Abstract
Venous thromboembolism (VTE) is a frequent complication of cancer and cancer treatment and is associated with multiple clinical consequences including recurrent VTE, bleeding and an increase in risk of death. While the risks associated with VTE has been well recognized in surgical cancer patients, there is also considerable and increasing risk in medical cancer patients. VTE risk factors in medical cancer patients include the type and stage of cancer, major comorbid illnesses, current hospitalization, active chemotherapy, hormonal therapy, and antiangiogenic agents. Low-molecular-weight heparins (LMWHs) are commonly recommended for the prevention of VTE in hospitalized cancer patients and higher-risk ambulatory cancer patients due to their favorable risk-to-benefit profile. These agents have been shown to be effective in both the primary and secondary prevention of VTE in medical cancer patients. Extended-duration anticoagulant therapy is often recommended to reduce the risk of VTE recurrence in patients with cancer. LMWHs are often utilized for long-term prophylaxis due to a reduced need for coagulation monitoring, few major bleeding episodes, and once-daily dosing. Despite clinical and practical benefits, a substantial proportion of medical cancer patients do not receive VTE prophylaxis. To improve the appropriate prevention and treatment of VTE in cancer patients, guidelines have been published recently by the American Society of Clinical Oncology and the National Comprehensive Cancer Network. Widespread dissemination and application of these guidelines are encouraged to improve the appropriate use of these agents and improve clinical outcomes in medical cancer patients at risk for VTE and its complications.
Condensed abstract
To improve appropriate prevention of venous thromboembolism in cancer patients and clinical outcomes widespread dissemination and utilization of evidence-based guidelines such as those from the American Society of Clinical Oncology and the National Comprehensive Cancer Network are needed. Low-molecular-weight heparins are commonly recommended for the prevention of venous thromboembolism in hospitalized cancer patients and higher-risk ambulatory cancer patients.
Keywords: venous thromboembolism, cancer, anticoagulation, low-molecular-weight heparin, prophylaxis
INTRODUCTION
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a frequent complication of cancer and cancer treatment. VTE in patients with cancer is strongly associated with reduced survival,1-3 such that these patients are over three times more likely to die within 6 months of VTE, compared with patients with VTE without cancer.1,4 Furthermore, cancer diagnosed at the same time or within a year of a VTE event is more often associated with advanced stage and poor prognosis compared with cancer patients diagnosed without a preceding VTE event.2 Cancer patients with VTE are also more likely to develop recurrent VTE and major bleeding during anticoagulant treatment compared with VTE patients without malignancy.5
The low-molecular-weight heparins (LMWHs) have been shown to reduce the incidence of VTE and prevent recurrent VTE events in cancer patients.6-8 LMWH is frequently prescribed for the treatment and secondary prevention of VTE in patients with cancer due to the favorable benefit-to-risk profile and minimal requirement for monitoring.9-13 While still requiring further investigation, several randomized clinical trials have also suggested that LMWH may improve survival in certain populations of cancer patients.14-19
An association between active cancer, increased risk for VTE and the need for thromboprophylaxis is commonly acknowledged in cancer patients undergoing major surgical procedures. However, the incidence and impact of VTE are also considerable in patients undergoing non-surgical cancer treatment.13,20,21 Hospitalized neutropenic patients with cancer and VTE have a greater mortality rate compared with cancer patients without VTE (odds ratio [OR] = 2.01; 95% confidence interval [CI] 1.83-2.22; P < .0001), with a similar increased risk in patients with either non-metastatic or metastatic disease.22 In a recent study of ambulatory patients with cancer receiving chemotherapy, the cumulative risk of VTE was approximately 4% and its occurrence was a significant independent predictor for early mortality in multivariate analysis.4 Clinical risk models and new genetic and molecular biomarkers are under active investigation in an effort to better identify cancer patients undergoing medical treatment at increased risk for VTE and may be candidates for thromboprophylaxis in the ambulatory setting. This review will discuss the incidence and risk factors associated with VTE in medical patients with cancer, discuss the appropriate indications for LMWH thromboprophylaxis and highlight the current underutilization of VTE prophylaxis in cancer patients at increased risk.
INCIDENCE OF VTE IN MEDICAL PATIENTS WITH CANCER
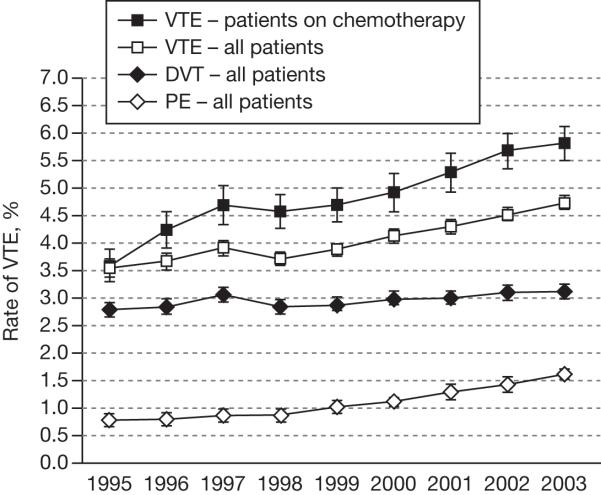
Clinically detectable VTE is diagnosed in approximately 15% of all cancer patients, and the number is likely to be even higher when subclinical thromboembolism is taken into account.23 Several studies have investigated the clinical incidence of VTE in medical cancer patients.22,24,25 In a large retrospective study of patients hospitalized between 1979 and 1999 (n = 40,787,000), the rate of VTE was 2% in patients with cancer compared with 1% in similar patients without malignancy.24 In an analysis of neutropenic cancer patients hospitalized between 1995 and 2002, 5.4% of patients developed VTE during their first hospitalization and rates increased significantly over the study period.21 Khorana and colleagues also noted the increasing frequency of VTE in a recent study of 1,015,598 hospitalized cancer patients included in the discharge database of the University HealthSystem Consortium.25 The proportion of patients with VTE increased from 3.6% per hospitalization in 1995, to 4.6% in 2002-2003, a rise of 28% (P < .0001; Fig. 1). A near doubling of the rate of PE in this study was reported from 0.8% in 1995 to 1.5% in 2002-2003.
FIGURE 1.
The rate of venous thromboembolism (VTE) over time in patients with cancer (adapted from Khorana AA et al. 2007).25 Error bars represent 95% confidence intervals. DVT, deep vein thrombosis; PE, pulmonary embolism.
