Abstract
Methamphetamine (Meth) abuse exacerbates HIV-1-associated neurocognitive disorders (HAND). The underlying mechanism for this effect is not entirely clear but likely involves cooperation between Meth and HIV-1 virotoxins, such as the transactivator of transcription, Tat. HIV-1 Tat mediates damage in the CNS by inducing inflammatory processes including astrogliosis. Wnt/β catenin signaling regulates survival processes for both neurons and astrocytes. Here, we evaluated the impact of Meth on the Wnt/β-catenin pathway in astrocytes transfected with Tat. Meth and Tat downregulated Wnt/β-catenin signaling by >50%, as measured by TOPflash reporter activity in both an astrocytoma cell line and primary human fetal astrocytes. Meth and Tat also down-regulated LEF-1 transcript by >30%. LEF-1 is a key partner of β-catenin to regulate cognate gene expression. Interestingly, estrogen, which induces β-catenin signaling in a cell-type specific manner, at physiological concentrations of 1.5 and 3 nM normalized individual Meth and Tat effects on β-catenin signaling but not their combined effects. These findings suggest that Meth and Tat likely exert different mechanisms to mediate down regulation of β-catenin signaling. The consequences of which may contribute to the pathophysiologic effects of HIV-1 and Meth co-morbidity in the CNS.
Keywords: Methamphetamine, HIV, Tat, β-catenin signaling, Astrocytes
Introduction
In approximately 50% of HIV-1 infected individuals, HIV-1 causes a spectrums of neurologic disorders that range from mild cognitive impairment to more severe HIV- associated dementia (HAD). These disorders are collectively known as HIV associated neurocognitive disorders (HAND), which persist despite Highly Active Antiretroviral Therapy (Letendre et al. 2009; Letendre et al. 2010). HAND is more severe in those who abuse drugs than in those who do not (Reyes et al. 1991; Hauser et al. 2007; Ferris et al. 2008). This co-morbidity applies to Methamphetamine (Meth), a potent psychostimulant that has exceeded cocaine abuse in the United States (Hser et al. 2008) and is also frequently abused in the HIV/AIDS population (Koutsilieri et al. 1997; Maragos et al. 2002; Nath et al. 2002). Meth exacerbates HAND as Meth abusers who are HIV-1 infected have more severe neuropathology than those who are none Meth abusers (Koutsilieri et al. 1997; Maragos et al. 2002; Nath et al. 2002). The mechanisms underlying these exacerbated co-morbidity is not entirely clear.
Although HIV-1-mediated neuropathogenesis is a complex process, largely driven by direct and indirect effects of HIV-1 infection in the CNS, the HIV-1 transactivator of transcription (Tat, M.W. 8335) causes profound damage in the CNS (New et al. 1997; Kruman et al. 1998; Bansal et al. 2000; Maragos et al. 2003; King et al. 2006). Tat induces astrogliosis by activating glial fibrillary acidic protein (GFAP) expression through a regulatory process involving Tat induction of p300 through its early growth response 1 (egr-1) element, which then activates GFAP expression (Zhou et al. 2004; Zou et al. 2010). Tat dysregulation of astrocytes, which depending on the dose and the system used, may also include apoptosis of astrocytes (Kim et al. 2003; Khurdayan et al. 2004; Eugenin et al., 2007). Because Tat is a small protein that is released from infected cells and can enter bystander cells or be transported axonally (Bruce-Keller et al. 2003), Tat can exert its effects even at distant sites from HIV-1 infection.
Given the critical role of astrocytes in maintaining CNS homeostasis, we evaluated the impact of Meth and Tat on the Wnt/β-catenin pathway in astrocytes. The Wnt/β-catenin pathway is a negative regulator of HIV-1 replication in astrocytes (Carroll-Anzinger et al. 2007; Li et al. 2011). It is a complex signal transduction pathway that regulates the transcription of hundreds of genes, including those involved in cell differentiation, communication, apoptosis/ survival and proliferation (Miller and Moon 1996; Moon et al. 1997). The Wnt/β-catenin signaling is initiated by binding of Wnt protein (soluble glycoproteins) to the seven transmembrane Frizzled family of receptors. Binding of Wnt to Frizzled often requires the recruitment of low-density lipoprotein receptor-related protein (LRP) 5/6. This leads to a signal transduction cascade that destabilizes a β-catenin destruction complex. This destruction complex consists of several proteins including axin, adenomatous polyposis coli (APC), casein kinase 1α (Ck1α), and glycogen synthase kinase 3β (GSK3β) and, when stable, leads to the phosphorylation of β-catenin at Ser33, Ser37, Ser45 and Thr 41, its ubiquitination by βTrcp and degradation through the proteasomal pathway. Destabilization of the destruction complex allows hypophosphorylated β-catenin to translocate to the nucleus where it binds to T-cell factor/lymphoid enhancer (TCF/LEF) transcription factors and leads to target gene transcription. β-catenin may also be membrane-associated by binding to cadherins to stabilize the actin cytoskeleton, mediate cell adhesion, and regulates synaptic strength. β-catenin can also be activated independent of a Wnt ligand initiating cascade, whereby signals that inhibit the destruction complex lead to stabilization and accumulation of β-catenin.
Considerable data point to the neuroprotective role of the Wnt/β-catenin pathway in the CNS (Henderson and Al-Harthi, 2011). Astrocytes are a vital source for Wnt ligands, which in turn initiate a Wnt signaling cascade to mediate anti-inflammatory responses, neuroprotection, and adult neurogenesis. Activated astrocytes in ventral midbrain secret Wnt 1 and via Wnt signaling mediate neuroprotection against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) neurotoxicity (Toledo and Inestrosa 2010). Further, Wnt signaling in astrocytes promotes dopaminergic neurogenesis from adult midbrain neuroprogenitor cells and proliferation of astrocytes post injury (White et al. 2010; L'Episcopo et al. 2011).
Given the importance of the Wnt/β-catenin pathway in astrocyte function and in mediating anti-inflammatory processes and because of the critical role that astrocytes play in maintaining CNS integrity, we evaluated the impact of Meth and HIV-1 Tat on β-catenin signaling and determined whether the female sex hormone, 17β estradiol (estrogen), which in a cell-specific manner induces β-catenin signaling (Cardona-Gomez et al. 2004; Kouzmenko et al. 2004; Zhang et al. 2008; Varea et al. 2009) can modulate these effects in astrocytes.
Materials and methods
Cell culture
U87MG astrocytoma cells were obtained from the NIH AIDS Research and Reference Reagent Program (Frederick, MD). The cells were propagated in Dulbecco’s modified eagle’s medium (DMEM, Gibco Invitrogen,) supplemented with 10% heat inactivated fetal bovine serum (HI-FBS; Sigma-Aldrich, St. Louis, MO) and 1% penicillin-streptomycin (Gibco Invitrogen, Carlsbad, CA) in 5% CO2 humidified atmosphere at 37°C. Progenitor-derived astrocytes (PDAs) were generated from neural progenitor cells provided by Dr. Eugene Major (NINDS, NIH, MD) and differentiated into astrocytes as previously described (Lamba et al. 2009). Briefly, progenitor cells were seeded on poly-D-lysine coated T-75 tissue culture flasks at 2×106 cells/flask and maintained in progenitor medium consisting of neurobasal media (Gibco Invitrogen) supplemented with 0.5% bovine albumin (Sigma), neuro-survival factor (NSF) (Lonza, Walkersville, MD), N2 components (Gibco Invitrogen), 25 ng/ml fibroblast growth factor (bFGF), 20 ng/ml epidermal growth factor (EGF) (R & D Systems, Minneapolis MN), 50 µg/ml gentamicin (Lonza) and 2 mM L-glutamine (Gibco Invitrogen). To induce differentiation, progenitor medium was replaced with PDA medium containing DMEM supplemented with 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine and 50 µg/ml gentamicin. Cultures were >90% positive for GFAP after 30 days of differentiation.
