Mycobacterium bovis increases SOCS1 and SOCS3 to inhibit IFNγ-induced STAT1 [136]. Furthermore, bacterial flagellin was shown to act via TLR5 to induce SOCS1 thereby suppressing TCR-mediated T cell activation [137]. In contrast, hepatitis C core protein down-regulates SOCS1, resulting in enhanced STAT5 signaling in B cells [138].
SOCS2
SOCS2 was shown to be highly expressed in the fetal kidney [139], as well as adult kidney, lung, testes, liver [36,139,140], pancreatic islets [141], peripheral blood mononuclear leukocytes [142], and to a lesser extent in adult heart, muscle and brain [139,140]. SOCS2 is most closely related to CISH, and like CISH is induced by cytokines that activate STAT5, including GH [32,143], EPO [36,144], PRL [34] IL-2 [145], IL-3, GM-CSF and G-CSF [36]. However, it is also induced by CNTF [46], IFNα [43], IFNγ [36,142], leukemia inhibitory factor (LIF) [36], IL-1β [142], IL-4 [36], IL-6 [142], IL-15 [146] and insulin (INS) [72].
SOCS2 acts to regulate signaling induced by the cytokines GH [26], PRL [147], LIF [145], IL-2, IL-3 [148] and IL-6 [21], but also by growth factors, such as epidermal growth factor (EGF) [149] and insulin-like growth factor (IGF)-1 [139]. SOCS2 differs from other SOCS family members in two important and interesting ways. Firstly, SOCS2 appears to play a dualistic regulatory role, both inhibiting and potentiating signaling dependent on its concentration and cellular context [21,34,150]. In vitro studies have demonstrated that low levels of SOCS2 led to a reduction in GH signaling, while higher levels actually increased GH signaling [150]. Secondly, SOCS2 has been shown to possess the ability to antagonize other SOCS family members [50]. Thus, SOCS1 inhibition of GH signaling was reduced with increasing doses of co-transfected SOCS2 [150], while SOCS2 was shown to exert an antagonistic role in the SOCS1- and SOCS3-mediated negative regulation of IL-2 and IL-3 signaling, respectively [148]. SOCS2 primarily exerts its effects by stimulating ubiquitination of target proteins, including receptors, such as GHR [151], and signaling proteins, such as SOCS3 [148].
Socs2 knockout mice were indistinguishable from their littermates until 3 weeks of age, at which point they began to demonstrate increased overall growth, being 40% heavier than wild-type (WT) littermates by adulthood [140]. The increase in weight was due to increased bone length and enlargement of internal organs [140]. Constitutive expression of SOCS2 produced a similar phenotype, with SOCS2 transgenic mice being significantly larger than WT animals [152], consistent with the dualistic nature of SOCS2. A naturally-occurring mouse mutant, high growth (hg), which exhibited a 30-50% increase in postnatal growth, has been mapped to the Socs2 region, indicating that hg is most likely an allele of Socs2 – although whether it is hypomorphic or hypermorphic allele remains to be determined [153]. Socs2 KO mice exhibited prolonged STAT5B activation in response to GH, and crossing with Stat5b KO mice partially relieved the growth enhancement [143]. This suggests that SOCS2 regulates the GH/IGF-1 axis through negative regulation of the downstream STAT5B [140,143]. SOCS2 also has a role in controlling prolactin-induced mammary gland development, which appears to be the result of enhanced STAT5A activation [147]. SOCS2 also exerts a dualistic role in the regulation of EGF signaling, with increased intestinal growth in Socs2 KO mice due to enhanced responsiveness to EGF [154], and increased neural outgrowth of cortical neurons derived from SOCS2 transgenic mice, apparently also due to enhanced EGF signaling [155]. Transgenic mice overexpressing SOCS2 specifically in pancreatic β-cells using the rat insulin promoter displayed hyperglycemia and glucose intolerance, but did not exhibit overt diabetes [156]. In contrast, the pancreatic β-cells of Socs2 KO mice showed unaltered insulin and glucose tolerance when compared to WT mice [157].
SOCS2 also functions in immune cells, with roles in both DCs as well as CD4+ T cells. When SOCS2 was silenced in DCs, maturation was disrupted and a reduction in LPS stimulated MAPK activation was observed [158], suggesting a requirement for SOCS2 in TLR-induced DC activation. However, others have argued that SOCS2 is a TLR-responsive gene with its delayed expression providing a mechanism for late-phase counter-regulation to limit inflammation-driving DC activity [159]. SOCS2 silencing in CD4+ T cells resulted in increased preference for helper T cell (Th)2 differentiation, which is consistent with elevated Th2 responses observed in SOCS2 KO mice [160]. In human NK cells, SOCS2 was shown to be induced by IL-15 and targeted PYK2 for degradation, with SOCS2 knockdown resulting in defective NK cell effector functions [146].
Like its close homologue CISH, SOCS2 has been linked to osteoarthritis, with SOCS2 mRNA levels also found to be 10-fold lower in osteoarthritic samples when compared to control samples, with increased expression seen following cytokine treatment [60]. A SNP in the 5’ region of the SOCS2 gene was associated with type 2 diabetes (T2D) in a Japanese population. Adenovirus-mediated expression of the SOCS2 gene in pancreatic islets significantly suppressed glucose stimulated insulin secretion, suggesting a likely mechanism by which SOCS2 may influence susceptibility to T2D [61]. SOCS2 also appears to be a cellular target of the HIV-1 transactivator protein (Tat) in primary human monocytes, leading to increased SOCS2 levels, which was shown to suppress IFNγ-activated STAT1 phosphorylation, resulting in dysregulated cytokine production and immune evasion [161]. Finally, SOCS2 has been implicated in oncogenesis, where it also shows a dualistic nature. Patients with active acromegaly and colonic polyps showed a significantly increased SOCS2 expression, which mediated a reduction in SOCS1, leading to elevated STAT5B levels, potentially resulting in upregulation of GH-mediated proliferation of colonic epithelial cells [162]. In contrast, SOCS2 expression was shown to have a favorable prognostic value in breast cancer [163], and hypermethylation of SOCS2 was detected in ovarian but not breast cancer [128].
SOCS3
SOCS3 has been shown to be expressed in a wide variety of murine and human tissues. In mice, SOCS3 was found to be expressed in the spleen, thymus, and lung [36], while in humans, SOCS3 was expressed in the colon, spleen, bladder, peripheral blood leukocytes, trachea and placenta, with very high expression in the lung, adipose tissue, ovary and aorta [61]. SOCS3 has been demonstrated to be induced by the cytokines IL-1β [164], IL-2 [165], IL-3 [166], IL-4 [63], IL-6 [167], IL-9 [35], IL-10 [42], IL-11 [168], IL-13 [36], IL-22 [169], IFN-γ [67], IFNα [67], EPO [170], LIF [32], PRL [34], GH [32], leptin [171], G-CSF [36], GM-CSF [172], CNTF [46], TPO [66], TNFα [173], cardiotrophin (CT)1 [174], OSM [175]. It is also induced by several growth factors, including EGF [176,177], platelet-derived growth factor (PDGF) [176], thyroid stimulating hormone (TSH) [73], insulin [72], and basic fibroblast growth factor (BFGF) [178].
SOCS3 has been demonstrated to play a regulatory role in signaling downstream of a wide range of cytokine receptors, including those for IL-2 [165], IL-4 [63], IL-6 [21], IL-9 [35], IL-11 [168], IL-23 [179], IL-27 [180], IFNα/β [67], IFNγ [67], G-CSF [181], EPO [170], PRL [33,34], GH [32,51], LIF [32], leptin [171], CNTF [46], IL-1β [182], OSM [175] and CT1 [174], as well as IGF-1 [81], INS [183], CD28 [184] and calcineurin [185]. Like its closest homologue, SOCS1, the SOCS3 protein can directly inhibit receptor-bound JAKs, although it achieves this via a high-affinity interaction between its SH2 domain and a phosphotyrosine residue on the receptor (e.g. GP130), rather than the JAK [170], binding simultaneously to both [186]. SOCS3 also regulates signaling via binding site competition. Thus, SOCS3 has been shown to bind to the same site as the SH2-domain hematopoietic phosphatase (SHP)-2 on several receptors [27] and with STAT4 on others [187]. Finally, like other members of the SOCS family, SOCS3 can also regulate signaling by targeting proteins for degradation, however this is not its primary mechanism of action, as it can still regulate signaling without its SOCS box [21]. It has been demonstrated that an IL-6 transcriptional response can be converted to one mimicking that of interferons when SOCS3 is absent [167,188], while IL-7-induced viral clearance occurred via a mechanism that required both the induction of IL-6 and inhibition of SOCS3 expression [189]. Collectively, this suggests that SOCS3 primarily functions by dampening cytokine-induced STAT3 and STAT1 activation. Induction of SOCS3 is most pronounced by cytokines that strongly activate STAT3, with its regulatory specificity determined by the presence of high-affinity SOCS3-binding sites on target receptors.
Socs3 mouse KO embryos exhibited fatal placental defects during embryonic development and although anatomically sound, they did not survive past 13 days of gestation. These embryos showed expanded numbers of giant trophoblast cells in the placenta, as well as abnormities in the spongiotrophoblast and labyrinth placental layers [190]. LIFR deficit was able to rescue the Socs3 KO placental defect and embryonic lethality, establishing SOCS3 as an essential regulator of LIFR signaling during placental formation [191]. However, these double KO mice died by 190 days of age due to neutrophilia accompanied by inflammatory-cell tissue infiltration [192]. Hematopoietic-specific Socs3 KO mice developed a variety of inflammatory conditions, including a prolonged and enhanced responses to G-CSF that facilitated neutrophilia and enhanced progenitor cell survival, suggesting SOCS3 is an important in vivo regulator of G-CSFR signaling [181]. Interestingly, differentiation of SOCS3-deficient progenitors was skewed toward a macrophage state in response to G-CSF and IL-6 stimulation, suggesting that SOCS3 is also important in maintaining the specificity of biological responses mediated by cytokine signaling [193]. SOCS3 was also shown to be a positive regulator of TLR4 responses in macrophages via inhibition of IL-6R-mediated STAT3 activation [194] as well as endogenous TGFβ-mediated/SMAD3 [195]. Socs3 deficiency in either hepatocytes or macrophages resulted in prolonged IL-6-induced activation of STAT1 and STAT3, but normal IFNγ and IL-10 signaling [167,188,194]. These observations strongly suggest that SOCS3 targets GP130 dependent signal transduction pathways in vivo.
SOCS3 has been shown to be selectively expressed in Th2 cells [196] and required for Th2 development. Mice heterozygous for Socs3 or expressing a dominant-negative SOCS3 showed reduced Th2 development, while those expressing a SOCS3 transgene exhibited enhanced Th2 polarity [197]. T cell-specific Socs3 KO mice showed increased CD8(+) T cell proliferation via enhanced IL-6 and IL-27 signaling [180]. It has been suggested that the ability of SOCS3 to skew T cell differentiation to the Th2 phenotype may be due to an ability to compete for the STAT4-binding site on the IL-12Rβ2 chain, thus inhibiting IL-12/STAT4-driven polarization to the alternative Th1 phenotype [187], or alternatively via its inhibition of interferon-induced STAT1 activation that is also associated with Th1 polarization [167].
SOCS3 has diverse roles outside of the immune and hematopoietic lineages. Mammary stem/progenitor cell-specific Socs3 KO mice exhibited impaired lactation resulting from reduced proliferation [198]. Loss of SOCS3 from differentiated luminal cells resulted in accelerated tissue remodeling upon weaning [198]. SOCS3 has also been shown to play a role in fine-tuning photoreceptor cell differentiation [199], while SOCS3 transgenic mice showed reduced pancreatic β-cell mass and proliferation [200]. SOCS3 was also shown to be required for normal wound healing, again via action on GP130 signaling [201]. Finally, elevated levels of SOCS3 in the arcuate nucleus of the hypothalamus have been associated with leptin resistance and obesity in mice [171].
SOCS3 has also been associated with the progression of a number of inflammatory conditions. SOCS3 expression has been reported in synovial tissue from mice during experimental arthritis, and in peripheral blood mononuclear cells in patients with rheumatoid arthritis (RA) [202]. Mice with a GP130 receptor mutation that ablates SOCS3 binding develop a RA-like joint disease [203]. Similarly, hematopoietic and endothelial cell-specific Socs3 KO mice exhibited severe phenotypes in experimental arthritis models, most likely due to enhanced responsiveness of IL-6, G-CSF and possibly IL-1 [204]. Moreover, adenoviral delivered SOCS3 could eliminate joint inflammation in mice with experimental autoimmune arthritis, mediate via inhibition of IL-6 signaling [205]. SOCS3 was found to be highly expressed in lamina propria and epithelial cells in the colon of mice with inflammatory bowel disease (IBD), as well in human patients with both ulcerative colitis and Crohn’s disease (CD), again suggested to be due to enhanced IL-6 signaling (via STAT3) [206]. In contrast, SOCS3 has been found to be upregulated in the peripheral T cells from patients with Th2 type diseases, such as atopic asthma and dermatitis, where its expression has found to correlate tightly with disease severity [197]. Similarly, SOCS3 has been associated with allergic conjunctivitis (AC), with high expression at the site of disease, with reduction of SOCS3 leading to decreased clinical severity [207].
SOCS3 appears to also play a tumor-suppressor/anti-proliferative role. For example, overcoming SOCS3 regulation seems to be a common theme in proliferative syndromes. Thus, the myeloproliferative disease-associated JAK2V617F mutant is no longer able to be negatively-regulated by SOCS3 [208]. Similarly, G-CSFR truncations associated with severe congenital neutropenia leading to acute myeloid leukemia have lost the sequences required for SOCS3-mediated control of STAT5 activation [209]. Once again though, the exact role for SOCS3 is complex. For example, overexpression of SOCS3 associated with decreased survival in a cohort of patients with de novo follicular lymphoma [210], while SOCS3 may in fact potentiate the JAK2V617F mutation [208]. However, hypermethylation of SOCS3 occurs frequently in both Barrett’s adenocarcinoma [125] and hepatocellular carcinoma [211], in the latter case leading to increased JAK2/STAT3 activation [211]. Hypermethylation mediated reduction in SOCS3 expression has also been observed in malignant human melanoma [212], while constitutive SOCS3 expression was shown to confer a proliferative advantage to a human melanoma cell line [213]. SOCS3 was also found to be frequently silenced by hypermethylation in human lung cancer where it suppressed cell growth [214]. SOCS3 was able to limit inflammation-associated tumorigenesis in the colon, via regulation of STAT3 and NFκB [215], while in ulcerative colitis, loss of SOCS3 expression was observed in the areas of colon dysplasia [216]. SOCS3 was protective against hepatitis-induced hepatocellular carcinoma, with loss of SOCS3 leading to resistance to apoptosis and increased proliferation [217]. Similar epigenetic silencing of SOCS3 has been seen in cholangiocarcinoma cells, resulting in enhanced IL-6/STAT3 signaling and reduced apoptosis [218]. SOCS3 hypermethylation was also seen in glioma [219], and prostate cancer tissues – although not in benign prostate hyperplasia [220]. Finally, reduced expression of SOCS3 was also specifically observed in breast cancer with lymph node metastasis, suggesting a role in tumor spread [221].
