Abstract
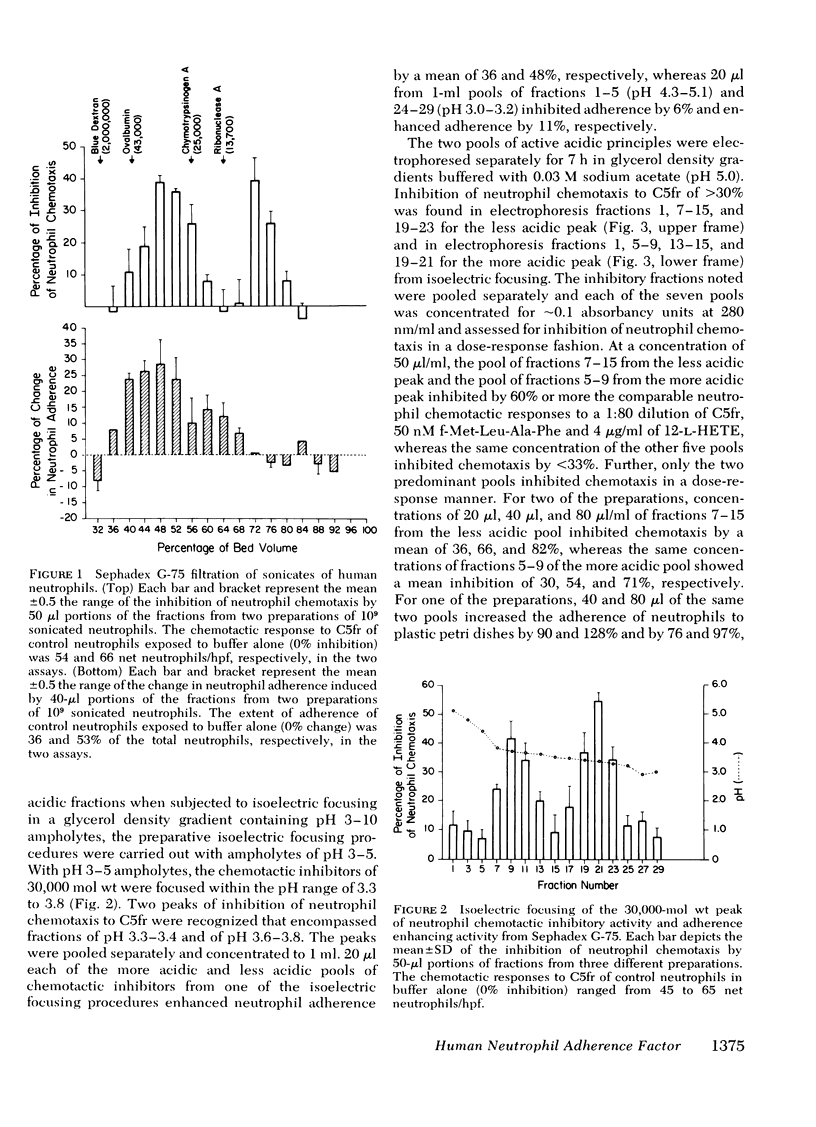
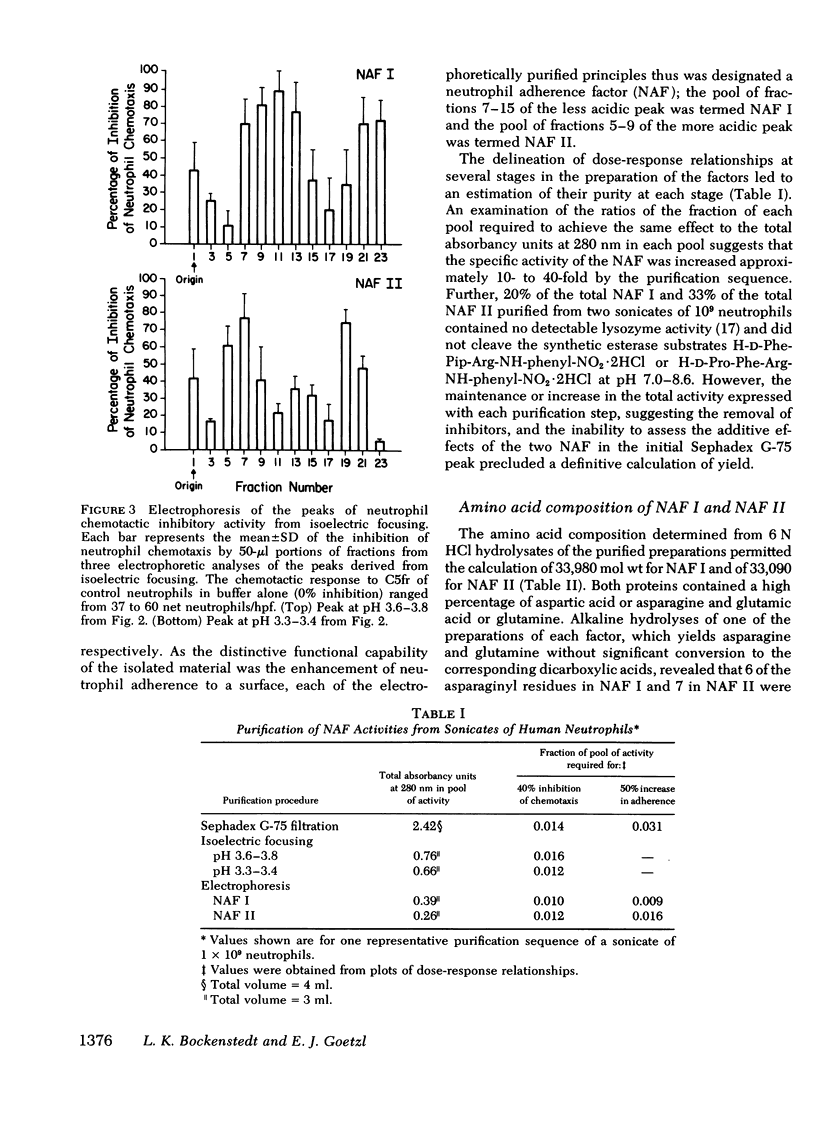
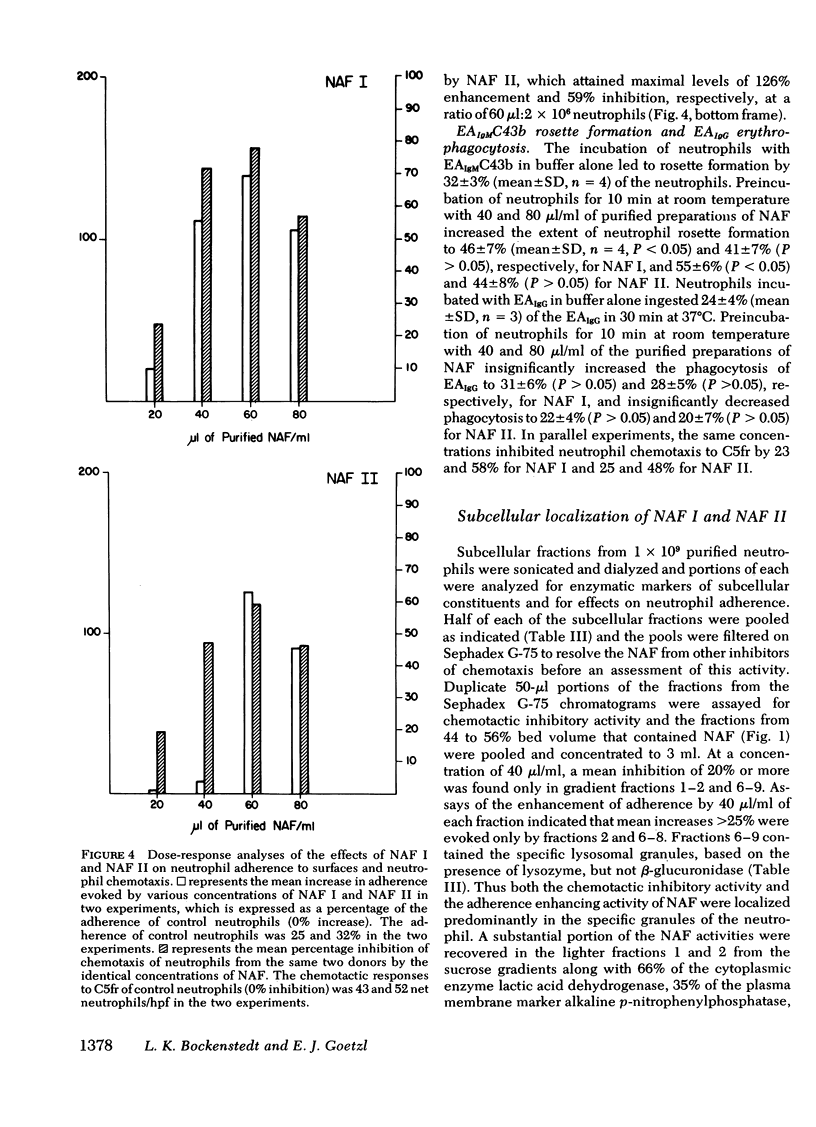
The endogenous constituents of human neutrophils that enhance the adherence of the neutrophils to surfaces have been isolated from sonicates of purified neutrophils. The predominant adherence-enhancing activity in the neutrophil sonicates cofiltered on Sephadex G-75 with a major peak of chemotactic inhibitory activity and exhibited ∼30,000 mol wt. Sequential isoelectric focusing and electrophoresis in glycerol gradients of the 30,000-mol wt activities resolved two distinct acidic protein with isoelectric points of 3.6-3.8 and 3.3-3.4 that were designated the neutrophil adherence factor (NAF) I and II, respectively. Glutamic acid and aspartic acid together accounted for a total of 18 and 19% of the amino acids in purified preparations of NAF I and NAF II, respectively, whereas the basic amino acids lysine, arginine, and histidine represented <2 and 3% of the total residues. The preincubation of portions of 2 × 106 neutrophils with as little as 6 pmol of NAF I or 9 pmol of NAF II enhanced adherence to plastic petri dishes and inhibited chemotactic migration to a maximal extent, with comparable dose-response relationships for the two effects. Neither of the NAF was cytotoxic, exhibited substantial neutrophil chemotactic or chemokinetic activity, or influenced the phagocytosis of sheep erythrocytes sensitized with immunoglobulin (Ig)G. Analyses of subcellular fractions of neutrophils indicated that the NAF are contained predominantly in the specific granules. These distinctive acidic proteins of the specific granules of human neutrophils represent a new class of endogenous