Abstract
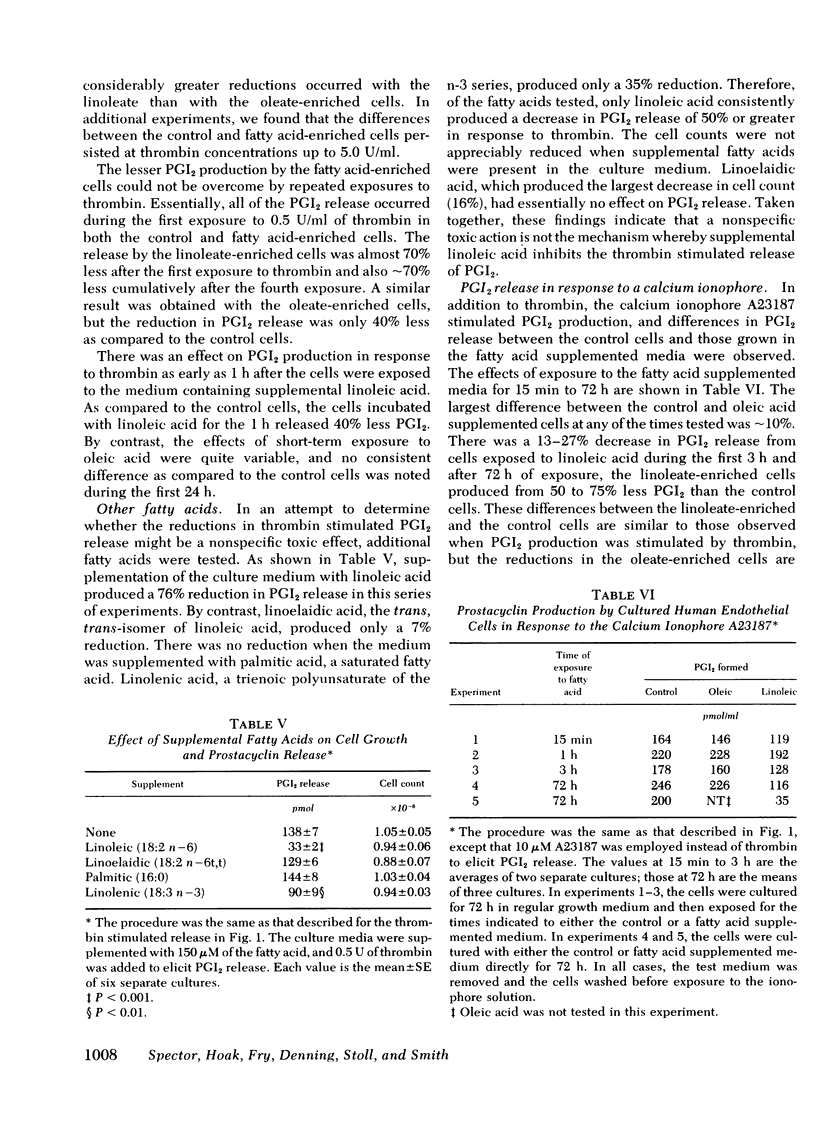
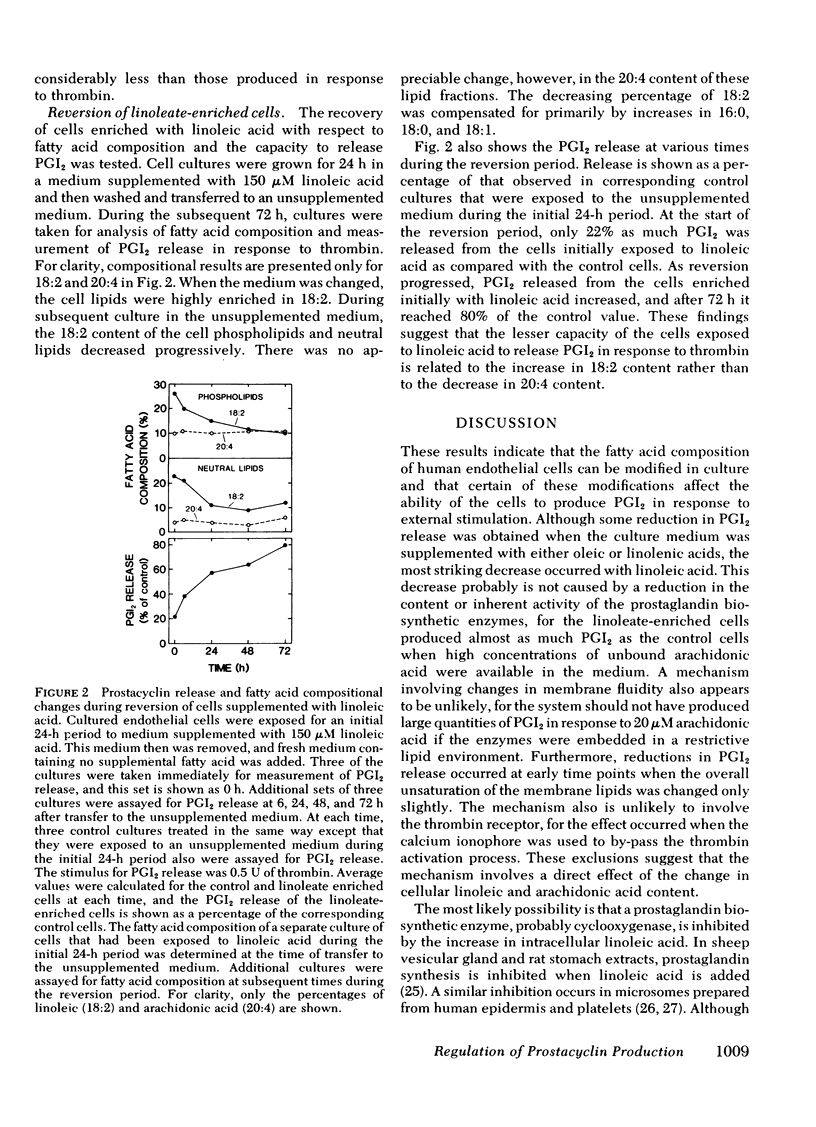
We have investigated whether changes in cellular fatty acid saturation can influence prostacyclin (PGI2) production by cultured human umbilical vein endothelial cells. As compared to control cells, those enriched with linoleic acid released 60--75% less PGI2 in response to thrombin or the calcium ionophore A23187. A similar but considerably smaller effect was observed when the cells were enriched with oleic or linolenic acid, but no reduction occurred with palmitic or linoelaidic acids. Some reduction in PGI2 release was noted as early as 1 h after exposure to linoleic acid. When the culture medium was supplemented with linoleic acid, the cell phospholipids contained four to five times more linoleate and 25--40% less arachidonate. These changes were most marked in the choline and serine plus inositol phosphoglyceride fractions. When the fatty acid composition of the cells enriched with linoleic acid was allowed to revert, there was a progressive increase in the capacity of the cells to release PGI2 in response to thrombin. The increase correlated with a reduction in linoleate content of the cell lipids, but there was no change in arachidonate content. This suggests that linoleic acid may act as an inhibitor of PGI2 production. The cultured endothelial cells were also able to produce PGI2 directly from added arachidonic acid. As the arachidonic acid concentration of the medium was raised, PGI2 formation by the linoleate-enriched cells increased relative to control cells, suggesting that the inhibition produced by linoleic acid may be competitive.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aeberhard E. E., Corbo L., Menkes J. H. Polyenoic acid metabolism in cultured human skin fibroblasts. Lipids. 1978 Nov;13(11):758–767. doi: 10.1007/BF02533473. [DOI] [PubMed] [Google Scholar]
- Bell R. L., Kennerly D. A., Stanford N., Majerus P. W. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3238–3241. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bills T. K., Smith J. B., Silver M. J. Metabolism of [14C]arachidonic acid by human platelets. Biochim Biophys Acta. 1976 Feb 23;424(2):303–314. doi: 10.1016/0005-2760(76)90198-3. [DOI] [PubMed] [Google Scholar]
