Abstract
OBJECTIVE
To evaluate the relationship between long-term glycemia, traditional cardiovascular disease (CVD) risk factors, and ascending aortic stiffness in type 1 diabetes.
RESEARCH DESIGN AND METHODS
Eight hundred seventy-nine subjects in the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) study were evaluated. The stiffness/distensibility of the ascending thoracic aorta (AA) was measured with magnetic resonance imaging. Associations of AA distensibility and CVD risk factors, mean HbA1c, and cardiovascular complications including macroalbuminuria were assessed using multivariate linear regression models.
RESULTS
The mean age of the subjects was 50 ± 7 years (47% women, mean diabetes duration of 28 years). Over 22 years of follow-up, 27% of participants had cardiovascular complications. After adjusting for gender and cohort, AA distensibility was lower with increasing age, mean systolic blood pressure, LDL, and HbA1c measured over an average of 22 years (−26.3% per 10 years, −11.0% per 10 mmHg SBP, −1.8% per 10 mg/dL of LDL, and −9.3% per unit mean HbA1c [%], respectively). Patients with macroalbuminuria had 25% lower AA distensibility compared with those without (P < 0.0001). Lower AA distensibility also was associated with greater ratio of left ventricular mass to volume (−3.4% per 0.1 g/mL; P < 0.0001).
CONCLUSIONS
Our findings indicate strong adverse effects of hypertension, chronic hyperglycemia and macroalbuminuria on AA stiffness in type 1 diabetes in the DCCT/EDIC cohort.
Increased arterial stiffness is an important marker of increased left ventricular load and an independent predictor of cardiovascular morbidity and mortality both in asymptomatic humans (1) and in disease including renal failure (2), hypertension (3), and diabetes (4). Increased aortic stiffness has been shown to be an independent predictor of 10-year mortality in diabetic patients (5).
Aortic stiffness is a marker of vascular age and is notably greater after the fifth decade of life in healthy men and women (6,7). The main mechanism for age-related aortic stiffening is fracture and fragmentation of elastin fibers with repetitive stretch, leading to the transfer of stress to less extensible collagenous fibers in the arterial wall (8). Arterial–ventricular coupling is an important determinant of circulatory function (9). Aortic stiffness corresponds to a chronic increased afterload leading to concentric left ventricular remodeling and hypertrophy and potentially heart failure (10). Age-related aortic changes are accelerated by cardiovascular disease (CVD) and potentially modifiable cardiovascular risk factors including hypertension (11) and glucose status (12). Age and diabetes have been shown to lead to aortic stiffness through arterial wall glycation processes (13) that potentiate accelerated aortic alterations in younger individuals (12).
The adverse impact of type 1 diabetes on the stiffness/distensibility of large arteries may depend on a number of factors, such as concurrent CVD risk factors, the presence of macrovascular or microvascular complications, and duration of diabetes (5). Whether the degree of chronic glycemic control also affects distensibility is not known. A better understanding of factors that contribute to the development of a less distensible aorta in type 1 diabetes may provide a useful start point to formulate strategies designed to improve arterial health. In this study, we used magnetic resonance imaging (MRI) to characterize aortic distensibility (14) in the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) cohort of patients with type 1 diabetes (15). The DCCT/EDIC cohort is uniquely suited to evaluate risk factors that contribute to decreased aortic distensibility in type 1 diabetes because of its large sample size and long period of close patient follow-up.
RESEARCH DESIGN AND METHODS
Study sample
The DCCT and its follow-up study, the EDIC study, have been previously described in detail (16,17). In brief, between 1983 and 1989, 1,441 patients (aged 13–39 years) with type 1 diabetes were recruited to compare the effects of an intensive insulin therapy with conventional therapy on long-term complications. At baseline, all patients were free of history of CVD, hypertension, and hypercholesterolemia. DCCT participants were recruited into a primary prevention cohort with 1–5 years of diabetes duration and no retinopathy or microalbuminuria at baseline, or into a secondary intervention cohort with 1–15 years of duration, minimal to moderate retinopathy, and albuminuria <200 mg per 24 h at baseline. After the DCCT, all patients were encouraged to continue or implement intensive therapy and returned to their own health care providers for ongoing diabetes care. The EDIC study was designed as a prospective observational follow-up study of the DCCT cohort in 1994. Ninety-six percent (1,375) of the surviving 1,428 participants joined the EDIC study and 1,301 participants were active in EDIC during years 14–16 at the time of the MRI examination. The study was approved by the Institutional Review Boards of all participating centers, and all subjects gave written informed consent.
Study procedures
During DCCT, participants underwent an annual medical history and physical examination, electrocardiography, and laboratory testing for fasting lipid levels, serum creatinine values, and other risk factors for CVD (16). Glycated hemoglobin (HbA1c) values were measured quarterly during DCCT and annually during EDIC (18). During EDIC, the lipid profile and urinary albumin excretion rate were measured in alternate years (17). Weighted mean laboratory values over the study duration for 22 years were computed with weights proportional to the time interval between values because of differences in frequency of measurement during DCCT and EDIC.
Hypertension was defined as blood pressure ≥140/90 mmHg or use of antihypertensive medications (17). Hypercholesterolemia was defined as LDL levels ≥130 mg/dL or use of lipid-lowering medication.
Assessment of diabetes complications
All complications were cumulative from the beginning of DCCT to the current study except for neuropathy. CVD included clinical myocardial infarction (MI; nonfatal) or electrocardiogram (ECG)-diagnosed silent MI. To establish the occurrence of major cardiovascular events, medical records of participants including ECG findings and cardiac enzyme levels were submitted for adjudication to a committee masked to treatment group assignment, HbA1c, and glucose levels. Clinical MI events classified as definite are included in these analyses. Silent MIs were identified based on serial changes in Minnesota codes among all available ECGs during DCCT/EDIC as reported previously (19). ECGs were obtained at baseline, every 2 years during DCCT, at closeout of DCCT, and annually during EDIC. For the current study, retinopathy was defined as any proliferative diabetic retinopathy or worse. Nephropathy included sustained microalbuminuria, defined as urinary albumin excretion rate ≥30 mg/24 h at any two consecutive visits; macroalbuminuria, defined as albumin excretion rate ≥300 mg/24 h at any visit; or end-stage renal disease (dialysis or kidney transplant). Neuropathy included cardiac autonomic neuropathy from autonomic nervous system testing and confirmed clinical neuropathy from nerve conduction testing at EDIC year 13 or 14. Cardiovascular complications were defined as having clinical or silent MI, stroke, peripheral vascular disease, any proliferative diabetic retinopathy, or nephropathy (macroalbuminuria or end-stage renal disease). Macrovascular complications were defined as having clinical/silent MI, stroke, or peripheral vascular disease, and microvascular complications were defined as having any proliferative diabetic retinopathy, macroalbuminuria, end-stage renal disease, or autonomic neuropathy.