Plasmids
TOPflash reporter plasmid was purchased from Adgene (Cambridge, MA). Topflash is an indicator of β catenin driven gene expression. It consists of seven native TCF/LEF binding sites linked to firefly luciferase reporter under the control of a minimal TA viral promoter. NFκB-luciferase reporter plasmid was purchased from Stratagene/ Agilent Technologies (Santa Clara, CA). It consists of putative NFκB binding sites linked to firefly luciferase under the control of minimal TATA box promoter. Tat cDNA (Adgene plasmid 14654) was purchased from Adgene (Cambridge, MA) and contains wild Tat gene corresponding to nucleotides 1–360. Renilla plasmid was purchased from Promega (Madison, WI) and was used as an internal transfection control.
Transfection and dual luciferase reporter assay
Transfections of the indicated reporter plasmids and Tat cDNA were performed using TransIT-LT1 transfection reagent performed as recommended by the manufacturer (Mirus Bio LLC, Madison, WI). Briefly, the transfection reagent was mixed with serum-free media to which the recommended amount of DNA was added (0.5 µg/100,000 cells). The resulting transfection mix was then incubated for 20– 30 min at room temperature. Following the incubation, the transfection mix was added to the cells and the respective assay performed after 24 h of incubation. Twenty hours after transfection, the medium was removed and the cells were lysed in 1x passive lysis buffer for 20 min at 37°C. 20 µL of lysate was used in a dual luciferase reporter assay (Promega, Madison, WI) using a single injector luminometer.
MTS assay
MTS assay is a colometric cell viability assay from Promega (Madison, WI). It is based on the ability of viable cells to reduce a tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H–tetrazolium, inner salt; MTS] and an electron coupling reagent (phenazine methosulfate) PMS into a formazan product which is absorbed at 490 nm and detected by a ELISA plate reader. This assay was performed according to the manufacturer’s recommendations.
Real-time RT-PCR
Total RNA was isolated using RNeasy mini kit (Qiagen, Valencia, CA). cDNA synthesis was performed using qScript cDNA supermix using a protocol from Quanta BioSciences (Gaithersburg, MD). qRTPCR was performed using SsoFast EvaGreen Supermix with low ROX (BioRad, Hercules, CA) in a 7500 Real Time PCR System (Applied Biosystems by Life Technologies, Carlsbad, CA) using 7500 software v2.0.1 for data analysis. Primers used are: (β-catenin: 5′TCTTGCCCTTTGTCCCGCAAATCA3′, Reverse 5′TCCACAAATTGCTGCTGTGTCCCA3′. TCF4: Forward 5′TCGGCAGAGAGGGATTTAGCTGATGT3′, Re verse 5′CTTTCCCGGGATTTGTCTCGGAAACT3′. LEF1: Forward 5′AAGCATCCAGATGGAGGCCTCTACAA3′, Re verse 5’TGATGTTCTCGGGATGGGTGGAGAAA3’; MCP1: Forward 5’TCGCTCAGCCAGATGCAATCAATG3’, Reverse 5’TTCTTTGGGACACTTGCTGCTGGT3’. IL6: Forward 5’ TGGTGTTGCCTGCTGCCTT3’, Reverse 5’ TTCGTTCTGAAGAGGTGAGTGGCT3’.
Statistical analysis
Descriptive statistics, mean or median +/− Standard deviation and Student’s t-test analyses (including unpaired and paired tests) were performed using Microsoft excel software. p≤0.05 was considered significant.
Results
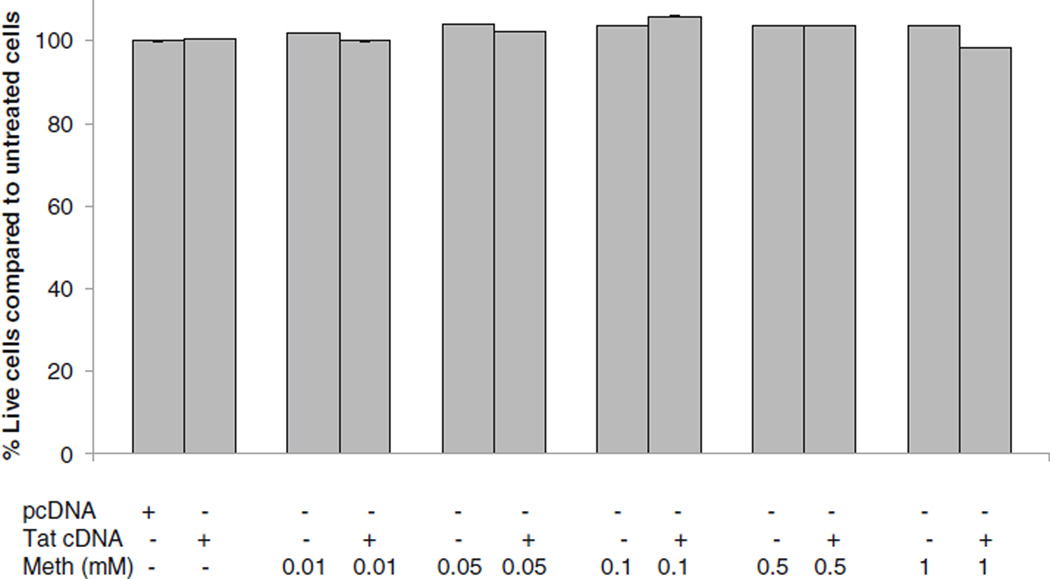
Meth and Tat, at the concentrations tested, did not lead to cytotoxicity of astrocytes
The concentration of Meth in postmortem brains of chronic Meth abusers is reported to be as high as 0.8–1 mM (Talloczy et al. 2008). We evaluated the impact of Meth on survivability of astrocytes using 0.01 mM to 1 mM doses of Meth. The MTS assay was performed at 24 h post-exposure. At concentrations between 0.01 mM and 1 mM, Meth had no effect on survival of astrocytes. Likewise, transfection of astrocytes with up to 0.5 µg Tat cDNA had no effect on astrocyte survival, as determined by the MTS assay (Fig. 1).
Fig. 1.
Effects of Tat and Meth on astrocytoma viability. U87MG were treated with Meth (M) at 0.01, 0.05, 0.1, 0.5 and 1 mM and/or transfected with Tat cDNA, or backbone DNA (pcDNA) or left untreated. At 24 h post-treatment, MTS assay was performed. Data represent a minimum of two experiments
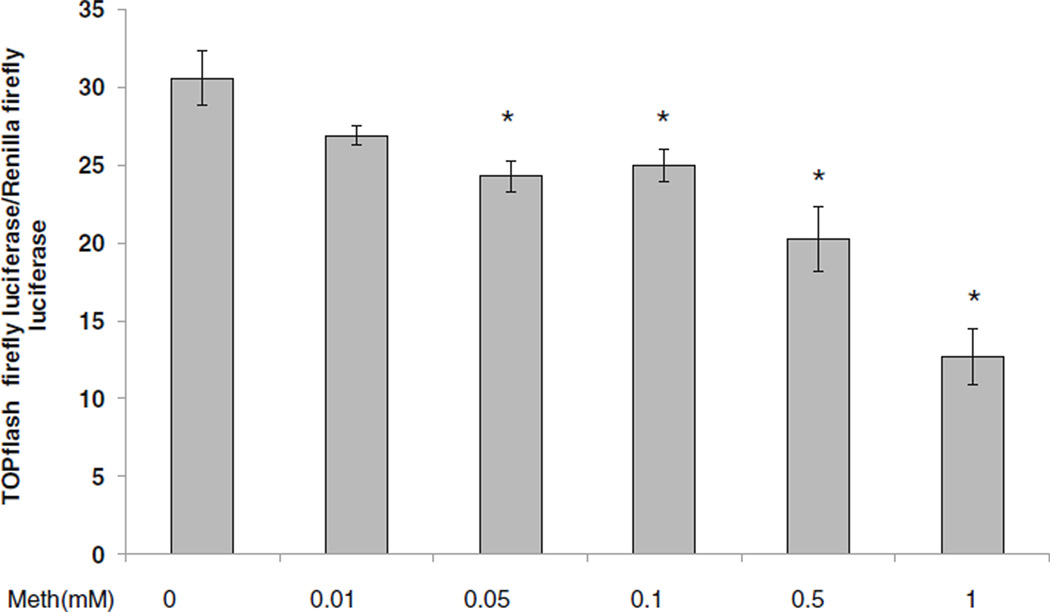
Meth down-regulated β-catenin signaling
To assess the impact of Meth on β-catenin-dependent signaling, astrocytes were transfected with a TOPflash construct consisting of putative TCF/LEF binding sites linked to firefly luciferase reporter or irrelevant/backbone vector (pCDNA) along with Renilla luciferase to normalize for transfection efficiency. Cells were then treated with Meth at doses ranging from 0.01 mM to 1 mM. At 24 h post transfection, dual luciferase assay was performed. Starting at 0.05 mM, Meth caused a dose-dependent reduction in TOPflash activity, which reached an approximately 2.5 fold reduction in TOPflash reporter activity at 1 mM Meth. These data demonstrate that Meth diminished β-catenin-mediated signaling in a concentration-dependent manner (Fig. 2).