SOCS3 has also been associated with infectious diseases. For example, the severe inflammation mediated by SARS virus infection was found to correlate with lower expression of SOCS3 in infected cells [222]. In contrast, SOCS3 was able to inhibit the antiviral response to influenza [133]. Indeed, pathogenic strains of Salmonella sp. could increase SOCS3 expression in macrophages to mediate suppression of immune responses [135]. Similarly, M. bovis was able to induce SOCS3 to mediate inhibition of IFNγ-induced STAT1 [136]. Finally, high levels of SOCS3 have been found to be associated with non-responsiveness to combined IFN antiviral therapy [223].
SOCS4
SOCS4 remains the least studied member of the SOCS family. It has been shown to be particularly highly expressed in the intestine and thymus of adult pig [224], with the zebrafish socs4a homologue expressed in the embryonic nervous system (MCT and ACW, unpublished). Available mouse data suggests it is widely expressed, with higher expression in the olfactory bulb (http://biogps.org/#goto=genereport&id=122809). To date, SOCS4 has been shown to be induced only by EGF, at least in vitro [225]. Similar to CISH, miR-98 and let-7 are thought to post-transcriptionally regulate SOCS4, facilitating translational repression by targeting its 3’UTR region. Infection of binary epithelial cells with C. parvum decreased miR-98 and let-7, leading to increased SOCS4 expression [226].
SOCS4 has been demonstrated to regulate EGFR signaling in vitro [6,225]. This appears to be mediated through docking of SOCS4 to phosphotyrosine residues on the activated EGFR, subsequently targeting the receptor for proteasomal degradation by recruitment of E3 ubiquitin ligase activity [6,11]. However, SOCS4 binds with high affinity to the same EGFR phosphotyrosine as STAT3 and therefore may also inhibit STAT3 activation directly by blocking its ability to dock to EGFR [11]. SOCS4 also has a low micromolar affinity for JAK2 and c-KIT, the biological consequences of which remain to be determined [11]. A recent report has suggested a role for SOCS4 in the regulation of primordial follicle activation, a process initiated by LIF activation of the JAK1/STAT3 pathway. This occurred concurrently with SOCS4 induction, with SOCS4 shown to interact with several proteins involved in ovarian follicular development [227]. No Socs4 KO mouse has been reported.
Several studies have suggested a tumor suppressor role for SOCS4. An inverse relationship between SOCS4 expression levels and tumor node metastasis stage has been reported in human breast cancer [228]. SOCS4 expression was also found to be significantly lower in cancerous tissue compared to non-cancerous tissue in a patient with gastric cancer, mediated by hypermethylation of CpG sites in the promoter region of the SOCS4 gene leading to SOCS4 silencing [229]. Mouse studies also suggest a tumor suppressor in epithelial cells via RUNX1-mediated repression of the SOCS4 promoter, leading to decreased SOCS4 levels and increased STAT3 activity, thereby contributing to tumor development [230].
SOCS5
SOCS5 is most closely related to SOCS4, and also has not been fully characterised. SOCS5 has been shown to be expressed in a variety of adult tissues including heart, brain, retina, lung, colon, bladder, testis and skeletal muscle as well as the placenta [5,225,231,232]. Expression was particularly high in lymphoid organs including the spleen, lymph nodes, thymus, and bone marrow, with specific expression in primary B and T cells [233], suggesting possible immune-related functions [231]. Like SOCS4, SOCS5 expression has been shown to be induced by EGF in vitro [225].
SOCS5 appears able to regulate both RTK and cytokine receptor signaling. Thus, SOCS5 has been shown to negatively regulate EGFR in vitro [6,225], and more weakly IL-6R, LIFR [21] and IL-4R signaling [28]. This seems to be a conserved function since overexpression of the Drosophila melanogaster SOCS5 homologue, SOCS36E, resulted in several phenotypes consistent with reduction in both cytokine receptor and EGFR signaling. SOCS5 is thought to regulate signaling by initiating the proteasomal degradation of its target proteins, as seen in the regulation of EGFR, where both its SH2 domain and SOCS box required for initiation of degradation [6,225]. Interestingly, the SOCS5 protein has been found to associate with EGFR independent of ligand stimulation, binding via its N-terminal domain [225].
Consistent with its expression in lymphoid organs and in vitro effects on IL-4 signaling, SOCS5 has been implicated in T helper cell differentiation, particularly in the balance between Th1 and Th2 cells, with SOCS5 protein preferentially expressed in Th1 cells. SOCS5 Tg mice showed a significant reduction in Th2 development, thought to be facilitated by the ability of SOCS5 to inhibit IL-4R mediated STAT6 activation that normally stimulates differentiation of naïve T cells toward a Th2 fate [28]. Interestingly, SOCS5 was found to associate with the IL-4R regardless of tyrosine phosphorylation [28,225]. However, Socs5 KO mice showed no abnormalities in Th1/Th2 differentiation, indicating possible redundancy in its lymphoid role [233].
SOCS5 has been implicated in a variety of disease states, such as allergic conjunctivitis [207], atopic dermatitis [234], asthma [235] and uveitis [236], although some of these associations have so far only been identified in rodent models. These studies also provide further support for a role for SOCS5 in regulating the balance between Th1 and Th2 cells. For example, SOCS5 expression was decreased in patients with the Th2 dominant disease atopic dermatitis (AD) compared to healthy controls, with patients demonstrating eosinophilia showing even lower levels of SOCS5 [234]. Moreover, constitutive SOCS5 expression has been found to reduce eosinophil infiltration in allergic conjunctivitis [207], further implicating SOCS5 in the balance of Th1/Th2 cells, since eosinophil production is stimulated by Th2 cytokines, including IL-4. This notion was further supported in a murine model for allergic conjunctivitis, an ocular disease that is characterized by IL-4-mediated eosinophil infiltration. In mice constitutively expressing SOCS5 under the control of the lck proximal promoter and Eμ enhancer, decreased conjunctival eosinophil infiltration was observed [207]. These mice also showed decreased lethality to septic peritonitis and significantly lower bacterial burden compared to WT controls. This was associated with accumulation of neutrophils and macrophages, with these cells showing increased bactericidal properties and impaired IL-4-induced STAT6 activation [237]. Since STAT6 knockout mice have also been found to be resistant to septic peritonitis [238], this suggests a regulatory role for SOCS5 on the IL-4/STAT6 pathway. SOCS5 transgenic mice also showed increased peritoneal IL-2 and IFN-γ, cytokines involved in the promotion of Th1 differentiation [237]. Finally, a reduction in SOCS5 expression was observed in cancer of the thyroid gland [239], suggesting a possible tumor-suppressor function.
SOCS6
SOCS6 has been shown to be ubiquitously expressed during mouse embryonic development, while in the adult mouse expression has been reported in areas of the bone marrow containing monocytes and immature granulocytes [7] and in the retina [232]. SOCS6 was found to be induced by both INS [240,241] and IGF-2 [242].
SOCS6 has been demonstrated to negatively regulate signaling by IGF-1 [242], INS [240], FLT3 [243], Stem Cell Factor (SCF) [244] and TCR [245]. Like other SOCS proteins, SOCS6 likely exerts its regulatory effects primarily through ubiquitination and degradation of target proteins [7]. However, SOCS6 interacts with an alternate E3 ligase component, heme-oxidized IRP2 ubiquitin ligase-1 (HOIL-1), which induces the poly-ubiquitination and degradation of SOCS6-associated proteins [244]. Like SOCS2, SOCS6 also has the ability to degrade other SOCS proteins, including SOCS7 [246]. The SOCS6 N-terminal domain has been shown to drive localisation to the nucleus, where it appears to negatively regulate STAT3, although the exact mechanism by which SOCS6 regulates STAT3 has not been identified [14].
SOCS6 has been shown to control TCR-mediated T cell activation in vitro through negative regulation of p56lck. SOCS6 was shown to bind to the kinase domain of active p56lck, targeting it for ubiquitination and subsequent degradation, with SOCS6 overexpression resulting in repression of TCR-dependent IL-2 promoter activity [245]. SOCS6 also appears to negatively regulate signaling of several important hematopoietic receptor tyrosine kinases. SOCS6 binds to the juxtamembrane region of c-KIT following stimulation with SCF, thereby regulating activation of members of the MAPK pathway, such as ERK1/2 and p38 [244]. SOCS6 can also bind to FLT3 and negatively regulate its signaling, reducing downstream ERK1/2 signaling and concomitant cell proliferation [243].
A potential role for SOCS6 in neural stem cell differentiation has also been suggested. Expression of SOCS6 was upregulated during differentiation of these cells. SOCS6 overexpression resulted in enhanced neurite outgrowth cells, while siRNA-mediated knockdown of SOCS6 decreased neurite extension [242]. Neurite outgrowth was also enhanced by IGF-1, which increased SOCS6 levels, but reduced in the presence of a JAK/STAT pathway inhibitor that could not be rescued by IGF-1 treatment [242]. There is also a large body of in vitro data supporting a role for SOCS6 in glucose homeostasis. SOCS6 has been shown to inhibit pathways downstream of the INS and IGF-1 receptors [240]. This was facilitated by direct binding of SOCS6 to the IRS-4 adaptor protein following its phosphorylation in response to IGF-1 or insulin and more weakly to IRS-2 in response to IGF-1, allowing it to indirectly associate with the p85 regulatory subunit of PI3K in response to IGF-1 or insulin stimulation [7,241]. It has been suggested that the mechanism of regulation in this case might be via preventing recruitment of other downstream signaling proteins [7]. SOCS6 has also been found to interact with PIM3, a protein upregulated in β-cells in response to glucose stimulation. Pim3 KO mice showed greatly reduced levels SOCS6 expression in their pancreatic islets, while overexpression of SOCS6 inhibited glucose-induced ERK1/2 activation, suggesting a role for SOCS6 and PIM3 in the negative regulation of ERK1/2 in response to glucose stimulation [247]. Reduced endogenous SOCS6 in retinal pigment epithelia cells was found to coincide with inhibition of insulin signaling. It has therefore been suggested that SOCS6 expression may serve to maintain high basal insulin/AKT signaling in retina and improve glucose metabolism [232].
Socs6 KO mice displayed an 8-10% reduction in body weight compared to WT littermates, thought to be due to perturbation of IGF-1R signaling [7]. However, despite the in vitro data, Socs6 knockout mice did not display any alterations in glucose metabolism [7]. It has been suggested that this may be due to compensation by other SOCS family members implicated in the regulation of insulin receptor signaling, such as SOCS7 [7] or SOCS1 [80]. However, SOCS6 Tg mice utilizing the elongation factor I promoter displayed enhanced AKT activation in response to INS and increased glucose metabolism, supporting an in vivo role for SOCS6 in the regulation of INS signaling [241]. Finally, despite its expression in the bone marrow, no hematological phenotypes could be identified in Socs6 KO or SOCS6 Tg mice [7,241]. Again, redundancy between SOCS family members may play a role in the absence of a phenotype in these mice.
Altered SOCS6 expression has been described in several disease states, including cancer. However, similar to other SOCS proteins, SOCS6 does not appear to function exclusively as a tumor suppressor. Thus, low SOCS6 expression has been associated with recurrent primary lung squamous cell carcinoma [248] and cancers of the liver and thyroid gland [239]. Loss of SOCS6 was also observed in over 50% of patients with gastric or colorectal cancer, with SOCS6 inactivation predominantly caused by allelic loss or promoter hypermethylation [249,250]. However, in the case of colorectal cancer, this did not correlate with disease-free survival or overall survival [251]. Ectopic SOCS6 expression supressed gastric cancer cell growth and colony formation in vitro [249]. However, a recent study found that levels of SOCS6 expression in colon and rectum tissue samples taken from healthy individuals varied widely, and demonstrated that SOCS6 expression was increased in gastric cancer [239].
SOCS7
SOCS7 has been shown to be expressed in many murine tissues [7], but the relative levels vary between different mouse strains [8]. In the C57BL strain, Socs7 expression was highest in isolated pancreatic islets, whole brain, and skeletal muscle, with lower levels detected in the liver, perigonadal fat, skin, whole pancreas, testis and spleen [7,8]. Expression in the 129S6 strain was similar overall, but with a 5-fold decrease in whole brain expression, a 6-fold increase in spleen expression and a 2,000-fold decrease in expression in isolated pancreatic islets, when compared to the C57BL strain [8]. Other sites of expression in this strain were the testes, kidney and eye [252]. SOCS7 has been shown to be induced by the cytokines GH and PRL [142], as well as EGF [253], INS and IGF-1 [7].
SOCS7 has been found to regulate signaling by GH, PRL, leptin [29] and INS [8]. SOCS7 appears to control signaling in a number of ways. It was able to inhibit PRL and leptin mediated activation of STAT5 and STAT3, respectively [29], achieved by direct interaction of SOCS7 with phosphorylated STAT3 and STAT5, which in the case of STAT3 prevented its nuclear translocation [29]. SOCS7 can similarly inhibit the nuclear transport of the adaptor protein NCK [16]. SOCS7 was also demonstrated to interact via its SH2 domain to EGFR [253] and INS receptor [8], along with the adaptor proteins IRS-1 [8], IRS-2 [7], IRS-4 [7], the p85 subunit of PI3K [7] and GRB2 [253]. In these instances, SOCS7 likely regulates signaling activity through recruitment of E3 ubiquitin ligase activity and subsequent proteasomal targeting of associated proteins [8].
There have been conflicting reports regarding the in vivo function of SOCS7, probably due to differences in the genetic background of the respective mouse knockouts. One Socs7 KO mouse line exhibited a 7-10% reduction in body size compared to wild type littermates, with no abnormalities in circulating glucose or insulin levels [252]. Approximately 50% of these Socs7 KO mice died by week 15 due to hydrocephaly [252]. However, the hydrocephaly was not consistent in other mouse strains [8]. When the Socs7 KO allele was on a mixed genetic background the hydrocephalus was obviated, which revealed increased insulin sensitivity when compared to WT mice [8]. These mice also showed an increase in the number of pancreatic islets and a hyperplasia of islets that was not present at birth but developed with age [8]. These observations suggested an active role of SOCS7 in insulin signaling, consistent with the findings that SOCS7 can interact with the INS receptor and their adaptor proteins [7,8]. More recently it was demonstrated that approximately 50% of hydrocephaly-resistant Socs7 KO mice developed a severe cutaneous disease by 16 months of age, with the dermis appearing hyperplastic with an infiltration of leukocytes. The skin of both affected and unaffected Socs7 KO mice possessed significantly increased mast cell numbers compared to controls, which were hyperactive to IgE-mediated stimuli. This resulted in increased production of the pro-inflammatory cytokines IL-13, IL-6 and TNFα, with levels of TSLP and a component of its receptor also upregulated [254].
There has only been very limited examination of the role of SOCS7 in human disease. However, a potential tumor-suppressor role has again been indicated, with one study demonstrating higher SOCS7 expression was significantly associated with earlier stages of cancer and overall survival [228]. A recent study has also found there to be associations between SOCS7 haplotypes and various metabolic traits, including obesity, insulin resistance and lipid metabolism [255].