Assessment of coronary artery calcium score and common carotid intima-media thickness
Measurements of coronary artery calcium (CAC) score and common carotid intima-media thickness (IMT) have been described (20,21). In brief, CAC score was assessed and quantified from computed tomography scans performed between November 2000 and March 2003 at a single reading center (Harbor UCLA Research and Education Institute, Torrance, CA). CAC score was calibrated according to the readings of phantom using the Agatston score. Common carotid IMT was measured from a single longitudinal lateral view of the distal 10 mm of the right and left common carotid arteries performed between October 1998 and November 2000 at a single reading center (Tufts Medical Center, Boston, MA). The maximum IMT of the common carotid artery was defined as the mean of the maximum IMT for near and far walls on both right and left sides. In both reading centers, readers were unaware of the subjects' clinical information.
MRI
MRI examination was performed for 1,028 subjects at 27 centers between July 2007 and April 2009 with 1.5-T magnets, with the exception of one center that had a 3-T magnet (Espree or Avanto, Siemens Medical Systems, Erlangen, Germany; Intera, Philips Medical Systems, Best, the Netherlands; and Signa, GE Medical Systems, Waukesha, WI). Anterior and posterior surface coils were used for signal reception at all centers.
Phase-contrast cine images of ascending aorta (AA) were obtained in axial plane at the level of the right pulmonary artery with ECG gating (repetition time/echo time, minimized; flip angle, 20 degrees; field of view, 340 × 340 mm; matrix, 256 × 256; maximal velocity encoding, 150 cm/s, through plane; slice thickness, 8 mm; number of phases, 50; temporal resolution, 30 ms; and number of averages, 2). Supine brachial cuff blood pressure was measured immediately before and after the MRI examination on the scanner gantry; the average of two measurements was used as the final blood pressure measurement.
All aortic MRI studies were evaluated and quantified at the reading center (Johns Hopkins University, Baltimore, MD) using QFLOW software (version 5.1; Medis, Leiden, the Netherlands). AA contours were traced at all phases of the cardiac cycle automatically after selecting the center point in the first image. Contours were checked and corrected manually if needed. Minimum and maximum cross-sectional areas were determined. AA distensibility was calculated as follows: [(maximum area − minimum area) / (minimum area × ΔP)] × 1,000, where ΔP is the pulse pressure (12,22). Pulse pressure (millimeters of mercury) was the difference between mean systolic and diastolic blood pressures in the scanner. Noninterpretable 149 scans were excluded. Reasons for exclusion were missing series (2.8%) and anatomic localization errors (4.3%), low image quality (2.3%), and technical failure (5.0%) (Supplementary Figure 1). Quality-control measures included intrareader and inter-reader evaluations. MRIs of 87 participants were read separately by two readers with an inter-reader intraclass correlation of 0.82 (95% CI, 0.74–0.88). Those MRIs also were reread by one reader with an intrareader intraclass correlation of 0.91 (0.86–0.94). MRI analysts were blinded to other study data.
Left ventricular mass, end-diastolic volume, and ejection fraction were obtained from short-axis steady-state free-precession cine images with temporal resolution 30–50 ms as described previously (23).