RISK FACTORS FOR VTE IN MEDICAL CANCER PATIENTS
Identifying clinical characteristics that place cancer patients at increased risk of initial and recurrent VTE and its complications is important if their outcomes are to be improved. The main risk factors for VTE in cancer patients can be categorized as either related to individual patient’s characteristics, to the presence of cancer itself, or to the cancer treatment received (Table 1).13
TABLE 1. Rick Factors for Venous Throm boem bolism (VTE) in Patients with Cancer.
From Lyman GH, Khorana AA, Falanga A, et al. J Clin Oncol. 2007;25: 5490-5505.13 Adapted with permission. © 2007 American Society of Clinical Oncology. All rights reserved.
| Patient-Related Factors |
| Advanced age |
| Ethnicity (higher in AfricanAmericans; lower in Asian-Pacific Islanders) |
| Co-morbid conditions (obesity, infection, renal disease, pulmonary disease, arterial thromboembolism) |
| Prior history of VTE |
| Elevated pre-chemotherapy platelet count |
| Heritable prothrombotic mutations |
| Cancer-Related Factors |
| Primary site of cancer (gastrointestinal, brain, lung, gynecological, renal, hematological) |
| Initial 3-6 months after diagnosis |
| Current metastatic disease |
| Treatment-Related Factors |
| Recent major surgery |
| Current hospitalization |
| Active chemotherapy |
| Active hormonal therapy |
| Current or recent angiogenic therapy (thalidomide, lenalidomide, bevacizumab*) |
| Current erythropoiesis-stimulating agents |
| Presence of central venous catheters |
Bevacizumab is clearly associated with an increased risk of arterial thrombotic events; an association with venous thrombosis is not fully established.
In the study of hospitalized cancer patients by Khorana et al., advanced age (≥ 65 years), female gender, and black ethnicity were patient-related risk factors associated with VTE in multivariate analysis (P < .0001).25 The presence of co-morbid conditions, in particular neutropenic complications, infection, obesity, anemia, pulmonary disease and renal disease, also contribute to the risk of VTE.25 Other patient-related risk factors include prior history of VTE or inherited prothrombotic mutations, such as factor V Leiden and prothrombin 20210A mutations.26
The primary site of cancer is an important risk factor for VTE, with high-rates of VTE reported in hospitalized patients with cancer of the pancreas (8.1%), kidney (5.6%), ovary (5.6%), lung (5.1%), stomach (4.9%), and brain (4.7%).25 A high incidence of VTE has also been reported in patients with hematological malignancies, such as multiple myeloma (5.0%), non-Hodgkin lymphoma (4.8%), and Hodgkin disease (4.6%).25 Patients with cancer have a particularly increased risk of VTE in the first few months after diagnosis and when distant metastases are present.26,27
Active medical treatments, such as chemotherapy and hormone therapy, have been shown to increase the risk of VTE including the risk of recurrent VTE. A population survey demonstrated a 6-fold increase in the risk of VTE in cancer patients undergoing chemotherapy, compared with a 4-fold increase in cancer patients without chemotherapy.20 Moreover, the risk of recurrent VTE increased by over 4-fold in cancer patients receiving chemotherapy and 2-fold in those without chemotherapy.28 It has been estimated that cancer patients receiving chemotherapy represent 12% of the total cases of VTE in the community whereas cancer patients not receiving chemotherapy constitute some 6%.29
Furthermore, the rate of VTE in patients receiving chemotherapy in the ambulatory setting has reportedly increased over time from 3.9% in 1995, to 5.7% in 2002-2003, a significant rise of 46% (P < .0001).25 Although the reasons for this increase are largely unknown, they may relate, in part, to the increased utilization and sensitivity of imaging procedures used in staging cancer patients, as well as the use of more intensive combination systemic therapies for the treatment of cancer.30,31 The observed rise in VTE events may also relate, in part, to the introduction of new antiangiogenic cancer treatments, such as bevacizumab and thalidomide or lenalidomide-based regimens, which have been shown to increase the risk of VTE complications.32,33 In a meta-analysis of the use of thalidomide in patients with multiple myeloma, thalidomide and dexamethasone were found to increase the risk of VTE by 2.6 and 2.8 times, respectively; whereas the combination of agents along with chemotherapy and corticosteroids increased VTE risk 8-fold.31 An increased risk of VTE has also been demonstrated in cancer patients treated with hormonal therapy. VTE rates as high as 8% have been reported in a meta-analysis of patients with breast cancer treated with tamoxifen.34 Supportive treatment with the erythropoiesis-stimulating agents, epoetin and darbepoetin, has been associated with an increase in VTE (relative risk = 1.67; 95% CI 1.35-2.06).35 However, a recent study of 504,208 cancer patients hospitalized between 1995 and 2003 demonstrated an increased risk of VTE with a diagnosis of anemia (OR = 1.24; 95% CI 1.19-1.29; P < .001) as well as with the use of red blood cell transfusions (OR = 1.60; 95% CI 1.53-1.67; P < .001) following adjustment for other risk factors for VTE.36
A simple risk model for predicting VTE has been developed using data from a multicenter prospective observational study of ambulatory cancer outpatients receiving chemotherapy, using baseline clinical and laboratory variables.27 Risk factors for VTE were studied in a randomly selected development cohort of 2701 ambulatory patients with cancer. A risk score for VTE was derived in this population and then validated in a separate group of 1365 patients from the same study. Five predictive variables were identified as significant independent risk factors for VTE in a multivariate model as shown in Table 2.27 Three risk categories were defined based on the score from the risk model (low [0], intermediate [1-2], and high [≥ 3]). The observed rates of VTE according to risk category were found to be similar in the development and validation cohorts, with approximately 7% of patients deemed at high-risk of VTE (Fig. 2).27 The risk model may be used by clinicians to assess risk for VTE in clinical practice and also in the selection of cancer outpatients for trials of thromboprophylaxis. Additional investigation is underway to prospectively validate this risk model as well as demonstrate the influence of prophylactic anticoagulation on rates of VTE in high-risk ambulatory cancer patients. The potential impact of adding various biomarkers such as tissue factor on the predictive accuracy of the model are also being studied. The VTE risk score has also recently been demonstrated to discriminate patients at low, intermediate and high risk for early all-cause mortality in ambulatory cancer patients receiving chemotherapy.37
TABLE 2. Predictive Model for Chemotherapy-Associated Venous Thromboembolism.