Fig. 2.
Effects of Meth on β-catenin-dependent signaling. U87MG cells were transfected with TOPflash and Renilla lucif-erase constructs and treated with Meth (M) at 0.01, 0.05, 0.1, 0.5 and 1 mM or left untreated. At 24 h post-treatment, dual lucifer-ase activity was measured and normalized as relative light units (RLU) of TOPflash firefly lucif-erase activity/Renilla luciferase activity. Data represent a minimum of three experiments. Error bars represent mean +/−standard deviation. Asterisks denote p≤ 0.05 in comparison to Meth untreated control
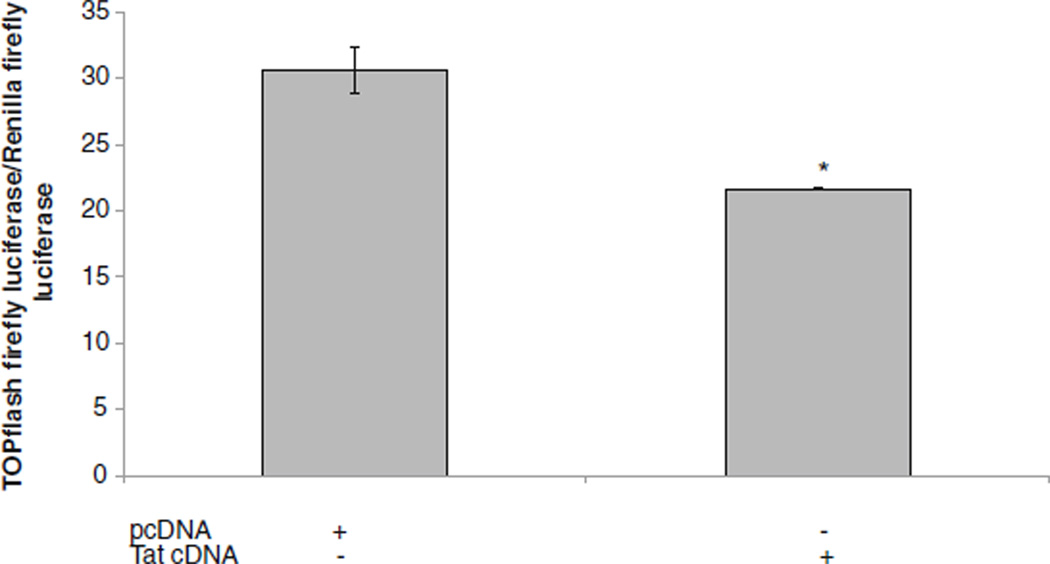
Tat down-regulated β-catenin signaling
We also determined the impact of Tat on β-catenin signaling using TOPflash reporter activity. Tat cDNA was transfected into astrocytes along with TOPflash, backbone vector (pCDNA) and Renilla luciferase plasmid. At 24 h post transfection, Tat down regulated β-catenin activity by approximately 1.5 fold in comparison to pcDNA transfected cultures (Fig. 3).
Fig. 3.
Effects of Tat on β-catenin-dependent signaling. U87MG were transfected with TOPflash and Renilla luciferase and transfected with Tat cDNA, backbone pcDNA vector, or left untreated. At 24 h post-treatment, dual luciferase activity was measured and normalized as relative light units (RLU) of TOPflash firefly luciferase activity/ Renilla luciferase activity. Data represent a minimum of three experiments. Error bars represent mean +/−standard deviation. Asterisks denote p≤0.05 in comparison to pcDNA transfected cultures
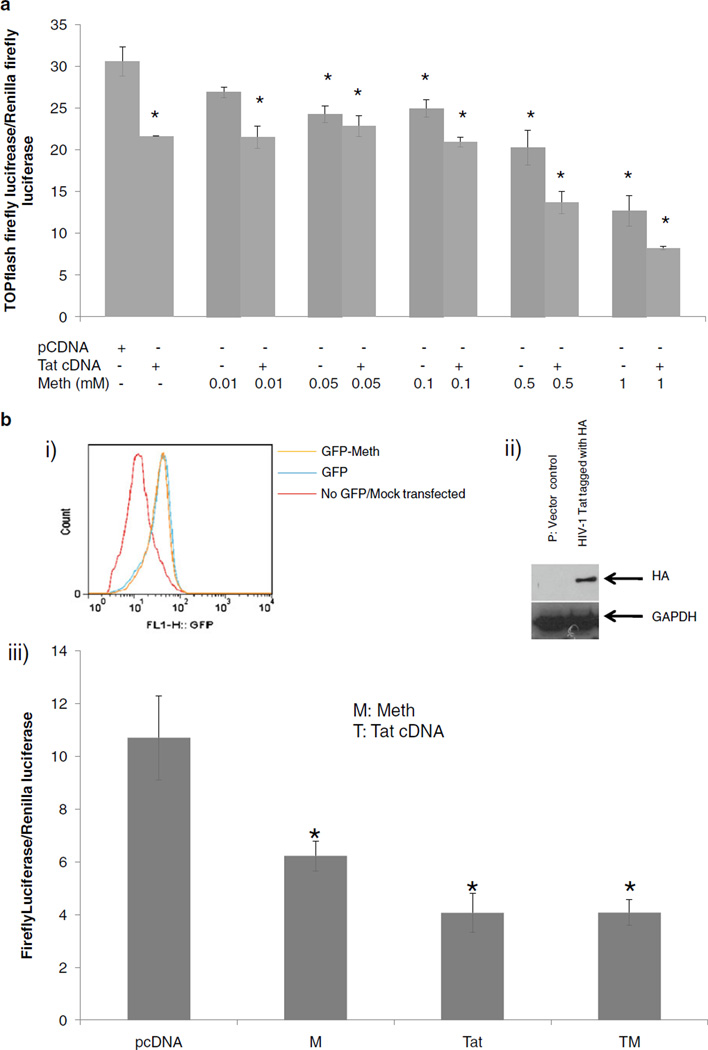
Meth cooperated with Tat to down regulate β-catenin signaling in U87MG cells
To assess the combined effect of Tat and Meth on β-catenin signaling, an astrocytoma cell line (U87MG) was transfected with TOPflash luciferase and Renilla luciferase and treated with Meth at 0.01, 0.05, 0.1, 0.5, 1 mM, and/or transfected with Tat cDNA, or left untreated. While individual Tat and Meth treatments down-regulated β-catenin reporter activity by 35% and 55%, respectively. Together, Meth and Tat downregulated β-catenin signaling reporter activity by >60% in U87MG cells (Fig. 4a). These data suggest that the effect of Tat and Meth on β-catenin signaling in U87MG cells were additive, engaging separate pathways to repress β-catenin-mediated signaling.