Statistical analysis
Clinical characteristics of DCCT/EDIC participants, measured immediately before or at the time of MRI scan, are reported as mean ± SD or percentage. Groups of subjects were compared using Wilcoxon rank-sum tests for quantitative variables, and χ2 tests or Fisher exact test was used for categorical variables.
The distribution of AA distensibility was skewed. A natural log transformation was used to obtain homoscedastic and approximately normal residual distribution. The geometric mean of AA distensibility is presented. The association of AA distensibility with risk factors was assessed using multiple linear regression models. Models adjusted for age, gender, cohort (secondary intervention versus primary prevention), and machine type (Siemens versus Philips versus GE). All two-way interactions between covariates were assessed. The percent difference and percent change in AA distensibility were reported for binary and continuous variables, respectively. All analyses were performed using SAS software (version 9.2; SAS Institute, Cary, NC). P < 0.05 was considered statistically significant.
RESULTS
Study population
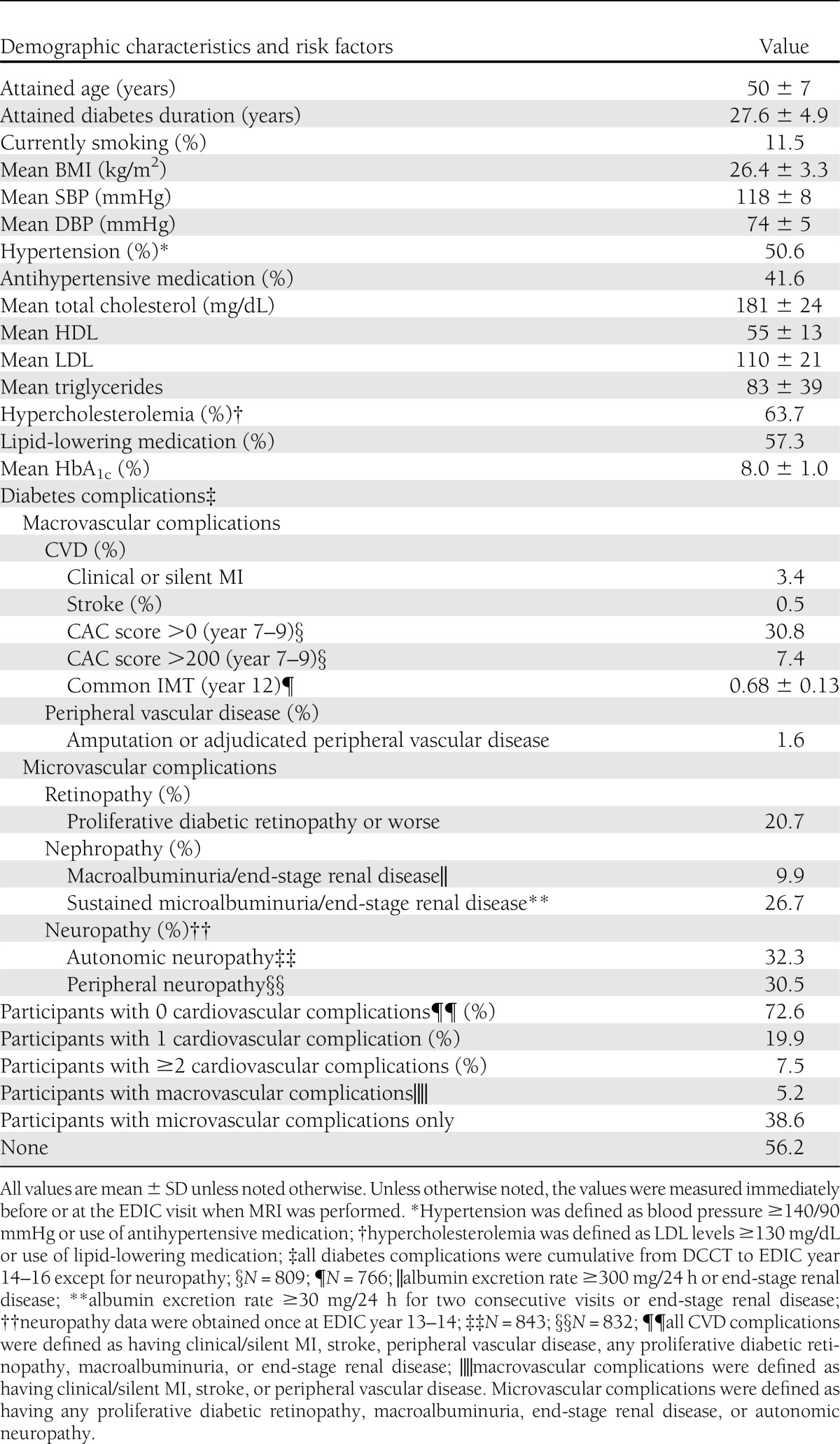
Of the 1,028 DCCT/EDIC participants, 879 (86.4%) had technically acceptable phase-contrast cine aorta MRI (Supplementary Fig. 1). Among the 879 subjects included in these analyses, the mean age at the time of the MRI examination was 50 ± 7 years, 47% were female, and the mean diabetes duration was 28 ± 5 years. Fifty-one percent of the participants were hypertensive, 12% were current smokers, and 64% had hypercholesterolemia. Mean HbA1c was 8.0 ± 1.0%. Thirty-one percent of subjects had a nonzero CAC score and 7.4% of them had a CAC score >200. The mean common carotid IMT was 0.68 ± 0.13 mm; 3.4% of subjects had clinical or silent MI, 0.5% had stroke, 1.6% had peripheral vascular disease, 20.7% had retinopathy, 9.9% had nephropathy with a history of macroalbuminuria or end-stage renal disease, 72.6% of participants had no cardiovascular complications, 19.9% had only one cardiovascular complication, and 7.5% had two or more cardiovascular complications (Table 1).
Table 1.
Clinical characteristics and diabetes complications among 879 EDIC participants
The cardiovascular risk profiles of participants who had technically acceptable aorta MRIs were similar to those of those who agreed to undergo MRI, except they had slightly lower BMI (1.1 kg/m2 less) and less frequent history of clinical or silent MI (not shown).
AA distensibility in relation to age
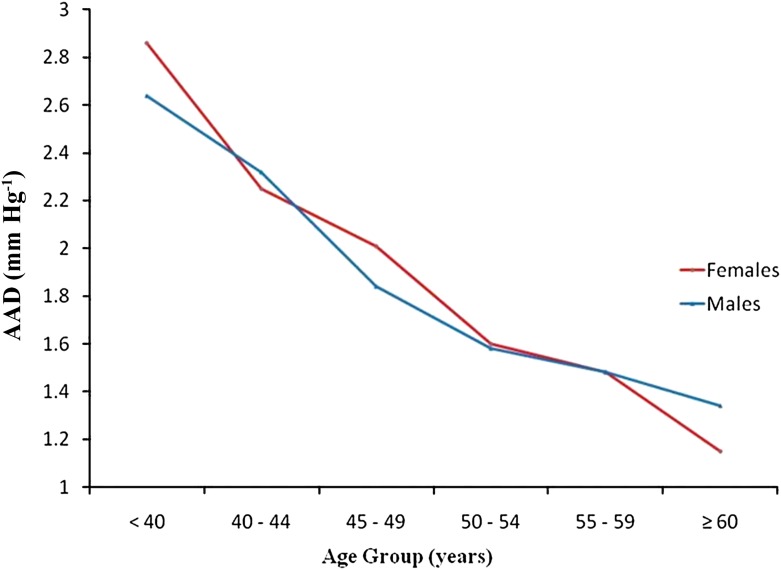
The geometric mean and standard deviation of AA distensibility was 1.8 ± 1.66 mmHg−1. Age, gender, cohort, and machine type accounted for 22% of the variability in AA distensibility. AA distensibility showed a linear decrease with age (Fig. 1), with no gender difference. AA distensibility was 26.3% less per 10 years of older age after adjusting for gender, cohort, and machine type (95% CI, −29.5% to −22.9%; P < 0.0001) (Table 2). The association between age and AA distensibility remained significant after further adjusting for other risk factors (Table 3).
Figure 1.
The geometric mean of ascending aortic distensibility by age in patients with type 1 diabetes of the EDIC study. (A high-quality color representation of this figure is available in the online issue.)
Table 2.
Minimally adjusted linear regression models of AA distensibility in relation to individual CVD risk factors
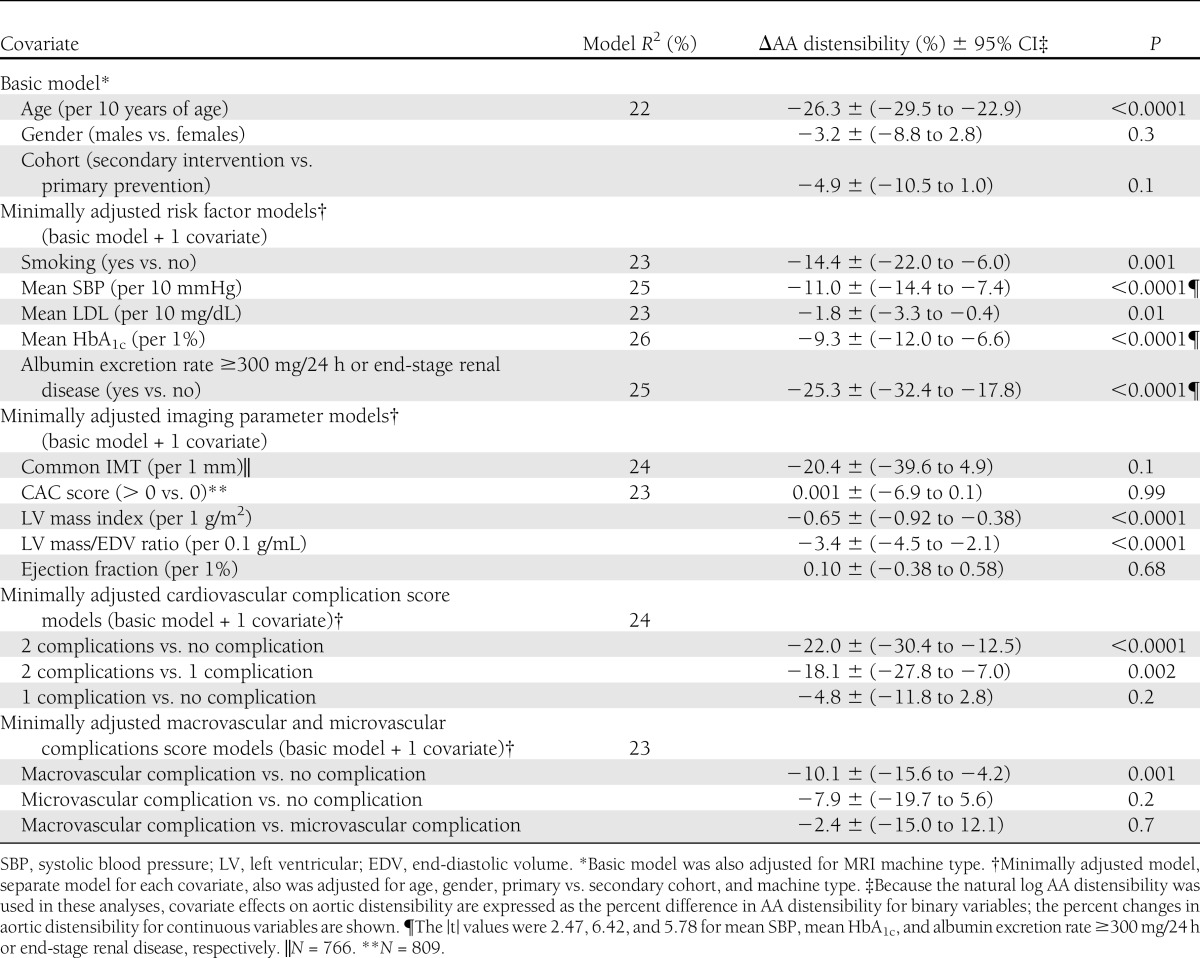
Table 3.
Multivariate linear regression model of AA distensibility in relation to composite CVD risk factors
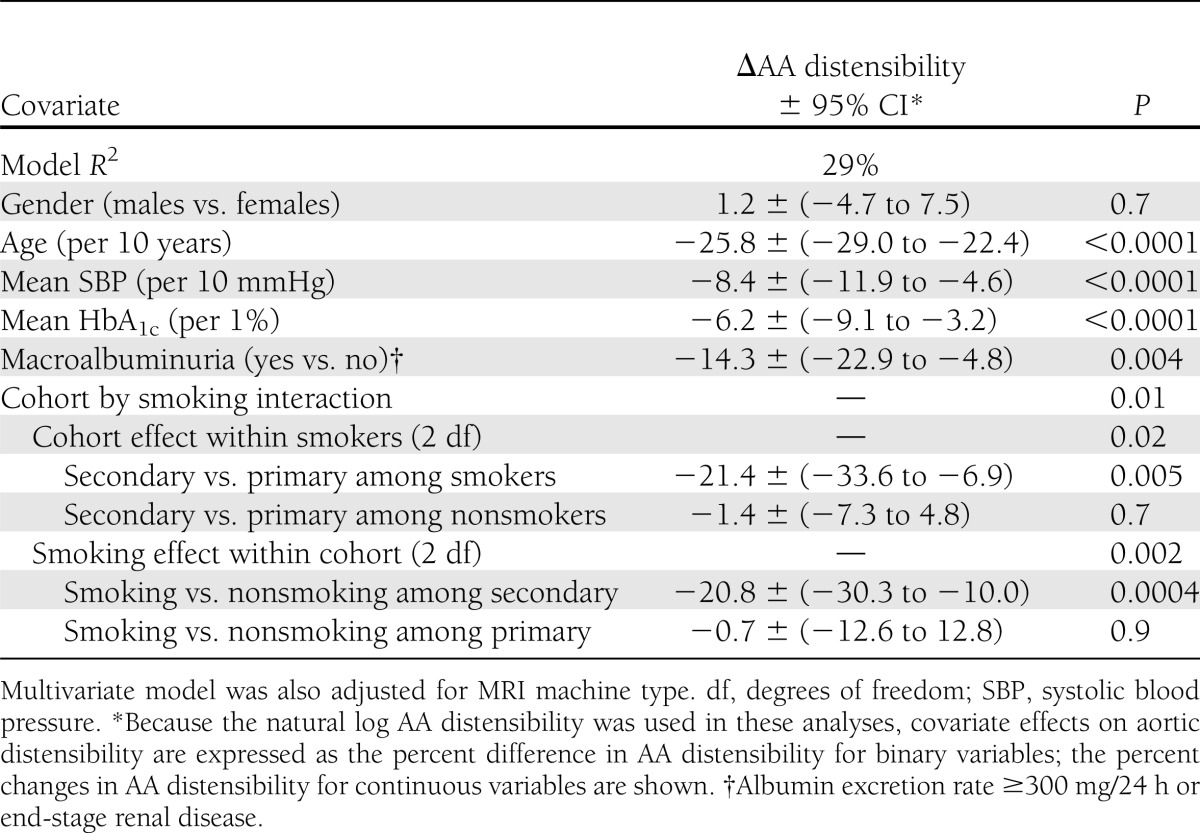
Aortic distensibility in relation to CVD risk factors
Table 2 describes the association of each CVD risk factor separately with AA distensibility when adjusted for basic factors (age, gender, cohort, and machine type). There was no significant association of gender (P = 0.2836) or diabetes duration (P = 0.59) with AA distensibility. AA distensibility was 11% and 1.8% lower per 10-mmHg greater mean systolic blood pressure and per 10 mg/dL greater mean LDL. Mean HbA1c also was inversely associated with AA distensibility (−9.3% lower per-unit increase in HbA1c; 95% CI, −12 to −6.6). The geometric mean values of AA distensibility with HbA1c quartiles and stratified by age groups and by systolic blood pressure quartiles are in line with linear regression models (Supplementary Table 1A and B). Subjects with macroalbuminuria had 25% (95% CI, −32.4 to −17.8) less distensible aortas compared with those without macroalbuminuria. AA distensibility was 14% less for participants who were smokers compared with those who were nonsmokers (Table 2).