Adapted with permission from Khorana AA et al. Blood. 2008;111:4902-4907.27 © the American Society of Hematology.
| Risk score | |
|---|---|
| Site of cancer | |
| Very high-risk (stomach, pancreas) | 2 |
| High risk (lung, lymphoma, gynecological, bladder, testicular) | 1 |
| Pre-chemotherapy platelet count ≥ 350 × 109/L | 1 |
| Hemoglobin < 100 g/L or use of red blood cell growth factors | 1 |
| Pre-chemotherapy leukocyte count > 11 × 109/L | 1 |
| Body mass index ≥ 35 kg/m2 | 1 |
FIGURE 2.
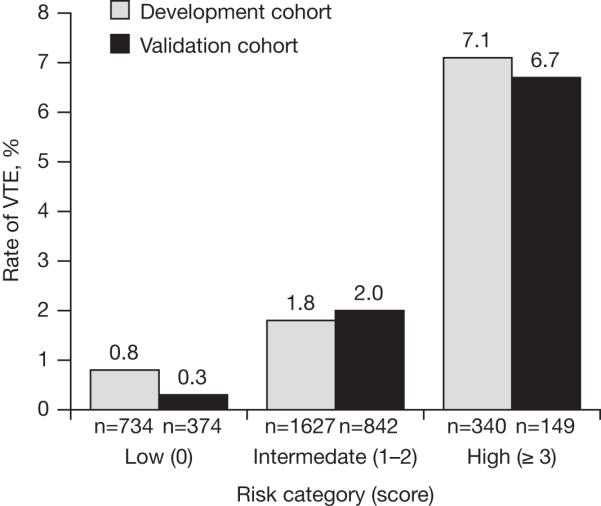
Observed rates of venous thromboembolism (VTE) according to risk category for derivation and validation cohorts in the development of a predictive model for chemotherapy-associated VTE.27 Reproduced with permission from Khorana AA et al. Blood. 2008;111:4902-4907. © the American Society of Hematology.
PROPHYLAXIS RATES IN MEDICAL PATIENTS WITH CANCER
Evidence from population-based surveys indicates that rates of VTE prophylaxis are low or, when provided, VTE prophylaxis is often inappropriately prescribed for cancer patients in general, and medical patients with cancer in particular (Table 3).38-42 In an analysis of 1096 patients with active cancer from a prospective US DVT registry, 28.2% of medical oncology patients received prophylaxis prior to developing DVT which was significantly less than in patients without cancer (34.6%; P < .0001).38 A 4-year survey of prophylaxis rates in more than 2 million US medical patient discharges found that hospitalized cancer patients had the lowest rates of prophylaxis (18-25%) compared with other medical conditions, including acute myocardial infarction (71-74%), heart failure (29-38%), severe lung disease (24-32%), and ischemic stroke (27-32%).39 In addition, a Canadian audit of hospital VTE prophylaxis reported that, among 1894 patients, those with cancer had a significantly reduced likelihood of receiving prophylaxis compared with acutely ill medical patients without cancer (OR = 0.40; 95% CI 0.24-0.68; P = .0007).42 Likewise, the Fundamental Research in Oncology and Thrombosis (FRONTLINE) survey assessed prophylaxis by clinicians involved in cancer care, and found marked differences in their use for surgical and medical cancer patients.41 Over 50% of surgeons reported that they initiated prophylaxis routinely, whereas most medical oncologists stated they used prophylaxis in less than 5% of patients. The use of appropriate prophylaxis was assessed in a recent survey of 196,104 medical patient discharges from 227 US hospitals, including 30,708 patients with cancer.40 Although approximately half of the patients with cancer (56.4%) received some prophylaxis, only 27.6% of patients received prophylaxis in accordance with the Sixth American College of Chest Physicians (ACCP) guidelines. Possible reasons for the poor prophylaxis rates seen in cancer patients include concerns over the risks of bleeding and thrombocytopenia, belief that the cancer-associated VTE risk is low, the perception that treatments are not very effective and cost considerations.41,42 As a result, physicians may be reluctant to prescribe anticoagulation despite evidence that appropriate prophylaxis can confer greater benefits than risks. To address such concerns, clinical practice guidelines specific to VTE prophylaxis in cancer patients have been developed based on emerging evidence from a number of randomized controlled clinical trials designed to evaluate the efficacy and safety as well as the optimal approach to prophylactic anticoagulation in medical patients with cancer.
TABLE 3. Rates of Use of Prophylaxis in Patients with Cancer At Risk of Venous Thromboembolism.
| Reference | Total at-risk medical patients studied who received any prophylaxis, % | At-risk medical cancer patients who received any prophylaxis, % |
|---|---|---|
| Seddighzadeh A et al. 200738 | 34.6* | 28.2 |
| Burleigh E et al. 200639 | 26-33 | 18-25 |
| Amin A et al. 200740 | 61.8 | 56.4 |
| Kakkar AK et al.200341 | NA | < 5 |
Total medical patients without cancer.
NA, not applicable.
IMPACT OF VTE PROPHYLAXIS
Efficacy and safety of prophylaxis with LMWH
Studies have demonstrated the efficacy and safety of LMWH versus unfractionated heparin (UFH) or placebo in surgical cancer patients including the Enoxaparin and Cancer (ENOXACAN) studies I and II.6,43 There are fewer data, however, on the efficacy of LMWH as primary prophylaxis in medical cancer patients. Nevertheless, three large RCTs of hospitalized acutely ill medical patients have demonstrated that enoxaparin (MEDENOX), dalteparin (PREVENT) and fondaparinux (ARTEMIS) are effective in the prevention of screen-detected VTE.7,8,44
A number of studies have assessed the efficacy and safety of LMWH treatment in the secondary prevention of VTE (Table 4).45-49 In the Randomized Comparison of LMWH versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) study, patients with cancer who had acute, symptomatic proximal DVT, PE, or both were randomized to receive the LMWH, dalteparin for 5-7 days plus a coumarin derivative for 6 months, or dalteparin alone for 6 months (Table 4).46 The hazard ratio (HR) for recurrent VTE in the dalteparin group compared with the oral anticoagulant group was 0.48 (95% CI 0.30-0.77; P = .002), with no significant differences in the rate of major bleeding. Likewise, when the long-term effects of usual care versus tinzaparin were investigated, cancer patients receiving tinzaparin experienced a lower risk of recurrent VTE than those receiving usual care (RR = 0.44; absolute difference -9.0%; 95% CI -21.7 to -0.7; P = .044), with a similar number of patients experiencing major or fatal bleeding complications.47 However, the US Food and Drug Administration cautioned against the use of tinzaparin to treat VTE in elderly patients with renal insufficiency. Celgene has issued a letter describing a controlled clinical study suggesting that tinzaparin may increase the risk for death, compared to unfractionated heparin in elderly patients with renal insufficiency. ASCO Guidelines recommend alternatives to tinzaparin when treating such patients for DVT with or without PE50
TABLE 4. Randomized Controlled Trials for Treatment of Cancer-Related Venous Thromboembolism (VTE).