Conclusions
Research to date has highlighted a number of important roles for SOCS proteins in both development and disease. Indeed, all have been directly or indirectly implicated in immunity and/or hematopoiesis. Many have been shown to be involved in disease pathogenesis, including inflammatory and other immune disorders, susceptibility to infectious diseases and cancer. This suggests that there is considerable potential for the development of therapeutics based on augmenting (or antagonizing) SOCS function.
Acknowledgments
MCT was supported by an Australian Postgraduate Awards. ACW was supported by funding from the Australian Research Council Discovery Grant and Linkage-Equipment Infrastructure Fund schemes.
References
- 1.Robb L. Cytokine receptors and hematopoietic differentiation. Oncogene. 2007;26:6715–6723. doi: 10.1038/sj.onc.1210756. [DOI] [PubMed] [Google Scholar]
- 2.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wormald S, Hilton D. Inhibitors of cytokine signal tranduction. J Biol Chem. 2003;279:821–824. doi: 10.1074/jbc.R300030200. [DOI] [PubMed] [Google Scholar]
- 4.O’Sullivan LA, Liongue C, Lewis RS, Stephenson SEM, Ward AC. Cytokine receptor signaling through the Jak-Stat-Socs pathway in disease. Mol Immunol. 2007;44:2497–2506. doi: 10.1016/j.molimm.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Hilton DJ, Richardson RT, Alexander WS, Viney EM, Willson TA, Sprigg NS, Starr R, Nicholson SE, Metcalf D, Nicola NA. Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc Natl Acad Sci USA. 1998;95:114–119. doi: 10.1073/pnas.95.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kario E, Marmor MD, Adamsky K, Citri A, Amit I, Amariglio N, Rechavi G, Yarden Y. Suppressors of cytokine signaling 4 and 5 regulate epidermal growth factor receptor signaling. J Biol Chem. 2005;280:7038–7048. doi: 10.1074/jbc.M408575200. [DOI] [PubMed] [Google Scholar]
- 7.Krebs DL, Uren RT, Metcalf D, Rakar S, Zhang JG, Starr R, De Souza DP, Hanzinikolas K, Eyles J, Connolly LM, Simpson RJ, Nicola NA, Nicholson SE, Baca M, Hilton DJ, Alexander WS. SOCS-6 binds to insulin receptor substrate 4, and mice lacking the SOCS-6 gene exhibit mild growth retardation. Mol Cell Biol. 2002;22:4567–4578. doi: 10.1128/MCB.22.13.4567-4578.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banks AS, Li J, McKeag L, Hribal ML, Kashiwada M, Accili D, Rothman PB. Deletion of SOCS7 leads to enhanced insulin action and enlarged islets of Langerhans. J Clin Invest. 2005;115:2462–2471. doi: 10.1172/JCI23853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liongue C, O’Sullivan LA, Trengove MC, Ward AC. Evolution of JAK-STAT pathway components: mechanisms and role in immune system development. PLoS One. 2012;7:e32777. doi: 10.1371/journal.pone.0032777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liongue C, Ward AC. Evolution of the Jak-Stat pathway. JAK-STAT. 2013;2:1–8. doi: 10.4161/jkst.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bullock AN, Rodriguez MC, Debreczeni JE, Songyang Z, Knapp S. Structure of the SOCS4-ElonginB/C complex reveals a distinct SOCS box interface and the molecular basis for SOCS-dependent EGFR degradation. Structure. 2007;15:1493–1504. doi: 10.1016/j.str.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yasukawa H, Misawa H, Sakamoto H, Masuhara M, Sasaki A, Wakioka T, Ohtsuka S, Imaizumi T, Matsuda T, Ihle JN, Yoshimura A. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J. 1999;18:1309–1320. doi: 10.1093/emboj/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng ZP, Chandrashekaran IR, Low A, Speed TP, Nicholson SE, Norton RS. The N-terminal domains of SOCS proteins: a conserved region in the disordered N-termini of SOCS4 and 5. Proteins. 2012;80:946–957. doi: 10.1002/prot.23252. [DOI] [PubMed] [Google Scholar]
- 14.Hwang MN, Min CH, Kim HS, Lee H, Yoon KA, Park SY, Lee ES, Yoon S. The nuclear localization of SOCS6 requires the N-terminal region and negatively regulates Stat3 protein levels. Biochem Biophys Res Commun. 2007;360:333–338. doi: 10.1016/j.bbrc.2007.06.062. [DOI] [PubMed] [Google Scholar]
- 15.Martens N, Wery M, Wang P, Braet F, Gertler A, Hooghe R, Vandenhaute J, Hooghe-Peters EL. The suppressor of cytokine signaling (SOCS)-7 interacts with the actin cytoskeleton through vinexin. Exp Cell Res. 2004;298:239–248. doi: 10.1016/j.yexcr.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Kremer BE, Adang LA, Macara IG. Septins regulate actin organization and cell-cycle arrest through nuclear accumulation of NCK mediated by SOCS7. Cell. 2007;130:837–850. doi: 10.1016/j.cell.2007.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang JG, Farley A, Nicholson SE, Willson TA, Zugaro LM, Simpson RJ, Moritz RL, Cary D, Richardson R, Hausmann G, Kile BJ, Kent SB, Alexander WS, Metcalf D, Hilton DJ, Nicola NA, Baca M. The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc Natl Acad Sci USA. 1999;96:2071–2076. doi: 10.1073/pnas.96.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamura T, Maenaka K, Kotoshiba S, Matsumoto M, Kohda D, Conaway RC, Conaway JW, Nakayama KI. VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases. Genes Dev. 2004;18:3055–3065. doi: 10.1101/gad.1252404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larsen L, Ropke C. Suppressors of cytokine signalling: SOCS. APMIS. 2002;110:833–844. doi: 10.1034/j.1600-0463.2002.1101201.x. [DOI] [PubMed] [Google Scholar]
- 20.Babon JJ, Sabo JK, Zhang JG, Nicola NA, Norton RS. The SOCS box encodes a hierarchy of affinities for Cullin5: implications for ubiquitin ligase formation and cytokine signalling suppression. J Mol Biol. 2009;387:162–174. doi: 10.1016/j.jmb.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholson SE, Willson TA, Farley A, Starr R, Zhang JG, Baca M, Alexander WS, Metcalf D, Hilton DJ, Nicola NA. Mutational analyses of the SOCS proteins suggest a dual domain requirement but distinct mechanisms for inhibition of LIF and IL-6 signal transduction. EMBO J. 1999;18:375–385. doi: 10.1093/emboj/18.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, Akira S, Kishimoto T. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- 23.Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, Miyazaki T, Leonor N, Taniguchi T, Fujita T, Kanakura Y, Komiya S, Yoshimura A. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki A, Yasukawa H, Suzuki A, Kamizono S, Syoda T, Kinjyo I, Sasaki M, Johnston JA, Yoshimura A. Cytokine-inducible SH2 protein-3 (CIS3/SOCS3) inhibits Janus tyrosine kinase by binding through the N-terminal kinase inhibitory region as well as SH2 domain. Genes Cells. 1999;4:339–351. doi: 10.1046/j.1365-2443.1999.00263.x. [DOI] [PubMed] [Google Scholar]
- 25.Verdier F, Rabionet R, Gouilleux F, Beisenherz-Huss C, Varlet P, Muller O, Mayeux P, Lacombe C, Gisselbrecht S, Chretien S. A sequence of the CIS gene promoter interacts preferentially with two associated STAT5A dimers: a distinct biochemical difference between STAT5A and STAT5B. Mol Cell Biol. 1998;18:5852–5860. doi: 10.1128/mcb.18.10.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ram PA, Waxman DJ. SOCS/CIS protein inhibition of growth hormone-stimulated STAT5 signaling by multiple mechanisms. J Biol Chem. 1999;274:35553–35561. doi: 10.1074/jbc.274.50.35553. [DOI] [PubMed] [Google Scholar]
- 27.Nicholson SE, De Souza D, Fabri LJ, Corbin J, Willson TA, Zhang JG, Silva A, Asimakis M, Farley A, Nash AD, Metcalf D, Hilton DJ, Nicola NA, Baca M. Suppressor of cytokine signaling-3 preferentially binds to the SHP-2-binding site on the shared cytokine receptor subunit gp130. Proc Natl Acad Sci USA. 2000;97:6493–6498. doi: 10.1073/pnas.100135197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seki Y, Hayashi K, Matsumoto A, Seki N, Tsukada J, Ransom J, Naka T, Kishimoto T, Yoshimura A, Kubo M. Expression of the suppressor of cytokine signaling-5 (SOCS5) negatively regulates IL-4-dependent STAT6 activation and Th2 differentiation. Proc Natl Acad Sci USA. 2002;99:13003–13008. doi: 10.1073/pnas.202477099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martens N, Uzan G, Wery M, Hooghe R, Hooghe-Peters EL, Gertler A. Suppressor of cytokine signaling 7 inhibits prolactin, growth hormone, and leptin signaling by interacting with STAT5 or STAT3 and attenuating their nuclear translocation. J Biol Chem. 2005;280:13817–13823. doi: 10.1074/jbc.M411596200. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimura A, Ohkubo T, Kiguchi T, Jenkins NA, Gilbert DJ, Copeland NG, Hara T, Miyajima A. A novel cytokine-inducible gene CIS encodes an SH2-containing protein that binds to tyrosine-phosphorylated interleukin 3 and erythropoietin receptors. EMBO J. 1995;14:2816–2826. doi: 10.1002/j.1460-2075.1995.tb07281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto A, Masuhara M, Mitsui K, Yokouchi M, Ohtsubo M, Misawa H, Miyajima A, Yoshimura A. CIS, a cytokine inducible SH2 protein, is a target of the JAK-STAT5 pathway and modulates STAT5 activation. Blood. 1997;89:3148–3154. [PubMed] [Google Scholar]
- 32.Adams TE, Hansen JA, Starr R, Nicola NA, Hilton DJ, Billestrup N. Growth hormone preferentially induces the rapid, transient expression of SOCS-3, a novel inhibitor of cytokine receptor signaling. J Biol Chem. 1998;273:1285–1287. doi: 10.1074/jbc.273.3.1285. [DOI] [PubMed] [Google Scholar]
- 33.Helman D, Sandowski Y, Cohen Y, Matsumoto A, Yoshimura A, Merchav S, Gertler A. Cytokine-inducible SH2 protein (CIS3) and JAK2 binding protein (JAB) abolish prolactin receptor-mediated STAT5 signaling. FEBS Lett. 1998;441:287–291. doi: 10.1016/s0014-5793(98)01555-5. [DOI] [PubMed] [Google Scholar]
- 34.Pezet A, Favre H, Kelly PA, Edery M. Inhibition and restoration of prolactin signal transduction by suppressors of cytokine signaling. J Biol Chem. 1999;274:24497–24502. doi: 10.1074/jbc.274.35.24497. [DOI] [PubMed] [Google Scholar]
- 35.Lejeune D, Demoulin JB, Renauld JC. Interleukin 9 induces expression of three cytokine signal inhibitors: cytokine-inducible SH2-containing protein, suppressor of cytokine signalling (SOCS)-2 and SOCS-3, but only SOCS-3 overexpression suppresses interleukin 9 signalling. Biochem J. 2001;353:109–116. [PMC free article] [PubMed] [Google Scholar]
- 36.Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 37.Okabe S, Tauchi T, Morita H, Ohashi H, Yoshimura A, Ohyashiki K. Thrombopoietin induces an SH2-containing protein, CIS1, which binds to Mpl: involvement of the ubiquitin proteosome pathway. Exp Hematol. 1999;27:1542–1547. doi: 10.1016/s0301-472x(99)00094-6. [DOI] [PubMed] [Google Scholar]
- 38.Hunter MG, Jacob A, O’Donnell LC, Agler A, Druhan LJ, Coggeshall KM, Avalos BR. Loss of SHIP and CIS recruitment to the granulocyte colony-stimulating factor receptor contribute to hyperproliferative responses in severe congenital neutropenia/acute myelogenous leukemia. J Immunol. 2004;173:5036–5045. doi: 10.4049/jimmunol.173.8.5036. [DOI] [PubMed] [Google Scholar]
- 39.Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 40.Moriggl R, Topham DJ, Teglund S, Sexl V, McKay C, Wang D, Hoffmeyer A, van Deursen J, Sangster MY, Bunting KD, Grosveld GC, Ihle JN. Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity. 1999;10:249–259. doi: 10.1016/s1074-7613(00)80025-4. [DOI] [PubMed] [Google Scholar]
- 41.Moriggl R, Sexl V, Kenner L, Duntsch C, Stangl K, Gingras S, Hoffmeyer A, Bauer A, Piekorz R, Wang D, Bunting KD, Wagner EF, Sonneck K, Valent P, Ihle JN, Beug H. Stat5 tetramer formation is associated with leukemogenesis. Cancer Cell. 2005;7:87–99. doi: 10.1016/j.ccr.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Shen X, Hong F, Nguyen VA, Gao B. IL-10 attenuates IFN-alpha-activated STAT1 in the liver: involvement of SOCS2 and SOCS3. FEBS Lett. 2000;480:132–136. doi: 10.1016/s0014-5793(00)01905-0. [DOI] [PubMed] [Google Scholar]
- 43.Brender C, Nielsen M, Ropke C, Nissen MH, Svejgaard A, Billestrup N, Geisler C, Odum N. Interferon-alpha induces transient suppressors of cytokine signalling expression in human T cells. Exp Clin Immunogenet. 2001;18:80–85. doi: 10.1159/000049186. [DOI] [PubMed] [Google Scholar]
- 44.Isaksen DE, Baumann H, Trobridge PA, Farr AG, Levin SD, Ziegler SF. Requirement for Stat5 in thymic stromal lymphopoietin-mediated signal transduction. J Immunol. 1999;163:5971–5977. [PubMed] [Google Scholar]
- 45.Emilsson V, Arch JR, de Groot RP, Lister CA, Cawthorne MA. Leptin treatment increases suppressors of cytokine signaling in central and peripheral tissues. FEBS Lett. 1999;455:170–174. doi: 10.1016/s0014-5793(99)00874-1. [DOI] [PubMed] [Google Scholar]