constituents that may regulate the involvement of neutrophils in inflammation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYDEN S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962 Mar 1;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E. L., Showell H. J., Henson P. M., Hsu L. S. The ability of chemotactic factors to induce lysosomal enzyme release. I. The characteristics of the release, the importance of surfaces and the relation of enzyme release to chemotactic responsiveness. J Immunol. 1974 Jun;112(6):2047–2054. [PubMed] [Google Scholar]
- Bretz U., Baggiolini M. Biochemical and morphological characterization of azurophil and specific granules of human neutrophilic polymorphonuclear leukocytes. J Cell Biol. 1974 Oct;63(1):251–269. doi: 10.1083/jcb.63.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of leucocytes from human blood. Further observations. Methylcellulose, dextran, and ficoll as erythrocyteaggregating agents. Scand J Clin Lab Invest Suppl. 1968;97:31–50. [PubMed] [Google Scholar]
- Fehr J., Dahinden C. Modulating influence of chemotactic factor-induced cell adhesiveness on granulocyte function. J Clin Invest. 1979 Jul;64(1):8–16. doi: 10.1172/JCI109466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigli I., Wintroub B. U., Goetzl E. J. A phagocytosis-enhancing factor in human plasma. Immunology. 1976 Jun;30(6):915–924. [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. A neutrophil-immobilizing factor derived from human leukocytes. I. Generation and partial characterization. J Exp Med. 1972 Dec 1;136(6):1564–1580. doi: 10.1084/jem.136.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Brash A. R., Tauber A. I., Oates J. A., Hubbard W. C. Modulation of human neutrophil function by monohydroxy-eicosatetraenoic acids. Immunology. 