- Bills T. K., Smith J. B., Silver M. J. Selective release of archidonic acid from the phospholipids of human platelets in response to thrombin. J Clin Invest. 1977 Jul;60(1):1–6. doi: 10.1172/JCI108745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunting S., Gryglewski R., Moncada S., Vane J. R. Arterial walls generate from prostaglandin endoperoxides a substance (prostaglandin X) which relaxes strips of mesenteric and coeliac ateries and inhibits platelet aggregation. Prostaglandins. 1976 Dec;12(6):897–913. doi: 10.1016/0090-6980(76)90125-8. [DOI] [PubMed] [Google Scholar]
- Czervionke R. L., Hoak J. C., Fry G. L. Effect of aspirin on thrombin-induced adherence of platelets to cultured cells from the blood vessel wall. J Clin Invest. 1978 Oct;62(4):847–856. doi: 10.1172/JCI109197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czervionke R. L., Smith J. B., Fry G. L., Hoak J. C., Haycraft D. L. Inhibition of prostacyclin by treatment of endothelium with aspirin. Correlation with platelet adherence. J Clin Invest. 1979 May;63(5):1089–1092. doi: 10.1172/JCI109379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czervionke R. L., Smith J. B., Hoak J. C., Fry G. L., Haycraft D. L. Use of a radioimmunoassay to study thrombin-induced release of PGI2 from cultured endothelium. Thromb Res. 1979;14(4-5):781–786. doi: 10.1016/0049-3848(79)90132-4. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gerrard J. M., White J. G., Krivit W. Labile aggregation stimulating substance, free fatty acids, and platelet aggregation. J Lab Clin Med. 1976 Jan;87(1):73–82. [PubMed] [Google Scholar]
- Heimberg M., Dunn G. D., Wilcox G. The derivation of plasma-free fatty acids from dietary neutral fat in man. J Lab Clin Med. 1974 Mar;83(3):393–402. [PubMed] [Google Scholar]
- Hoak J. C., Czervionke R. L., Lewis L. J. Uptake and utilization of free fatty acids (FFA) by human endothelial cells. Thromb Res. 1974 Jun;4(6):879–883. doi: 10.1016/0049-3848(74)90030-9. [DOI] [PubMed] [Google Scholar]
- Hong S. L., Patton G., Deykin D. Arachidonic acid level in cellular lipids determines the amount of prostaglandins synthesized during cell growth in tissue culture. Prostaglandins. 1979 Jan;17(1):53–59. doi: 10.1016/0090-6980(79)90074-1. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Weksler B. B. Recovery of endothelial cell prostacyclin production after inhibition by low doses of aspirin. J Clin Invest. 1979 Mar;63(3):532–535. doi: 10.1172/JCI109332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAYDEN H. J., KARMEN A., DUMONT A. ALTERATIONS IN THE FATTY ACID COMPOSITION OF HUMAN LYMPH AND SERUM LIPOPROTEINS BY SINGLE FEEDINGS. J Clin Invest. 