- 46.Bjorbaek C, Elmquist JK, El-Haschimi K, Kelly J, Ahima RS, Hileman S, Flier JS. Activation of SOCS-3 messenger ribonucleic acid in the hypothalamus by ciliary neurotrophic factor. Endocrinology. 1999;140:2035–2043. doi: 10.1210/endo.140.5.6736. [DOI] [PubMed] [Google Scholar]
- 47.Li S, Chen S, Xu X, Sundstedt A, Paulsson KM, Anderson P, Karlsson S, Sjogren HO, Wang P. Cytokine-induced Src homology 2 protein (CIS) promotes T cell receptor-mediated proliferation and prolongs survival of activated T cells. J Exp Med. 2000;191:985–994. doi: 10.1084/jem.191.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu G, Zhou R, Liu J, Gong AY, Eischeid AN, Dittman JW, Chen XM. MicroRNA-98 and let-7 confer cholangiocyte expression of cytokine-inducible Src homology 2-containing protein in response to microbial challenge. J Immunol. 2009;183:1617–1624. doi: 10.4049/jimmunol.0804362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aman MJ, Migone TS, Sasaki A, Ascherman DP, Zhu M, Soldaini E, Imada K, Miyajima A, Yoshimura A, Leonard WJ. CIS associates with the interleukin-2 receptor beta chain and inhibits interleukin-2-dependent signaling. J Biol Chem. 1999;274:30266–30272. doi: 10.1074/jbc.274.42.30266. [DOI] [PubMed] [Google Scholar]
- 50.Dif F, Saunier E, Demeneix B, Kelly PA, Edery M. Cytokine-inducible SH2-containing protein suppresses PRL signaling by binding the PRL receptor. Endocrinology. 2001;142:5286–5293. doi: 10.1210/endo.142.12.8549. [DOI] [PubMed] [Google Scholar]
- 51.Hansen JA, Lindberg K, Hilton DJ, Nielsen JH, Billestrup N. Mechanism of inhibition of growth hormone receptor signaling by suppressor of cytokine signaling proteins. Mol Endocrinol. 1999;13:1832–1843. doi: 10.1210/mend.13.11.0368. [DOI] [PubMed] [Google Scholar]
- 52.Hanada T, Yoshida H, Kato S, Tanaka K, Masutani K, Tsukada J, Nomura Y, Mimata H, Kubo M, Yoshimura A. Suppressor of cytokine signaling-1 is essential for suppressing dendritic cell activation and systemic autoimmunity. Immunity. 2003;19:437–450. doi: 10.1016/s1074-7613(03)00240-1. [DOI] [PubMed] [Google Scholar]
- 53.Piessevaux J, De Ceuninck L, Catteeuw D, Peelman F, Tavernier J. Elongin B/C recruitment regulates substrate binding by CIS. J Biol Chem. 2008;283:21334–21346. doi: 10.1074/jbc.M803742200. [DOI] [PubMed] [Google Scholar]
- 54.Matsumoto A, Seki Y, Kubo M, Ohtsuka S, Suzuki A, Hayashi I, Tsuji K, Nakahata T, Okabe M, Yamada S, Yoshimura A. Suppression of STAT5 functions in liver, mammary glands, and T cells in cytokine-inducible SH2-containing protein 1 transgenic mice. Mol Cell Biol. 1999;19:6396–6407. doi: 10.1128/mcb.19.9.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miah MA, Yoon CH, Kim J, Jang J, Seong YR, Bae YS. CISH is induced during DC development and regulates DC-mediated CTL activation. Eur J Immunol. 2012;42:58–68. doi: 10.1002/eji.201141846. [DOI] [PubMed] [Google Scholar]
- 56.Khor CC, Vannberg FO, Chapman SJ, Guo H, Wong SH, Walley AJ, Vukcevic D, Rautanen A, Mills TC, Chang KC, Kam KM, Crampin AC, Ngwira B, Leung CC, Tam CM, Chan CY, Sung JJ, Yew WW, Toh KY, Tay SK, Kwiatkowski D, Lienhardt C, Hien TT, Day NP, Peshu N, Marsh K, Maitland K, Scott JA, Williams TN, Berkley JA, Floyd S, Tang NL, Fine PE, Goh DL, Hill AV. CISH and susceptibility to infectious diseases. N Engl J Med. 2010;362:2092–2101. doi: 10.1056/NEJMoa0905606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tong HV, Toan NL, Song le H, Kremsner PG, Kun JF, Tp V. Association of CISH -292A/T genetic variant with hepatitis B virus infection. Immunogenetics. 2012;64:261–265. doi: 10.1007/s00251-011-0584-y. [DOI] [PubMed] [Google Scholar]
- 58.Periasamy S, Dhiman R, Barnes PF, Paidipally P, Tvinnereim A, Bandaru A, Valluri VL, Vankayalapati R. Programmed death 1 and cytokine inducible SH2-containing protein dependent expansion of regulatory T cells upon stimulation With Mycobacterium tuberculosis. J Infect Dis. 2011;203:1256–1263. doi: 10.1093/infdis/jir011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsao JT, Kuo CC, Lin SC. The analysis of CIS, SOCS1, SOSC2 and SOCS3 transcript levels in peripheral blood mononuclear cells of systemic lupus erythematosus and rheumatoid arthritis patients. Clin Exp Med. 2008;8:179–185. doi: 10.1007/s10238-008-0006-0. [DOI] [PubMed] [Google Scholar]
- 60.de Andres MC, Imagawa K, Hashimoto K, Gonzalez A, Goldring MB, Roach HI, Oreffo RO. Suppressors of cytokine signalling (SOCS) are reduced in osteoarthritis. Biochem Biophys Res Commun. 2011;407:54–59. doi: 10.1016/j.bbrc.2011.02.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kato H, Nomura K, Osabe D, Shinohara S, Mizumori O, Katashima R, Iwasaki S, Nishimura K, Yoshino M, Kobori M, Ichiishi E, Nakamura N, Yoshikawa T, Tanahashi T, Keshavarz P, Kunika K, Moritani M, Kudo E, Tsugawa K, Takata Y, Hamada D, Yasui N, Miyamoto T, Shiota H, Inoue H, Itakura M. Association of single-nucleotide polymorphisms in the suppressor of cytokine signaling 2 (SOCS2) gene with type 2 diabetes in the Japanese. Genomics. 2006;87:446–458. doi: 10.1016/j.ygeno.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 62.Sporri B, Kovanen PE, Sasaki A, Yoshimura A, Leonard WJ. JAB/SOCS1/SSI-1 is an interleukin-2-induced inhibitor of IL-2 signaling. Blood. 2001;97:221–226. doi: 10.1182/blood.v97.1.221. [DOI] [PubMed] [Google Scholar]
- 63.Losman JA, Chen XP, Hilton D, Rothman P. SOCS-1 is a potent inhibitor of IL-4 signal transduction. J Immunol. 1999;162:3770–3774. [PMC free article] [PubMed] [Google Scholar]
- 64.Hebenstreit D, Luft P, Schmiedlechner A, Regl G, Frischauf AM, Aberger F, Duschl A, Horejs-Hoeck J. IL-4 and IL-13 induce SOCS-1 gene expression in A549 cells by three functional STAT6-binding motifs located upstream of the transcription initiation site. J Immunol. 2003;171:5901–5907. doi: 10.4049/jimmunol.171.11.5901. [DOI] [PubMed] [Google Scholar]
- 65.Crespo A, Filla MB, Russell SW, Murphy WJ. Indirect induction of suppressor of cytokine signalling-1 in macrophages stimulated with bacterial lipopolysaccharide: partial role of autocrine/paracrine interferon-alpha/beta. Biochem J. 2000;349:99–104. doi: 10.1042/0264-6021:3490099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Q, Miyakawa Y, Fox N, Kaushansky K. Interferon-alpha directly represses megakaryopoiesis by inhibiting thrombopoietin-induced signaling through induction of SOCS-1. Blood. 2000;96:2093–2099. [PubMed] [Google Scholar]
- 67.Song MM, Shuai K. The suppressor of cytokine signaling (SOCS) 1 and SOCS3 but not SOCS2 proteins inhibit interferon-mediated antiviral and antiproliferative activities. J Biol Chem. 1998;273:35056–35062. doi: 10.1074/jbc.273.52.35056. [DOI] [PubMed] [Google Scholar]
- 68.Morita Y, Naka T, Kawazoe Y, Fujimoto M, Narazaki M, Nakagawa R, Fukuyama H, Nagata S, Kishimoto T. Signals transducers and activators of transcription (STAT)-induced STAT inhibitor-1 (SSI-1)/suppressor of cytokine signaling-1 (SOCS-1) suppresses tumor necrosis factor alpha-induced cell death in fibroblasts. Proc Natl Acad Sci USA. 2000;97:5405–5410. doi: 10.1073/pnas.090084797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kinjyo I, Hanada T, Inagaki-Ohara K, Mori H, Aki D, Ohishi M, Yoshida H, Kubo M, Yoshimura A. SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity. 2002;17:583–591. doi: 10.1016/s1074-7613(02)00446-6. [DOI] [PubMed] [Google Scholar]
- 70.Nakagawa R, Naka T, Tsutsui H, Fujimoto M, Kimura A, Abe T, Seki E, Sato S, Takeuchi O, Takeda K, Akira S, Yamanishi K, Kawase I, Nakanishi K, Kishimoto T. SOCS-1 participates in negative regulation of LPS responses. Immunity. 2002;17:677–687. doi: 10.1016/s1074-7613(02)00449-1. [DOI] [PubMed] [Google Scholar]
- 71.Dalpke AH, Opper S, Zimmermann S, Heeg K. Suppressors of cytokine signaling (SOCS)-1 and SOCS-3 are induced by CpG-DNA and modulate cytokine responses in APCs. J Immunol. 2001;166:7082–7089. doi: 10.4049/jimmunol.166.12.7082. [DOI] [PubMed] [Google Scholar]
- 72.Sadowski CL, Choi TS, Le M, Wheeler TT, Wang LH, Sadowski HB. Insulin induction of SOCS-2 and SOCS-3 mRNA expression in C2C12 skeletal muscle cells Is mediated by Stat5. J Biol Chem. 2001;276:20703–20710. doi: 10.1074/jbc.M101014200. [DOI] [PubMed] [Google Scholar]
- 73.Park ES, Kim H, Suh JM, Park SJ, Kwon OY, Kim YK, Ro HK, Cho BY, Chung J, Shong M. Thyrotropin induces SOCS-1 (suppressor of cytokine signaling-1) and SOCS-3 in FRTL-5 thyroid cells. Mol Endocrinol. 2000;14:440–448. doi: 10.1210/mend.14.3.0433. [DOI] [PubMed] [Google Scholar]
- 74.Wu T, Xie M, Wang X, Jiang X, Li J, Huang H. miR-155 modulates TNF-alpha-inhibited osteogenic differentiation by targeting SOCS1 expression. Bone. 2012;51:498–505. doi: 10.1016/j.bone.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 75.Lu LF, Thai TH, Calado DP, Chaudhry A, Kubo M, Tanaka K, Loeb GB, Lee H, Yoshimura A, Rajewsky K, Rudensky AY. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity. 2009;30:80–91. doi: 10.1016/j.immuni.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trop S, De Sepulveda P, Zuniga-Pflucker JC, Rottapel R. Overexpression of suppressor of cytokine signaling-1 impairs pre-T-cell receptor-induced proliferation but not differentiation of immature thymocytes. Blood. 2001;97:2269–2277. doi: 10.1182/blood.v97.8.2269. [DOI] [PubMed] [Google Scholar]
- 77.Eyles JL, Metcalf D, Grusby MJ, Hilton DJ, Starr R. Negative regulation of interleukin-12 signaling by suppressor of cytokine signaling-1. J Biol Chem. 2002;277:43735–43740. doi: 10.1074/jbc.M208586200. [DOI] [PubMed] [Google Scholar]
- 78.Ilangumaran S, Ramanathan S, La Rose J, Poussier P, Rottapel R. Suppressor of cytokine signaling 1 regulates IL-15 receptor signaling in CD8+CD44high memory T lymphocytes. J Immunol. 2003;171:2435–2445. doi: 10.4049/jimmunol.171.5.2435. [DOI] [PubMed] [Google Scholar]
- 79.Motta M, Accornero P, Baratta M. Leptin and prolactin modulate the expression of SOCS-1 in association with interleukin-6 and tumor necrosis factor-alpha in mammary cells: a role in differentiated secretory epithelium. Regul Pept. 2004;121:163–170. doi: 10.1016/j.regpep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 80.Kawazoe Y, Naka T, Fujimoto M, Kohzaki H, Morita Y, Narazaki M, Okumura K, Saitoh H, Nakagawa R, Uchiyama Y, Akira S, Kishimoto T. Signal transducer and activator of transcription (STAT)-induced STAT inhibitor 1 (SSI-1)/suppressor of cytokine signaling 1 (SOCS1) inhibits insulin signal transduction pathway through modulating insulin receptor substrate 1 (IRS-1) phosphorylation. J Exp Med. 2001;193:263–269. doi: 10.1084/jem.193.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zong CS, Chan J, Levy DE, Horvath C, Sadowski HB, Wang LH. Mechanism of STAT3 activation by insulin-like growth factor I receptor. J Biol Chem. 2000;275:15099–15105. doi: 10.1074/jbc.M000089200. [DOI] [PubMed] [Google Scholar]
- 82.Mansell A, Smith R, Doyle SL, Gray P, Fenner JE, Crack PJ, Nicholson SE, Hilton DJ, O’Neill LA, Hertzog PJ. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat Immunol. 2006;7:148–155. doi: 10.1038/ni1299. [DOI] [PubMed] [Google Scholar]
- 83.Vuong BQ, Arenzana TL, Showalter BM, Losman J, Chen XP, Mostecki J, Banks AS, Limnander A, Fernandez N, Rothman PB. SOCS-1 localizes to the microtubule organizing complex-associated 20S proteasome. Mol Cell Biol. 2004;24:9092–9101. doi: 10.1128/MCB.24.20.9092-9101.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ungureanu D, Saharinen P, Junttila I, Hilton DJ, Silvennoinen O. Regulation of Jak2 through the ubiquitin-proteasome pathway involves phosphorylation of Jak2 on Y1007 and interaction with SOCS-1. Mol Cell Biol. 2002;22:3316–3326. doi: 10.1128/MCB.22.10.3316-3326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kamizono S, Hanada T, Yasukawa H, Minoguchi S, Kato R, Minoguchi M, Hattori K, Hatakeyama S, Yada M, Morita S, Kitamura T, Kato H, Nakayama K, Yoshimura A. The SOCS box of SOCS-1 accelerates ubiquitin-dependent proteolysis of TEL-JAK2. J Biol Chem. 2001;276:12530–12538. doi: 10.1074/jbc.M010074200. [DOI] [PubMed] [Google Scholar]
- 86.Frantsve J, Schwaller J, Sternberg DW, Kutok J, Gilliland DG. Socs-1 inhibits TEL-JAK2-mediated transformation of hematopoietic cells through inhibition of JAK2 kinase activity and induction of proteasome-mediated degradation. Mol Cell Biol. 2001;21:3547–3557. doi: 10.1128/MCB.21.10.3547-3557.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Sepulveda P, Ilangumaran S, Rottapel R. Suppressor of cytokine signaling-1 inhibits VAV function through protein degradation. J Biol Chem. 2000;275:14005–14008. doi: 10.1074/jbc.c000106200. [DOI] [PubMed] [Google Scholar]
- 88.Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem. 2002;277:42394–42398. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- 89.Narazaki M, Fujimoto M, Matsumoto T, Morita Y, Saito H, Kajita T, Yoshizaki K, Naka T, Kishimoto T. Three distinct domains of SSI-1/SOCS-1/JAB protein are required for its suppression of interleukin 6 signaling. Proc Natl Acad Sci USA. 1998;95:13130–13134. doi: 10.1073/pnas.95.22.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Starr R, Metcalf D, Elefanty AG, Brysha M, Willson TA, Nicola NA, Hilton DJ, Alexander WS. Liver degeneration and lymphoid deficiencies in mice lacking suppressor of cytokine signaling-1. Proc Natl Acad Sci USA. 1998;95:14395–14399. doi: 10.1073/pnas.95.24.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Naka T, Matsumoto T, Narazaki M, Fujimoto M, Morita Y, Ohsawa Y, Saito H, Nagasawa T, Uchiyama Y, Kishimoto T. Accelerated apoptosis of lymphocytes by augmented induction of Bax in SSI-1 (STAT-induced STAT inhibitor-1) deficient mice. Proc Natl Acad Sci USA. 1998;95:15577–15582. doi: 10.1073/pnas.95.26.15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alexander WS, Starr R, Fenner JE, Scott CL, Handman E, Sprigg NS, Corbin JE, Cornish AL, Darwiche R, Owczarek CM, Kay TWH, Nicola NA, Hertzog PJ, Metcalf D, Hilton DJ. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell. 1999;98:597–608. doi: 10.1016/s0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- 93.Metcalf D, Mifsud S, Di Rago L, Nicola NA, Hilton DJ, Alexander WS. Polycystic kidneys and chronic inflammatory lesions are the delayed consequences of loss of the suppressor of cytokine signaling-1 (SOCS-1) Proc Natl Acad Sci USA. 2002;99:943–948. doi: 10.1073/pnas.022628499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cornish AL, Davey GM, Metcalf D, Purton JF, Corbin JE, Greenhalgh CJ, Darwiche R, Wu L, Nicola NA, Godfrey DI, Heath WR, Hilton DJ, Alexander WS, Starr R. Suppressor of cytokine signaling-1 has IFN-gamma-independent actions in T cell homeostasis. J Immunol. 2003;170:878–886. doi: 10.4049/jimmunol.170.2.878. [DOI] [PubMed] [Google Scholar]
- 95.Lee C, Kolesnik TB, Caminschi I, Chakravorty A, Carter W, Alexander WS, Jones J, Anderson GP, Nicholson SE. Suppressor of cytokine signalling 1 (SOCS1) is a physiological regulator of the asthma response. Clin Exp Allergy. 2009;39:897–907. doi: 10.1111/j.1365-2222.2009.03217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Horino J, Fujimoto M, Terabe F, Serada S, Takahashi T, Soma Y, Tanaka K, Chinen T, Yoshimura A, Nomura S, Kawase I, Hayashi N, Kishimoto T, Naka T. Suppressor of cytokine signaling-1 ameliorates dextran sulfate sodium-induced colitis in mice. Int Immunol. 2008;20:753–762. doi: 10.1093/intimm/dxn033. [DOI] [PubMed] [Google Scholar]
- 97.Cornish AL, Chong MM, Davey GM, Darwiche R, Nicola NA, Hilton DJ, Kay TW, Starr R, Alexander WS. Suppressor of cytokine signaling-1 regulates signaling in response to interleukin-2 and other gamma c-dependent cytokines in peripheral T cells. J Biol Chem. 2003;278:22755–22761. doi: 10.1074/jbc.M303021200. [DOI] [PubMed] [Google Scholar]
- 98.Chong MM, Cornish AL, Darwiche R, Stanley EG, Purton JF, Godfrey DI, Hilton DJ, Starr R, Alexander WS, Kay TW. Suppressor of cytokine signaling-1 is a critical regulator of interleukin-7-dependent CD8+ T cell differentiation. Immunity. 2003;18:475–487. doi: 10.1016/s1074-7613(03)00078-5. [DOI] [PubMed] [Google Scholar]
- 99.Zhan Y, Davey GM, Graham KL, Kiu H, Dudek NL, Kay TW, Lew AM. SOCS1 negatively regulates the production of Foxp3+ CD4+ T cells in the thymus. Immunol Cell Biol. 2009;87:473–480. doi: 10.1038/icb.2009.23. [DOI] [PubMed] [Google Scholar]
- 100.Diehl S, Anguita J, Hoffmeyer A, Zapton T, Ihle JN, Fikrig E, Rincon M. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity. 2000;13:805–815. doi: 10.1016/s1074-7613(00)00078-9. [DOI] [PubMed] [Google Scholar]
- 101.Tanaka K, Ichiyama K, Hashimoto M, Yoshida H, Takimoto T, Takaesu G, Torisu T, Hanada T, Yasukawa H, Fukuyama S, Inoue H, Nakanishi Y, Kobayashi T, Yoshimura A. Loss of suppressor of cytokine signaling 1 in helper T cells leads to defective Th17 differentiation by enhancing antagonistic effects of IFN-gamma on STAT3 and Smads. J Immunol. 2008;180:3746–3756. doi: 10.4049/jimmunol.180.6.3746. [DOI] [PubMed] [Google Scholar]
- 102.Abe T, Nomura S, Nakagawa R, Fujimoto M, Kawase I, Naka T. Osteoblast differentiation is impaired in SOCS-1-deficient mice. J Bone Miner Metab. 2006;24:283–290. doi: 10.1007/s00774-006-0685-0. [DOI] [PubMed] [Google Scholar]
- 103.Jamieson E, Chong MM, Steinberg GR, Jovanovska V, Fam BC, Bullen DV, Chen Y, Kemp BE, Proietto J, Kay TW, Andrikopoulos S. Socs1 deficiency enhances hepatic insulin signaling. J Biol Chem. 2005;280:31516–31521. doi: 10.1074/jbc.M502163200. [DOI] [PubMed] [Google Scholar]
- 104.Fenner JE, Starr R, Cornish AL, Zhang JG, Metcalf D, Schreiber RD, Sheehan K, Hilton DJ, Alexander WS, Hertzog PJ. Suppressor of cytokine signaling 1 regulates the immune response to infection by a unique inhibition of type I interferon activity. Nat Immunol. 2006;7:33–39. doi: 10.1038/ni1287. [DOI] [PubMed] [Google Scholar]
- 105.Gingras S, Parganas E, de Pauw A, Ihle JN, Murray PJ. Re-examination of the role of suppressor of cytokine signaling 1 (SOCS1) in the regulation of toll-like receptor signaling. J Biol Chem. 2004;279:54702–54707. doi: 10.1074/jbc.M411043200. [DOI] [PubMed] [Google Scholar]
- 106.O’Sullivan LA, Noor SM, Trengove MC, Lewis RS, Liongue C, Sprigg NS, Nicholson SE, Ward AC. Suppressor of cytokine signaling 1 regulates embryonic myelopoiesis independently of its effects on T cell development. J Immunol. 2011;186:4751–4761. doi: 10.4049/jimmunol.1000343. [DOI] [PubMed] [Google Scholar]
- 107.Egan PJ, Lawlor KE, Alexander WS, Wicks IP. Suppressor of cytokine signaling-1 regulates acute inflammatory arthritis and T cell activation. J Clin Invest. 2003;111:915–924. doi: 10.1172/JCI16156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakashima T, Yokoyama A, Onari Y, Shoda H, Haruta Y, Hattori N, Naka T, Kohno N. Suppressor of cytokine signaling 1 inhibits pulmonary inflammation and fibrosis. J Allergy Clin Immunol. 2008;121:1269–1276. doi: 10.1016/j.jaci.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 109.Inagaki-Ohara K, Sasaki A, Matsuzaki G, Ikeda T, Hotokezaka M, Chijiiwa K, Kubo M, Yoshida H, Nawa Y, Yoshimura A. Suppressor of cytokine signalling 1 in lymphocytes regulates the development of intestinal inflammation in mice. Gut. 2006;55:212–219. doi: 10.1136/gut.2004.062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Harada M, Nakashima K, Hirota T, Shimizu M, Doi S, Fujita K, Shirakawa T, Enomoto T, Yoshikawa M, Moriyama H, Matsumoto K, Saito H, Suzuki Y, Nakamura Y, Tamari M. Functional polymorphism in the suppressor of cytokine signaling 1 gene associated with adult asthma. Am J Respir Cell Mol Biol. 2007;36:491–496. doi: 10.1165/rcmb.2006-0090OC. [DOI] [PubMed] [Google Scholar]
- 111.Barral AM, Thomas HE, Ling EM, Darwiche R, Rodrigo E, Christen U, Ejrnaes M, Wolfe T, Kay TW, von Herrath MG. SOCS-1 protects from virally-induced CD8 T cell mediated type 1 diabetes. J Autoimmun. 2006;27:166–173. doi: 10.1016/j.jaut.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 112.Ueki K, Kadowaki T, Kahn CR. Role of suppressors of cytokine signaling SOCS-1 and SOCS-3 in hepatic steatosis and the metabolic syndrome. Hepatol Res. 2005;33:185–192. doi: 10.1016/j.hepres.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 113.Weniger MA, Melzner I, Menz CK, Wegener S, Bucur AJ, Dorsch K, Mattfeldt T, Barth TF, Moller P. Mutations of the tumor suppressor gene SOCS-1 in classical Hodgkin lymphoma are frequent and associated with nuclear phospho-STAT5 accumulation. Oncogene. 2006;25:2679–2684. doi: 10.1038/sj.onc.1209151. [DOI] [PubMed] [Google Scholar]
- 114.Mottok A, Renne C, Willenbrock K, Hansmann ML, Brauninger A. Somatic hypermutation of SOCS1 in lymphocyte-predominant Hodgkin lymphoma is accompanied by high JAK2 expression and activation of STAT6. Blood. 2007;110:3387–3390. doi: 10.1182/blood-2007-03-082511. [DOI] [PubMed] [Google Scholar]
- 115.Melzner I, Bucur AJ, Bruderlein S, Dorsch K, Hasel C, Barth TF, Leithauser F, Moller P. Biallelic mutation of SOCS-1 impairs JAK2 degradation and sustains phospho-JAK2 action in the MedB-1 mediastinal lymphoma line. Blood. 2005;105:2535–2542. doi: 10.1182/blood-2004-09-3701. [DOI] [PubMed] [Google Scholar]
- 116.Chen CY, Tsay W, Tang JL, Shen HL, Lin SW, Huang SY, Yao M, Chen YC, Shen MC, Wang CH, Tien HF. SOCS1 methylation in patients with newly diagnosed acute myeloid leukemia. Genes Chromosomes Cancer. 2003;37:300–305. doi: 10.1002/gcc.10222. [DOI] [PubMed] [Google Scholar]
- 117.Watanabe D, Ezoe S, Fujimoto M, Kimura A, Saito Y, Nagai H, Tachibana I, Matsumura I, Tanaka T, Kanegane H, Miyawaki T, Emi M, Kanakura Y, Kawase I, Naka T, Kishimoto T. Suppressor of cytokine signalling-1 gene silencing in acute myeloid leukaemia and human haematopoietic cell lines. Br J Haematol. 2004;126:726–735. doi: 10.1111/j.1365-2141.2004.05107.x. [DOI] [PubMed] [Google Scholar]
- 118.Liu TC, Lin SF, Chang JG, Yang MY, Hung SY, Chang CS. Epigenetic alteration of the SOCS1 gene in chronic myeloid leukaemia. Br J Haematol. 2003;123:654–661. doi: 10.1046/j.1365-2141.2003.04660.x. [DOI] [PubMed] [Google Scholar]
- 119.Jost E, do ON, Dahl E, Maintz CE, Jousten P, Habets L, Wilop S, Herman JG, Osieka R, Galm O. Epigenetic alterations complement mutation of JAK2 tyrosine kinase in patients with BCR/ABL-negative myeloproliferative disorders. Leukemia. 2007;21:505–510. doi: 10.1038/sj.leu.2404513. [DOI] [PubMed] [Google Scholar]
- 120.Bock O, Hussein K, Brakensiek K, Buhr T, Schlue J, Wiese B, Kreipe H. The suppressor of cytokine signalling-1 (SOCS-1) gene is overexpressed in Philadelphia chromosome negative chronic myeloproliferative disorders. Leuk Res. 2007;31:799–803. doi: 10.1016/j.leukres.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 121.Roman-Gomez J, Jimenez-Velasco A, Castillejo JA, Cervantes F, Barrios M, Colomer D, Heiniger A, Torres A. The suppressor of cytokine signaling-1 is constitutively expressed in chronic myeloid leukemia and correlates with poor cytogenetic response to interferon-alpha. Haematologica. 2004;89:42–48. [PubMed] [Google Scholar]
- 122.Hatirnaz O, Ure U, Ar C, Akyerli C, Soysal T, Ferhanoglu B, Ozcelik T, Ozbek U. The SOCS-1 gene methylation in chronic myeloid leukemia patients. Am J Hematol. 2007;82:729–730. doi: 10.1002/ajh.20886. [DOI] [PubMed] [Google Scholar]
- 123.Sobti RC, Singh N, Hussain S, Suri V, Nijhawan R, Bharti AC, Bharadwaj M, Das BC. Aberrant promoter methylation and loss of suppressor of cytokine signalling-1 gene expression in the development of uterine cervical carcinogenesis. Cell Oncol (Dordr) 2011;34:533–543. doi: 10.1007/s13402-011-0056-2. [DOI] [PubMed] [Google Scholar]
- 124.Hussain S, Singh N, Salam I, Bandil K, Yuvaraj M, Akbar Bhat M, Muzaffar Mir M, Siddiqi MA, Sobti RC, Bharadwaj M, Das BC. Methylation-mediated gene silencing of suppressor of cytokine signaling-1 (SOCS-1) gene in esophageal squamous cell carcinoma patients of Kashmir valley. J Recept Signal Transduct Res. 2011;31:147–156. doi: 10.3109/10799893.2011.553836. [DOI] [PubMed] [Google Scholar]
- 125.Tischoff I, Hengge UR, Vieth M, Ell C, Stolte M, Weber A, Schmidt WE, Tannapfel A. Methylation of SOCS-3 and SOCS-1 in the carcinogenesis of Barrett’s adenocarcinoma. Gut. 2007;56:1047–1053. doi: 10.1136/gut.2006.111633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nagai H, Kim YS, Konishi N, Baba M, Kubota T, Yoshimura A, Emi M. Combined hypermethylation and chromosome loss associated with inactivation of SSI-1/SOCS-1/JAB gene in human hepatocellular carcinomas. Cancer Lett. 2002;186:59–65. doi: 10.1016/s0304-3835(02)00244-6. [DOI] [PubMed] [Google Scholar]
- 127.Zhou H, Miki R, Eeva M, Fike FM, Seligson D, Yang L, Yoshimura A, Teitell MA, Jamieson CA, Cacalano NA. Reciprocal regulation of SOCS 1 and SOCS3 enhances resistance to ionizing radiation in glioblastoma multiforme. Clin Cancer Res. 2007;13:2344–2353. doi: 10.1158/1078-0432.CCR-06-2303. [DOI] [PubMed] [Google Scholar]
- 128.Sutherland KD, Lindeman GJ, Choong DY, Wittlin S, Brentzell L, Phillips W, Campbell IG, Visvader JE. Differential hypermethylation of SOCS genes in ovarian and breast carcinomas. Oncogene. 2004;23:7726–7733. doi: 10.1038/sj.onc.1207787. [DOI] [PubMed] [Google Scholar]
- 129.Hanada T, Kobayashi T, Chinen T, Saeki K, Takaki H, Koga K, Minoda Y, Sanada T, Yoshioka T, Mimata H, Kato S, Yoshimura A. IFNgamma-dependent, spontaneous development of colorectal carcinomas in SOCS1-deficient mice. J Exp Med. 2006;203:1391–1397. doi: 10.1084/jem.20060436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gui Y, Yeganeh M, Ramanathan S, Leblanc C, Pomerleau V, Ferbeyre G, Saucier C, Ilangumaran S. SOCS1 controls liver regeneration by regulating HGF signaling in hepatocytes. J Hepatol. 2011;55:1300–1308. doi: 10.1016/j.jhep.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 131.Rottapel R, Ilangumaran S, Neale C, La Rose J, Ho JM, Nguyen MH, Barber D, Dubreuil P, de Sepulveda P. The tumor suppressor activity of SOCS-1. Oncogene. 2002;21:4351–4362. doi: 10.1038/sj.onc.1205537. [DOI] [PubMed] [Google Scholar]
- 132.Yang T, Stark P, Janik K, Wigzell H, Rottenberg ME. SOCS-1 protects against Chlamydia pneumoniae-induced lethal inflammation but hampers effective bacterial clearance. J Immunol. 2008;180:4040–4049. doi: 10.4049/jimmunol.180.6.4040. [DOI] [PubMed] [Google Scholar]
- 133.Pothlichet J, Chignard M, Si-Tahar M. Innate immune response triggered by influenza A virus is negatively regulated by SOCS1 and SOCS3 through a RIG-I/IFNAR1-dependent pathway. J Immunol. 2008;180:2034–2038. doi: 10.4049/jimmunol.180.4.2034. [DOI] [PubMed] [Google Scholar]
- 134.Masood KI, Rottenberg ME, Carow B, Rao N, Ashraf M, Hussain R, Hasan Z. SOCS1 gene expression is increased in severe pulmonary tuberculosis. Scand J Immunol. 2012;76:398–404. doi: 10.1111/j.1365-3083.2012.02731.x. [DOI] [PubMed] [Google Scholar]
- 135.Zimmermann S, Murray PJ, Heeg K, Dalpke AH. Induction of suppressor of cytokine signaling-1 by Toxoplasma gondii contributes to immune evasion in macrophages by blocking IFN-gamma signaling. J Immunol. 2006;176:1840–1847. doi: 10.4049/jimmunol.176.3.1840. [DOI] [PubMed] [Google Scholar]
- 136.Imai K, Kurita-Ochiai T, Ochiai K. Mycobacterium bovis bacillus Calmette-Guerin infection promotes SOCS induction and inhibits IFN-gamma-stimulated JAK/STAT signaling in J774 macrophages. FEMS Immunol Med Microbiol. 2003;39:173–180. doi: 10.1016/S0928-8244(03)00231-1. [DOI] [PubMed] [Google Scholar]
- 137.Okugawa S, Yanagimoto S, Tsukada K, Kitazawa T, Koike K, Kimura S, Nagase H, Hirai K, Ota Y. Bacterial flagellin inhibits T cell receptor-mediated activation of T cells by inducing suppressor of cytokine signalling-1 (SOCS-1) Cell Microbiol. 2006;8:1571–1580. doi: 10.1111/j.1462-5822.2006.00731.x. [DOI] [PubMed] [Google Scholar]
- 138.Yao ZQ, Prayther D, Trabue C, Dong ZP, Moorman J. Differential regulation of SOCS-1 signalling in B and T lymphocytes by hepatitis C virus core protein. Immunology. 2008;125:197–207. doi: 10.1111/j.1365-2567.2008.02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dey BR, Spence SL, Nissley P, Furlanetto RW. Interaction of human suppressor of cytokine signaling (SOCS)-2 with the insulin-like growth factor-I receptor. J Biol Chem. 1998;273:24095–24101. doi: 10.1074/jbc.273.37.24095. [DOI] [PubMed] [Google Scholar]
- 140.Metcalf D, Greenhalgh CJ, Viney E, Willson TA, Starr R, Nicola NA, Hilton DJ, Alexander WS. Gigantism in mice lacking suppressor of cytokine signalling-2. Nature. 2000;405:1069–1073. doi: 10.1038/35016611. [DOI] [PubMed] [Google Scholar]
- 141.Santangelo C, Scipioni A, Marselli L, Marchetti P, Dotta F. Suppressor of cytokine signaling gene expression in human pancreatic islets: modulation by cytokines. Eur J Endocrinol. 2005;152:485–489. doi: 10.1530/eje.1.01856. [DOI] [PubMed] [Google Scholar]
- 142.Dogusan Z, Hooghe-Peters EL, Berus D, Velkeniers B, Hooghe R. Expression of SOCS genes in normal and leukemic human leukocytes stimulated by prolactin, growth hormone and cytokines. J Neuroimmunol. 2000;109:34–39. doi: 10.1016/s0165-5728(00)00300-3. [DOI] [PubMed] [Google Scholar]
- 143.Greenhalgh CJ, Bertolino P, Asa SL, Metcalf D, Corbin JE, Adams TE, Davey HW, Nicola NA, Hilton DJ, Alexander WS. Growth enhancement in suppressor of cytokine signaling 2 (SOCS-2)-deficient mice is dependent on signal transducer and activator of transcription 5b (STAT5b) Mol Endocrinol. 2002;16:1394–1406. doi: 10.1210/mend.16.6.0845. [DOI] [PubMed] [Google Scholar]
- 144.Wang L, Zhang Z, Zhang R, Hafner MS, Wong HK, Jiao Z, Chopp M. Erythropoietin upregulates SOCS2 in neuronal progenitor cells derived from SVZ of adult rat. Neuroreport. 2004;15:1225–1229. doi: 10.1097/01.wnr.0000127636.15181.c1. [DOI] [PubMed] [Google Scholar]
- 145.Minamoto S, Ikegame K, Ueno K, Narazaki M, Naka T, Yamamoto H, Matsumoto T, Saito H, Hosoe S, Kishimoto T. Cloning and functional analysis of new members of STAT Induced STAT Inhibitor (SSI) family: SSI-2 and SSI-3. Biochem Biophys Res Comm. 1997;237:79–83. doi: 10.1006/bbrc.1997.7080. [DOI] [PubMed] [Google Scholar]
- 146.Lee SH, Yun S, Piao ZH, Jeong M, Kim DO, Jung H, Lee J, Kim MJ, Kim MS, Chung JW, Kim TD, Yoon SR, Greenberg PD, Choi I. Suppressor of cytokine signaling 2 regulates IL-15-primed human NK cell function via control of phosphorylated Pyk2. J Immunol. 2010;185:917–928. doi: 10.4049/jimmunol.1000784. [DOI] [PubMed] [Google Scholar]
- 147.Harris J, Stanford PM, Sutherland K, Oakes SR, Naylor MJ, Robertson FG, Blazek KD, Kazlauskas M, Hilton HN, Wittlin S, Alexander WS, Lindeman GJ, Visvader JE, Ormandy CJ. Socs2 and Elf5 mediate prolactin-induced mammary gland development. Mol Endocrinol. 2006;20:1177–1187. doi: 10.1210/me.2005-0473. [DOI] [PubMed] [Google Scholar]
- 148.Tannahill GM, Elliott J, Barry AC, Hibbert L, Cacalano NA, Johnston JA. SOCS2 can enhance interleukin-2 (IL-2) and IL-3 signaling by accelerating SOCS3 degradation. Mol Cell Biol. 2005;25:9115–9126. doi: 10.1128/MCB.25.20.9115-9126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Goldshmit Y, Walters CE, Scott HJ, Greenhalgh CJ, Turnley AM. SOCS2 induces neurite outgrowth by regulation of epidermal growth factor receptor activation. J Biol Chem. 2004;279:16349–16355. doi: 10.1074/jbc.M312873200. [DOI] [PubMed] [Google Scholar]
- 150.Favre H, Benhamou A, Finidori J, Kelly PA, Edery M. Dual effects of suppressor of cytokine signaling (SOCS-2) on growth hormone signal transduction. FEBS Lett. 1999;453:63–66. doi: 10.1016/s0014-5793(99)00681-x. [DOI] [PubMed] [Google Scholar]
- 151.Vesterlund M, Zadjali F, Persson T, Nielsen ML, Kessler BM, Norstedt G, Flores-Morales A. The SOCS2 ubiquitin ligase complex regulates growth hormone receptor levels. PLoS One. 2011;6:e25358. doi: 10.1371/journal.pone.0025358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Greenhalgh CJ, Metcalf D, Thaus AL, Corbin JE, Uren R, Morgan PO, Fabri LJ, Zhang JG, Martin HM, Willson TA, Billestrup N, Nicola NA, Baca M, Alexander WS, Hilton DJ. Biological evidence that SOCS-2 can act either as an enhancer or suppressor of growth hormone signaling. J Biol Chem. 2002;277:40181–40184. doi: 10.1074/jbc.C200450200. [DOI] [PubMed] [Google Scholar]
- 153.Horvat S, Medrano JF. Lack of Socs2 expression causes the high-growth phenotype in mice. Genomics. 2001;72:209–212. doi: 10.1006/geno.2000.6441. [DOI] [PubMed] [Google Scholar]
- 154.Michaylira CZ, Simmons JG, Ramocki NM, Scull BP, McNaughton KK, Fuller CR, Lund PK. Suppressor of cytokine signaling-2 limits intestinal growth and enterotrophic actions of IGF-I in vivo. Am J Physiol Gastrointest Liver Physiol. 2006;291:G472–481. doi: 10.1152/ajpgi.00218.2005. [DOI] [PubMed] [Google Scholar]
- 155.Goldshmit Y, Greenhalgh CJ, Turnley AM. Suppressor of cytokine signalling-2 and epidermal growth factor regulate neurite outgrowth of cortical neurons. Eur J Neurosci. 2004;20:2260–2266. doi: 10.1111/j.1460-9568.2004.03698.x. [DOI] [PubMed] [Google Scholar]
- 156.Lebrun P, Cognard E, Gontard P, Bellon-Paul R, Filloux C, Berthault MF, Magnan C, Ruberte J, Luppo M, Pujol A, Pachera N, Herchuelz A, Bosch F, Van Obberghen E. The suppressor of cytokine signalling 2 (SOCS2) is a key repressor of insulin secretion. Diabetologia. 2010;53:1935–1946. doi: 10.1007/s00125-010-1786-9. [DOI] [PubMed] [Google Scholar]
- 157.Puff R, Dames P, Weise M, Goke B, Parhofer K, Lechner A. No non-redundant function of suppressor of cytokine signaling 2 in insulin producing beta-cells. Islets. 2010;2:252–257. doi: 10.4161/isl.2.4.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hu J, Winqvist O, Flores-Morales A, Wikstrom AC, Norstedt G. SOCS2 influences LPS induced human monocyte-derived dendritic cell maturation. PLoS One. 2009;4:e7178. doi: 10.1371/journal.pone.0007178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Posselt G, Schwarz H, Duschl A, Horejs-Hoeck J. Suppressor of cytokine signaling 2 is a feedback inhibitor of TLR-induced activation in human monocyte-derived dendritic cells. J Immunol. 2011;187:2875–2884. doi: 10.4049/jimmunol.1003348. [DOI] [PubMed] [Google Scholar]
- 160.Knosp CA, Carroll HP, Elliott J, Saunders SP, Nel HJ, Amu S, Pratt JC, Spence S, Doran E, Cooke N, Jackson R, Swift J, Fitzgerald DC, Heaney LG, Fallon PG, Kissenpfennig A, Johnston JA. SOCS2 regulates T helper type 2 differentiation and the generation of type 2 allergic responses. J Exp Med. 2011;208:1523–1531. doi: 10.1084/jem.20101167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cheng SM, Li JC, Lin SS, Lee DC, Liu L, Chen Z, Lau AS. HIV-1 transactivator protein induction of suppressor of cytokine signaling-2 contributes to dysregulation of IFNγ signaling. Blood. 2009;113:5192–5201. doi: 10.1182/blood-2008-10-183525. [DOI] [PubMed] [Google Scholar]
- 162.Bogazzi F, Ultimieri F, Raggi F, Russo D, Costa A, Marciano E, Bartalena L, Martino E. Changes in the expression of suppressor of cytokine signalling (SOCS) 2 in the colonic mucosa of acromegalic patients are associated with hyperplastic polyps. Clin Endocrinol (Oxf) 2009;70:898–906. doi: 10.1111/j.1365-2265.2008.03431.x. [DOI] [PubMed] [Google Scholar]
- 163.Haffner MC, Petridou B, Peyrat JP, Revillion F, Muller-Holzner E, Daxenbichler G, Marth C, Doppler W. Favorable prognostic value of SOCS2 and IGF-I in breast cancer. BMC Cancer. 2007;7:136. doi: 10.1186/1471-2407-7-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Boisclair YR, Wang J, Shi J, Hurst KR, Ooi GT. Role of the suppressor of cytokine signaling-3 in mediating the inhibitory effects of interleukin-1beta on the growth hormone-dependent transcription of the acid-labile subunit gene in liver cells. J Biol Chem. 2000;275:3841–3847. doi: 10.1074/jbc.275.6.3841. [DOI] [PubMed] [Google Scholar]
- 165.Cohney SJ, Sanden D, Cacalano NA, Yoshimura A, Mui A, Migone TS, Johnston JA. SOCS-3 is tyrosine phosphorylated in response to interleukin-2 and suppresses STAT5 phosphorylation and lymphocyte proliferation. Mol Cell Biol. 1999;19:4980–4988. doi: 10.1128/mcb.19.7.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Magrangeas F, Boisteau O, Denis S, Jacques Y, Minvielle S. Negative cross-talk between interleukin-3 and interleukin-11 is mediated by suppressor of cytokine signalling-3 (SOCS-3) Biochem J. 2001;353:223–230. doi: 10.1042/0264-6021:3530223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Forster I, Clausen BE, Nicola NA, Metcalf D, Hilton DJ, Roberts AW, Alexander WS. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 168.Auernhammer CJ, Melmed S. Interleukin-11 stimulates proopiomelanocortin gene expression and adrenocorticotropin secretion in corticotroph cells: evidence for a redundant cytokine network in the hypothalamo-pituitary-adrenal axis. Endocrinology. 1999;140:1559–1566. doi: 10.1210/endo.140.4.6636. [DOI] [PubMed] [Google Scholar]
- 169.Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, Pestka S. Identification, cloning, and characterization of a novel soluble receptor that binds IL-22 and neutralizes its activity. J Immunol. 2001;166:7096–7103. doi: 10.4049/jimmunol.166.12.7096. [DOI] [PubMed] [Google Scholar]
- 170.Sasaki A, Yasukawa H, Shouda T, Kitamura T, Dikic I, Yoshimura A. CIS3/SOCS-3 suppresses erythropoietin (EPO) signaling by binding the EPO receptor and JAK2. J Biol Chem. 2000;275:29338–29347. doi: 10.1074/jbc.M003456200. [DOI] [PubMed] [Google Scholar]
- 171.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 172.Faderl S, Harris D, Van Q, Kantarjian HM, Talpaz M, Estrov Z. Granulocyte-macrophage colony-stimulating factor (GM-CSF) induces antiapoptotic and proapoptotic signals in acute myeloid leukemia. Blood. 2003;102:630–637. doi: 10.1182/blood-2002-06-1890. [DOI] [PubMed] [Google Scholar]
- 173.Hong F, Nguyen VA, Gao B. Tumor necrosis factor alpha attenuates interferon alpha signaling in the liver: involvement of SOCS3 and SHP2 and implication in resistance to interferon therapy. FASEB J. 2001;15:1595–1597. doi: 10.1096/fj.00-0908fje. [DOI] [PubMed] [Google Scholar]
- 174.Hamanaka I, Saito Y, Yasukawa H, Kishimoto I, Kuwahara K, Miyamoto Y, Harada M, Ogawa E, Kajiyama N, Takahashi N, Izumi T, Kawakami R, Masuda I, Yoshimura A, Nakao K. Induction of JAB/SOCS-1/SSI-1 and CIS3/SOCS-3/SSI-3 is involved in gp130 resistance in cardiovascular system in rat treated with cardiotrophin-1 in vivo. Circ Res. 2001;88:727–732. doi: 10.1161/hh0701.088512. [DOI] [PubMed] [Google Scholar]
- 175.Magrangeas F, Boisteau O, Denis S, Jacques Y, Minvielle S. Negative regulation of oncostatin M signaling by suppressor of cytokine signaling (SOCS-3) Eur Cytokine Netw. 2001;12:309–315. [PubMed] [Google Scholar]
- 176.Cacalano NA, Sanden D, Johnston JA. Tyrosine-phosphorylated SOCS-3 inhibits STAT activation but binds to p120 RasGAP and activates Ras. Nat Cell Biol. 2001;3:460–465. doi: 10.1038/35074525. [DOI] [PubMed] [Google Scholar]
- 177.Tonko-Geymayer S, Goupille O, Tonko M, Soratroi C, Yoshimura A, Streuli C, Ziemiecki A, Kofler R, Doppler W. Regulation and function of the cytokine-inducible SH-2 domain proteins, CIS and SOCS3, in mammary epithelial cells. Mol Endocrinol. 2002;16:1680–1695. doi: 10.1210/mend.16.7.0872. [DOI] [PubMed] [Google Scholar]
- 178.Terstegen L, Gatsios P, Bode JG, Schaper F, Heinrich PC, Graeve L. The inhibition of interleukin-6-dependent STAT activation by mitogen-activated protein kinases depends on tyrosine 759 in the cytoplasmic tail of glycoprotein 130. J Biol Chem. 2000;275:18810–18817. doi: 10.1074/jbc.M904148199. [DOI] [PubMed] [Google Scholar]
- 179.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O’Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci USA. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Brender C, Tannahill GM, Jenkins BJ, Fletcher J, Columbus R, Saris CJ, Ernst M, Nicola NA, Hilton DJ, Alexander WS, Starr R. Suppressor of cytokine signaling 3 regulates CD8 T-cell proliferation by inhibition of interleukins 6 and 27. Blood. 2007;110:2528–2536. doi: 10.1182/blood-2006-08-041541. [DOI] [PubMed] [Google Scholar]
- 181.Croker BA, Metcalf D, Robb L, Wei W, Mifsud S, DiRago L, Cluse LA, Sutherland KD, Hartley L, Williams E, Zhang JG, Hilton DJ, Nicola NA, Alexander WS, Roberts AW. SOCS3 is a critical physiological negative regulator of G-CSF signaling and emergency granulopoiesis. Immunity. 2004;20:153–165. doi: 10.1016/s1074-7613(04)00022-6. [DOI] [PubMed] [Google Scholar]
- 182.Karlsen AE, Ronn SG, Lindberg K, Johannesen J, Galsgaard ED, Pociot F, Nielsen JH, Mandrup-Poulsen T, Nerup J, Billestrup N. Suppressor of cytokine signaling 3 (SOCS-3) protects beta -cells against interleukin-1beta - and interferon-gamma -mediated toxicity. Proc Natl Acad Sci USA. 2001;98:12191–12196. doi: 10.1073/pnas.211445998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Emanuelli B, Peraldi P, Filloux C, Sawka-Verhelle D, Hilton D, Van Obberghen E. SOCS-3 is an insulin-induced negative regulator of insulin signaling. J Biol Chem. 2000;275:15985–15991. doi: 10.1074/jbc.275.21.15985. [DOI] [PubMed] [Google Scholar]
- 184.Matsumoto A, Seki Y, Watanabe R, Hayashi K, Johnston JA, Harada Y, Abe R, Yoshimura A, Kubo M. A role of suppressor of cytokine signaling 3 (SOCS3/CIS3/SSI3) in CD28-mediated interleukin 2 production. J Exp Med. 2003;197:425–436. doi: 10.1084/jem.20020939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Banerjee A, Banks AS, Nawijn MC, Chen XP, Rothman PB. Suppressor of cytokine signaling 3 inhibits activation of NFATp. J Immunol. 2002;168:4277–4281. doi: 10.4049/jimmunol.168.9.4277. [DOI] [PubMed] [Google Scholar]
- 186.Babon JJ, Kershaw NJ, Murphy JM, Varghese LN, Laktyushin A, Young SN, Lucet IS, Norton RS, Nicola NA. Suppression of cytokine signaling by SOCS3: characterization of the mode of inhibition and the basis of its specificity. Immunity. 2012;36:239–250. doi: 10.1016/j.immuni.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yamamoto K, Yamaguchi M, Miyasaka N, Miura O. SOCS-3 inhibits IL-12-induced STAT4 activation by binding through its SH2 domain to the STAT4 docking site in the IL-12 receptor beta2 subunit. Biochem Biophys Res Commun. 2003;310:1188–1193. doi: 10.1016/j.bbrc.2003.09.140. [DOI] [PubMed] [Google Scholar]
- 188.Lang R, Pauleau AL, Parganas E, Takahashi Y, Mages J, Ihle JN, Rutschman R, Murray PJ. SOCS3 regulates the plasticity of gp130 signaling. Nat Immunol. 2003;4:546–550. doi: 10.1038/ni932. [DOI] [PubMed] [Google Scholar]
- 189.Pellegrini M, Calzascia T, Toe JG, Preston SP, Lin AE, Elford AR, Shahinian A, Lang PA, Lang KS, Morre M, Assouline B, Lahl K, Sparwasser T, Tedder TF, Paik JH, DePinho RA, Basta S, Ohashi PS, Mak TW. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144:601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 190.Roberts AW, Robb L, Rakar S, Hartley L, Cluse L, Nicola NA, Metcalf D, Hilton DJ, Alexander WS. Placental defects and embryonic lethality in mice lacking suppressor of cytokine signaling 3. Proc Natl Acad Sci USA. 2001;98:9324–9329. doi: 10.1073/pnas.161271798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Takahashi Y, Carpino N, Cross JC, Torres M, Parganas E, Ihle JN. SOCS3: an essential regulator of LIF receptor signaling in trophoblast giant cell differentiation. EMBO J. 2003;22:372–384. doi: 10.1093/emboj/cdg057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Robb L, Boyle K, Rakar S, Hartley L, Lochland J, Roberts AW, Alexander WS, Metcalf D. Genetic reduction of embryonic leukemia-inhibitory factor production rescues placentation in SOCS3-null embryos but does not prevent inflammatory disease. Proc Natl Acad Sci USA. 2005;102:16333–16338. doi: 10.1073/pnas.0508023102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Croker BA, Mielke LA, Wormald S, Metcalf D, Kiu H, Alexander WS, Hilton DJ, Roberts AW. Socs3 maintains the specificity of biological responses to cytokine signals during granulocyte and macrophage differentiation. Exp Hematol. 2008;36:786–798. doi: 10.1016/j.exphem.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, Hanada T, Takeda K, Akira S, Hoshijima M, Hirano T, Chien KR, Yoshimura A. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4:551–556. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- 195.Liu X, Zhang Y, Yu Y, Yang X, Cao X. SOCS3 promotes TLR4 response in macrophages by feedback inhibiting TGF-beta1/Smad3 signaling. Mol Immunol. 2008;45:1405–1413. doi: 10.1016/j.molimm.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 196.Egwuagu CE, Yu CR, Zhang M, Mahdi RM, Kim SJ, Gery I. Suppressors of cytokine signaling proteins are differentially expressed in Th1 and Th2 cells: implications for Th cell lineage commitment and maintenance. J Immunol. 2002;168:3181–3187. doi: 10.4049/jimmunol.168.7.3181. [DOI] [PubMed] [Google Scholar]
- 197.Seki Y, Inoue H, Nagata N, Hayashi K, Fukuyama S, Matsumoto K, Komine O, Hamano S, Himeno K, Inagaki-Ohara K, Cacalano N, O’Garra A, Oshida T, Saito H, Johnston JA, Yoshimura A, Kubo M. SOCS-3 regulates onset and maintenance of T(H)2-mediated allergic responses. Nat Med. 2003;9:1047–1054. doi: 10.1038/nm896. [DOI] [PubMed] [Google Scholar]
- 198.Robinson GW, Pacher-Zavisin M, Zhu BM, Yoshimura A, Hennighausen L. Socs 3 modulates the activity of the transcription factor Stat3 in mammary tissue and controls alveolar homeostasis. Dev Dyn. 2007;236:654–661. doi: 10.1002/dvdy.21058. [DOI] [PubMed] [Google Scholar]
- 199.Ozawa Y, Nakao K, Shimazaki T, Shimmura S, Kurihara T, Ishida S, Yoshimura A, Tsubota K, Okano H. SOCS3 is required to temporally fine-tune photoreceptor cell differentiation. Dev Biol. 2007;303:591–600. doi: 10.1016/j.ydbio.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 200.Lindberg K, Ronn SG, Tornehave D, Richter H, Hansen JA, Romer J, Jackerott M, Billestrup N. Regulation of pancreatic beta-cell mass and proliferation by SOCS-3. J Mol Endocrinol. 2005;35:231–243. doi: 10.1677/jme.1.01840. [DOI] [PubMed] [Google Scholar]
- 201.Zhu BM, Ishida Y, Robinson GW, Pacher-Zavisin M, Yoshimura A, Murphy PM, Hennighausen L. SOCS3 negatively regulates the gp130-STAT3 pathway in mouse skin wound healing. J Invest Dermatol. 2008;128:1821–1829. doi: 10.1038/sj.jid.5701224. [DOI] [PubMed] [Google Scholar]
- 202.Isomaki P, Alanara T, Isohanni P, Lagerstedt A, Korpela M, Moilanen T, Visakorpi T, Silvennoinen O. The expression of SOCS is altered in rheumatoid arthritis. Rheumatology (Oxford) 2007;46:1538–1546. doi: 10.1093/rheumatology/kem198. [DOI] [PubMed] [Google Scholar]
- 203.Atsumi T, Ishihara K, Kamimura D, Ikushima H, Ohtani T, Hirota S, Kobayashi H, Park SJ, Saeki Y, Kitamura Y, Hirano T. A point mutation of Tyr-759 in interleukin 6 family cytokine receptor subunit gp130 causes autoimmune arthritis. J Exp Med. 2002;196:979–990. doi: 10.1084/jem.20020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wong PK, Egan PJ, Croker BA, O’Donnell K, Sims NA, Drake S, Kiu H, McManus EJ, Alexander WS, Roberts AW, Wicks IP. SOCS-3 negatively regulates innate and adaptive immune mechanisms in acute IL-1-dependent inflammatory arthritis. J Clin Invest. 2006;116:1571–1581. doi: 10.1172/JCI25660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shouda T, Yoshida T, Hanada T, Wakioka T, Oishi M, Miyoshi K, Komiya S, Kosai K, Hanakawa Y, Hashimoto K, Nagata K, Yoshimura A. Induction of the cytokine signal regulator SOCS3/CIS3 as a therapeutic strategy for treating inflammatory arthritis. J Clin Invest. 2001;108:1781–1788. doi: 10.1172/JCI13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Suzuki A, Hanada T, Mitsuyama K, Yoshida T, Kamizono S, Hoshino T, Kubo M, Yamashita A, Okabe M, Takeda K, Akira S, Matsumoto S, Toyonaga A, Sata M, Yoshimura A. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med. 2001;193:471–481. doi: 10.1084/jem.193.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ozaki A, Seki Y, Fukushima A, Kubo M. The control of allergic conjunctivitis by suppressor of cytokine signaling (SOCS)3 and SOCS5 in a murine model. J Immunol. 2005;175:5489–5497. doi: 10.4049/jimmunol.175.8.5489. [DOI] [PubMed] [Google Scholar]
- 208.Hookham MB, Elliott J, Suessmuth Y, Staerk J, Ward AC, Vainchenker W, Percy MJ, McMullin MF, Constantinescu SN, Johnston JA. The myeloproliferative disorder-associated JAK2 V617F mutant escapes negative regulation by suppressor of cytokine signaling 3. Blood. 2007;109:4924–4929. doi: 10.1182/blood-2006-08-039735. [DOI] [PubMed] [Google Scholar]
- 209.van de Geijn GJ, Gits J, Aarts LH, Heijmans-Antonissen C, Touw IP. G-CSF receptor truncations found in SCN/AML relieve SOCS3-controlled inhibition of STAT5 but leave suppression of STAT3 intact. Blood. 2004;104:667–674. doi: 10.1182/blood-2003-08-2913. [DOI] [PubMed] [Google Scholar]
- 210.Krishnadasan R, Bifulco C, Kim J, Rodov S, Zieske AW, Vanasse GJ. Overexpression of SOCS3 is associated with decreased survival in a cohort of patients with de novo follicular lymphoma. Br J Haematol. 2006;135:72–75. doi: 10.1111/j.1365-2141.2006.06248.x. [DOI] [PubMed] [Google Scholar]
- 211.Niwa Y, Kanda H, Shikauchi Y, Saiura A, Matsubara K, Kitagawa T, Yamamoto J, Kubo T, Yoshikawa H. Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK/STAT and FAK signalings in human hepatocellular carcinoma. Oncogene. 2005;24:6406–6417. doi: 10.1038/sj.onc.1208788. [DOI] [PubMed] [Google Scholar]
- 212.Tokita T, Maesawa C, Kimura T, Kotani K, Takahashi K, Akasaka T, Masuda T. Methylation status of the SOCS3 gene in human malignant melanomas. Int J Oncol. 2007;30:689–694. [PubMed] [Google Scholar]
- 213.Komyod W, Bohm M, Metze D, Heinrich PC, Behrmann I. Constitutive suppressor of cytokine signaling 3 expression confers a growth advantage to a human melanoma cell line. Mol Cancer Res. 2007;5:271–281. doi: 10.1158/1541-7786.MCR-06-0274. [DOI] [PubMed] [Google Scholar]
- 214.He B, You L, Uematsu K, Zang K, Xu Z, Lee AY, Costello JF, McCormick F, Jablons DM. SOCS-3 is frequently silenced by hypermethylation and suppresses cell growth in human lung cancer. Proc Natl Acad Sci USA. 2003;100:14133–14138. doi: 10.1073/pnas.2232790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rigby RJ, Simmons JG, Greenhalgh CJ, Alexander WS, Lund PK. Suppressor of cytokine signaling 3 (SOCS3) limits damage-induced crypt hyper-proliferation and inflammation-associated tumorigenesis in the colon. Oncogene. 2007;26:4833–4841. doi: 10.1038/sj.onc.1210286. [DOI] [PubMed] [Google Scholar]