From Falanga A, Lee AY, Streiff MB, Lyman GH. 2008 ASCO Education Book: 258-268.45 Adapted with permission. © 2008 American Society of Clinical Oncology. All rights reserved.
| Study | No. of patients | Treatment regimens | Proportion of patients experiencing event during treatment period (%) | Statistical inference | ||
|---|---|---|---|---|---|---|
| Recurrent VTE | Major bleeding | Death | ||||
| Lee et al. 200346 | 336 | Dalteparin (200 IU/kg OD) for 5-7 days + warfarin (target INR 2-3) for 6 months | 16 | 4 | 41 | P = .002 for recurrent VTE; NS for major bleeding and for death |
| 336 | Dalteparin (200 IU/kg OD) for 1 month + dalteparin (~150 IU/kg OD) for 5 months | 8 | 6 | 39 | ||
| Hull et al. 200647 | 100 | IV UFH (APTT adjusted) for 6 days + warfarin (target INR 2-3) for 3 months | 10 | 7* | 19 | NS |
| 100 | Tinzaparin (175 IU/kg OD) for 3 months | 6 | 7* | 20 | ||
| Meyer et al. 200248 | 71 | Enoxaparin (1.5 mg/kg OD) for ≥ 4 days + warfarin (target INR2-3) for 3 months | 21.1 † | 16 | 22.7 | NS |
| 67 | Enoxaparin (1.5 mg/kg OD) for 3 months | 10.5 † | 7 | 11.3 | ||
| Deitcher et al. 200649 | 30 | Enoxaparin (1 mg/kg BID) for ≥ 5 days + warfarin (target INR2-3) for 178 days | 10.0 | 2.9 | 32.4 | ND for recurrent VTE or death; NS for major bleeding |
| 29 | Enoxaparin (1 mg/kg BID) for 5 days + enoxaparin (1 mg/kg OD) for 175 days | 6.9 | 6.5 | 22.6 | ||
| 32 | Enoxaparin (1 mg/kg BID) for 5 days + enoxaparin (1.5 mg/kg OD) for 175 days | 6.3 | 11.1 | 41.7 | ||
Major bleeding rate does not include fatal bleeding.
Rate of recurrent VTE or major hemorrhage.
APTT, activated partial thromboplastin time; BID, twice-daily; INR, international normalized ratio; IV, intravenous; OD, once-daily; ND, not determined; NS, not significant; UFH, unfractionated heparin.
Another study of patients with cancer and VTE compared treatment with enoxaparin for three months with enoxaparin bridged to warfarin therapy (Table 4).48 A total of 21% of patients who received warfarin experienced the composite outcome of major hemorrhage or recurrent VTE (95% CI 12.3-32.4) compared with 10.5% in patents who received enoxaparin alone (95% CI 4.3-20.3; P = .09). When time to major hemorrhage or recurrent VTE event was analyzed, enoxaparin was more effective than warfarin (P = .04 by log-rank test). Major bleeding occurred in 16% of patients in the warfarin group compared with 7% in the enoxaparin alone arm (P = .09). It should be noted, however, that the definition of major bleeding varied between the trials cited.46-49 Unlike the other studies considered, Hull and colleagues did not classify bleeding resulting in death as major bleeding, but as a separate outcome.47 In addition, Deitcher and colleagues considered major bleeding the need for surgery or decompression of a closed space, and ecchymosis or hematoma greater than 10 cm in diameter.49
The potential role of LMWH in VTE prophylaxis in ambulatory cancer patients remains an area of active investigation. Of the RCTs of LMWH in ambulatory cancer patients reported to date, none have demonstrated a significant reduction in VTE and only two have been published.15,51-53 Of note, the recently presented PROTECHT study did observe a significant reduction in all thrombotic events combined.53 The relatively low risk of VTE in unselected ambulatory patients and the relative small sample size of most studies preclude definitive conclusions and further suggest that better methods for risk stratification in this patient population are needed.
Effects of LMWH on survival
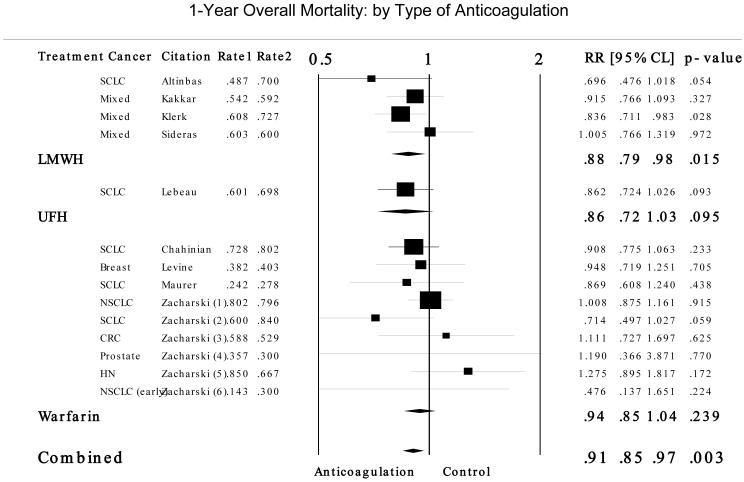
In addition to preventing VTE complications, a number of randomized controlled trials and meta-analyses of these trials have address the question of whether treatment with a LMWH in cancer patients without recognized VTE may improve the survival of patients.18,19,54 One-year mortality rates from studies included in the meta-analysis performed in support of the ASCO Guidelines Panel are presented in Figure 3.19 For cancer patients without a concurrent diagnosis of VTE, the relative risk for mortality compared to controls was 0.877 (95% CI 0.789-0.975; P = .015) for LMWH, and 0.942 (95% CI 0.854-1.040; P = .239) for warfarin. The estimated absolute risk difference (ARD) compared to controls was 8% for LMWH and 3% for warfarin. While studied in different patient populations, the estimated absolute increase in major bleeding episodes was 1% [95% CI: 0.3 - 2.3%] in studies of LMWH and 11.5% [95% CI: 8.5 - 14.5%] in studies of warfarin.
FIGURE 3.