Further analysis jointly adjusted for multiple factors (Table 3) confirmed these findings with small changes in the magnitude of covariate effects except for mean LDL, which became nonsignificant. Additional adjustment for CVD complication score had little effect on regression coefficients (not shown). Also, there was a significant interaction between smoking and study cohort. AA distensibility was 20.8% less among smokers than nonsmokers within the secondary intervention cohort (P < 0.001), with no difference within the primary prevention cohort (P = 0.91). All associations remained significant after further adjustments for antihypertensive and lipid-lowering medication use in the model.
Aortic distensibility in relation to left ventricular parameters and indices of atherosclerosis
AA distensibility was 0.7% less per 1 g/m2 greater left ventricular mass and 3.4% per 0.1 g/mL increment in left ventricular mass–to–end-diastolic volume ratio after adjusting for basic factors. These associations remained significant with a decline in the magnitude of covariate effects after additional adjustments for systolic blood pressure, LDL, and mean HbA1c (−0.4% lower per 1 g/m2 greater left ventricular mass; 95% CI, −0.7 to −0.2; −1.7% lower per 0.1 g/mL greater ratio of left ventricular mass to volume; 95% CI, −3.1 to −0.03).
There was no significant association between AA distensibility and common carotid IMT or participants with nonzero CAC score compared with those with zero CAC score and left ventricular ejection fraction (Table 2).
Aortic distensibility in relation to cardiovascular complications
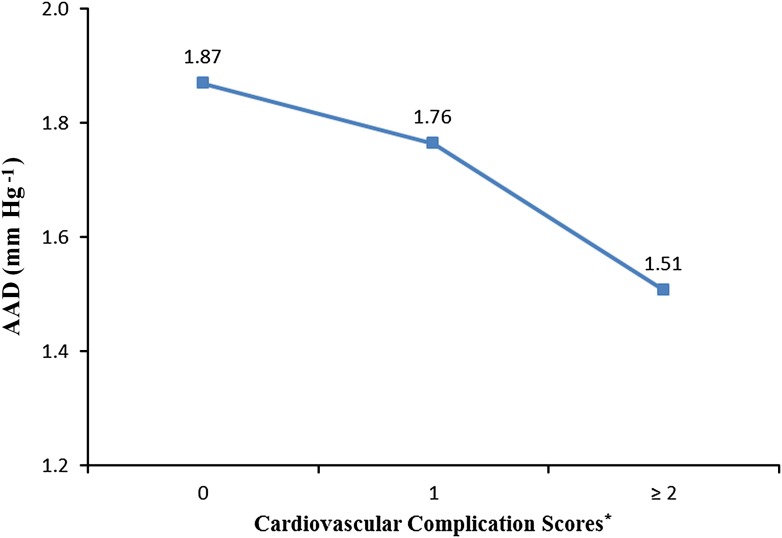
AA distensibility was lower with increasing number of CVD complications after adjusting for basic factors (age, gender, cohort, and machine type; P for trend <0.0001). The decline in aortic distensibility was nonsignificant when we compared patients with one cardiovascular complication with patients with no complications, whereas it became significant when we compared patients with one complication with those with two or more complications (Fig. 2).
Figure 2.
The geometric mean of ascending aortic distensibility by cardiovascular complication scores in patients with type 1 diabetes of the EDIC study. (A high-quality color representation of this figure is available in the online issue.)
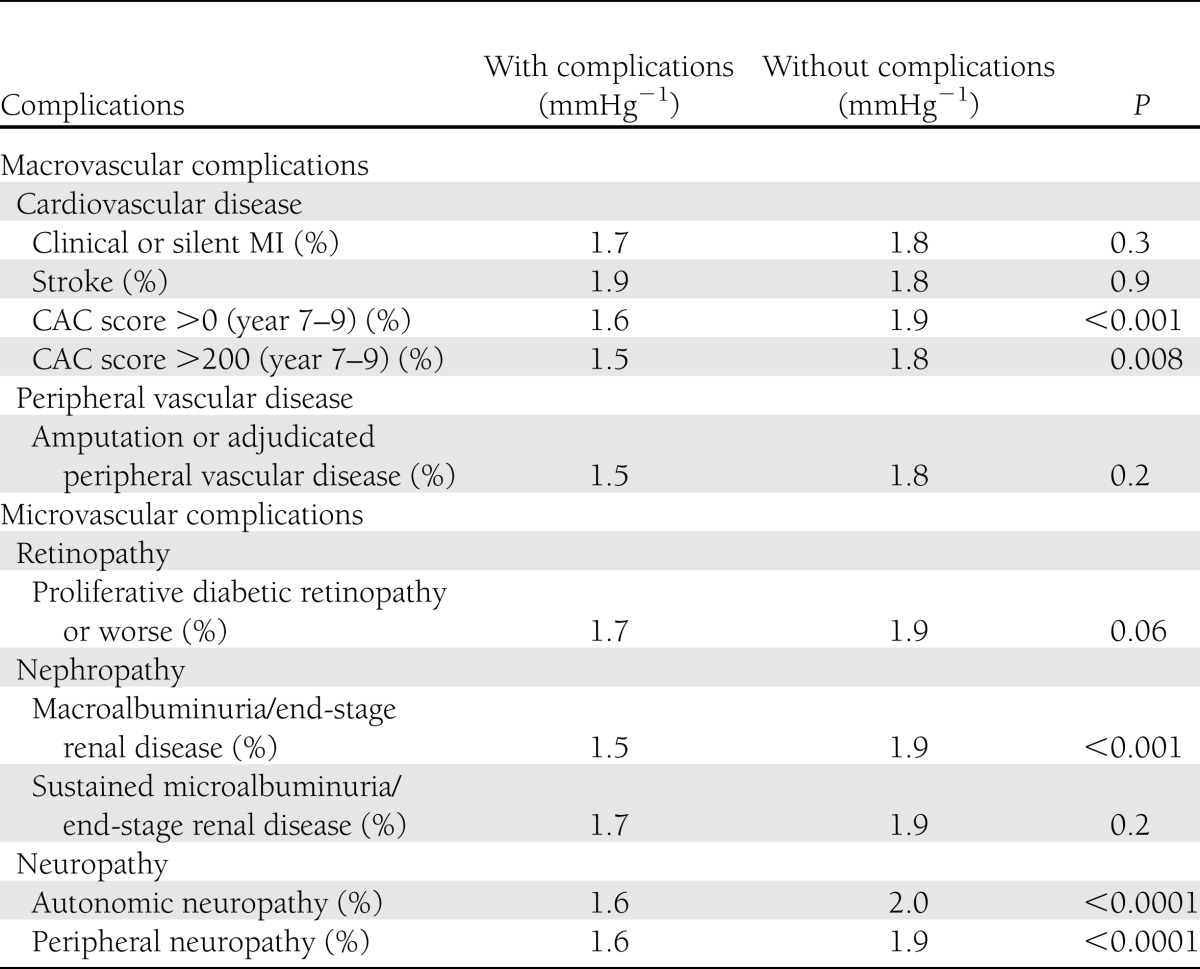
The geometric mean of AA distensibility was significantly lower in patients with nonzero CAC score, CAC score >200, macroalbuminuria, or end-stage renal disease and neuropathy compared with those without (Table 4). After adjusting for basic factors, AA distensibility was 10% lower in patients with macrovascular complications compared with those without, whereas it was not significantly different when comparing participants with microvascular complications versus those with no complications and participants with macrovascular complications versus microvascular complications (Table 2).