Fig. 4.
Effects of Tat and Meth on β-catenin-dependent signaling. a U87MG were transfected with TOPflash and Renilla luciferase and treated with Meth (M) at 0.01, 0.05, 0.1, 0.5 and 1 mM and/or transfected with Tat cDNA, or with pcDNA vector control. At 24 h post-treatment, dual luciferase activity was measured and normalized as relative light units (RLU) of TOPflash firefly luciferase activity/Renilla luciferase activity. Data represent a minimum of three experiments. Error bars represent mean +/−standard deviation. Asterisks denote p< 0.05 in comparison to vector control. bi) Human progenitor derived fetal astrocytes (PDAs) were transfected with GFP construct or mock transfected (no GFP DNA) and left untreated or treated with Meth (1 mM). At 24 h post transfection, GFP expression was monitored by conventional flow cytometry. bii) PDAs were transfected with backbone vector alone or with HIV Tat cDNA that is HA tagged. 24 h post-transfection cell lystates were extracted and western blot was performed using HA antibodies. biii) PDAs were transfected with TOPflash and Renilla construct with either backbone vector DNA (pcDNA) or Tat cDNA then treated with Meth at 1 mM or left untreated. 24 h post treatment, Dual luciferase assay was performed. Data represent at least three independent experiments. M denote Meth; T denote Tat cDNA, asterisks denote p≤0.05 in comparison to pcDNA transfected cultures
To assess whether this effect also occurs in primary human astrocytes, we used human progenitor derived fetal astrocytes (PDA). PDAs were efficiently transfected as monitored by a GFP construct and Meth did not interfere with their transfection efficiency (Fig. 4bi). Further, the transfected Tat into PDAs, which is HA tagged, can be detected by western blot using anti-HA antibodies (Fig. 4ii). Under these conditions both Meth and Tat individually downregulated β-catenin signaling but no additive effect was observed in PDAs (Fig. 4biii). These findings suggest that Meth and Tat in PDAs engage separate pathways to promote inhibition of β-catenin signaling.
Meth and Tat down-regulated downstream effectors of β-catenin signaling
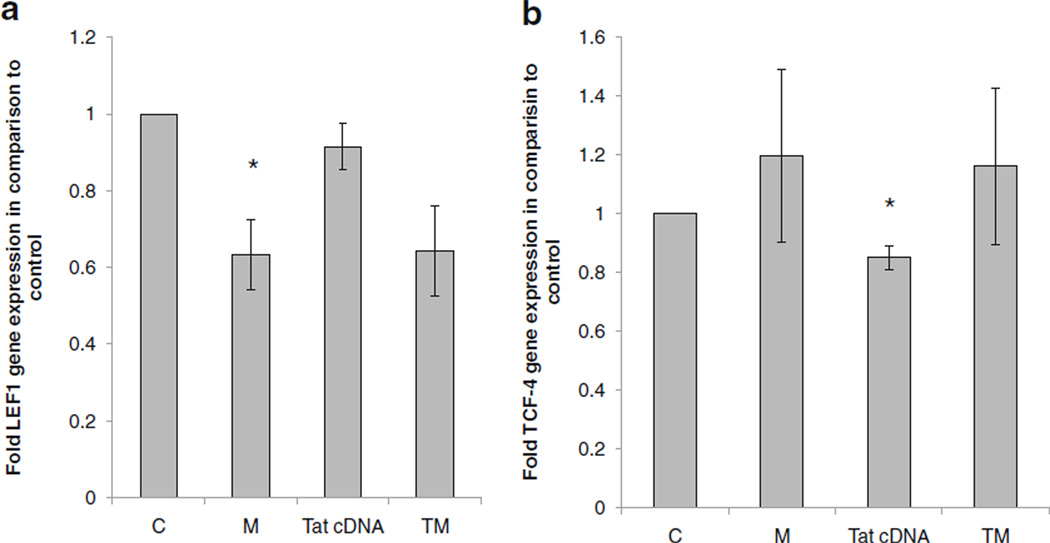
TCF-4 and LEF1 are key downstream effectors of the canonical β-catenin pathway. β-catenin binds TCF-4 or LEF-1 to regulate cognate gene expression. β-catenin itself also regulates the expression of these transcription factors at the transcription level. To assess the impact of Meth and Tat on these critical transcriptional effectors of β-catenin signaling, we measured TCF-4 and LEF-1 transcripts post Meth and Tat treatment in astrocytes by qRTPCR. We show that Meth down regulated LEF-1 expression by approximately 40% but it had no effect on TCF-4 (Fig. 5a,b). Tat, on the other hand, had no effect on LEF-1 and it modestly inhibited TCF-4 expression (Fig. 5a, b). After combined treatment, LEF-1 mRNA downregulation was evident whereas TCF-4mRNA did not statistically change (Fig. 5a, b).
Fig. 5.
Effects of Meth and Tat on LEF1 and TCF4 mRNA. U87MG cells were left untreated or treated with 1 mM Meth, transfected with pcDNA vector control or Tat cDNA or treated with Meth (1 mM) and transfected with Tat. At day one post-treatment, RNA was isolated and level of LEF-1 a and TCF4 b mRNA was measured by qRT-PCR. Data are normalized to GAPDH. Data represent a minimum of three experiments. Error bars represent mean +/−standard deviation. Asterisks denote p≤0.05 in comparison to control (C)
Estrogen rescued the inhibitory effect of meth and Tat on the β-catenin pathway
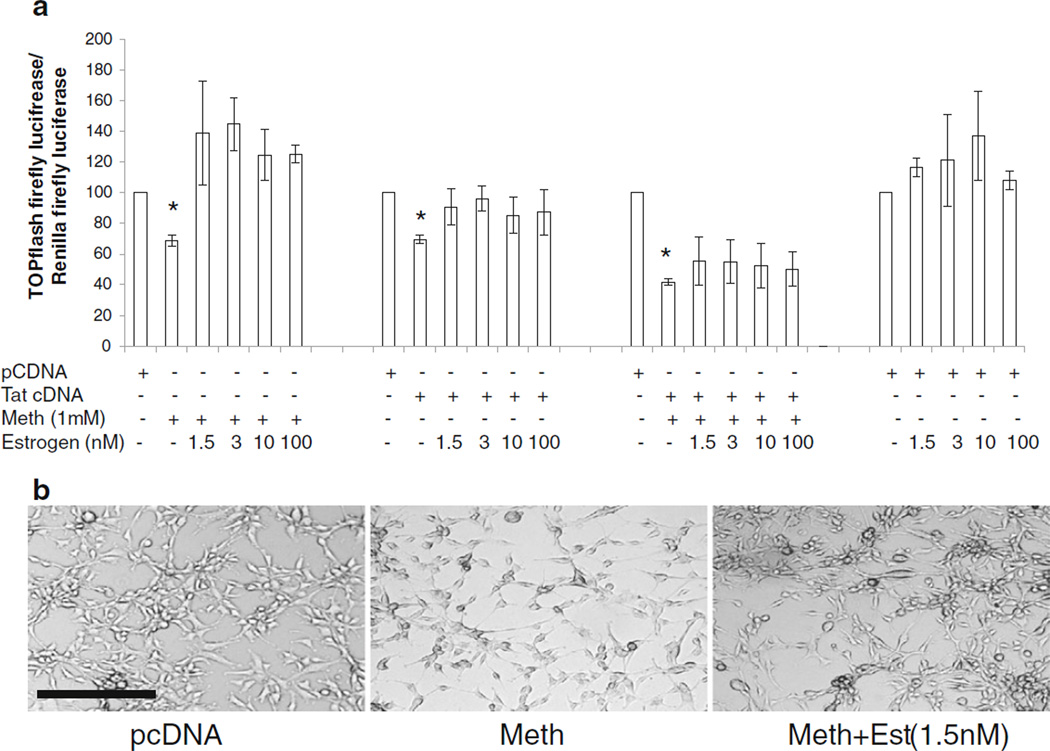
We investigated whether estrogen can overcome the downregulation of β-catenin signaling mediated by Meth and Tat. Estrogen was added at concentrations between 1.5–100 nM to astrocytes transfected with TOPflash with or without Tat cDNA and treated with Meth. Estrogen, in concentrations as low as 1.5 nM (400 pg/ml), which is reported in serum of women of healthy reproductive age (Straub 2007; Breu et al. 2011) normalized the individual effects of Meth and Tat on down regulation of β-catenin signaling, but did not overcome the combined inhibitory effect of Meth and Tat on β-catenin signaling (Fig. 6a). Treatment of astrocytes with Meth also caused the cells to assume abnormal beaded morphology with long thread like processes indicative of a stress response (Fig. 6b). Estrogen at physiological concentrations reversed this stress-induced beaded morphology (Fig. 6b). These data demonstrate the potential of estrogen to normalize individual Tat and Meth effects on β-catenin, but at physiologic concentrations, estrogen was not sufficient to overcome the combined effects of these co-morbid factors on the β-catenin pathway.
Fig. 6.
Effect of estrogen on Meth- and Tat-mediated reduction of β-catenin signaling. a U87MG cells were transfected with TOPflash and Renilla luciferase or backbone vector of TOPflash and renilla then treated with Meth (M) at 1 mM with or without 17β-estradiol (estrogen; E) at 1.5 nM, 3 nM, 10 nM, or 100 nM. Similarly, cells were transfected with TOPflash and Renilla and Tat cDNA (T) alone or in combination with Meth (1 mM) and treated with or without estrogen at the concentrations indicated. Asterisks denote p≤0.05 in comparison to pcDNA control. b U87MG cells were treated with Meth (1 mM) alone or in combination of Estrogen (1.5 nM). At 24 h post treatment, images were taken using Carl Zeiss Axio Observor.Z1 microscope. Magnification bar corresponds to 200 µM
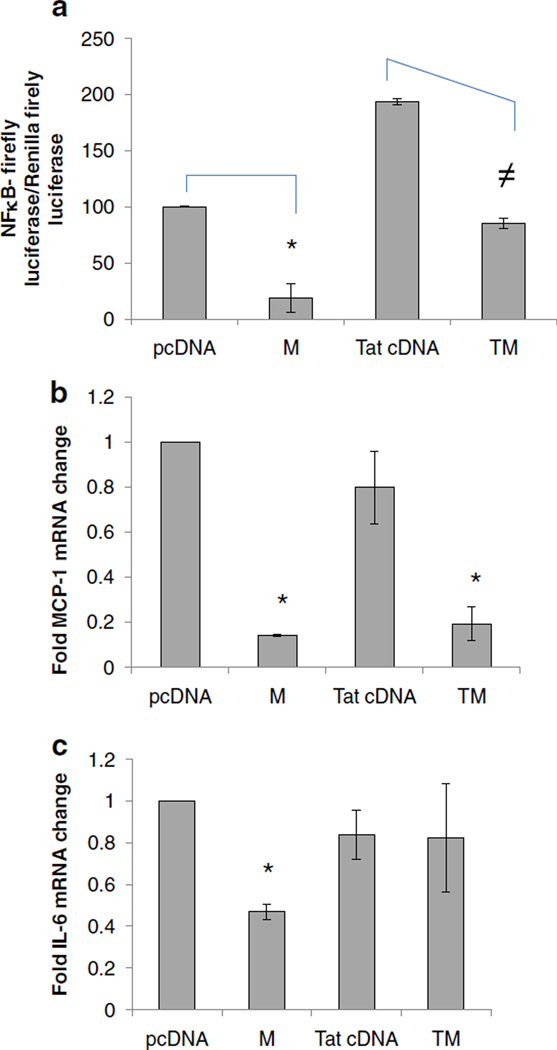
Meth down-regulated NFκB pathway
In some cell types, β-catenin antagonizes NFκB signaling (Deng et al. 2002). Given our observation that Meth and Tat down-regulated β- catenin, it may be that Meth and/or Tat also effect the NFκB pathway. To test this possibility, we evaluated the impact of Meth and Tat on NFκB signaling in astrocytes. To assess for NFκB signaling activity, we used an NFκB reporter constructs as well as measured down-stream target genes of NFkB pathway such as IL-6 and MCP-1 (Liebermann and Baltimore, 1990; Thompson and Van Eldik 2009). We show that Meth at 1 mM dramatically down regulated NFκB reporter activity, while Tat, as expected, induced NFκB activity (Fig. 7a). Consistent with these data, Meth treatment led to a significant reduction in MCP-1 and IL-6 mRNAs by approximately 80% and 60%, respectively (Fig. 7b, c). Down regulation of NFκB reporter activity and MCP-1 transcript persisted in presence of Meth and Tat while IL-6 expression normalized in presence of Meth and Tat co-exposure, indicating that Tat induction of NFκB was a stronger signal to overcome Meth mediated down regulation of IL-6 but not MCP-1.
Fig. 7.
Effects of Meth and Tat on NFκB signaling: a U87MG were transfected with NFkB luciferase reporter with or without Tat cDNA (T) or backbone vector (pcDNA) and left untreated or treated with Meth (M) at 1 mM. At 24 h post-treatment, dual luciferase activity was measured and normalized as relative light units (RLU) of NFκB firefly luciferase activity/Renilla luciferase activity. U87MG RNA was isolated after treatments described in a and expression of MCP1 b and IL-6 c transcripts was measured by qRT-PCR. Data were normalized to GAPDH. Asterisks and≠denote p≤0.05 in comparison to pCDNA control and Tat cDNA treatment, respectively
Discussion
Individuals co-morbid for Meth abuse and HIV-1 demonstrate exacerbated neurocognitive impairment than either condition alone (Reyes et al. 1991; Hauser et al. 2007; Ferris et al. 2008)). While the combined effects of Meth and HIV-1 on disruption of brain function is well reported in the literature, the mechanism(s) by which Meth promotes HIV-1-induced neuropathology is not entirely clear (Koutsilieri et al. 1997; Nath et al. 2002; Chang et al. 2005; Ferris et al. 2008). The Wnt/β-catenin signaling pathway is an important neuroprotective pathway. Its dysregulation has been linked to a number of neurodegenerative diseases, including Alzheimer’s, Parkinson’s disease, and psychiatric disorders such as bipolar disorder and depression (Caricasole et al. 2003; Caricasole et al. 2005; De Ferrari and Moon 2006; Inestrosa et al. 2007). The pathway plays a vital role in regulation of various functions in CNS ranging from neurogenesis to neurotransmitter release, to vesical recycling, and to induction of long term potentiation and depolarization resulting in increased synaptic strengths (Inestrosa and Arenas 2010; Henderson and Al-Harthi 2011b). Disruption of β-catenin signaling has profound biologic consequences that impacts synaptic connections in neurons and neurogenesis. In astrocytes, disruption of β-catenin leads to alterations of hundreds of genes, some of which are essential for the neuroprotective role of astrocyte such as alterations in the glutamate transport network within astrocytes (Henderson and Al-Harthi, unpublished observations). Further, transgenic mice models indicate that β-catenin expression is required for memory consolidation in astrocytes (Maguschak and Ressler 2008). Therefore, disruption of β- catenin signaling in astrocytes is likely to compromise the integrity of astrocytes that can in turn compromise their ability to promote neuronal health and maintain CNS homeostasis. We show here for the first time that Meth diminished β-catenin signaling. The Meth effect was more pronounced at 24 h post treatment. Meth and Tat in adult astrocytoma cells exhibited an additive effect on inhibition of β-catenin. While this additive effect was not observed in using primary human progenitor fetal astrocytes, each agent alone lead to substantial inhibition of β-catenin signaling. These findings suggest that the mechanism engaged by Meth and Tat to down regulate β-catenin is independent. Along these lines, Meth and Tat exhibited differential effects on transcript level of the two most prominent transcription factors of β-catenin pathway, LEF-1 and TCF-4. Meth down regulated LEF-1 transcript without impacting TCF-4 mRNA, while Tat had no effect on LEF-1 transcript but demonstrated a modest inhibition of TCF-4 mRNA.