1980 Apr;39(4):491–501. [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Gigli I., Wasserman S., Austen K. F. A neutrophil immobilizing factor derived from human leukocytes. II. Specificity of action on polymorphonuclear leukocyte mobility. J Immunol. 1973 Sep;111(3):938–945. [PubMed] [Google Scholar]
- Goetzl E. J., Rocklin R. E. Amplification of the activity of human leukocyte inhibitory factor (LIF) by the generation of a low molecular weight inhibitor of PMN leukocyte chemotaxis. J Immunol. 1978 Sep;121(3):891–896. [PubMed] [Google Scholar]
- Gordon L. I., Douglas S. D., Kay N. E., Yamada O., Osserman E. F., Jacob H. S. Modulation of neutrophil function by lysozyme. Potential negative feedback system of inflammation. J Clin Invest. 1979 Jul;64(1):226–232. doi: 10.1172/JCI109443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugli T. E., Moore S. Determination of the tryptophan content of proteins by ion exchange chromatography of alkaline hydrolysates. J Biol Chem. 1972 May 10;247(9):2828–2834. [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Lachmann P. J., Kay A. B., Thompson R. A. The chemotactic activity for neutrophil and eosinophil leucocytes of the trimolecular complex of the fifth, sixth and seventh components of human complement (C567) prepared in free solution by the 'reactive lysis' procedure. Immunology. 1970 Dec;19(6):895–899. [PMC free article] [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., McCormack R. T., Fiegel V. D., Simmons R. L. Chemotactic deactivation of human neutrophils: evidence for nonspecific and specific components. Infect Immun. 1978 Nov;22(2):441–444. doi: 10.1128/iai.22.2.441-444.