- 216.Li Y, Deuring J, Peppelenbosch MP, Kuipers EJ, de Haar C, van der Woude CJ. IL-6-induced DNMT1 activity mediates SOCS3 promoter hypermethylation in ulcerative colitis-related colorectal cancer. Carcinogenesis. 2012;33:1889–1896. doi: 10.1093/carcin/bgs214. [DOI] [PubMed] [Google Scholar]
- 217.Ogata H, Kobayashi T, Chinen T, Takaki H, Sanada T, Minoda Y, Koga K, Takaesu G, Maehara Y, Iida M, Yoshimura A. Deletion of the SOCS3 gene in liver parenchymal cells promotes hepatitis-induced hepatocarcinogenesis. Gastroenterology. 2006;131:179–193. doi: 10.1053/j.gastro.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 218.Isomoto H, Mott JL, Kobayashi S, Werneburg NW, Bronk SF, Haan S, Gores GJ. Sustained IL-6/STAT-3 signaling in cholangiocarcinoma cells due to SOCS-3 epigenetic silencing. Gastroenterology. 2007;132:384–396. doi: 10.1053/j.gastro.2006.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lindemann C, Hackmann O, Delic S, Schmidt N, Reifenberger G, Riemenschneider MJ. SOCS3 promoter methylation is mutually exclusive to EGFR amplification in gliomas and promotes glioma cell invasion through STAT3 and FAK activation. Acta Neuropathol. 2011;122:241–251. doi: 10.1007/s00401-011-0832-0. [DOI] [PubMed] [Google Scholar]
- 220.Pierconti F, Martini M, Pinto F, Cenci T, Capodimonti S, Calarco A, Bassi PF, Larocca LM. Epigenetic silencing of SOCS3 identifies a subset of prostate cancer with an aggressive behavior. Prostate. 2011;71:318–325. doi: 10.1002/pros.21245. [DOI] [PubMed] [Google Scholar]
- 221.Nakagawa T, Iida S, Osanai T, Uetake H, Aruga T, Toriya Y, Takagi Y, Kawachi H, Sugihara K. Decreased expression of SOCS-3 mRNA in breast cancer with lymph node metastasis. Oncol Rep. 2008;19:33–39. [PubMed] [Google Scholar]
- 222.Okabayashi T, Kariwa H, Yokota S, Iki S, Indoh T, Yokosawa N, Takashima I, Tsutsumi H, Fujii N. Cytokine regulation in SARS coronavirus infection compared to other respiratory virus infections. J Med Virol. 2006;78:417–424. doi: 10.1002/jmv.20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Persico M, Capasso M, Russo R, Persico E, Croce L, Tiribelli C, Iolascon A. Elevated expression and polymorphisms of SOCS3 influence patient response to antiviral therapy in chronic hepatitis C. Gut. 2008;57:507–515. doi: 10.1136/gut.2007.129478. [DOI] [PubMed] [Google Scholar]
- 224.Delgado-Ortega M, Melo S, Meurens F. Expression of SOCS1-7 and CIS mRNA in porcine tissues. Vet Immunol Immunopathol. 2011;144:493–498. doi: 10.1016/j.vetimm.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 225.Nicholson SE, Metcalf D, Sprigg NS, Columbus R, Walker F, Silva A, Cary D, Willson TA, Zhang JG, Hilton DJ, Alexander WS, Nicola NA. Suppressor of cytokine signaling (SOCS)-5 is a potential negative regulator of epidermal growth factor signaling. Proc Natl Acad Sci USA. 2004;102:2328–2333. doi: 10.1073/pnas.0409675102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hu G, Zhou R, Liu J, Gong AY, Chen XM. MicroRNA-98 and let-7 regulate expression of suppressor of cytokine signaling 4 in biliary epithelial cells in response to Cryptosporidium parvum infection. J Infect Dis. 2010;202:125–135. doi: 10.1086/653212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sutherland JM, Keightley RA, Nixon B, Roman SD, Robker RL, Russell DL, McLaughlin EA. Suppressor of cytokine signaling 4 (SOCS4): moderator of ovarian primordial follicle activation. J Cell Physiol. 2012;227:1188–1198. doi: 10.1002/jcp.22837. [DOI] [PubMed] [Google Scholar]
- 228.Sasi W, Jiang WG, Sharma A, Mokbel K. Higher expression levels of SOCS 1,3,4,7 are associated with earlier tumour stage and better clinical outcome in human breast cancer. BMC Cancer. 2010;10:178. doi: 10.1186/1471-2407-10-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kobayashi D, Nomoto S, Kodera Y, Fujiwara M, Koike M, Nakayama G, Ohashi N, Nakao A. Suppressor of cytokine signaling 4 detected as a novel gastric cancer suppressor gene using double combination array analysis. World J Surg. 2012;36:362–372. doi: 10.1007/s00268-011-1358-2. [DOI] [PubMed] [Google Scholar]
- 230.Scheitz CJ, Lee TS, McDermitt DJ, Tumbar T. Defining a tissue stem cell-driven Runx1/Stat3 signalling axis in epithelial cancer. EMBO J. 2012;31:4124–4139. doi: 10.1038/emboj.2012.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Magrangeas F, Apiou F, Denis S, Weidle U, Jacques Y, Minvielle S. Cloning and expression of CIS6, chromosome assignment to 3p22 and 2p21 by in situ hybridization. Cytogenet Cell Genet. 2000;88:78–81. doi: 10.1159/000015490. [DOI] [PubMed] [Google Scholar]
- 232.Liu X, Mameza MG, Lee YS, Eseonu CI, Yu CR, Kang Derwent JJ, Egwuagu CE. Suppressors of cytokine-signaling proteins induce insulin resistance in the retina and promote survival of retinal cells. Diabetes. 2008;57:1651–1658. doi: 10.2337/db07-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Brender C, Columbus R, Metcalf D, Handman E, Starr R, Huntington N, Tarlinton D, Odum N, Nicholson SE, Nicola NA, Hilton DJ, Alexander WS. SOCS5 is expressed in primary B and T lymphoid cells but is dispensable for lymphocyte production and function. Mol Cell Biol. 2004;24:6094–6103. doi: 10.1128/MCB.24.13.6094-6103.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Arakawa S, Hatano Y, Katagiri K. Differential expression of mRNA for Th1 and Th2 cytokine-associated transcription factors and suppressors of cytokine signalling in peripheral blood mononuclear cells of patients with atopic dermatitis. Clin Exp Immunol. 2004;135:505–510. doi: 10.1111/j.1365-2249.2004.02405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ohshima M, Yokoyama A, Ohnishi H, Hamada H, Kohno N, Higaki J, Naka T. Overexpression of suppressor of cytokine signalling-5 augments eosinophilic airway inflammation in mice. Clin Exp Allergy. 2007;37:735–742. doi: 10.1111/j.1365-2222.2007.02707.x. [DOI] [PubMed] [Google Scholar]
- 236.Egwuagu CE, Yu CR, Li Z, Nussenblatt RB. SOCS5 mRNA levels in peripheral blood mononuclear cells (PBMC): a potential bio-marker for monitoring response of uveitis patients to Daclizumab therapy. J Autoimmun. 2005;24:39–46. doi: 10.1016/j.jaut.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 237.Watanabe H, Kubo M, Numata K, Takagi K, Mizuta H, Okada S, Ito T, Matsukawa A. Overexpression of suppressor of cytokine signaling-5 in T cells augments innate immunity during septic peritonitis. J Immunol. 2006;177:8650–8657. doi: 10.4049/jimmunol.177.12.8650. [DOI] [PubMed] [Google Scholar]
- 238.Matsukawa A, Kaplan MH, Hogaboam CM, Lukacs NW, Kunkel SL. Pivotal role of signal transducer and activator of transcription (Stat)4 and Stat6 in the innate immune response during sepsis. J Exp Med. 2001;193:679–688. doi: 10.1084/jem.193.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yoon S, Yi YS, Kim SS, Kim JH, Park WS, Nam SW. SOCS5 and SOCS6 have similar expression patterns in normal and cancer tissues. Tumour Biol. 2012;33:215–221. doi: 10.1007/s13277-011-0264-4. [DOI] [PubMed] [Google Scholar]
- 240.Mooney RA, Senn J, Cameron S, Inamdar N, Boivin LM, Shang Y, Furlanetto RW. Suppressors of cytokine signaling-1 and -6 associate with and inhibit the insulin receptor. A potential mechanism for cytokine-mediated insulin resistance. J Biol Chem. 2001;276:25889–25893. doi: 10.1074/jbc.M010579200. [DOI] [PubMed] [Google Scholar]
- 241.Li L, Gronning LM, Anderson PO, Li S, Edvardsen K, Johnston J, Kioussis D, Shepherd PR, Wang P. Insulin induces SOCS-6 expression and its binding to the p85 monomer of phosphoinositide 3-kinase, resulting in improvement in glucose metabolism. J Biol Chem. 2004;279:34107–34114. doi: 10.1074/jbc.M312672200. [DOI] [PubMed] [Google Scholar]
- 242.Gupta S, Mishra K, Surolia A, Banerjee K. Suppressor of cytokine signalling-6 promotes neurite outgrowth via JAK2/STAT5-mediated signalling pathway, involving negative feedback inhibition. PLoS One. 2011;6:e26674. doi: 10.1371/journal.pone.0026674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kazi JU, Sun J, Phung B, Zadjali F, Flores-Morales A, Ronnstrand L. Suppressor of cytokine signaling 6 (SOCS6) negatively regulates Flt3 signal transduction through direct binding to phosphorylated tyrosines 591 and 919 of Flt3. J Biol Chem. 2012;287:36509–36517. doi: 10.1074/jbc.M112.376111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bayle J, Letard S, Frank R, Dubreuil P, De Sepulveda P. Suppressor of cytokine signaling 6 associates with KIT and regulates KIT receptor signaling. J Biol Chem. 2004;279:12249–12259. doi: 10.1074/jbc.M313381200. [DOI] [PubMed] [Google Scholar]
- 245.Choi YB, Son M, Park M, Shin J, Yun Y. SOCS-6 negatively regulates T cell activation through targeting p56lck to proteasomal degradation. J Biol Chem. 2010;285:7271–7280. doi: 10.1074/jbc.M109.073726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Piessevaux J, Lavens D, Montoye T, Wauman J, Catteeuw D, Vandekerckhove J, Belsham D, Peelman F, Tavernier J. Functional cross-modulation between SOCS proteins can stimulate cytokine signaling. J Biol Chem. 2006;281:32953–32966. doi: 10.1074/jbc.M600776200. [DOI] [PubMed] [Google Scholar]
- 247.Vlacich G, Nawijn MC, Webb GC, Steiner DF. Pim3 negatively regulates glucose-stimulated insulin secretion. Islets. 2010;2:308–317. doi: 10.4161/isl.2.5.13058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Sriram KB, Larsen JE, Savarimuthu Francis SM, Wright CM, Clarke BE, Duhig EE, Brown KM, Hayward NK, Yang IA, Bowman RV, Fong KM. Array-comparative genomic hybridization reveals loss of SOCS6 is associated with poor prognosis in primary lung squamous cell carcinoma. PLoS One. 2012;7:e30398. doi: 10.1371/journal.pone.0030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lai RH, Hsiao YW, Wang MJ, Lin HY, Wu CW, Chi CW, Li AF, Jou YS, Chen JY. SOCS6, downregulated in gastric cancer, inhibits cell proliferation and colony formation. Cancer Lett. 2010;288:75–85. doi: 10.1016/j.canlet.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 250.Storojeva I, Boulay JL, Heinimann K, Ballabeni P, Terracciano L, Laffer U, Mild G, Herrmann R, Rochlitz C. Prognostic and predictive relevance of microsatellite instability in colorectal cancer. Oncol Rep. 2005;14:241–249. [PubMed] [Google Scholar]
- 251.Storojeva I, Boulay JL, Ballabeni P, Buess M, Terracciano L, Laffer U, Mild G, Herrmann R, Rochlitz C. Prognostic and predictive relevance of DNAM-1, SOCS6 and CADH-7 genes on chromosome 18q in colorectal cancer. Oncology. 2005;68:246–255. doi: 10.1159/000086781. [DOI] [PubMed] [Google Scholar]
- 252.Krebs DL, Metcalf D, Merson TD, Voss AK, Thomas T, Zhang JG, Rakar S, O’Bryan MK, Willson TA, Viney EM, Mielke LA, Nicola NA, Hilton DJ, Alexander WS. Development of hydrocephalus in mice lacking SOCS7. Proc Natl Acad Sci USA. 2004;101:15446–15451. doi: 10.1073/pnas.0406870101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Matuoka K, Miki H, Takahashi K, Takenawa T. A novel ligand for an SH3 domain of the adaptor protein Nck bears an SH2 domain and nuclear signaling motifs. Biochem Biophys Res Commun. 1997;239:488–492. doi: 10.1006/bbrc.1997.7492. [DOI] [PubMed] [Google Scholar]
- 254.Knisz J, Banks A, McKeag L, Metcalfe DD, Rothman PB, Brown JM. Loss of SOCS7 in mice results in severe cutaneous disease and increased mast cell activation. Clin Immunol. 2009;132:277–284. doi: 10.1016/j.clim.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 255.Tellechea ML, Steinhardt AP, Rodriguez G, Taverna MJ, Poskus E, Frechtel G. Common variants in SOCS7 gene predict obesity, disturbances in lipid metabolism and insulin resistance. Nutr Metab Cardiovasc Dis. 2012 Mar 6; doi: 10.1016/j.numecd.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 256.Marine JC, Topham DJ, McKay C, Wang D, Parganas E, Stravopodis D, Yoshimura A, Ihle JN. SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell. 1999;98:609–616. doi: 10.1016/s0092-8674(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 257.Fujimoto M, Naka T, Nakagawa R, Kawazoe Y, Morita Y, Tateishi A, Okumura K, Narazaki M, Kishimoto T. Defective thymocyte development and perturbed homeostasis of T cells in STAT-induced STAT inhibitor-1/suppressors of cytokine signaling-1 transgenic mice. J Immunol. 2000;165:1799–1806. doi: 10.4049/jimmunol.165.4.1799. [DOI] [PubMed] [Google Scholar]
- 258.Marine JC, McKay C, Wang D, Topham DJ, Parganas E, Nakajima H, Pendeville H, Yasukawa H, Sasaki A, Yoshimura A, Ihle JN. SOCS3 is essential in the regulation of fetal liver erythropoiesis. Cell. 1999;98:617–627. doi: 10.1016/s0092-8674(00)80049-5. [DOI] [PubMed] [Google Scholar]
- 259.Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004;10:739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]