Results for 1-year overall mortality by type of anticoagulation treatment from a meta-analysis of patients with cancer without venous thromboembolism. Adapted from Kuderer NM, Khorana AA, Lyman GH, Francis CW. Cancer. 2007;110:1149-1160.19 Rate 1 indicates anticoagulation group and Rate 2 the control group. CRC, colorectal cancer; HN, head and neck cancer; LMWH, low-molecular-weight heparin; Mixed, mixture of solid tumors; NSCLC, non-small cell lung cancer; RR, relative risk; SCLC, small cell lung cancer; UFH, unfractionated heparin.
In the Fragmin Advanced Malignancy Outcome Study (FAMOUS), a significant difference in survival was not observed with dalteparin 5000 IU once-daily versus placebo when the entire study population was analyzed.15 However, a post hoc analysis of a subgroup of patients with a better prognosis who were alive 17 months after randomization, suggested that 2- and 3-year survival were improved for patients receiving dalteparin versus placebo (78% vs. 55%; and 60% vs. 36%, respectively; P = .03). Likewise, a subgroup analysis was conducted of the CLOT trial to assess the effect of dalteparin on survival in patients with and without metastases.17 In patients without metastases, the probability of death at 12 months was 20% in the dalteparin group compared with 36% in the oral anticoagulant group (HR = 0.50; 95% CI 0.27-0.95; P = .03) whereas no significant difference was observed in patients with metastatic disease (HR = 1.1; 95% CI 0.87-1.4; P = .46). Such post hoc subgroup analyses must be interpreted with caution and considered hypothesis generating. Of note, a recent meta-analysis of five randomized controlled trials of LMWH in patients with limited disease small cell lung cancer suggests a survival benefit with LMWH.55
Studies of the impact of LMWHs on survival in patients with metastatic disease have produced inconsistent results. An 18-week study compared chemotherapy alone (n = 42) with chemotherapy plus the LMWH dalteparin 5000 IU once-daily (n = 42) in the treatment of patients with small cell lung cancer.14 Median overall survival was 8 months with chemotherapy alone and 13 months with the addition of dalteparin (P = .01), with similar improvements in survival observed in patients with both limited and extensive disease stages. In a 6-week study of a LMWH in patients with advanced cancer, median survival was 6.6 months in the placebo group compared to 8.0 months in the LMWH group (HR for mortality = 0.75; 95% CI 0.59-0.96; P = .021).16 However, Sideras et al. found no difference in survival with LMWH therapy versus placebo in cancer patients with advanced disease.51 Further studies are required to better define the clinical setting, including stage of disease, where LMWHs might be considered. Additional research is also warranted to investigate the effects of LMWHs across different cancer sites. Zacharski et al. concluded that warfarin treatment was particularly beneficial in patients with small cell lung cancer compared with non-small cell lung, colorectal, head and neck, and prostate cancers.56 However, the comparative effects of the LMWHs on different tumor types remain to be elucidated.
Antitumor effects
Heparin and heparin-like compounds appear to possess anticancer properties. Heparin may influence malignant cell growth through different interrelated mechanisms, including inhibiting heparin-binding growth factors that drive malignant cell growth, inhibiting tumor cell heparinases that mediate tumor cell invasion and metastasis, and blocking cell surface selectin-mediated tumor cell metastasis and blood coagulation.57 A number of experimental studies have suggested that the LMWHs may inhibit angiogenesis, block thrombin-induced platelet aggregation, inhibit platelet interaction with vascular endothelium, and stimulate platelet production.58 In contrast to UFH, it has been shown that the LMWHs can hinder the binding of growth factors to their high-affinity receptors.57 For example, small molecular heparin fractions have been shown to inhibit vascular endothelial growth factor- and basic fibroblast growth factor-mediated angiogenesis in vivo.57 Further evidence from clinical trials in patients with cancer is needed to confirm these findings, and to further clarify the potential impact of LMWH on the natural history of the disease. Currently, anticoagulants including the LMWHs are not indicated for use as anti-cancer treatment.13
Practical Aspects of LMWH prophylaxis
While this review has focused on LMWH, vitamin K antagonists and unfractionated heparin (UFH) continue to be used in some settings. Extended-duration anticoagulant treatment is often recommended to reduce the risk of recurrence of VTE in patients with cancer. While less costly, long-term treatment with vitamin K antagonists may not only be less effective but may also be impractical in many patients.59 Unpredictable anticoagulant responses can result from warfarin treatment due to multiple food and drug interactions, and this is particularly likely in cancer patients receiving multiple additional medications, including chemotherapeutic agents and anti-emetics.31 Responses to vitamin K antagonists are affected by liver dysfunction, borderline vitamin K deficiency, and gastrointestinal disorders (e.g., vomiting, diarrhea), which are commonly observed in patients being treated for cancer. Vitamin K antagonists are also difficult to manage in patients who need anticoagulant interruptions due to chemotherapy-induced thrombocytopenia or invasive procedures, such as spinal taps, paracentesis, and various surgical procedures.60
Unlike vitamin K antagonists, LMWHs have a longer half-life, greater bioavailability, and a more predictable anticoagulant effect with dose monitoring and adjustment normally only required in patients with severe renal impairment or obesity.61 A reduced need for regular coagulation monitoring for the majority of patients receiving LMWH therapy makes these agents suitable for outpatient treatment,62 and extended-duration VTE prophylaxis.42 Outpatient treatment and freedom from coagulation monitoring offer improved convenience for cancer patients. LMWH therapy appears to be cost-effective for long-term secondary prophylaxis in cancer patients as higher drug costs appear to be partially offset by the potential for reduced hospital stays, reduced need for coagulation monitoring, and fewer bleeding complications.63
Unfortunately, there are very limited data on the use of UFH for primary prophylaxis in either hospitalized or ambulatory cancer patients. Prophylaxis studies of UFH in acute ill hospitalized medical patients have yielded variable results but none have been conducted specifically in a cancer population.68-69 While UFH is still utilized in some settings, the ASCO VTE Guidelines recommend LMWHs for both the initial and extended treatment of VTE in cancer patients with established VTE. There have been no trials of UFH for primary prophylaxis in ambulatory cancer patients.