Table 4.
Geometric mean of AA distensibility by diabetes complications
CONCLUSIONS
The DCCT/EDIC study is the first to evaluate AA distensibility in patients with type 1 diabetes using MRI. The main conclusions in our type 1 diabetes cohort are as follows: 1) AA distensibility was inversely proportional to age in a dose-related manner for both men and women; 2) elevated HbA1c levels, macroalbuminuria, and blood pressure measured over an average of 22 years were each independently associated with lower AA distensibility; 3) patients with lower AA distensibility had higher left ventricular mass and ratio of end-diastolic mass to volume, confirming the deleterious role of increased left ventricular afterload through arterial–ventricular coupling in patients with type 1 diabetes; and 4) AA distensibility was lower in patients with type 1 diabetes who had a greater number of diabetic cardiovascular complications.
Aortic stiffness increases substantially with advancing age in the general population (15,24). This association is independent of traditional CVD risk factors such as hypertension, diabetes, dyslipidemia, and smoking, suggesting the impact of the aging process itself and potential exposure to undefined nontraditional risk factors. Increased aortic stiffness also is related to hypertension and smoking (15,25). Similar to the general population, the EURODIAB study showed an association between age and pulse pressure (an indirect measure of arterial stiffness) in young type 1 diabetic individuals (mean age, 33 years) (26). Brandts et al. (25) reported a predominant contributive effect of hypertension on aortic stiffness in patients with type 1 diabetes by measuring aortic pulse wave velocity using MRI. Giannattasio et al. (27) reported similar findings in type 1 diabetes by measuring abdominal aortic distensibility. The results of the current study instead indicate that smoking was associated with increased aortic stiffness beyond the effect of hypertension, but only in subjects who had development of microvascular complications (i.e., the secondary prevention cohort). This suggests that smoking may accelerate the development of aortic stiffness in type 1 diabetic patients in the presence of core risk factors that are well-known to cause microvascular damage.
Both aging and diabetes share some common pathophysiologic pathways for aortic stiffening, in part through formation of nonenzymatic glycosylation of proteins (glycation) (13). In addition to glycation, poor glycemic control accelerates the risk of vascular complications in type 1 diabetes, possibly by generating oxidative stress and an inflammatory response (28,29). These mechanisms support the concept of accelerated physiological aging of the cardiovascular system in diabetes. Previous studies supported the same concept by showing elevated arterial stiffness in patients with type 1 diabetes compared with nondiabetic controls in studies with small sample sizes using a variety of methods, including pulse wave analysis, echo-tracking techniques by ultrasound, and aortic pulse wave velocity by MRI (27,30–32). We previously reported higher aortic distensibility in nondiabetic individuals of similar age (mean, 2.25 mmHg−1) than that observed in the current study of patients with type 1 diabetes (mean, 1.8 mmHg−1) (7). In contrast, smaller studies (25,33) reported no difference between aortic stiffness and age in patients with diabetes (30 subjects with a mean age of 26 years and 20 subjects with a mean age of 48 years, respectively). However, the current study is more definitive regarding the distribution of proximal aortic distensibility in type 1 diabetes, encompassing 879 subjects who have been comprehensively studied for >20 years.
The single time point estimate of HbA1c was not associated with aortic stiffness as shown in the EURODIAB prospective diabetes complications study (34). In the current study, we calculated the mean HbA1c value over an average of 22 years. We found that AA distensibility is lower in subjects who have greater mean HbA1c levels. The DCCT/EDIC study has established the role of hyperglycemia in the development and progression of microvascular complications and CVD events (35,36). We found that proximal aortic function is adversely affected in patients with a long history of hyperglycemia with type 1 diabetes mellitus. Interestingly, we found no independent association between imaging biomarkers of atherosclerosis such as carotid IMT and CAC and AA distensibility, suggesting that AA distensibility is not simply a surrogate measure of atherosclerotic burden but rather an integrative measure of large artery alterations strongly influenced by glucose control in patients with diabetes beyond the anticipated effects of age, blood pressure, and other atherosclerosis risk factors.
Because of the coupling of ventricular and aortic function, the left ventricle compensates for the decline in arterial elasticity by adverse concentric remodeling (37). This same pathophysiology is present in individuals with long-standing hypertension (37) and early chronic kidney disease (38). In the EDIC cohort, reduced aortic distensibility also was related to adverse concentric remodeling (increased left ventricular mass-to-volume ratio) in adjusted models. The coupling of arterial stiffness and ventricular concentric remodeling may contribute to greater cardiovascular morbidity and mortality in patients with type 1 diabetes. Therapies that help preserve aortic function thus also may prove beneficial to left ventricular geometry and function and may reduce adverse events in diabetic patients.
Greater aortic stiffness was associated with an increased incidence of cardiovascular complications in our study. This finding is in line with previous studies reporting more pronounced stiffening in patients with diabetes complications such as microalbuminuria, retinopathy, or neuropathy (27,39,40) and parasympathetic function in women only (41). Increased cardiac output and excessive pressure pulsatility associated with diabetes may impair microvascular function, but adverse effects of aortic stiffening on microvascular function also may contribute to the pathogenesis of diabetes-related vascular disease. Specifically, the loss of pulsatile flow damping by the proximal aorta may result in increased structural and functional microvascular damage, particularly in the heart, brain, retina, and kidney. Recent studies have shown the beneficial effect of aerobic exercise in reversing aortic stiffness in older adults with type 2 diabetes and hypertension (42). However, further studies are needed to further define the implications of these relations in diabetes and to determine whether interventions aimed at preventing or reversing arterial stiffening will prevent the morbidity associated with damage in microvascular beds of major target organs.