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson I., Venge P. Cationic proteins of human granulocytes. II. Separation of the cationic proteins of the granules of leukemic myeloid cells. Blood. 1974 Aug;44(2):235–246. [PubMed] [Google Scholar]
- SMOLELIS A. N., HARTSELL S. E. The determination of lysozyme. J Bacteriol. 1949 Dec;58(6):731–736. doi: 10.1128/jb.58.6.731-736.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showell H. J., Freer R. J., Zigmond S. H., Schiffmann E., Aswanikumar S., Corcoran B., Becker E. L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976 May 1;143(5):1154–1169. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C., Patrick R. A., Hassett C. Motility and adhesiveness in human neutrophils. Effects of chemotactic factors. J Clin Invest. 1979 Feb;63(2):221–229. doi: 10.1172/JCI109293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber A. I., Goetzl E. J. Structural and catalytic properties of the solubilized superoxide-generating activity of human polymorphonuclear leukocytes. Solubilization, stabilization in solution, and partial characterization. Biochemistry. 1979 Dec 11;18(25):5576–5584. doi: 10.1021/bi00592a009. [DOI] [PubMed] [Google Scholar]
- Venge P. Kinetic studies of cell migration in a modified Boyden chamber: dependence on cell concentration and effects of the chymotrypsin-like cationic protein of human granulocytes. J Immunol. 1979 Apr;122(4):1180–1184. [PubMed] [Google Scholar]
- Ward P. A., Becker E. L. The deactivation of rabbit neutrophils by chemotactic factor and the nature of the activatable esterase. J Exp Med. 1968 Apr 1;127(4):693–709. doi: 10.1084/jem.127.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]