CLINICAL PRACTICE GUIDELINES
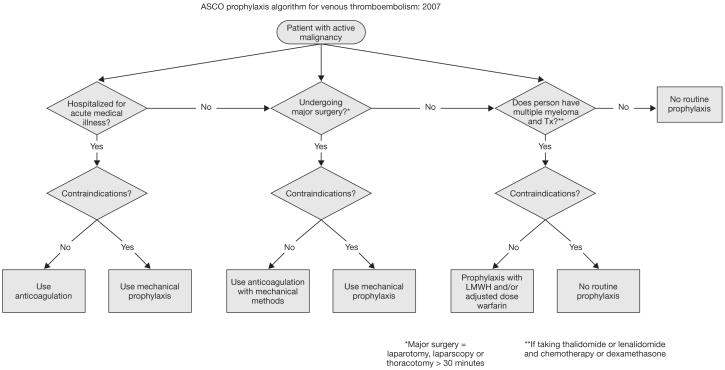
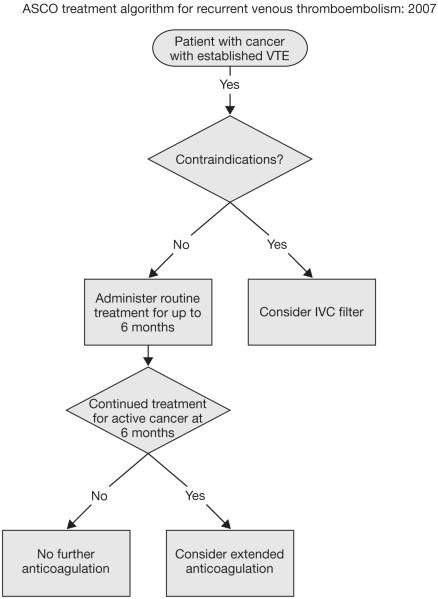
Despite compelling evidence, a significant proportion of medical cancer patients at increased risk for VTE do not receive appropriate VTE prophylaxis. In recognition of the importance of VTE prevention in patients with cancer, specific VTE management guidelines have recently been developed by the American Society of Clinical Oncology (ASCO)13 and National Comprehensive Cancer Network,12 in addition to the updated recommendations from the ACCP9 and the International Union of Angiology.11 A summary of the ASCO guideline recommendations is presented in Table 5,13 and algorithms for the prevention and treatment of VTE for patients with cancer are shown in Figure 4.13,64 ASCO recommendations include: (1) all hospitalized cancer patients should be considered for VTE prophylaxis with anticoagulants in the absence of bleeding or other contraindications; (2) routine prophylaxis of ambulatory cancer patients with anticoagulation is not recommended, with the exception of myeloma patients receiving thalidomide or lenalidomide with chemotherapy or dexamethasone; (3) patients undergoing major surgery for malignant disease should be considered for pharmacological thromboprophylaxis; (4) LMWH represents the preferred agent for both the initial and extended treatment of cancer patients with established VTE; and (5) the impact of anticoagulants on cancer patient survival requires additional study and cannot be recommended at present (Table 5).13,65 Widespread, active dissemination of these guidelines is needed to improve appropriate prescribing rates for prophylactic anticoagulation in cancer patients at risk of VTE. Improved thromboprophylaxis in medical cancer patients should significantly reduce patient morbidity and consumption of healthcare resources while improving the delivery of cancer therapy and cancer-related outcomes including, most importantly, cancer patient survival.66
TABLE 5. Summary of American Society of Clinical Oncology 2007 Guidelines on Venous Thromboembolism (VTE) Prophylaxis and Treatment in Patients with Cancer.13.
| Patient group | Recommended | Not recommended |
|---|---|---|
| Hospitalized patients with cancer | VTE prophylaxis with anticoagulants (LMWH, UFH, or fondaparinux) | If presence of bleeding or other contraindications to anticoagulation |
| Ambulatory patients with cancer receiving chemotherapy | LMWH or adjusted-dose warfarin for patients with multiple myeloma receiving thalidomide or lenalidomide plus chemotherapy or dexamethasone | Otherwise, no routine VTE prophylaxis |
| Patients with cancer undergoing surgery | Prophylaxis with low-dose UFH, LMWH, or fondaparinux for at least 7-10 days Combined prophylaxis with mechanical methods for patients at very high risk |
If presence of bleeding or other contraindications to anticoagulation Consider mechanical methods alone for those with contraindications to pharmacological methods |
| Patients with cancer with established VTE | LMWH for the initial 5-10 days LMWH for at least 6 months or vitamin K antagonists (target INR 2-3) when LMWH unavailable Consider continued anticoagulation beyond 6 months in those with active cancer |
|
| Patients with cancer without VTE, to improve survival | Prophylaxis not recommended |
INR, international normalized ratio; LMWH, low-molecular-weight heparin; UFH, unfractionated heparin.
FIGURE 4.

Algorithms for the prevention and treatment of venous thromboembolism (VTE) for patients with cancer from American Society of Clinical Oncology. J Oncol Practice. 2007;3: 326-329.64 Reproduced with permission from American Society of Clinical Oncology. 2007. © 2007 American Society of Clinical Oncology. All rights reserved. (A) Primary prophylaxis, (B) secondary prophylaxis and (C) Contraindications applicable to primary and secondary prophylaxis. IVC, inferior vena cava; LMWH, low-molecular-weight heparin; Tx, treatment.
FURTHER STUDIES
Further study of the potential roles for these agents in patients with cancer is clearly needed. Very few available data are available on the prevention of VTE in ambulatory patients with cancer. Further studies are also needed to better define the optimum dose and duration of LMWH therapy in specific clinical settings in cancer patients.49,67 Further studies are needed to better define the benefits and risks associated with prolonged anti-coagulation, especially in high-risk patients, such as the elderly or those with central nervous system malignancies.13 Finally, while intriguing data have emerged from several trials, further research is needed to explore the potential impact of the LMWHs on cancer patient survival.
CONCLUSIONS
Although the value of primary prophylaxis with LMWHs in patients with cancer undergoing surgical treatment is well established, medical cancer patients also represent a population at significant risk for VTE and its complications. The risk of VTE in this population is increasing in frequency, in part, due to treatment with more aggressive systemic cancer therapies, including new targeted antiangiogenic agents. The LMWHs have demonstrated clinical efficacy in medical patients with cancer, including as primary VTE prophylaxis in high risk patients, for secondary prevention of recurrent episodes of VTE, and potentially for improvement in overall survival. The LMWHs appear to be suitable for long-term secondary prophylaxis as a result of the reduced need for coagulation monitoring, low rates of bleeding complications, and once-daily dosing. Clinical practice guidelines from ASCO and other professional organizations have provided recommendations for the appropriate and safe use of VTE prophylaxis for the high-risk cancer patient.
Acknowledgements
The author would like to acknowledge the countless discussions and many useful suggestions provided by Dr Nicole Kuderer in the preparation of this manuscript.
The author received editorial support in the preparation of this manuscript, funded by sanofi-aventis, NJ, USA. However, the author had complete independence in defining content and in all editorial decisions in respect of this manuscript.