In our system, we transfected astrocytes with Tat cDNA (0.05 µg/100,000 cells). The level of Tat was deliberately maintained at a low level in an attempt to mimic presumably low level of CNS Tat associated with HAART. Meth concentrations in the CNS is highly variable in humans, depending on several factors including use pattern (e.g., chronic binging) and CNS concentrations can be 10 fold higher than sera (Talloczy et al. 2008). Post mortem concentrations of Meth reported from fatal overdose reach up to 1 mM Meth (Talloczy et al. 2008). Nonetheless, starting at 0.01 mM, we document dose-depended reduction in β-catenin mediated signaling. While the binding site for Meth in astrocytes is not known, considerable evidence exists documenting profound biologic effects of Meth exposure on astrocytes (Narita et al. 2008). Some of these effects include long term astrocyte activation as well as hyperlocomation, as evaluated in small animal drug dependence behavioral models. These effects persisted even after withdraw of Meth treatment for 2 months. Given that Wnt/β-catenin signaling reduces inflammatory responses, even in astrocytes, suggest that its inhibition by Meth may potentiate proinflammatory/hyperactivation of astrocytes. It is interesting to note that Meth caused a morphological change in U87MG cells that is reminiscent of a beading appearance. This morphologic change does not reflect cell death as evaluated by the MTS assay. Most probably, because β-catenin also binds to cadherins at the cell membrane and is associated with the cytoskeleton of cells, its downregulation by Meth or Tat may also lead to alteration in cell adhesion properties of astrocytes that is reflected by the morphologic change observed.
17 β-estradiol (estrogen) has substantial neuroprotective properties, including in the setting of HIV-1 and Meth (Turchan et al. 2001; Wallace et al. 2006), yet the mechanism by which estrogen exerts these effects are not entirely clear. The reports that estrogen increases β-catenin signaling suggests that its neuroprotective properties maybe partly mediated by its induction of this neuroprotective pathway. In the current study, estrogen reversed the down regulatory effects of Meth and Tat on β-catenin signaling; however, at the doses tested, it was unable to reverse the combined effect of Meth and Tat. The estrogen concentration used in our studies (i.e., between 1 and 3 nM) was consistent with sera concentrations in healthy women of reproductive age during the menstrual cycle. Nevertheless, these concentrations were not sufficient to reverse the combined Tat and Meth effects. Other activators of β-catenin signaling, such as lithium, may be more beneficial in overcoming Meth- and Tat-mediated inhibition of β-catenin signaling. Activation of Wnt signaling by lithium chloride, which inhibits the negative regulator of the Wnt/ β-catenin pathway (GSK3β), or Rosiglitazone (a PPAR-γ agonist) reduce astrocytic activation in neurodegenerative diseases and are thought to do so through their ability to induce Wnt signaling (Toledo and Inestrosa 2010).
To the best of our knowledge, there are no studies that evaluated the interaction between Meth and Wnt/β-catenin signaling, and studies with other psychostimulants are sparse. Moreover, most focused on the interaction of chronic cocaine and β-catenin expression (Freeman et al. 2001a; Freeman et al. 2001b; Zhang et al. 2002; Gil et al. 2003; Alimohamad et al. 2005; Dunbar et al. 2006; Lynch et al. 2008). Chronic cocaine increases total β-catenin levels in dopaminergic regions in rodents (Zhang et al. 2002; Novikova et al. 2005) and non human primates (Freeman et al. 2001b). However, these reports did not discriminate between active and inactive forms of β-catenin. In another study, the cocaine-induced increase in total β-catenin was accompanied by increase in levels of proteins that degrade β-catenin (Novikova et al. 2005). Cumulatively, the studies suggest that cocaine does not affect active β-catenin levels but rather causes degradation of inactive β-catenin. It is important to bear in mind that while cocaine and Meth share the capacity to enhance monoaminergic transmission, in contrast to Meth, cocaine is not thought to be neurotoxic, thus making direct comparisons tenuous.
Meth down regulated NFκB reporter activity in astrocytes; which was consistent with inhibition of MCP-1 and IL-6, two downstream target genes of the NFκB pathway. NFκB is expressed in brain regions actively undergoing neurogenesis, particularly in cells committed to the astrocytic lineage (O'Neill and Kaltschmidt 1997; Denis-Donini et al. 2005), it is neuroprotective against the apoptotic insults of HIV-1 verotoxin Tat (Ramirez et al. 2001), and functions as a pro-survival signaling molecule in the CNS. Our finding that Meth and Tat cooperate to down regulate two neuroprotectove pathways, β-catenin and NFκB, suggests that Meth- and HIV-1-exposed astrocytes may be more susceptible to toxic signals than unexposed cells. In a cell-specific manner, β-catenin antagonizes NFκB activity (Buss et al. 2004). Particularly, in neurons β-catenin negatively regulates NFκB. However, in astrocytes, Meth-induced down regulation of β-catenin was not associated with induction of NFκB, suggesting that mechanism does not exist in astrocytes.
Together, HIV-1 Tat and Meth down regulate neuro-protective pathways, β-catenin and NFκB, the consequence of which may drive the pathophysiologic dysregulation associated with Meth and HIV-1 co-morbidity. A better understanding of the interplay between HIV-1, Meth, and the Wnt/ β -catenin is likely to expedite the development of activators of β-catenin signaling to protect against Tat and Meth insults on the brain.
Acknowledgements
We thank Dr. Eugene O. Major (NINDS, NIH, MD) for providing us the human neuronal progenitor cells and Lisa J. Henderson for their differentiation into astrocytes. This work is supported by NIDA R03 DA026723 (LA), R03 DA026746 (X-T H), the Center for Compulsive Behavior and Addiction, and the Chicago D-CFAR P30 AI082151. We thank Dr. Srinivas Narasipura (Rush University Medical Center, Chicago, IL) and Dr. Priyanka Sharma (Wright State University, Dayton, OH) for helpful discussions. We thank Ms. Lisa Henderson for support with handling of astrocytoma cell lines.
Footnotes
Disclaimers None.
Contributor Information
Amit Sharma, Department of Immunology/Microbiology, Rush University Medical Center, 1735 W. Harrison Street, 614 Cohn, Chicago, IL 60612, USA; Center for Compulsive Behavior and Addiction, Rush University Medical Center, Chicago, IL, USA.
Xiu-Ti Hu, Department of Pharmacology, Rush University Medical Center, Chicago, IL, USA; Chicago Center for AIDS Research, Rush University Medical Center, Chicago, IL, USA; Center for Compulsive Behavior and Addiction, Rush University Medical Center, Chicago, IL, USA.
T. Celeste Napier, Department of Pharmacology, Rush University Medical Center, Chicago, IL, USA; Chicago Center for AIDS Research, Rush University Medical Center, Chicago, IL, USA; Center for Compulsive Behavior and Addiction, Rush University Medical Center, Chicago, IL, USA.
Lena Al-Harthi, Email: Lena_Al-Harthi@rush.edu, Department of Immunology/Microbiology, Rush University Medical Center, 1735 W. Harrison Street, 614 Cohn, Chicago, IL 60612, USA; Chicago Center for AIDS Research, Rush University Medical Center, Chicago, IL, USA; Center for Compulsive Behavior and Addiction, Rush University Medical Center, Chicago, IL, USA.
