Abstract
Arsenic exposure represents a major health concern increasing cancer risks, yet the mechanism of arsenic carcinogenesis has not been elucidated. We and others recently reported that cell malignant transformation by arsenic is accompanied by epithelial to mesenchymal transition (EMT). However, the role of EMT in arsenic carcinogenesis is not well understood. Although previous studies showed that short term exposure of endothelial cells to arsenic stimulated angiogenesis, it remains to be determined whether cells that were malignantly transformed by long term arsenic exposure have a pro-angiogenic effect. The objective of this study was to investigate the effect of arsenic-transformed human bronchial epithelial cells that underwent EMT on angiogenesis and the underlying mechanism. It was found that the conditioned medium from arsenic-transformed cells strongly stimulated tube formation by human umbilical vein endothelial cells (HUVECs). Moreover, enhanced angiogenesis was detected in mouse xenograft tumor tissues resulting from inoculation of arsenic-transformed cells. Mechanistic studies revealed that β-catenin was activated in arsenic-transformed cells up-regulating its target gene expression including angiogenic-stimulating vascular endothelial growth factor (VEGF). Stably expressing microRNA-200b in arsenic-transformed cells that reversed EMT inhibited β-catenin activation, decreased VEGF expression and reduced tube formation by HUVECs. SiRNA knockdown β-catenin decreased VEGF expression. Adding a VEGF neutralizing antibody into the conditioned medium from arsenic-transformed cells impaired tube formation by HUVECs. Reverse transcriptase-PCR analysis revealed that the mRNA levels of canonical Wnt ligands were not increased in arsenic-transformed cells. These findings suggest that EMT in arsenic-transformed cells promotes angiogenesis through activating β-catenin-VEGF pathway.
Keywords: Arsenic-transformed cells, angiogenesis, β-catenin, epithelial-to-mesenchymal transition (EMT), microRNA-200b (miR-200b), Wnt
Introduction
Arsenic is a well-recognized human carcinogen and arsenic exposure represents a major health concern. Epidemiological and experimental studies have shown that chronic arsenic exposure through contaminated drinking water increases the risk of skin, lung, bladder, liver, and prostate cancer (Celik et al. 2008; Benbrahim-Tallaa and Waalkes 2008; Liu and Waalkes 2008; Tapio and Grosche 2006; Tokar et al. 2012). However, the mechanism of arsenic carcinogenesis has not been elucidated (Chervona et al. 2012; Pi et al. 2008; Rossman and Klein, 2011).
Epithelial to mesenchymal transition (EMT) is a crucial developmental program that converts epithelial cells into mesenchymal-like ones, featuring a loss of epithelial properties such as cell adhesion and expression of the epithelial marker E-cadherin, and an acquisition of mesenchymal properties such as increased cell motility and expression of the mesenchymal marker vimentin (Lee et al. 2006). EMT is usually induced during development by EMT-inducing transcription factors such as snail, slug, twist, zinc finger E-box-binding homeobox 1 (ZEB1) and ZEB2. It was recently found that EMT could also be regulated by microRNAs (miRNAs) (Gregory et al. 2008b). MiRNAs are an abundant class of small non-coding RNAs that down-regulate protein-coding gene expression post-transcriptionally by interacting with messenger RNAs (mRNAs) and inducing translation suppression and mRNA degradation (Iorio and Croce 2012). Among a few of EMT-repressive miRNAs, miRNA-200 family members inhibit EMT through down-regulating the expression of ZEB1 and ZEB2 and increasing the expression of E-cadherin (Gregory et al., 2008a; Korpal et al., 2008). In addition to regulating cell motility, EMT of mammary tumor epithelial cells also promotes tumor angiogenesis (Ma et al. 2010). Angiogenesis refers to the process of forming new blood vessels from pre-existing ones and is required for tumor growth and metastasis (Weis and Cheresh 2011).
Studies have shown that arsenic-induced malignant transformation of animal and human epithelial cells is often accompanied by cellular morphology changes resembling EMT. It was initially reported by Zhao et al. (1997) that a 18-week exposure of arsenite (0.125-0.5 μM) induced malignant transformation of the rat liver epithelial TRL 1215 cells accompanied by a dramatic morphological change of cells from epithelioid to fibroblast-like. Our recent study demonstrated for the first time that a 16-week arsenite (2.5 μM) exposure caused EMT and malignant transformation of immortalized p53-knocked down human bronchial epithelial cells (p53lowHBECs) as evidenced by: (i) The majority of p53lowHBECs obtained the spindle-like mesenchymal morphology by 16 weeks of arsenite exposure; (ii) Western blot analysis showed that arsenite induced expression of the mesenchymal marker vimentin in p53lowHBECs starting from 8 weeks of exposure and that the epithelial marker E-cadherin expression was completely lost in p53lowHBECs exposed to arsenite for 16 weeks (Wang et al. 2011). We further determined that arsenic exposure triggered EMT through increasing the expression of EMT-inducing transcription factors ZEB1 and ZEB2 and depleting the expression of EMT-repressing miRNA-200s (Wang et al. 2011). Recent other independent studies also showed that a 15-week arsenite (1 μM) exposure resulted in malignant transformation of human bronchial epithelial cells and keratinocytes accompanied by EMT (Jiang et al. 2012; Xu et al. 2012). Although it has been observed that chronic arsenic exposure caused EMT, the role of EMT in arsenic carcinogenesis is not well understood. While it was previously reported that acute short term arsenic treatment of human umbilical vein endothelial cells (HUVECs) stimulated angiogenesis (Kao et al. 2003; Soucy et al. 2003; Meng et al. 2010; Liu et al. 2011), the exact mechanism of arsenic stimulating angiogenesis has not been clearly defined. Furthermore, it is not known whether cells that were transformed by chronic arsenic exposure have a pro-angiogenic activity.
This study was performed to investigate the effect of arsenic-transformed human bronchial epithelial cells that underwent EMT on angiogenesis and the underlying mechanism. We found that the conditioned medium from arsenic-transformed cells cultured in the absence of arsenic significantly increased tube formation by HUVECs. Moreover, enhanced angiogenesis was detected in mouse xenograft tumor tissues resulting from subcutaneous inoculation of arsenic-transformed cells. Stably expressing miRNA-200b that caused mesenchymal to epithelial transition (MET) inhibited angiogenesis induced by arsenic-transformed cells. Further mechanistic studies revealed that EMT in arsenic-transformed cells promotes angiogenesis through activating β-catenin-vascular endothelial growth factor (VEGF) pathway. The findings from this study provide additional novel evidence suggesting that EMT plays an important role in arsenic carcinogenesis by promoting angiogenesis, in addition to previously reported its potential role in arsenic-induced malignant transformation and its role in promoting arsenic-transformed cell migration and invasion.
Materials and methods
Cell lines and cell culture
Human bronchial epithelial cells (HBECs) with p53 expression stably knocked down (defined as p53lowHBECs) were generated from the telomerase- and Cdk4-immortalized p53 intact HBECs and generously provided by Dr. John D. Minna (University of Texas Southwestern Medical Center, Dallas, TX) (Sato et al. 2006). Our previous study showed that chronic arsenic exposure caused EMT and malignant transformation of p53lowHBECs but not of p53 intact HBECs (Wang et al. 2011). Arsenic-transformed p53lowHBECs (defined as As-p53lowHBECs) were generated in our previous study by exposing p53lowHBECs to sodium arsenite (2.5 μM) for 16 weeks (Wang et al. 2011). The green fluorescence protein (GFP) vector control and miRNA-200b stable expressing As-p53lowHBECs were also generated in our previous study and named as As-p53lowHBEC-GFP and As-p53lowHBEC-GFP-200b, respectively (Wang et al. 2011). In current study, control p53lowHBECs, As-p53lowHBECs, As-p53lowHBEC-GFP and As-p53lowHBEC-GFP-200b cells were cultured in chemically defined serum-free medium (K-SFM) (Invitrogen, Carlsbad, CA) in the absence of arsenic as previously described (Wang et al. 2011). Primary human umbilical vein endothelial cells (HUVECs) were purchased from Lonza (Walkersville, MD) and cultured in endothelial cell growth medium-2 (EGM-2) supplemented with BulletKit (Lonza). All cells were cultured at 37 °C in a humidified 5% CO2 atmosphere.
Tube formation assay
The tube formation by HUVECs was determined in the presence of various conditioned medium from control and arsenic-transformed cells following the protocol described by Fang et al (2005) with modifications. To prepare the conditioned medium from control p53lowHBECs and arsenic-transformed p53lowHBECs, cells were cultured to about 90-100% confluence. The old complete growth medium was then removed, and cells were washed once with PBS and provided with fresh basic serum-free medium supplemented with 20 μg/ml of bovine pituitary extract but no epidermal growth factor. The cells were incubated for 20 h and the medium was collected as the conditioned medium and stored at -80C°. To collect the conditioned medium from control siRNA and β-catenin siRNA knocked down cells, the old medium was replaced with above-mentioned fresh basic medium 72 h after siRNA transfection as described below. To determine the effect of vascular endothelial growth factor (VEGF) neutralization on tube formation, control antibody or VEGF neutralizing antibody (R&D, Minneapolis, MN) were added to the conditioned medium collected from As-p53lowHBECs at a concentration of 2 μg/ml. To analyze tube formation, about 80-90% confluence of HUVECs were starved (in basic EGM-2 medium containing 0.25% fetal bovine serum) for 24 h and then collected and re-suspended in basic EGM-2 medium. The HUVECs were mixed with 50 μl of basic EGM-2 medium supplemented with 0.5% fetal bovine serum and 50 μl of each conditioned medium and seeded to growth factor-reduced Matrigel (BD Bioscience)-pre-coated 96-well plate at 1.5 × 104 cells/well. After 20 h incubation at 37C°, tube formation was examined and photographed under a light microscope connected with a digital camera (Qimaging, Surrey, BC, Canada). The total number of formed tube branches in each well was counted under the light microscope. The experiments were performed in triplicate wells and performed three times.
Mouse xenograft tissue section CD31 immunofluorescence staining
Nude mouse xenograft tissues were produced by subcutaneous injection of As-p53lowHBEC-GFP or As-p53lowHBEC-GFP-200b cells from our previous study (Wang et al. 2011). Tissue sections (5 μm) from 3 mouse xenograft tissues of each group (As-p53lowHBEC-GFP or As-p53lowHBEC-GFP-200b) were prepared and subjected to hematoxylin and eosin (H&E) and immunofluorescence staining as previously described (Zhao et al., 2010). The anti-CD31 primary antibody was from Abcam (Cambridge, MA). Slides were counterstained with 4’,6-diamidino-2-phenylindole (DAPI). The stained sections were visualized and photographed with a Nikon Eclipse TE2000-U fluorescence microscope (Nikon, Inc., Melville, NY). The captured red fluorescent images (CD31 positive staining) were overlaid with the blue fluorescent images (nucleus DAPI staining) using MetaMorph software (Molecular Devices Corp., Downingtown, PA). The anti-CD31 staining was quantified by counting CD31 positive vessel structures in five randomly chosen fields (magnification: × 200) from each mouse xenograft tissue section. Three-stained mouse tissue sections from each group were counted.
Cellular β-catenin immunofluorescence staining
After 72 h culture on cover slides placed inside 6-well plates, cells were washed once with phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde in PBS for 10 min, permeabilized using 0.15% Triton X-100 in PBS for 10 min, and blocked with 2% bovine serum albumin (BSA) in PBS for 1 h at room temperature. Cells were then incubated with an anti-β-catenin antibody (Cell Signaling Technology, Beverly, MA) in PBS containing 0.15% Triton X-100 and 2% BSA at 4 C° for overnight. After incubation cells were washed with PBS and incubated with an Alexa 546–labeled secondary antibody (Molecular Probes, Eugene, OR) at room temperature for 1 h. Cells were then washed with PBS and were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) for 10 min at room temperature. Cells were visualized and photographed with a Nikon TE2000-U fluorescence microscope. The captured red fluorescent images (β-catenin staining) were overlaid with the blue fluorescent images (nucleus DAPI staining) using MetaMorph software.
β-catenin nuclear localization analysis
Cell fractionation experiment was performed to analyze nuclear-localized β-catenin using the protocol described in our recent paper (Li et al. 2012). The α-tubulin protein was used as a marker for the cytoplasmic fraction and the core protein component Sm of the small nuclear ribonucleoprotein complexes (snRNPs) was used as a marker for the nuclear fraction as previous studies showed that Sm was presented only in the nuclear (Vyakarnam et al. 1998; Haudek et al. 2009). The anti-α-tubulin antibody was purchased from Cell Signaling Technology and the anti-Sm antibody was kindly provided by Dr. JL Wang (Department of Biochemistry and Molecular Biology, Michigan State University, East Lansing, MI).
TOPflash and FOPflash luciferase reporter assay
The TOPflash and FOPflash luciferase reporter plasmids were purchased from Millipore (Billerica, MA). Subconfluent cells in 6-well plates were transfected with either TOPflash or FOPflash reporter plasmid using FuGENE-6 Transfection Reagent (Indianapolis, IN) following the manufacturer's instructions. A pRL-TK Rellina luciferase plasmid was co-transfected. Forty-eight h after transfection, cells were collected for measuring luciferase reporter activity using a dual luciferase assay kit from Promega (Madison, WI) following the manufacturer's instructions. The relative TOPflash or FOPflash luciferase activity was calculated as the TOPflash or FOPflash firefly luciferase activity divided by the Renilla luciferase activity. The experiments were carried out in triplicate wells and performed three times.
RT (reverse transcriptase)-PCR analysis of Wnt ligands and their Frizzled receptors and Q (quantitative)-PCR analysis of β-catenin target genes (c-Myc, cyclin D1 and VEGF)
Total RNA was prepared using Trizol and reverse transcribed using SuperScriptTM III RT according to the manufacturer's protocol (Invitrogen). The resulting cDNA was used for PCR amplification of Wnt lignads, Frizzled receptors and a house keeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase) using the primers listed in Supplementary Table 1. All Wnt lignads, Frizzled receptors and GAPDH-specific fragments were amplified by 30, 30 and 25 cycles of PCR, respectively, each cycle comprising 30 s at 95°C, 30 s at 55°C and 45 s at 72°C. The mRNA levels of β-catenin target genes (c-Myc, cyclin D1 and VEGF) were analyzed by Q-PCR using TagMan gene expression assays from Applied Biosystems (ABI) as described previously (Zhao et al. 2010)
Western blot analysis
Cells were lysed using tris-sodium dodecyl sulfate (SDS) and subjected to SDS-polyacrylamide gel electrophoresis as described previously (Wang et al. 2011). The following primary antibodies were used: anti-β-catenin and anti-C-Myc (Cell Signaling Technology); anti-cyclin D1 (BD Bioscience, San Jose, CA); anti-vascular endothelial growth factor (VEGF) (Santa Cruz Biotechnology, Santa Cruz, CA); and anti-β-actin (Sigma, St. Louis, MO).
β-catenin RNA interference and measurement of VEGF levels in the conditioned medium
Negative Control small interfering RNA (siRNA) and ON-TARGETplus SMARTpool siRNA for β-catenin were obtained from Thermo Scientific Dharmacon (Lafayette, CO). SiRNA duplexes (100 nM) were transfected into cells using Lipofectamine 2000 (Invitrogen) as described previously (Wang et al. 2011). Forty-eight h after transfection cells were collected for Western blot analysis as described above. To measure the secreted VEGF levels in the conditioned medium, control siRNA- and β-catenin siRNA-transfected cells were allowed to grow to about 90-100% confluence and the conditioned medium was collected as described above. The VEGF levels in the conditioned medium were measured using an ELISA kit from RayBiotech inc. (Norcross, GA) following the manufacturer's instructions and normalized to the remaining cell numbers.
Statistical analysis
The statistical analyses for the significance of differences in numerical data (means ± standard deviations) were performed using two-tailed t-tests for comparison of two data sets. A p value of <0.05 was considered statistically significant.
Results
Epithelial to mesenchymal transition (EMT) in arsenic-transformed cells (As-p53low HBECs) promotes angiogenesis
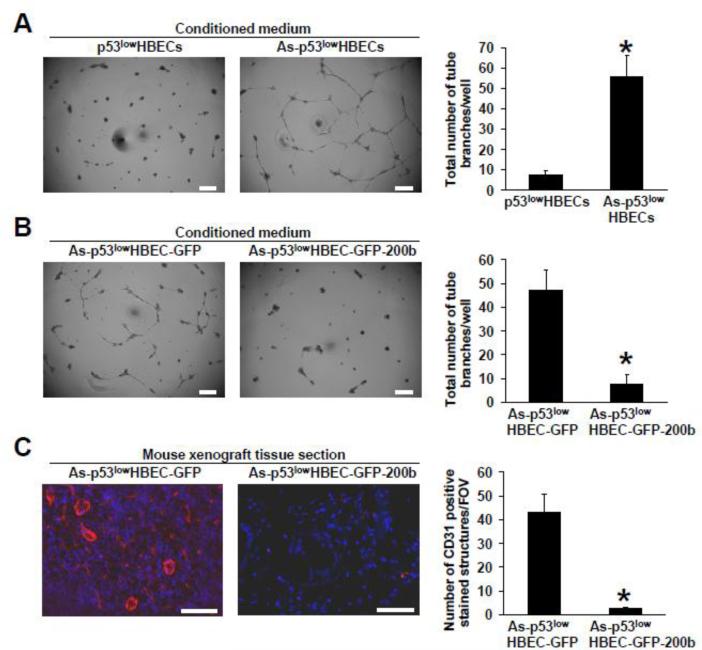
Our previous study showed that an irreversible EMT occurred in arsenic-transformed cells (As-p53lowHBECs) that displayed a spindle-like morphology expressing the mesenchymal marker vimentin and losing the epithelial marker E-cadherin (Wang et al 2011). Arsenic-transformed cells were cultured in the absence of arsenic and maintained the mesenchymal-like morphology. To investigate whether arsenic-transformed cells have a pro-angiogenic activity, we first examined the effect of the conditioned medium from As-p53lowHBECs on tube formation by human umbilical vein endothelial cells (HUVECs). Tube formation by HUVECs is a commonly used in vitro assay for studying the effect of various factors on angiogenesis. As shown in Figure 1A, the HUVECs cultured on Matrigel with the conditioned medium from control untransformed cells (p53lowHBECs) only formed very limited tubes, but tube formation by HUVECs was greatly induced by the conditioned medium from As-p53lowHBECs. These results suggest that arsenic-transformed cells have a pro-angiogenic activity.
Fig. 1. Epithelial to mesenchymal transition (EMT) in arsenic-transformed cells (As-p53lowHBECs) promotes angiogenesis.
(A) The conditioned medium from arsenic-transformed cells stimulates tube formation by HUVECs and (B) Stably expressing miR-200b in arsenic-transformed cells impairs tube formation by HUVECs. Representative images of tube formation by HUVECs induced by the conditioned media from indicated cells. The conditioned media were prepared for tube formation assay as described in Materials and Methods. The quantifications of formed tube branches was carried out as described in Materials and Methods and presented as total number of tube branches per well (means ± standard deviations, n=3). Scale bar=200 μm. * p<0.05, compared to p53lowHBECs (A) and to As-p53lowHBEC-GFP (B). Similar results were obtained in two additional experiments. (C) Enhanced angiogenesis is detected in mouse xenograft tumors produced by inoculation of arsenic-transformed cells (As-p53lowHBEC-GFP).Representative overlaid fluorescent images from anti-CD31 immunofluorescence staining (red color) and nucleus DAPI staining (blue color) in mouse xenograft tissues resulting from injection of As-p53lowHBEC-GFP or As-p53lowHBEC-GFP-200b cells. Tissue section preparation and anti-CD31 staining were carried out as described in Materials and Methods. The CD31 staining was quantified and presented as the number of CD31 positive-stained vessel structures per field of view (FOV) as described in Materials and Methods (means ± standard deviations, n=3). Scale bar=100 μm. * p<0.05, compared to the As-p53lowHBEC-GFP group.
We previously demonstrated that the expression of miRNA-200 family members was depleted in arsenic-transformed cells (As-p53lowHBECs) (Wang et al 2011). Stably re-expressing miRNA-200b (miR-200b) in As-p53lowHBECs caused mesenchymal to epithelial transition (MET) restoring the epithelial-like morphology and the expression of E-cadherin, and reversed their transformed phenotypes (Wang et al 2011). To determine the role of EMT in the angiogenic effect of arsenic-transformed cells, we examined the effect of the conditioned medium from previously-generated vector control (As-p53lowHBEC-GFP) and miR-200b stable expressing (As-p53lowHBEC-GFP-200b) cells (Wang et al 2011). It was found that the conditioned medium from the As-p53lowHBEC-GFP cells significantly stimulated tube formation by HUVECs comparable to the effect of the conditioned medium from As-p53lowHBECs (Figure 1B). In contrast, the tube formation by HUVECs cultured with the conditioned medium from the As-p53lowHBEC-GFP-200b cells was drastically reduced to the level induced by the conditioned medium from untransformed p53lowHBECs (Figure 1B). These results suggest that EMT plays a critical role in arsenic-transformed cells’ pro-angiogenic activity.
To further determine the angiogenic effect of arsenic-transformed cells, we next examined the angiogenesis in mouse xenograft tissues produced in our previous study by injection of As-p53lowHBEC-GFP or As-p53lowHBEC-GFP-200b cells. We recently reported that subcutaneous injection of As-p53lowHBEC-GFP cells into nude mice produced undifferentiated invasive epithelial tumors, but inoculation of As-p53lowHBEC-GFP-200b cells only produced scar-like tissues (Wang et al. 2011; Wang et al 2012; Yang 2011). Angiogenesis in mouse xenograft tissues was analyzed by performing immunofluorescence staining of an endothelial cell marker CD31. A good number of CD31 positive staining vessel structures were observed in mouse xenograft tumor tissues resulting from injection of As-p53lowHBEC-GFP cells (Figure 1C). In contrast, very little CD31 positive staining was found in mouse xenograft scar-like tissues resulting from injection of As-p53lowHBEC-GFP-200b cells (Figure 1C). These results indicate that arsenic-transformed cells also exhibit angiogenic effect in vivo.
EMT causes β-catenin activation in arsenic-transformed cells
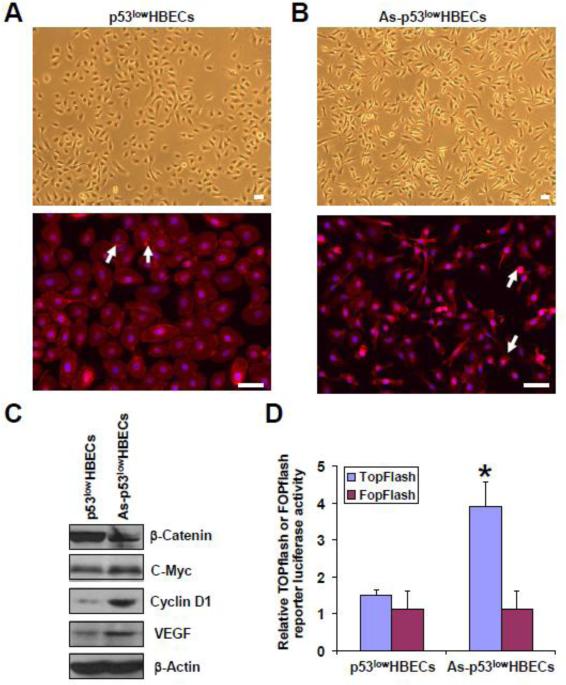
We next wanted to investigate the mechanism by which arsenic-transformed cells promote angiogenesis. β-catenin nuclear translocation and subsequent activation play an important role in tumor angiogenesis induced by mammary tumor epithelial cell EMT (Ma et al. 2010). We and others recently reported that EMT occurred in arsenic-transformed cells (Wang et al 2011; Jiang et al. 2012; Xu et al. 2012), so we set to examine β-catenin cellular localization and its transcriptional activity in arsenic-transformed cells. Figure 2A upper panel shows the epithelial morphology of control untransformed cells (p53lowHBECs) in a bright field. Typical epithelial cell cytoplasmic membrane β-catenin staining was observed in p53lowHBECs (Figure 2A, lower panel). Figure 2B upper panel shows the mesenchymal-like morphology of arsenic-transformed cells (As-p53lowHBECs) in a bright field. In contrast, β-catenin cytoplasmic membrane staining was lost and β-catenin nucleus localization was detected in the majority of As-p53lowHBECs (Figure 2B, lower panel). Higher magnification pictures of β-catenin immunofluorescence staining are presented in Supplementary Figure 1. Although the total β-catenin protein level in As-p53lowHBECs was lower than that in control p53lowHBECs (Figure 2C), cell fractionation experiment demonstrated that the nuclear β-catenin protein level in As-p53lowHBECs was much higher than that in control p53lowHBECs (Supplementary Figure 2). In the nuclear β-catenin functions as a co-transcription factor interacting with TCF/LEF family of transcription factors and promotes specific gene expression (Clevers and Nusse 2012). We next determined whether nuclear-localized β-catenin in As-p53lowHBECs is transcriptionally active using TOPflash (with wildtype TCF binding sites) and FOPflash (with mutated TCF binding sites) luciferase reporters. As shown in Figure 2D, the TOPflash luciferase reporter activity was significantly higher in As-p53lowHBECs than that in control cells. Furthermore, both the mRNA and protein levels of β-catenin's target genes including c-Myc, cyclin D1 and VEGF were dramatically increased in As-p53lowHBECs revealed by Q-PCR and Western blot analysis (Figure 2C and Supplementary Figure 3). Together, these results indicate that β-catenin is activated in arsenic-transformed human bronchial epithelial cells and the expressions of its target genes including angiogenic-stimulating growth factor VEGF are increased.
Fig. 2. β-Catenin is activated in arsenic-transformed human bronchial epithelial cells (As-p53lowHBECs).
(A) Representative images of control untransformed p53lowHBECs shown in a bright field (upper panel) and their β-catenin immunofluorescence staining (lower pane). White arrows point to representative cytoplasmic membrane stainings of β-catenin. (B) Representative images of arsenic-transformed cells (As-p53lowHBECs) shown in a bright field (upper panel) and their β-catenin immunofluorescence staining (lower pane). White arrows point to representative nucleus stainings of β-catenin. β-Catenin immunofluorescence staining was carried out as described in Materials and Methods. Scale bar=50 μm. (C) Representative Western blot analysis of cellular β-catenin, c-myc, cyclin D1 and VEGF protein levels. Western blot was carried out as described in Materials and Methods. (D) Quantification of cellular TOPflash and FOPflash reporter luciferase activity. The TOPflash and FOPflash reporter luciferase activity was measured using a dual luciferase reporter assay and calculated as described in Materials and Methods. The results are expressed as the ratio of the TOPflash or FOPflash luciferase activity divided by the Renilla luciferase activity (means ± standard deviations, n=3). * p<0.05, compared to p53lowHBECs. Similar results were obtained in two additional experiments.
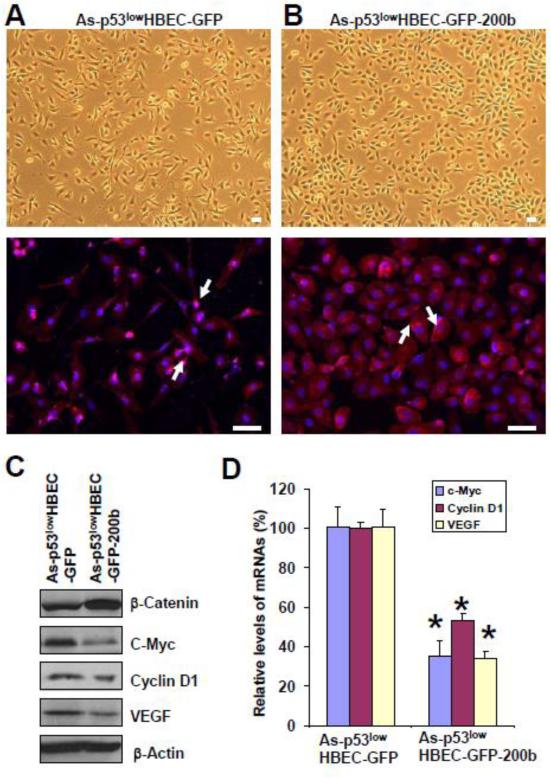
To determine whether EMT plays a role in β-catenin activation in arsenic-transformed cells, we next examined and compared β-catenin cellular localization and its target gene expression levels between As-p53lowHBEC-GFP and As-p53lowHBEC-GFP-200b cells. The upper panels in Figure 3A and 3B show the mesenchymal-like morphology of As-p53lowHBEC-GFP cells and the epithelial-like morphology of As-p53lowHBEC-GFP-200b cells in bright fields, respectively. In consistent with their respective morphology, As-p53lowHBEC-GFP cells showed similar β-catenin nucleus staining to that of As-p53lowHBECs (Fig. 3A, lower panel); but As-p53lowHBEC-GFP-200b cells displayed β-catenin mainly cytoplasmic membrane staining (Figure 3B, lower panel). Although the total β-catenin protein level in As-p53lowHBEC-GFP-200b cells was higher than that in As-p53lowHBEC-GFP cells (Figure 3C), further cell fractionation experiment demonstrated that As-p53lowHBEC-GFP-200b cells had much lower nuclear β-catenin protein level than As-p53lowHBEC-GFP cells (Supplementary Figure 2). Moreover, Q-PCR and Western blot analysis revealed that both the mRNA and protein expression levels of β-catenin's three target genes including c-Myc, cyclin D1 and VEGF in miR-200b stably expressing cells were dramatically decreased (Figure 3C and D). Since stably re-expressing miR-200b in arsenic-transformed cells reversed EMT (Wang et al 2011), these results suggest that EMT plays an important role in β-catenin activation in arsenic-transformed cells.