Sources of support
Dr. Lyman is supported by a grant from the National Heart, Lung and Blood Institute (1R01HL095109-01). The author received no compensation for the preparation of this manuscript. The author received editorial support in the preparation of this manuscript, funded by sanofi-aventis, NJ, USA. However, the author had complete independence in defining content and in all editorial decisions in respect of this manuscript.
REFERENCES
- 1.Levitan N, Dowlati A, Remick SC, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data. Medicine (Baltimore) 1999;78:285–291. doi: 10.1097/00005792-199909000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Sørensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 3.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632–634. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 4.Kuderer NM, Francis CW, Culakova E, et al. Venous thromboembolism and all-cause mortality in cancer patients receiving chemotherapy. J Clin Oncol. 2008 May 20;(Suppl) Abstract 9521. [Google Scholar]
- 5.Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 6.ENOXACAN Study Group Efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of deep vein thrombosis in elective cancer surgery. A double-blind randomized multicentre trial with venographic assessment. Br J Surg. 1997;84:1099–1103. [PubMed] [Google Scholar]
- 7.Samama MM, Cohen AT, Darmon JY, et al. Prophylaxis in Medical Patients with Enoxaparin Study Group A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. N Engl J Med. 1999;341:793–800. doi: 10.1056/NEJM199909093411103. [DOI] [PubMed] [Google Scholar]
- 8.Leizorovicz A, Cohen AT, Turpie AG, et al. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110:874–879. doi: 10.1161/01.CIR.0000138928.83266.24. [DOI] [PubMed] [Google Scholar]
- 9.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (8th Edition) 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 10.Kearon C, Kahn SR, Agnelli G, Goldhaber SZ, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (8th Edition) 2008;133:454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 11.Nicolaides AN, Fareed J, Kakkar AK, et al. Prevention and treatment of venous thromboembolism. International Consensus Statement (Guidelines according to scientific evidence) Int Angiol. 2006;25:101–161. [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Venous Thromboembolic Disease, version 2. [accessed Dec 9, 2008]. 2008. Available from URL: http://www.nccn.org/professionals/physician_gls/default.asp.
- 13.Lyman GH, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25:5490–5505. doi: 10.1200/JCO.2007.14.1283. [DOI] [PubMed] [Google Scholar]
- 14.Altinbas M, Coskun HS, Er O, et al. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemost. 2004;2:1266–1271. doi: 10.1111/j.1538-7836.2004.00871.x. [DOI] [PubMed] [Google Scholar]
- 15.Kakkar AK, Levine M, Kadziola Z, et al. Low molecular weight heparin, therapy with dalteparin,and survival in advanced cancer: the Fragmin Advanced Malignancy Outcomes Study (FAMOUS) J Clin Oncol. 2004;22:1944–1948. doi: 10.1200/JCO.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Klerk CP, Smorenburg SM, Otten HM, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130–2135. doi: 10.1200/JCO.2005.03.134. [DOI] [PubMed] [Google Scholar]
- 17.Lee AY, Rickles FR, Julian JA, et al. Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism. J Clin Oncol. 2005;23:2123–2129. doi: 10.1200/JCO.2005.03.133. [DOI] [PubMed] [Google Scholar]
- 18.Lazo-Langner A, Goss GD, Spaans JN, Rodger MA. The effect of low-molecular weight heparin on cancer survival. J Thromb Haemost. 2007;5:729–737. doi: 10.1111/j.1538-7836.2007.02427.x. [DOI] [PubMed] [Google Scholar]
- 19.Kuderer NM, Khorana AA, Lyman GH, Francis CW. A meta-analysis and systematic review of the efficacy and safety of anticoagulants as cancer treatment. Cancer. 2007;110:1149–1160. doi: 10.1002/cncr.22892. [DOI] [PubMed] [Google Scholar]
- 20.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Risk factors for deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med. 2000;160:809–815. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 21.Falanga A. The incidence and risk of venous thromboembolism associated with cancer and nonsurgical cancer treatment. Cancer Invest. 2009;27:105–115. doi: 10.1080/07357900802563028. [DOI] [PubMed] [Google Scholar]
- 22.Khorana AA, Francis CW, Culakova E, Fisher RI, Kuderer NM, Lyman GH. Thromboembolism in hospitalized neutropenic cancer patients. J Clin Oncol. 2006;24:484–490. doi: 10.1200/JCO.2005.03.8877. [DOI] [PubMed] [Google Scholar]
- 23.Caine GJ, Stonelake PS, Lip GY, Kehoe ST. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia. 2002;4:465–473. doi: 10.1038/sj.neo.7900263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein PD, Beemath A, Meyers FA, Skaf E, Sanchez J, Olson RE. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med. 2006;119:60–68. doi: 10.1016/j.amjmed.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 25.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110:2339–2346. doi: 10.1002/cncr.23062. [DOI] [PubMed] [Google Scholar]
- 26.Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–722. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 27.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heit JA, Mohr DN, Silverstein MD, Petterson TM, O’Fallon WM, Melton LJ., 3rd Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med. 2000;160:761–768. doi: 10.1001/archinte.160.6.761. [DOI] [PubMed] [Google Scholar]
- 29.Heit JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002;162:1245–1248. doi: 10.1001/archinte.162.11.1245. [DOI] [PubMed] [Google Scholar]
- 30.Hussein MA. Thromboembolism risk reduction in multiple myeloma patients treated with immunomodulatory drug combinations. Thromb Haemost. 2006;95:924–930. doi: 10.1160/TH06-02-0080. [DOI] [PubMed] [Google Scholar]
- 31.El Accaoui RN, Shamseddeen WA, Taher AT. Thalidomide and thrombosis - A meta-analysis. Thromb Haemost. 2007;97:1031–1036. [PubMed] [Google Scholar]
- 32.Shah M, Ilson D, Kelsen D. Thromboembolic events in gastric cancer: high incidence in patients receiving ironotecan-and bevacizumab-based therapy. J Clin Oncol. 2005;23:2574–2576. doi: 10.1200/JCO.2005.81.908. [DOI] [PubMed] [Google Scholar]