Study limitations
Patients in DCCT/EDIC were highly motivated and were knowledgeable about their disease condition. They have been followed up for >22 years and closely monitored by trained experts, which may have limited generalizability of results to the general population with type 1 diabetes. During study period, the number of deaths (overall 70 deaths, 17 of them attributable to CVD) was low. Additionally, blood pressure that was used for the calculation of pulse pressure was measured noninvasively in the brachial artery. Although these measurements were used as approximations of the central pressure, previous studies have indicated that such measures are usable (43). The average of two measurements of blood pressure inside the magnet was performed to minimize variability. Aorta MRIs were acquired with free breathing to increase the spatial and temporal resolutions. However, this caused blurring of images in some subjects, resulting in relatively higher rates of technical failure in image analysis and exclusion from analysis (5%). Finally, this cross-sectional study cannot assess temporality or prove causality.
In conclusion, age, blood pressure, elevated mean HbA1c levels, and nephropathy were independently associated with lower AA distensibility in type 1 diabetic patients. These findings indicate a strong role of hyperglycemia and macroalbuminuria in central arterial function that may contribute to other end-organ damage.
Acknowledgments
The DCCT/EDIC project is supported by contracts with the Division of Diabetes, Endocrinology, and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases, National Eye Institute, National Institute of Neurological Disorders and Stroke, the General Clinical Research Centers Program and the Clinical and Translation Science Centers Program, National Center for Research Resources, and by Genentech through a Cooperative Research and Development Agreement with the National Institute of Diabetes and Digestive and Kidney Diseases. The study is also supported in part by the intramural National Institutes of Health research program.
Industry contributors have had no role in the conduct of EDIC but have offered free or discounted supplies or equipment to participants: Abbott Diabetes Care, Animas, Bayer, Becton Dickinson, CanAm, Eli Lilly, Lifescan, Medtronic Diabetes, Omron, OmniPod Insulin Management System, Roche, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
E.B.T., A.R., A.C.S., and D.A.B. contributed to the study design, data interpretation, drafting of the manuscript, critical revision of the manuscript for intellectual content, and final approval of the submitted manuscript. J.-Y.C.B., P.A.C., and J.M.L. contributed to the study design, data analysis, data interpretation, critical revision of the manuscript for intellectual content, and final approval of the submitted manuscript. J.A.C.L. contributed to the study design, data interpretation, critical revision of the manuscript for intellectual content, and final approval of the submitted manuscript. D.A.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This study was presented at the American Heart Association 2011 Scientific Sessions, Orlando, Florida, 12–16 November 2011.
The authors acknowledge the data processing and technical assistance of Wanyu Hsu at The Biostatistics Center, The George Washington University.
Footnotes
A complete list of the members of the DCCT/EDIC Research Group is provided in the Supplementary Appendix published in New England Journal of Medicine (2011;365:2366–2376). A list of the participating radiologists and technologists is provided in Supplementary Material 1 available on the Circulation website.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0393/-/DC1.
Clinical trial reg. nos. NCT00360893 and NCT00360815, clinicaltrials.gov.
References
- 1.Laurent S, Tropeano AI, Boutouyrie P. Pulse pressure reduction and cardiovascular protection. J Hypertens Suppl 2006;24:S13–S8 [DOI] [PubMed]
- 2.Temmar M, Liabeuf S, Renard C, Czernichow S, Esper NE, Shahapuni I, et al. Pulse wave velocity and vascular calcification at different stages of chronic kidney disease. J Hypertension 2010;28:163–169 [DOI] [PubMed]
- 3.Safar ME, Blacher J, Jankowski P. Arterial stiffness, pulse pressure, and cardiovascular disease-is it possible to break the vicious circle? Atherosclerosis 2011;218:263–271 [DOI] [PubMed]
- 4.Theilade S, Lajer M, Jorsal A, Tarnow L, Parving HH, Rossing P. Arterial stiffness and endothelial dysfunction independently and synergistically predict cardiovascular and renal outcome in patients with type 1 diabetes. Diabet Med 2012;29:990–994 [DOI] [PubMed]
- 5.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation 2002;106:2085–2090 [DOI] [PubMed]
- 6.van der Heijden-Spek JJ, Staessen JA, Fagard RH, Hoeks AP, Boudier HA, van Bortel LM. Effect of age on brachial artery wall properties differs from the aorta and is gender dependent: a population study. Hypertension 2000;35:637–642 [DOI] [PubMed] [Google Scholar]
- 7.Redheuil A, Yu WC, Wu CO, Mousseaux E, de Cesare A, Yan R, et al. Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension 2010;55:319–326 [DOI] [PMC free article] [PubMed]
- 8.Benetos A, Waeber B, Izzo J, et al. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: clinical applications. Am J Hypertens 2002;15:1101–1108 [DOI] [PubMed] [Google Scholar]
- 9.Kass DA. Ventricular arterial stiffening: integrating the pathophysiology. Hypertension 2005;46:185–193 [DOI] [PubMed]
- 10.Chen CH, Nakayama M, Nevo E, Fetics BJ, Maughan WL, Kass DA. Coupled systolic-ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol 1998;32:1221–1227 [DOI] [PubMed]
- 11.O’Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens 2002;15:426–444 [DOI] [PubMed] [Google Scholar]
- 12.Stacey RB, Bertoni AG, Eng J, Bluemke DA, Hundley WG, Herrington D. Modification of the effect of glycemic status on aortic distensibility by age in the multi-ethnic study of atherosclerosis. Hypertension 2010;55:26–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertension. 2003;21:3–12 [DOI] [PubMed]
- 14.Metafratzi ZM, Efremidis SC, Skopelitou AS, De Roos A. The clinical significance of aortic compliance and its assessment with magnetic resonance imaging. J Cardiovasc Magn Reson 2002;4:481–491 [DOI] [PubMed] [Google Scholar]
- 15.Malayeri AA, Natori S, Bahrami H, et al. Relation of aortic wall thickness and distensibility to cardiovascular risk factors (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am J Cardiol 2008;102:491–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The DCCT Research Group The Diabetes Control and Complications Trial (DCCT). Design and methodologic considerations for the feasibility phase. Diabetes 1986;35:530–545 [PubMed] [Google Scholar]
- 17.Epidemiology of Diabetes Interventions and Complications (EDIC) Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feasibility of centralized measurements of glycated hemoglobin in the Diabetes Control and Complications Trial: a multicenter study. The DCCT Research Group. Clin Chem 1987;33:2267–2271 [PubMed]
- 19.Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cleary PA, Orchard TJ, Genuth S, Wong ND, Detrano R, Backlund JY, et al. The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes 2006;55:3556–3565 [DOI] [PMC free article] [PubMed]
- 21.Polak JF, Backlund JY, Cleary PA, Harrington AP, O'Leary DH, Lachin JM, et al. Progression of carotid artery intima-media thickness during 12 years in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes 2011;60:607–613 [DOI] [PMC free article] [PubMed]
- 22.Honda T, Yano K, Matsuoka H, Hamada M, Hiwada K. Evaluation of aortic distensibility in patients with essential hypertension by using cine magnetic resonance imaging. Angiology 1994;45:207–212 [DOI] [PubMed] [Google Scholar]
- 23.Turkbey EB, Backlund JY, Genuth S, Jain A, Miao C, Cleary PA, et al. Myocardial structure, function, and scar in patients with type 1 diabetes mellitus. Circulation 2011;124:1737–1746 [DOI] [PMC free article] [PubMed]
- 24.Mitchell GF, Guo CY, Benjamin EJ, et al. Cross-sectional correlates of increased aortic stiffness in the community: the Framingham Heart Study. Circulation 2007;115:2628–2636 [DOI] [PubMed] [Google Scholar]
- 25.Brandts A, van Elderen SG, Tamsma JT, Smit JW, Kroft LJ, Lamb HJ, et al. The effect of hypertension on aortic pulse wave velocity in type-1 diabetes mellitus patients: assessment with MRI. Int J Cardiovasc Imaging 2012;23:543–550 [DOI] [PMC free article] [PubMed]
- 26.Schram MT, Chaturvedi N, Fuller JH, Stehouwer CD. Pulse pressure is associated with age and cardiovascular disease in type 1 diabetes: the Eurodiab Prospective Complications Study. Hypertension 2003;21:2035–2044 [DOI] [PubMed]
- 27.Giannattasio C, Failla M, Piperno A, et al. Early impairment of large artery structure and function in type I diabetes mellitus. Diabetologia 1999;42:987–994 [DOI] [PubMed] [Google Scholar]
- 28.Ceriello A, Kumar S, Piconi L, Esposito K, Giugliano D. Simultaneous control of hyperglycemia and oxidative stress normalizes endothelial function in type 1 diabetes. Diabetes Care 2007;30:649–654 [DOI] [PubMed] [Google Scholar]
- 29.Schalkwijk CG, Poland DC, van Dijk W, et al. Plasma concentration of C-reactive protein is increased in type I diabetic patients without clinical macroangiopathy and correlates with markers of endothelial dysfunction: evidence for chronic inflammation. Diabetologia 1999;42:351–357 [DOI] [PubMed] [Google Scholar]
- 30.Giannattasio C, Failla M, Grappiolo A, Gamba PL, Paleari F, Mancia G. Progression of large artery structural and functional alterations in Type I diabetes. Diabetologia 2001;44:203–208 [DOI] [PubMed] [Google Scholar]
- 31.Wilkinson IB, MacCallum H, Rooijmans DF, et al. Increased augmentation index and systolic stress in type 1 diabetes mellitus. QJM 2000;93:441–448 [DOI] [PubMed] [Google Scholar]
- 32.van Elderen SG, Westenberg JJ, Brandts A, van der Meer RW, Romijn JA, Smit JW, et al. Increased aortic stiffness measured by MRI in patients with type 1 diabetes mellitus and relationship to renal function. AJR Am J Roentgenol. 2011;196:697–701 [DOI] [PubMed]
- 33.Kool MJ, Lambert J, Stehouwer CD, Hoeks AP, Struijker Boudier HA, Van Bortel LM. Vessel wall properties of large arteries in uncomplicated IDDM. Diabetes Care 1995;18:618–624 [DOI] [PubMed] [Google Scholar]
- 34.Schram MT, Schalkwijk CG, Bootsma AH, Fuller JH, Chaturvedi N, Stehouwer CD, EURODIAB Prospective Complications Study Group Advanced glycation end products are associated with pulse pressure in type 1 diabetes: the EURODIAB Prospective Complications Study. Hypertension 2005;46:232–237 [DOI] [PubMed] [Google Scholar]
- 35.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 36.The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med 2000;342:381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chantler PD, Lakatta EG. Arterial-ventricular coupling with aging and disease. Front Physiol 2012;3:90 [DOI] [PMC free article] [PubMed]
- 38.Edwards NC, Ferro CJ, Townend JN, Steeds RP. Aortic distensibility and arterial-ventricular coupling in early chronic kidney disease: a pattern resembling heart failure with preserved ejection fraction. Heart 2008;94:1038–1043 [DOI] [PubMed]
- 39.Secrest AM, Marshall SL, Miller RG, Prince CT, Orchard TJ. Pulse wave analysis and cardiac autonomic neuropathy in type 1 diabetes: A report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Technol Ther 2011;13:1264–1268 [DOI] [PMC free article] [PubMed]
- 40.Prince CT, Secrest AM, Mackey RH, Arena VC, Kingsley LA, Orchard TJ. Cardiovascular autonomic neuropathy, HDL cholesterol, and smoking correlate with arterial stiffness markers determined 18 years later in type 1 diabetes. Diabetes Care 2010;33:652–657 [DOI] [PMC free article] [PubMed]
- 41.Ahlgren AR, Sundkvist G, Wollmer P, Sonesson B, Länne T. Increased aortic stiffness in women with type 1 diabetes mellitus is associated with diabetes duration and autonomic nerve function. Diabet Med 1999;16:291–297 [DOI] [PubMed] [Google Scholar]
- 42.Madden KM, Lockhart C, Cuff D, Potter TF, Meneilly GS. Short-term aerobic exercise reduces arterial stiffness in older adults with type 2 diabetes, hypertension, and hypercholesterolemia. Diabetes Care 2009;32:1531–1535 [DOI] [PMC free article] [PubMed]
- 43.Fagard RH, Pardaens K, Staessen JA, Thijs L. The pulse pressure-to-stroke index ratio predicts cardiovascular events and death in uncomplicated hypertension. J Am Coll Cardiol 2001;38:227–231 [DOI] [PubMed] [Google Scholar]