1963 Sep;42:1373–1381. doi: 10.1172/JCI104821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry R. R., Tinsley I. J. Rapid colorimetric determination of free fatty acids. J Am Oil Chem Soc. 1976 Jul;53(7):470–472. doi: 10.1007/BF02636814. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Marcus A. J. The role of lipids in platelet function: with particular reference to the arachidonic acid pathway. J Lipid Res. 1978 Sep;19(7):793–826. [PubMed] [Google Scholar]
- Marcus A. J., Weksler B. B., Jaffe E. A. Enzymatic conversion of prostaglandin endoperoxide H2 and arachidonic acid to prostacyclin by cultured human endothelial cells. J Biol Chem. 1978 Oct 25;253(20):7138–7141. [PubMed] [Google Scholar]
- Mathers L., Bailey M. J. Enzyme deletions and essential fatty acid metabolism in cultured cells. J Biol Chem. 1975 Feb 10;250(3):1152–1153. [PubMed] [Google Scholar]
- Mathias M. M., Dupont J. The relationship of dietary fats to prostaglandin biosynthesis. Lipids. 1979 Feb;14(2):247–252. doi: 10.1007/BF02533877. [DOI] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Moncada S., Herman A. G., Higgs E. A., Vane J. R. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. Thromb Res. 1977 Sep;11(3):323–344. doi: 10.1016/0049-3848(77)90185-2. [DOI] [PubMed] [Google Scholar]
- Nordøy A., Svensson B., Hoak J. C. The effects of albumin bound fatty acids on the platelet inhibitory function of human endothelial cells. Eur J Clin Invest. 1979 Feb;9(1):5–10. doi: 10.1111/j.1365-2362.1979.tb01661.x. [DOI] [PubMed] [Google Scholar]
- Pace-Asciak C. R. Oxidative biotransformations of arachidonic acid. Prostaglandins. 1977 May;13(5):811–817. doi: 10.1016/0090-6980(77)90211-8. [DOI] [PubMed] [Google Scholar]
- Pace-Asciak C., Wolfe L. S. Inhibition of prostaglandin synthesis by oleic, linoleic and linolenic acids. Biochim Biophys Acta. 1968 Jul 1;152(4):784–787. doi: 10.1016/0005-2760(68)90126-4. [DOI] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S. Production of diglyceride from phosphatidylinositol in activated human platelets. J Clin Invest. 1979 Apr;63(4):580–587. doi: 10.1172/JCI109339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert J., Rebel G., Mandel P. Essential fatty acid metabolism in cultured astroblasts. Biochimie. 1977;59(4):417–423. doi: 10.1016/s0300-9084(77)80318-0. [DOI] [PubMed] [Google Scholar]
- Salmon J. A., Smith D. R., Flower R. J., Moncada S., Vane J. R. Further studies on the enzymatic conversion of prostaglandin endoperoxide into prostacyclin by porcine aorta microsomes. Biochim Biophys Acta. 1978 Mar 14;523(1):250–262. doi: 10.1016/0005-2744(78)90028-1. [DOI] [PubMed] [Google Scholar]