References
- Alimohamad H, Rajakumar N, Seah YH, Rushlow W. Anti-psychotics alter the protein expression levels of beta-catenin and GSK-3 in the rat medial prefrontal cortex and striatum. Biol Psychiatry. 2005;57:533–542. doi: 10.1016/j.biopsych.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res. 2000;879:42–49. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- Breu A, Sprinzing B, Merkl K, Bechmann V, Kujat R, Jenei-Lanzl Z, Prantl L, Angele P. Estrogen reduces cellular aging in human mesenchymal stem cells and chondrocytes. J Orthop Res. 2011 doi: 10.1002/jor.21424. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Chauhan A, Dimayuga FO, Gee J, Keller JN, Nath A. Synaptic transport of human immunodeficiency virus-Tat protein causes neurotoxicity and gliosis in rat brain. J Neurosci. 2003;23:8417–8422. doi: 10.1523/JNEUROSCI.23-23-08417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss H, Dorrie A, Schmitz ML, Frank R, Livingstone M, Resch K, Kracht M. Phosphorylation of serine 468 by GSK-3beta negatively regulates basal p65 NF-kappaB activity. J Biol Chem. 2004;279:49571–49574. doi: 10.1074/jbc.C400442200. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez P, Perez M, Avila J, Garcia-Segura LM, Wandosell F. Estradiol inhibits GSK3 and regulates interaction of estrogen receptors, GSK3, and beta-catenin in the hippocampus. Mol Cell Neurosci. 2004;25:363–373. doi: 10.1016/j.mcn.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Caricasole A, Copani A, Caruso A, Caraci F, Iacovelli L, Sortino MA, Terstappen GC, Nicoletti F. The Wnt pathway, cell-cycle activation and beta-amyloid: novel therapeutic strategies in Alzheimer's disease? Trends Pharmacol Sci. 2003;24:233–238. doi: 10.1016/s0165-6147(03)00100-7. [DOI] [PubMed] [Google Scholar]
- Caricasole A, Bakker A, Copani A, Nicoletti F, Gaviraghi G, Terstappen GC. Two sides of the same coin: Wnt signaling in neurodegeneration and neuro-oncology. Biosci Rep. 2005;25:309–327. doi: 10.1007/s10540-005-2893-6. [DOI] [PubMed] [Google Scholar]
- Carroll-Anzinger D, Kumar A, Adarichev V, Kashanchi F, Al-Harthi L. Human immunodeficiency virus-restricted replication in astrocytes and the ability of gamma interferon to modulate this restriction are regulated by a downstream effector of the Wnt signaling pathway. J Virol. 2007;81:5864–5871. doi: 10.1128/JVI.02234-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiatry. 2005;162:361–369. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ferrari GV, Moon RT. The ups and downs of Wnt signaling in prevalent neurological disorders. Oncogene. 2006;25:7545–7553. doi: 10.1038/sj.onc.1210064. [DOI] [PubMed] [Google Scholar]
- Deng J, Miller SA, Wang HY, Xia W, Wen Y, Zhou BP, Li Y, Lin SY, Hung MC. beta-catenin interacts with and inhibits NF-kappa B in human colon and breast cancer. Cancer Cell. 2002;2:323–334. doi: 10.1016/s1535-6108(02)00154-x. [DOI] [PubMed] [Google Scholar]
- Denis-Donini S, Caprini A, Frassoni C, Grilli M. Members of the NF-kappaB family expressed in zones of active neurogenesis in the postnatal and adult mouse brain. Brain Res Dev Brain Res. 2005;154:81–89. doi: 10.1016/j.devbrainres.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Dunbar SA, Karamian I, Roberts L, Zhang J. Increased prostaglandin E2 release and activated Akt/beta-catenin signaling pathway occur after opioid withdrawal in rat spinal cord. Anesthesiology. 2006;105:154–159. doi: 10.1097/00000542-200607000-00025. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, King JE, Nath A, Calderon TM, Zukin RS, Bennett MV, Berman JW. HIV-tat induces formation of an LRP-PSD-95- NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. Proc Natl Acad Sci U S A. 2007;104:3438–3443. doi: 10.1073/pnas.0611699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in Neuro-AIDS. Neurosci Biobehav Rev. 2008;32:883–909. doi: 10.1016/j.neubiorev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WM, Brebner K, Lynch WJ, Robertson DJ, Roberts DC, Vrana KE. Cocaine-responsive gene expression changes in rat hippocampus. Neuroscience. 2001a;108:371–380. doi: 10.1016/s0306-4522(01)00432-8. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Nader MA, Nader SH, Robertson DJ, Gioia L, Mitchell SM, Daunais JB, Porrino LJ, Friedman DP, Vrana KE. Chronic cocaine-mediated changes in non-human primate nucleus accumbens gene expression. J Neurochem. 2001b;77:542–549. doi: 10.1046/j.1471-4159.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- Gil M, Zhen X, Friedman E. Prenatal cocaine exposure alters glycogen synthase kinase-3beta (GSK3beta) pathway in select rabbit brain areas. Neurosci Lett. 2003;349:143–146. doi: 10.1016/s0304-3940(03)00852-8. [DOI] [PubMed] [Google Scholar]
- Hauser KF, El-Hage N, Stiene-Martin A, Maragos WF, Nath A, Persidsky Y, Volsky DJ, Knapp E. HIV-1 neuropatho-genesis: glial mechanisms revealed through substance abuse. J Neurochem. 2007;100:567–586. doi: 10.1111/j.1471-4159.2006.04227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LJ, Al-Harthi L. Role of beta-Catenin/TCF-4 Signaling in HIV Replication and Pathogenesis: Insights to Informing Novel Anti-HIV Molecular Therapeutics. J Neuroimmune Pharmacol. 2011;6(2):247–259. doi: 10.1007/s11481-011-9266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Evans E, Huang D, Brecht ML, Li L. Comparing the dynamic course of heroin, cocaine, and methamphetamine use over 10 years. Addict Behav. 2008;33(12):1581–1589. doi: 10.1016/j.addbeh.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci. 2010;11:77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Varela-Nallar L, Grabowski CP, Colombres M. Synaptotoxicity in Alzheimer's disease: the Wnt signaling pathway as a molecular target. IUBMB Life. 2007;59:316–321. doi: 10.1080/15216540701242490. [DOI] [PubMed] [Google Scholar]
- Khurdayan VK, Buch S, El-Hage N, Lutz SE, Goebel SM, Singh IN, Knapp PE, Turchan-Cholewo J, Nath A, Hauser KF. Preferential vulnerability of astroglia and glial precursors to combined opioid and HIV-1 Tat exposure in vitro. Eur J Neurosci. 2004;19:3171–3182. doi: 10.1111/j.0953-816X.2004.03461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol. 2003;162:1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JE, Eugenin EA, Buckner CM, Berman JW. HIV tat and neurotoxicity. Microbes Infect. 2006;8:1347–1357. doi: 10.1016/j.micinf.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Koutsilieri E, Gotz ME, Sopper S, Sauer U, Demuth M, ter Meulen V, Riederer P. Regulation of glutathione and cell toxicity following exposure to neurotropic substances and human immunodeficiency virus-1 in vitro. J Neurovirol. 1997;3:342–349. doi: 10.3109/13550289709030748. [DOI] [PubMed] [Google Scholar]
- Kouzmenko AP, Takeyama K, Ito S, Furutani T, Sawatsubashi S, Maki A, Suzuki E, Kawasaki Y, Akiyama T, Tabata T, Kato S. Wnt/beta-catenin and estrogen signaling converge in vivo. J Biol Chem. 2004;279:40255–40258. doi: 10.1074/jbc.C400331200. [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Lamba S, Ravichandran V, Major EO. Glial cell type-specific subcellular localization of 14-3-3 zeta: an implication for JCV tropism. Glia. 2009;57:971–977. doi: 10.1002/glia.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Cossetti C, D’Adamo P, Zardini E, Andreoni L, Ihekwaba AE, Serra PA, Franciotta D, Martino G, Pluchino S, Marchetti B. Reactive astrocytes and Wnt/beta-catenin signaling link nigrostriatal injury to repair in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Neurobiol Dis. 2011;41:508–527. doi: 10.1016/j.nbd.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre SL, Ellis RJ, Everall I, Ances B, Bharti A, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2009;17:46–56. [PMC free article] [PubMed] [Google Scholar]
- Letendre SL, Ellis RJ, Ances BM, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2010;18:45–55. [PMC free article] [PubMed] [Google Scholar]
- Li W, Henderson LJ, Major EO, Al-Harthi L. IFN-{gamma} mediates enhancement of HIV replication in astrocytes by inducing an antagonist of the {beta}-catenin pathway (DKK1) in a STAT 3-dependent manner. J Immunol. 2011;186:6771–6778. doi: 10.4049/jimmunol.1100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libermann TA, Baltimore D. Activation of interleukin-6 expression through NF- kappa B transcription factor. Mol Cel Biol. 1990;10:2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Girgenti MJ, Breslin FJ, Newton SS, Taylor JR. Gene profiling the response to repeated cocaine self-administration in dorsal striatum: a focus on circadian genes. Brain Res. 2008;1213:166–177. doi: 10.1016/j.brainres.2008.02.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguschak KA, Ressler KJ. Beta-catenin is required for memory consolidation. Nat Neurosci. 2008;11:1319–1326. doi: 10.1038/nn.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragos WF, Young KL, Turchan JT, Guseva M, Pauly JR, Nath A, Cass WA. Human immunodeficiency virus-1 Tat protein and methamphetamine interact synergistically to impair striatal dopaminergic function. J Neurochem. 2002;83:955–963. doi: 10.1046/j.1471-4159.2002.01212.x. [DOI] [PubMed] [Google Scholar]
- Maragos WF, Tillman P, Jones M, Bruce-Keller AJ, Roth S, Bell JE, Nath A. Neuronal injury in hippocampus with human immunodeficiency virus transactivating protein, Tat. Neuroscience. 2003;117:43–53. doi: 10.1016/s0306-4522(02)00713-3. [DOI] [PubMed] [Google Scholar]
- Miller JR, Moon RT. Signal transduction through beta-catenin and specification of cell fate during embryogenesis. Genes Dev. 1996;10:2527–2539. doi: 10.1101/gad.10.20.2527. [DOI] [PubMed] [Google Scholar]
- Moon RT, Brown JD, Torres M. WNTs modulate cell fate and behavior during vertebrate development. Trends Genet. 1997;13:157–162. doi: 10.1016/s0168-9525(97)01093-7. [DOI] [PubMed] [Google Scholar]
- Narita M, Suzuki M, Kuzumaki N, Miyatake M, Suzuki T. Implications of activated astrocytes in the development of drug dependence: differences between methamphetamine and morphine. Ann NY Acad Sci. 2008;1141:96–104. doi: 10.1196/annals.1441.032. [DOI] [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, Cass W, Turchan JT. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S62–S69. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- New DR, Ma M, Epstein LG, Nath A, Gelbard HA. Human immunodeficiency virus type 1 Tat protein induces death by apoptosis in primary human neuron cultures. J Neurovirol. 1997;3:168–173. doi: 10.3109/13550289709015806. [DOI] [PubMed] [Google Scholar]
- Novikova SI, He F, Bai J, Lidow MS. Neuropathology of the cerebral cortex observed in a range of animal models of prenatal cocaine exposure may reflect alterations in genes involved in the Wnt and cadherin systems. Synapse. 2005;56:105–116. doi: 10.1002/syn.20134. [DOI] [PubMed] [Google Scholar]
- O'Neill LA, Kaltschmidt C. NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20:252–258. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- Ramirez SH, Sanchez JF, Dimitri CA, Gelbard HA, Dewhurst S, Maggirwar SB. Neurotrophins prevent HIV Tat-induced neuronal apoptosis via a nuclear factor-kappaB (NF-kappaB)-dependent mechanism. J Neurochem. 2001;78:874–889. doi: 10.1046/j.1471-4159.2001.00467.x. [DOI] [PubMed] [Google Scholar]
- Reyes MG, Faraldi F, Senseng CS, Flowers C, Fariello R. Nigral degeneration in acquired immune deficiency syndrome (AIDS) Acta Neuropathol. 1991;82:39–44. doi: 10.1007/BF00310921. [DOI] [PubMed] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- Talloczy Z, Martinez J, Joset D, Ray Y, Gacser A, Toussi S, Mizushima N, Nosanchuk JD, Goldstein H, Loike J, Sulzer D, Santambrogio L. Methamphetamine inhibits antigen processing, presentation, and phagocytosis. PLoS Pathog. 2008;4:e28. doi: 10.1371/journal.ppat.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WL, Van Eldik LJ. Inflammatory cytokines stimulate the chemokines CCL2/MCP-1 and CCL7/MCP3 through NFkB and MAPK dependent pathways in rat astrocytes. Brains Res. 2009;1287:47–57. doi: 10.1016/j.brainres.2009.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo EM, Inestrosa NC. Activation of Wnt signaling by lithium and rosilitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of Alzheimer’s disease. Mol Psychiatry. 2010;15(3):272–285. doi: 10.1038/mp.2009.72. [DOI] [PubMed] [Google Scholar]
- Turchan J, Anderson C, Hauser KF, Sun Q, Zhang J, Liu Y, Wise PM, Kruman I, Maragos W, Mattson MP, Booze R, Nath A. Estrogen protects against the synergistic toxicity by HIV proteins, methamphetamine and cocaine. BMC Neurosci. 2001;2:3. doi: 10.1186/1471-2202-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varea O, Garrido JJ, Dopazo A, Mendez P, Garcia-Segura LM, Wandosell F. Estradiol activates beta-catenin dependent transcription in neurons. PLoS One. 2009;4:e5153. doi: 10.1371/journal.pone.0005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DR, Dodson S, Nath A, Booze RM. Estrogen attenuates gp120- and tat1-72-induced oxidative stress and prevents loss of dopamine transporter function. Synapse. 2006;59:51–60. doi: 10.1002/syn.20214. [DOI] [PubMed] [Google Scholar]
- White BD, Nathe RJ, Maris DO, Nguyen NK, Goodson JM, Moon RT, Horner PJ. Beta-catenin signaling increases in proliferating NG2+ progenitors and astrocytes during post-traumatic glio-genesis in the adult brain. Stem Cells. 2010;28:297–307. doi: 10.1002/stem.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhang L, Lou DW, Nakabeppu Y, Zhang J, Xu M. The dopamine D1 receptor is a critical mediator for cocaine-induced gene expression. J Neurochem. 2002;82:1453–1464. doi: 10.1046/j.1471-4159.2002.01089.x. [DOI] [PubMed] [Google Scholar]
- Zhang QG, Wang R, Khan M, Mahesh V, Brann DW. Role of Dickkopf-1, an antagonist of the Wnt/beta-catenin signaling pathway, in estrogen-induced neuroprotection and attenuation of tau phosphorylation. J Neurosci. 2008;28:8430–8441. doi: 10.1523/JNEUROSCI.2752-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BY, Liu Y, Kim B, Xiao Y, He JJ. Astrocyte activation and dysfunction and neuron death by HIV-1 Tat expression in astrocytes. Mol Cell Neurosci. 2004;27:296–305. doi: 10.1016/j.mcn.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Zou W, Wang Z, Liu Y, Fan Y, Zhou BY, Yang XF, He JJ. Involvement of p300 in constitutive and HIV-1 Tat-activated expression of glial fibrillary acidic protein in astrocytes. Glia. 2010;58:1640–1648. doi: 10.1002/glia.21038. [DOI] [PMC free article] [PubMed] [Google Scholar]