- 33.Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. 2008;300:2277–2285. doi: 10.1001/jama.2008.656. [DOI] [PubMed] [Google Scholar]
- 34.Deitcher SR, Gomes MPV. The risk of venous thromboembolic disease associated with adjuvant hormone therapy for breast carcinoma: A systematic review. Cancer. 2004;101:439–449. doi: 10.1002/cncr.20347. [DOI] [PubMed] [Google Scholar]
- 35.Bohlius J, Wilson J, Seidenfeld J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst. 2006;98:708–714. doi: 10.1093/jnci/djj189. [DOI] [PubMed] [Google Scholar]
- 36.Khorana AA, Francisw CW, Blumberg N, Culakova E, Refaal MA, Lyman GH. Blood transfusions, thrombosis and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168:2377–2381. doi: 10.1001/archinte.168.21.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuderer NM, Khorana AA, Franics CW, et al. Venous thromboembolism risk model predicts early progression and overall mortality in cancer patients receiving chemotherapy. Blood. 2008;112:71. Abstract 172. [Google Scholar]
- 38.Seddighzadeh A, Shetty R, Goldhaber SZ. Venous thromboembolism in patients with active cancer. Thromb Haemost. 2007;98:656–661. [PubMed] [Google Scholar]
- 39.Burleigh E, Wang C, Foster D, et al. Thromboprophylaxis in medically ill patients at risk for venous thromboembolism. Am J Health Syst Pharm. 2006;63(20 Suppl 6):S23–S29. doi: 10.2146/ajhp060390. [DOI] [PubMed] [Google Scholar]
- 40.Amin A, Stemkowski S, Lin J, Yang G. Thromboprophylaxis rates in US medical centers: success or failure? J Thromb Haemost. 2007;5:1610–1616. doi: 10.1111/j.1538-7836.2007.02650.x. [DOI] [PubMed] [Google Scholar]
- 41.Kakkar AK, Levine M, Pinedo HM, Wolff R, Wong J. Venous thrombosis in cancer patients: insights from the FRONTLINE survey. Oncologist. 2003;8:381–388. doi: 10.1634/theoncologist.8-4-381. [DOI] [PubMed] [Google Scholar]
- 42.Kahn SR, Panju A, Geerts W, et al. Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res. 2007;119:145–155. doi: 10.1016/j.thromres.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346:975–980. doi: 10.1056/NEJMoa012385. [DOI] [PubMed] [Google Scholar]
- 44.Francis CW. Clinical practice. Prophylaxis for thromboembolism in hospitalized medical patients. N Engl J Med. 2007 Apr 5;356:1438–1444. doi: 10.1056/NEJMcp067264. [DOI] [PubMed] [Google Scholar]
- 45.Falanga A, Lee AY, Streiff MB, Lyman GH. Cancer and Thrombosis 3: Anticoagulation in the Treatment of Venous Thromboembolism in Patients with Cancer. ASCO Education Book; 2008. pp. 258–268. [Google Scholar]
- 46.Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 47.Hull RD, Pineo GF, Brant RF, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119:1062–1072. doi: 10.1016/j.amjmed.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 48.Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162:1729–1735. doi: 10.1001/archinte.162.15.1729. [DOI] [PubMed] [Google Scholar]
- 49.Deitcher SR, Kessler CM, Merli G, et al. Secondary prevention of venous thromboembolic events in patients with active cancer: enoxaparin alone versus initial enoxaparin followed by warfarin for a 180-day period. Clin Appl Thromb Hemost. 2006;12:389–396. doi: 10.1177/1076029606293692. [DOI] [PubMed] [Google Scholar]
- 50.American Society of Clinical Oncology Guideline: Recommendations for Venous Thromboembolism Prophylaxis and Treatment in Patients with Cancer. [accessed Mar 4, 2009]. Available from URL: http://www.asco.org/ASCO/Quality+Care+&+Guidelines/Practice+Guidelines/Clinical+Practice+Guidelines/Supportive+Care+and+Quality+of+Life/American+Society+of+Clinical+Oncology+Guideline:+Recommendations+for+Venous+Thromboembolism+Prophylaxis+and+Treatment+in+Patients+with+Cancer. [DOI] [PubMed]
- 51.Sideras K, Schaefer PL, Okuno SH, et al. Low-molecular-weight heparin in patients with advanced cancer: a phase 3 clinical trial. Mayo Clin Proc. 2006;81:758–767. doi: 10.4065/81.6.758. [DOI] [PubMed] [Google Scholar]
- 52.Perry JR, Rogers L, Laperriere N, et al. PRODIGE: A phase III randomized placebo-controlled trial of thromboprophylaxis using dalteparin low molecular weight heparin in patients with newly diagnosed malignant glioma. J Clin Oncol. 2007;25:18. doi: 10.1111/j.1538-7836.2010.03973.x. Abstract 2011. [DOI] [PubMed] [Google Scholar]
- 53.Agnelli G, Gussoni G, Bianchini C, Verso M, Tonato M. A randomized double-blind placebo-controlled study on nadroparin for prophylaxis of thromboembolic events in cancer patients receiving chemotherapy: The PROTECHT study. Blood. 2008;112:5. Abstract 6. [Google Scholar]
- 54.Dolovich LR, Ginsberg JS, Douketis JD, Holbrook AM, Cheah G. A metaanalysis comparing low-molecular-weight heparins with unfractionated heparin in the treatment of venous thromboembolism: examining some unanswered questions regarding location of treatment, product type, and dosing frequency. Arch Intern Med. 2000;160:181–188. doi: 10.1001/archinte.160.2.181. [DOI] [PubMed] [Google Scholar]
- 55.Akl EA, van Doormaal FF, Barba M, et al. Parenteral anticoagulation may prolong the survival of patients with limited small cell lung cancer: a Cochrane systematic review. J Exp Clin Cancer Res. 2008;27:1–10. doi: 10.1186/1756-9966-27-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zacharski LR, Henderson WG, Rickles FR, et al. Effect of warfarin anticoagulation on survival in carcinoma of the lung, colon, head and neck, and prostate. Final report of VA Cooperative Study #75. Cancer. 1984;53:2046–2052. doi: 10.1002/1097-0142(19840515)53:10<2046::aid-cncr2820531007>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 57.Castelli R, Porro F, Tarsia P. The heparins and cancer: review of clinical trials and biological properties. Vasc Med. 2004;9:205–213. doi: 10.1191/1358863x04vm566ra. [DOI] [PubMed] [Google Scholar]
- 58.Cosgrove RH, Zacharski LR, Racine E, Andersen JC. Improved cancer mortality with low-molecular-weight heparin treatment: a review of the evidence. Semin Thromb Hemost. 2002;28:79–87. doi: 10.1055/s-2002-20566. [DOI] [PubMed] [Google Scholar]
- 59.Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (8th Edition) 2008;133:160S–198S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 60.Lee AY. Deep vein thrombosis and cancer: survival, recurrence, and anticoagulant choices. Dis Mon. 2005;51:150–157. doi: 10.1016/j.disamonth.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 61.Hirsh J, Bauer KA, Donati MB, Gould M, