Abstract
Endotoxin treatment of adult rats before hyperoxic exposure significantly increases their survival rate in >95% O2 (J. Clin. Invest.61: 269, 1978). In this study, we wished to determine: (a) whether endotoxin would protect against O2 toxicity if it were administered after the animals were already in >95% O2 for 12-48 h; and (b) the relationship between the endogenous antioxidant enzymes of the lung and the protective effect of endotoxin treatment.
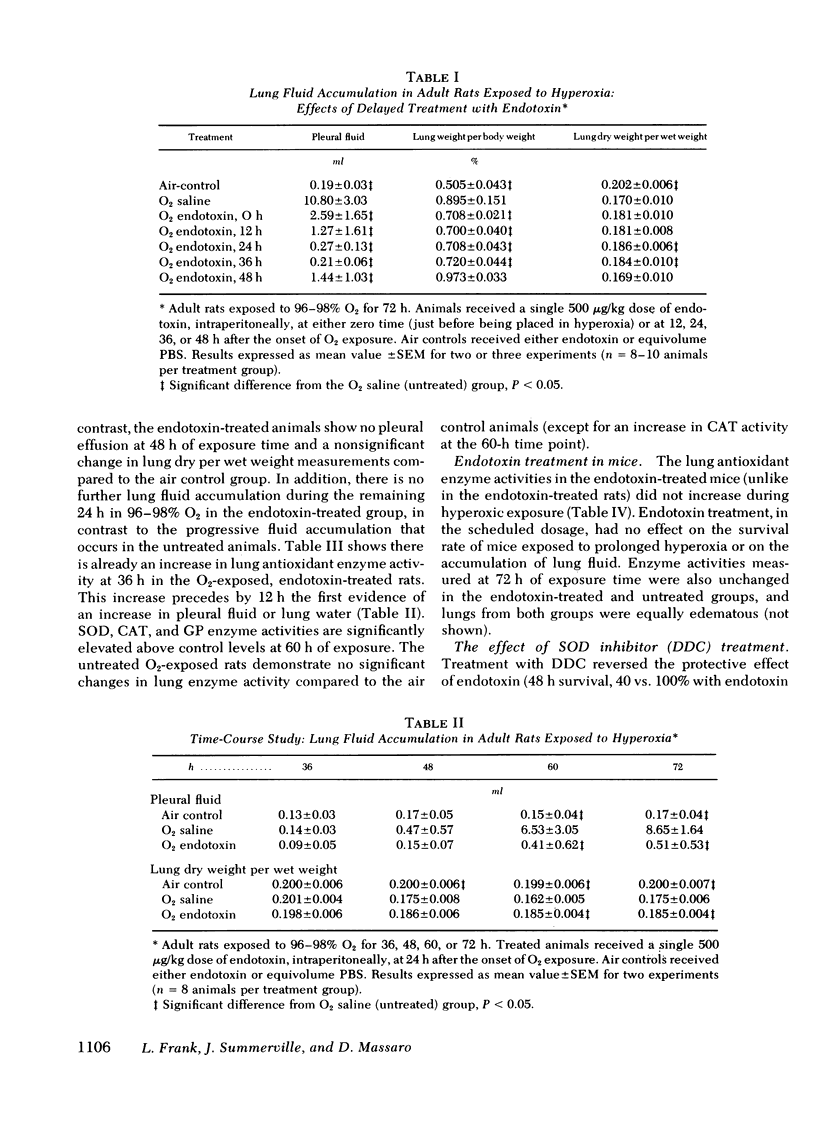
Our results showed that adult rats given a single 500 μg/kg dose of endotoxin up to 36 h after the onset of O2 exposure had significantly increased survival rates and decreased lung fluid accumulation compared to untreated animals in O2 (P < 0.05). (Survival, 16/49 [untreated rats]; 18/20 [endotoxin at 12 h after the start of O2 exposure]; 25/26 [endotoxin-24 h]; 15/20 [endotoxin-36 h].)
Endotoxin-treated animals in O2 showed increases in pulmonary superoxide dismutase, catalase, and glutathione peroxidase activities before the usual time of onset of measurable pulmonary edema in untreated animals in O2. When diethyldithiocarbamate was used to block the superoxide dismutase enzyme rise in the endotoxin-treated rats in O2, the protective action of endotoxin against pulmonary O2 toxicity was nullified. In endotoxin-treated, O2-exposed mice, there were no lung antioxidant enzyme increases, and no protective effect from O2 toxicity was achieved.
We conclude that, in the rat, a single dose of endotoxin given even 36 h after the onset of hyperoxic exposure results in marked protection against O2-induced lung damage; and the increased lung antioxidant enzyme activity in the endotoxin-treated rats appears to be an essential component of this protective action.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry L. J. Bacterial toxins. CRC Crit Rev Toxicol. 1977 Nov;5(3):239–318. doi: 10.3109/10408447709082601. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., McCord J. M. Oxygen-induced changes in pulmonary superoxide dismutase assayed by antibody titrations. Am J Physiol. 1976 Oct;231(4):1196–1203. doi: 10.1152/ajplegacy.1976.231.4.1196. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Tierney D. F. Superoxide dismutase and pulmonary oxygen toxicity. Am J Physiol. 1974 Jun;226(6):1401–1407. doi: 10.1152/ajplegacy.1974.226.6.1401. [DOI] [PubMed] [Google Scholar]
- Deneke S. M., Bernstein S. P., Fanburg B. L. Enhancement by disulfiram (Antabuse) of toxic effects of 95 to 97% O2 on the rat lung. J Pharmacol Exp Ther. 1979 Mar;208(3):377–380. [PubMed] [Google Scholar]
- Frank L., Bucher J. R., Roberts R. J. Oxygen toxicity in neonatal and adult animals of various species. J Appl Physiol Respir Environ Exerc Physiol. 1978 Nov;45(5):699–704. doi: 10.1152/jappl.1978.45.5.699. [DOI] [PubMed] [Google Scholar]
- Frank L., Roberts R. J. Endotoxin protection against oxygen-induced acute and chronic lung injury. J Appl Physiol Respir Environ Exerc Physiol. 1979 Sep;47(3):577–581. doi: 10.1152/jappl.1979.47.3.577. [DOI] [PubMed] [Google Scholar]
- Frank L., Roberts R. J. Oxygen toxicity: protection of the lung by bacterial lipopolysaccharide (endotoxin). Toxicol Appl Pharmacol. 1979 Sep 30;50(3):371–380. doi: 10.1016/0041-008x(79)90389-2. [DOI] [PubMed] [Google Scholar]
- Frank L., Wood D. L., Roberts R. J. Effect of diethyldithiocarbamate on oxygen toxicity and lung enzyme activity in immature and adult rats. Biochem Pharmacol. 1978 Jan 15;27(2):251–254. doi: 10.1016/0006-2952(78)90311-8. [DOI] [PubMed] [Google Scholar]
- Frank L., Yam J., Roberts R. J. The role of endotoxin in protection of adult rats from oxygen-induced lung toxicity. J Clin Invest. 1978 Feb;61(2):269–275. doi: 10.1172/JCI108936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Regulation of the synthesis of superoxide dismutase in Escherichia coli. Induction by methyl viologen. J Biol Chem. 1977 Nov 10;252(21):7667–7672. [PubMed] [Google Scholar]
- Heikkila R. E., Cabbat F. S., Cohen G. In vivo inhibition of superoxide dismutase in mice by diethyldithiocarbamate. J Biol Chem. 1976 Apr 10;251(7):2182–2185. [PubMed] [Google Scholar]
- Holmes R. S., Masters C. J. Epigenetic interconversions of the multiple forms of mouse liver catalase. FEBS Lett. 1970 Nov 9;11(1):45–48. doi: 10.1016/0014-5793(70)80488-4. [DOI] [PubMed] [Google Scholar]
- Moustafa Hassan H., Fridovich I. Regulation of superoxide dismutase synthesis in Escherichia coli: glucose effect. J Bacteriol. 1977 Nov;132(2):505–510. doi: 10.1128/jb.132.2.505-510.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglia D. E., Valentine W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967 Jul;70(1):158–169. [PubMed] [Google Scholar]
- Richards G. M. Modifications of the diphenylamine reaction giving increased sensitivity and simplicity in the estimation of DNA. Anal Biochem. 1974 Feb;57(2):369–376. doi: 10.1016/0003-2697(74)90091-8. [DOI] [PubMed] [Google Scholar]
- Robinson L. A., Wolfe W. G., Salin M. L. Alterations in cellular enzymes and tissue metabolism in the oxygen toxic primate lung. J Surg Res. 1978 May;24(5):359–365. doi: 10.1016/0022-4804(78)90027-6. [DOI] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Shtasel T. F., Berry L. J. Effect of endotoxin and cortisone on synthesis of ribonucleic acid and protein in livers of mice. J Bacteriol. 1969 Mar;97(3):1018–1025. doi: 10.1128/jb.97.3.1018-1025.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G., Winter P. M., Wheelis R. F. Increased normobaric oxygen tolerance of rabbits following oleic acid-induced lung damage. J Appl Physiol. 1973 Sep;35(3):395–400. doi: 10.1152/jappl.1973.35.3.395. [DOI] [PubMed] [Google Scholar]
- Stevens J. B., Autor A. P. Induction of superoxide dismutase by oxygen in neonatal rat lung. J Biol Chem. 1977 May 25;252(10):3509–3514. [PubMed] [Google Scholar]
- Tierney D. F., Ayers L., Kasuyama R. S. Altered sensitivity to oxygen toxicity. Am Rev Respir Dis. 1977 Jun;115(6 Pt 2):59–65. doi: 10.1164/arrd.1977.115.S.59. [DOI] [PubMed] [Google Scholar]
- Yam J., Frank L., Roberts R. J. Oxygen toxicity: comparison of lung biochemical responses in neonatal and adult rats. Pediatr Res. 1978 Feb;12(2):115–119. doi: 10.1203/00006450-197802000-00010. [DOI] [PubMed] [Google Scholar]
- ZWEIFACH B. W. Aspects of comparative physiology of laboratory animals relative to the problem of experimental shock. Fed Proc. 1961 Jul;20(Suppl 9):18–29. [PubMed] [Google Scholar]