- Schroit A. J., Gallily R. Macrophage fatty acid composition and phagocytosis: effect of unsaturation on cellular phagocytic activity. Immunology. 1979 Feb;36(2):199–205. [PMC free article] [PubMed] [Google Scholar]
- Shepherd J., Packard C. J., Patsch J. R., Gotto A. M., Jr, Taunton O. D. Effects of dietary polyunsaturated and saturated fat on the properties of high density lipoproteins and the metabolism of apolipoprotein A-I. J Clin Invest. 1978 Jun;61(6):1582–1592. doi: 10.1172/JCI109078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Ogletree M. L., Lefer A. M., Nicolaou J. C. Antibodies which antagonise the effects of prostacyclin. Nature. 1978 Jul 6;274(5666):64–65. doi: 10.1038/274064a0. [DOI] [PubMed] [Google Scholar]
- Spector A. A., Kiser R. E., Denning G. M., Koh S. W., DeBault L. E. Modification of the fatty acid composition of cultured human fibroblasts. J Lipid Res. 1979 May;20(4):536–547. [PubMed] [Google Scholar]
- Spector A. A. Lipids, hormones, and atherogenesis. The transport and utilization of free fatty acid. Ann N Y Acad Sci. 1968 Nov 21;149(2):768–783. doi: 10.1111/j.1749-6632.1968.tb53834.x. [DOI] [PubMed] [Google Scholar]
- Spector A. A., Steinberg D. Turnover and utilization of esterified fatty acids in Ehrlich ascites tumor cells. J Biol Chem. 1967 Jul 10;242(13):3057–3062. [PubMed] [Google Scholar]
- Spritz N., Mishkel M. A. Effects of dietary fats on plasma lipids and lipoproteins: an hypothesis for the lipid-lowering effect of unsaturated fatty acids. J Clin Invest. 1969 Jan;48(1):78–86. doi: 10.1172/JCI105976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F. F., Chapman J. P., McGuire J. C. Metabolism of prostaglandin endoperoxide in animal tissues. Prostaglandins. 1977;14(6):1055–1074. doi: 10.1016/0090-6980(77)90285-4. [DOI] [PubMed] [Google Scholar]
- Weksler B. B., Ley C. W., Jaffe E. A. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A 23187. J Clin Invest. 1978 Nov;62(5):923–930. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B. B., Marcus A. J., Jaffe E. A. Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3922–3926. doi: 10.1073/pnas.74.9.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziboh V. A. Biosynthesis of prostaglandin E 2 in human skin: subcellular localization and inhibition by unsaturated fatty acids and anti-inflammatory drugs. J Lipid Res. 1973 Jul;14(4):377–384. [PubMed] [Google Scholar]