Fig. 3. Stably re-expressing miR-200b in arsenic-transformed cells restores β-catenin cytoplasmic membrane localization and reduces its target gene expression.
(A) Representative images of arsenic-transformed vector control cells (As-p53lowHBEC-GFP) shown in a bright field (upper panel) and their β-catenin immunofluorescence staining (lower pane). White arrows point to representative nucleus stainings of β-catenin. (B) Representative images of arsenic-transformed miR-200b stably expressing cells (As-p53lowHBEC-GFP-200b) shown in a bright field (upper panel) and their β-catenin immunofluorescence staining (lower pane). White arrows point to representative cytoplasmic membrane stainings of β-catenin. β-Catenin immunofluorescence staining was carried out as described in Materials and Methods. Scale bar=50 μm. (C) Representative Western blot analysis of cellular β-catenin, c-myc, cyclin D1 and VEGF protein levels. Western blot was carried out as described in Materials and Methods. (D) Q-PCR analysis of cellular c-myc, cyclin D1 and VEGF mRNA levels (means ± standard deviations, n=3). Q-PCR analysis was performed as described in Materials and Methods. * p<0.05, compared to the As-p53lowHBEC-GFP cells. Similar results were obtained in two additional experiments.
The arsenic-transformed cells promote tube formation by HUVECs via β-catenin-VEGF pathway
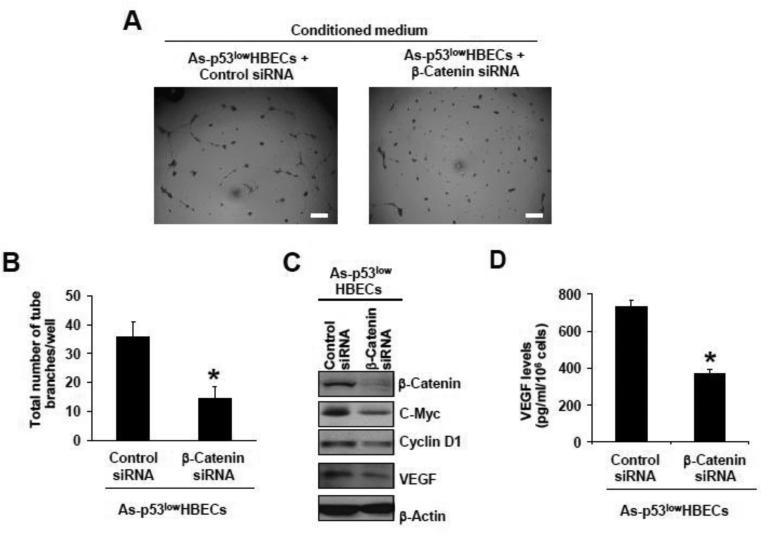
Since β-catenin was activated and the level of the angiogenic-stimulating growth factor VEGF was increased in arsenic-transformed cells, we next determined whether β-catenin activation plays a role in the up-regulation of VEGF expression and the pro-angiogenic effect of arsenic-transformed cells. First, siRNA knockdown of β-catenin expression was performed to determine its role in increased tube formation by HUVECs induced by the conditioned medium from arsenic-transformed cells. As shown in Figure 4A and 4B, the conditioned medium from As-p53lowHBECs transfected with an ON-TARGETplus SMARTpool siRNA targeting β-catenin failed to promote tube formation by HUVECs. These results indicate that β-catenin activation plays a crucial role in the pro-angiogenic effect of arsenic-transformed cells. Moreover, Western blot analysis revealed that β-catenin level was greatly knocked down by β-catenin siRNA, and the levels of its target genes and VEGF in the conditioned medium were dramatically reduced (Figure 4C and D), suggesting that up-regulation of VEGF expression in arsenic-transformed cells is mediated mainly by β-catenin activation.
Fig. 4. SiRNA knocking down β-catenin expression in arsenic-transformed cells reduces VEGF expression and impairs their conditioned medium-induced tube formation by HUVECs.
(A, B) Representative images of tube formation by HUVECs (A) and quantification of formed tube branches (means ± standard deviations, n=3) (B) induced by the conditioned medium from As-p53lowHBECs transfected with Control or β-catenin siRNA. Scale bar=200 μm. * p<0.05, compared to Control siRNA-transfected cells. (C) Representative Western blot analysis of cellular β-catenin, c-myc, cyclin D1 and VEGF protein levels. (D) Conditioned medium VEGF levels measured by ELISA (means ± standard deviations, n=3). * p<0.05, compared to Control siRNA-transfected cells. After overnight culture, As-p53lowHBECs were transfected with negative Control or β-catenin siRNA oligoes as described in Materials and Methods. Conditioned medium were collected for tube formation assay and ELISA measurement of VEGF levels and Western blot was performed as described in Materials and Methods. Similar results were obtained in two additional experiments.
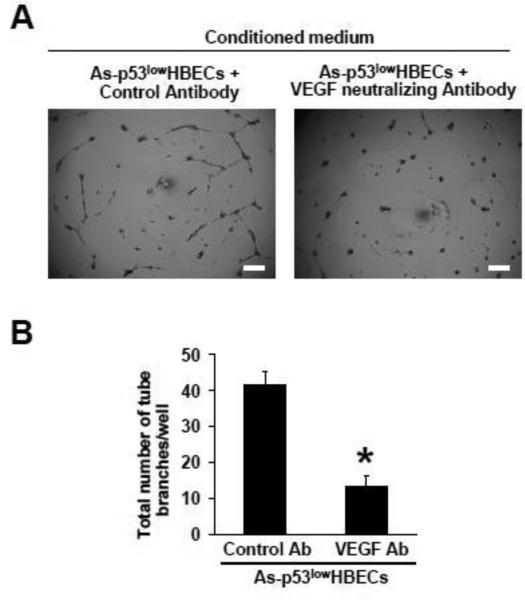
Finally, a VEGF neutralizing antibody was used to further determine the role of VEGF in the pro-angiogenic effect of arsenic-transformed cells. Compared to adding a control antibody, adding a VEGF neutralizing antibody into the conditioned medium from As-p53lowHBECs greatly impaired its tube forming capability (Figure 5A and 5B). These results indicate that knocking down β-catenin expression in arsenic-transformed cells impairs the capability of their conditioned medium promoting tube formation by HUVECs and this effect is mainly mediated through reducing the expression of the angiogenic-stimulating growth factor VEGF. Thus, arsenic-transformed cells promote tube formation by HUVECs via β-catenin-VEGF pathway.
Fig. 5. Adding a VEGF neutralizing antibody into the conditioned medium from arsenic-transformed cells reduces tube formation by HUVECs.
(A, B) Representative images of tube formation by HUVECs (A) and quantification of formed tube branches (means ± standard deviations, n=3) (B) induced by the conditioned medium from As-p53lowHBECs with the addition of a Control antibody or a VEGF neutralizing antibody. Scale bar=200 μm. * p<0.05, compared to Control antibody (Ab) group. Conditioned medium were collected for tube formation assay as described in Materials and Methods. Similar results were obtained in two additional experiments.
The expression levels of Wnt ligands and their Frizzled receptors (FZDs) in p53low HBECs, As-p53low HBECs, As-p53low HBEC-GFP and As-p53low HBEC-GFP-200b cells
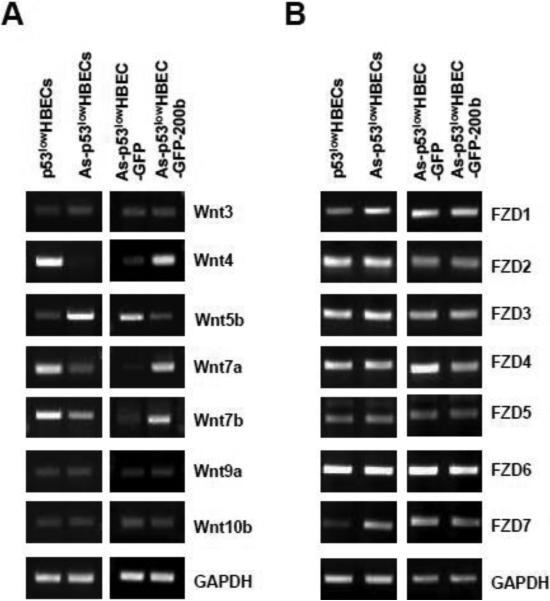
In addition to the pool of β-catenin located at the peripheral cytoplasmic membrane, another major pool of cellular β-catenin exists in the cytoplasm and can be activated in response to elevated canonical Wnt signaling (Clevers and Nusse, 2012). To further explore the potential mechanism responsible for β-catenin activation in arsenic-transformed cells, we wanted to analyze whether the expressions of any canonical Wnt ligands and their Frizzled receptors (FZDs) were up-regulated in arsenic-transformed cells. Semiquantitative RT–PCR analysis for the expression of human all 19 Wnts and 10 FZDs was performed. It was found that the expression of the majority of Wnt ligands was undetectable in both control p53lowHBECs and As-p53lowHBECs (Wnt1, 2, 2B, 3A, 5A, 6, 8A, 8B, 9B, 10A, 11, and 16, data not shown). For those Wnt ligands whose expressions were detected in control p53lowHBECs, some of them were either similarly expressed (Wnt3, 9a and 10b) or less expressed in As-p53lowHBECs (Wnt4, 7a and 7b) (Figure 6A). An increase of a non-canonical Wnt ligand Wnt5b expression was detected in As-p53lowHBECs (Figure 6A). While As-p53lowHBEC-GFP cells showed a similar pattern of Wnt ligand expressions to that of As-p53lowHBECs, stably expressing miR-200b in arsenic-transformed cells restored the Wnt ligand expression pattern similar to that of control cells (Figure 6A). These results indicate that the mRNA expression levels of canonical Wnt ligands are not increased in arsenic-transformed cells.
Fig. 6. RT-PCR analysis of cellular Wnt ligands and their Frizzled receptors (FZDs).
(A, B) Representative images of RT-PCR analysis of cellular Wnt ligands (A) and their Frizzled receptors (B). About 80-90% confluence of control untransformed cells (p53lowHBECs), arsenic-transformed cells (As-p53lowHBECs), vector control (As-p53lowHBEC-GFP) or miR-200b stably expressing (As-p53lowHBEC-200b) cells were used for extracting total RNA for RT-PCR analysis as described in Materials and Methods. Similar results were obtained in two additional experiments.
We next examined the mRNA expression levels of the Wnt ligand all 10 Frizzled receptors (FZDs). It was found that the FZDs1 to 7 were expressed in both control p53lowHBECs and As-p53lowHBECs (Figure 6B), but the expression of FZDs8 to10 was not detectable by RT-PCR (data not shown). While the levels of FZD1 and FZD7 were higher in As-p53lowHBECs than that in control p53lowHBECs, no dramatic differences of the levels of FZDs2 to 6 were found between control and arsenic-transformed cells (Figure 6B). It was further observed that stably re-expressing miR-200b in arsenic-transformed cells did not greatly affect the expression levels of FZDs1 to 7 as their similar expression levels were detected between As-p53lowHBEC-GFP and As-p53lowHBEC-GFP-200b cells (Figure 6B). Since stably re-expressing miR-200b inhibited β-catenin activation in arsenic-transformed cells (Figure 3) with no significant effect on up-regulated levels of FZD1 and FZD7 (Figure 6B), these results suggest that the differential expression of FZD1 and FZD7 between control p53lowHBECs and As-p53lowHBECs may not contribute significantly to β-catenin activation in arsenic-transformed cells.
Discussion
Chronic arsenic exposure is associated with increased risk of developing various types of cancers; however, the underlying mechanism of arsenic carcinogenesis has not been elucidated. Angiogenesis is a process of forming new blood vessels and plays important roles in carcinogenesis (Weis and Cheresh 2011). Although previous studies showed that acute short term arsenic treatment of HUVECs stimulated angiogenesis (Kao et al. 2003; Soucy et al. 2003; Meng et al. 2010; Liu et al. 2011), it remains to be determined whether cells that were transformed by long term arsenic exposure have an angiogenic effect. We and others recently reported that chronic arsenic exposure caused epithelial to mesenchymal transition (EMT) during arsenic-induced malignant transformation (Wang et al. 2011; Jiang et al. 2012; Xu et al. 2012), however, the significance of EMT in arsenic carcinogenesis has not been well understood. In this study, we demonstrated for the first time that arsenic-transformed cells that underwent EMT exhibit a pro-angiogenic activity; and EMT in arsenic-transformed cells promotes angiogenesis through activating β-catenin-VEGF pathway.
EMT converts epithelial cells into mesenchymal-like cells through disruption of cell-cell junctions and loss of apical-basolateral polarity enabling cells to migrate and invade (Lee et al. 2006). EMT plays key roles in the formation of many organs and is tightly regulated during embryonic development. Studies have shown that EMT is often re-activated in cancer and plays critical roles in cancer metastasis (Thiery et al. 2009). The human and animal epithelial cells malignantly transformed by chronic arsenic exposure not only displayed prominent mesenchymal-like morphology resembling EMT (Zhao et al. 1997; Wang et al. 2011; Jiang et al. 2012; Xu et al. 2012), when inoculated into nude mice, they were also capable of producing invasive and metastatic xenograft tumors (Achanzar et al. 2002; Wang et al. 2012; Zhao et al. 1997). However, the underlying mechanism is not clear. Given the important roles of EMT in cancer, it is thus essential to further investigate whether and how EMT contributes to arsenic carcinogenesis.
In addition to increase tumor cell migration and invasion, EMT of mammary tumor epithelial cells also promotes tumor angiogenesis. Tumor angiogenesis can be stimulated by elevated expression of angiogenic-stimulating growth factors such as VEGF among many others. One mechanism of up-regulating VEGF expression is through the activation of β-catenin (Zerlin et al. 2008). In epithelial cells, a significant pool of β-catenin is localized at the peripheral cytoplasmic membrane region. The cytoplasmic membrane β-catenin localization and its association with E-cadherin-mediated adherens junction complex plays a crucial role in maintaining epithelial cell-cell adhesion, polarity and limiting cell motility. Although we and others reported that arsenic-transformed human bronchial epithelial cells underwent EMT (Wang et al. 2011; Xu et al. 2012), it remains to be determined whether β-catenin is nuclear translocated and transcriptionally active in arsenic-transformed human cells. In this study, we demonstrated that β-catenin is activated in arsenic-transformed human bronchial epithelial cells evidenced by (i) its loss of cytoplasmic membrane localization but appearance in the nuclear; (ii) increased TOPflash luciferase reporter activity that indicates transcriptional activity of β-catenin; and (iii) up-regulated expression of its target genes at both mRNA and protein levels. We recently reported that stably expressing miR-200b in arsenic-transformed cells recovered the epithelial marker E-cadherin expression and reversed EMT (Wang et al. 2011). We now further demonstrated that stably expressing miR-200b in arsenic-transformed cells restored β-catenin cytoplasmic membrane localization and reduced its target gene expression. Together, these findings suggest that β-catenin is activated in arsenic-transformed cells that underwent EMT and EMT plays an important role in β-catenin activation.
Another major portion of the epithelial cell β-catenin exists in cytoplasm; and activation of cytoplasmic β-catenin could be achieved by elevated canonical Wnt signaling (Clevers and Nusse, 2012). In the absence of canonical Wnt signals, a multiprotein-containing destruction complex binds β-catenin and promotes its proteolytic degradation. Canonical Wnt signaling inhibits this degradation process and stabilizes β-catenin, which subsequently enters nuclear and promotes its target gene expression. However, it seems in this study that canonical Wnt signaling might not contribute significantly to β-catenin activation in arsenic-transformed cells based on the following observations: (i) No increase of canonical Wnt ligand mRNA expression was detected in arsenic-transformed cells. Compared to the control untransformed cells, the mRNA levels of canonical Wnt ligands were either no change or decreased in arsenic-transformed cells. (ii) The mRNA levels of the majority of the Wnt ligand Frizzled receptors (FZDs) were not changed in arsenic-transformed cells except FZD1 and FZD7. Nevertheless, stably expressing miR-200b did not reduce the mRNA levels of FZD1 and FZD7. miR-200b stable expressing reversed EMT and inhibited β-catenin activation as evidenced by cells’ epithelial-like morphology, β-catenin cytoplasmic membrane staining, and reduced expression of β-catenin target genes. Together, these findings suggest that higher levels of FZD1 and FZD7 might not result in a significant increase of canonical Wnt signaling in arsenic-transformed cells; or that β-catenin activation in arsenic-transformed cell could be independent of canonical Wnt signaling. More studies are needed to further examine the involvement of canonical Wnt signaling in β-catenin activation in arsenic-transformed cells. Interestingly, an increased expression of Wnt5b, one of the non-canonical Wnt members that do not activate β-catenin, has been observed in arsenic-transformed cells. Studies have shown that Wnt5b is highly expressed in invasive breast cancer and head and neck squamous cell carcinoma cells and knockdown Wnt5b expression significantly inhibits their migration and invasion (Deraz et al., 2011, Klemm et al. 2011). We recently reported that arsenic-transformed cells are highly migratory and invasive (Wang et al. 2012), it will be interesting to further investigate whether Wnt5b plays a role in this enhanced migratory and invasive behavior
How does β-catenin activation in arsenic-transformed cells promote angiogenesis? Previous studies showed that acute short term arsenic treatment stimulates angiogenesis through increasing the expression of VEGF in HUVECs (0.1-10 μM of arsenite, 4 h) (Kao et al. 2003), human microvascular endothelial cells (HMVECs) (0.5 μM of arsenite, 1-24 h) (Meng et al. 2010), immortalized human lung epithelial BEAS-2B cells (5 μM of arsenite, 24 h) (Liu et al. 2011), and colorectal adenocarcinoma DLD-1 cells (5 μM of arsenite, 24 h) (Wang et al. 2012). However, whether the expression of VEGF is up-regulated in the cells that were transformed by chronic arsenic exposure is not known. It has been proposed that short term arsenic treatment up-regulates VEGF expression through increasing the levels of heme oxygenase-1 and reactive oxygen species, activating AKT and ERK1/2 signaling pathways (Meng et al. 2010; Liu et al. 2011; Wang et al. 2012). In this study we showed for the first time that VEGF expression was up-regulated in arsenic-transformed cells. Stably expressing miR-200b that inhibited β-catenin activation or knocking down β-catenin expression dramatically reduced VEGF expression, suggesting that up-regulation of VEGF expression in arsenic-transformed cells is mainly mediated by β-catenin activation. Moreover, we further demonstrated that knocking down β-catenin expression or adding a VEGF neutralizing antibody into the conditioned medium from arsenic-transformed cells greatly reduced the tube formation by HUVECs, indicating that β-catenin-VEGF pathway plays a crucial role in this process.
In summary, in this study we examined the effect of arsenic-transformed cells on angiogenesis. We demonstrated for the first time that β-catenin is activated in arsenic-transformed human bronchial epithelial cells, which is due to the occurrence of EMT and might be independent of canonical Wnt signaling. β-Catenin activation increases the expression of the angiogenic-stimulating growth factor VEGF, which promotes angiogenesis. Since angiogenesis is critical for tumor growth, invasion and metastasis, the findings from this study provide additional novel evidence suggesting that EMT plays an important role in arsenic carcinogenesis by promoting angiogenesis, in addition to previously reported its potential role in arsenic-induced malignant transformation (Wang et al. 2011; Jiang et al. 2012; Xu et al. 2012) and its role in promoting arsenic-transformed cell migration and invasion (Wang et al. 2012).
Supplementary Material
Highlights.
Arsenic-transformed cells that underwent EMT displayed a pro-angiogenic effect
EMT in arsenic-transformed cells promotes angiogenesis via activating β-catenin
β-catenin activation increases VEGF expression in arsenic-transformed cells
β-catenin activation is likely independent of canonical Wnt signaling
EMT in arsenic-transformed cells promotes angiogenesis via β-catenin-VEGF pathway
Acknowledgments
Funding Information
This work was supported by the National Institutes of Health [1R01ES017777-01A1 to C.Y.]
Abbreviations and definitions
- EMT
epithelial-to-mesenchymal transition
- HUVEC
human umbilical vein endothelial cell
- p53lowHBECs
human bronchial epithelial cells (HBECs) with p53 expression stably knocked down
- IF
immunofluorescence
- miR-200b
microRNA 200b
- siRNA
small interfering RNA
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None declared.
References
- Achanzar WE, Brambila EM, Diwan BA, Webber MM, Waalkes MP. Inorganic arsenite-induced malignant transformation of human prostate epithelial cells. J. Natl. Cancer Inst. 2002;94:1888–1891. doi: 10.1093/jnci/94.24.1888. [DOI] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Waalkes MP. Inorganic arsenic and human prostate cancer. Environ. Health Perspect. 2008;116:158–164. doi: 10.1289/ehp.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik I, Gallicchio L, Boyd K, Lam TK, Matanoski G, Tao X, Shiels M, Hammond E, Chen L, Robinson KA, Caulfield LE, Herman JG, Guallar E, Alberg AJ. Arsenic in drinking water and lung cancer: a systematic review. Environ. Res. 2008;108:48–55. doi: 10.1016/j.envres.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Chervona Y, Arita A, Costa M. Carcinogenic metals and the epigenome: understanding the effect of nickel, arsenic, and chromium. Metallomics. 2012;4:619–27. doi: 10.1039/c2mt20033c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Deraz EM, Kudo Y, Yoshida M, Obayashi M, Tsunematsu T, Tani H, Siriwardena SB, Keikhaee MR, Qi G, Iizuka S, Ogawa I, Campisi G, Lo Muzio L, Abiko Y, Kikuchi A, Takata T. MMP-10/stromelysin-2 promotes invasion of head and neck cancer. PLoS One. 2011;6:e25438. doi: 10.1371/journal.pone.0025438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Xia C, Cao Z, Zheng JZ, Reed E, Jiang BH. Apigenin inhibits VEGF and HIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. FASEB J. 2005;19:342–353. doi: 10.1096/fj.04-2175com. [DOI] [PubMed] [Google Scholar]
- Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008a;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- Gregory PA, Bracken CP, Bert AG, Goodall GJ. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle. 2008b;7:3112–3118. doi: 10.4161/cc.7.20.6851. [DOI] [PubMed] [Google Scholar]
- Haudek KC, Voss PG, Locascio LE, Wang JL, Patterson RJ. A mechanism for incorporation of galectin-3 into the spliceosome through its association with U1 snRNP. Biochemistry. 2009;48:7705–7712. doi: 10.1021/bi900071b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio MV, Croce CM. MicroRNA involvement in human cancer. Carcinogenesis. 2012;33:1126–1133. doi: 10.1093/carcin/bgs140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R, Li Y, Xu Y, Zhou Y, Pang Y, Shen L, Zhao Y, Zhang J, Zhou J, Wang X, Liu Q. EMT and CSC-like properties mediated by the IKKβ/IκBα/RelA signal pathway via the transcriptional regulator, Snail, are involved in the arsenite-induced neoplastic transformation of human keratinocytes. Arch Toxicol. 2012 Oct 16; doi: 10.1007/s00204-012-0933-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kao YH, Yu CL, Chang LW, Yu HS. Low concentrations of arsenic induce vascular endothelial growth factor and nitric oxide release and stimulate angiogenesis in vitro. Chem. Res. Toxicol. 2003;16:460–468. doi: 10.1021/tx025652a. [DOI] [PubMed] [Google Scholar]
- Klemm F, Bleckmann A, Siam L, Chuang HN, Rietkötter E, Behme D, Schulz M, Schaffrinski M, Schindler S, Trümper L, Kramer F, Beissbarth T, Stadelmann C, Binder C, Pukrop T. β-catenin-independent WNT signaling in basal-like breast cancer and brain metastasis. Carcinogenesis. 2011;32:434–442. doi: 10.1093/carcin/bgq269. [DOI] [PubMed] [Google Scholar]
- Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J. Biol. Chem. 2008;283:14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J. Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Zhang C, Gao S, Chen F, Yang C, Luo R, Xiao H. TIP30 loss enhances cytoplasmic and nuclear EGFR signaling and promotes lung adenocarcinogenesis in mice. Oncogene. 2012 Jun 25; doi: 10.1038/onc.2012.253. doi: 10.1038/onc.2012.253. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Waalkes MP. Liver is a target of arsenic carcinogenesis. Toxicol. Sci. 2008;105:24–32. doi: 10.1093/toxsci/kfn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LZ, Jiang Y, Carpenter RL, Jing Y, Peiper SC, Jiang BH. Role and mechanism of arsenic in regulating angiogenesis. PLoS One. 2011;6:e20858. doi: 10.1371/journal.pone.0020858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S, Westermann F, Speleman F, Vandesompele J, Weinberg RA. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat. Cell Biol. 2010;12:247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng D, Wang X, Chang Q, Hitron A, Zhang Z, Xu M, Chen G, Luo J, Jiang B, Fang J, Shi X. Arsenic promotes angiogenesis in vitro via a heme oxygenase-1-dependent mechanism. Toxicol. Appl. Pharmacol. 2010;244:291–299. doi: 10.1016/j.taap.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Pi J, Diwan BA, Sun Y, Liu J, Qu W, He Y, Styblo M, Waalkes MP. Arsenic-induced malignant transformation of human keratinocytes: involvement of Nrf2. Free Radic Biol. Med. 2008;45:651–8. doi: 10.1016/j.freeradbiomed.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman TG, Klein CB. Genetic and epigenetic effects of environmental arsenicals. Metallomics. 2011;3:1135–41. doi: 10.1039/c1mt00074h. [DOI] [PubMed] [Google Scholar]
- Sato M, Vaughan MB, Girard L, Peyton M, Lee W, Shames DS, Ramirez RD, Sunaga N, Gazdar AF, Shay JW, Minna JD. Multiple oncogenic changes (K RAS(V12), p53 knockdown, mutant EGFRs, p16 bypass, telomerase) are not sufficient to confer a full malignant phenotype on human bronchial epithelial cells. Cancer Res. 2006;66:2116–2128. doi: 10.1158/0008-5472.CAN-05-2521. [DOI] [PubMed] [Google Scholar]
- Soucy NV, Ihnat MA, Kamat CD, Hess L, Post MJ, Klei LR, Clark C, Barchowsky A. Arsenic stimulates angiogenesis and tumorigenesis in vivo. Toxicol. Sci. 2003;76:271–279. doi: 10.1093/toxsci/kfg231. [DOI] [PubMed] [Google Scholar]
- Tapio S, Grosche B. Arsenic in the aetiology of cancer. Mutat. Res. 2006;612:215–246. doi: 10.1016/j.mrrev.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Tokar EJ, Diwan BA, Waalkes MP. Renal, hepatic, pulmonary and adrenal tumors induced by prenatal inorganic arsenic followed by dimethylarsinic acid in adulthood in CD1 mice. Toxicol. Lett. 2012;209:179–185. doi: 10.1016/j.toxlet.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyakarnam A, Lenneman AJ, Lakkides KM, Patterson RJ, Wang JL. A comparative nuclear localization study of galectin-1 with other splicing components. Exp. Cell Res. 1998;242:419–28. doi: 10.1006/excr.1998.4111. [DOI] [PubMed] [Google Scholar]
- Wang L, Son YO, Ding S, Wang X, Hitron JA, Budhraja A, Lee JC, Lin Q, Poyil P, Zhang Z, Luo J, Shi X. Ethanol enhances tumor angiogenesis in vitro induced by low-dose arsenic in colon cancer cells through hypoxia-inducible factor 1 alpha pathway. Toxicol. Sci. 2012;130:269–280. doi: 10.1093/toxsci/kfs242. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang Z, Yang J, Fisher T, Xiao H, Jiang Y, Yang C. Akt activation is responsible for enhanced migratory and invasive behavior of arsenic-transformed human bronchial epithelial cells. Environ. Health Perspect. 2012;120:92–97. doi: 10.1289/ehp.1104061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhao Y, Smith E, Goodall GJ, Drew PA, Brabletz T, Yang C. Reversal and Prevention of Arsenic-Induced Human Bronchial Epithelial Cell Malignant Transformation by microRNA-200b. Toxicol. Sci. 2011;121:110–122. doi: 10.1093/toxsci/kfr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat. Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- Xu Y, Li Y, Pang Y, Ling M, Shen L, Yang X, Zhang J, Zhou J, Wang X, Liu Q. EMT and stem cell-like properties associated with HIF-2α are involved in arsenite-induced transformation of human bronchial epithelial cells. PLoS One. 2012;7:e37765. doi: 10.1371/journal.pone.0037765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. Letter to the editor: Response to concerns about arsenic-induced epithelial to mesenchymal transition and malignant transformation of human bronchial epithelial cells. Toxicol. Sci. 2011;122:607–609. [Google Scholar]
- Zerlin M, Julius MA, Kitajewski J. Wnt/Frizzled signaling in angiogenesis. Angiogenesis. 2008;11:63–69. doi: 10.1007/s10456-008-9095-3. [DOI] [PubMed] [Google Scholar]
- Zhao CQ, Young MR, Diwan BA, Coogan TP, Waalkes MP. Association of arsenic-induced malignant transformation with DNA hypomethylation and aberrant gene expression. Proc. Natl. Acad. Sci. U. S. A. 1997;94:10907–10912. doi: 10.1073/pnas.94.20.10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Tan YS, Haslam SZ, Yang C. Perfluorooctanoic acid effects on steroid hormone and growth factor levels mediate stimulation of peripubertal mammary gland development in C57BL/6 mice. Toxicol. Sci. 2010;115:214–224. doi: 10.1093/toxsci/kfq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.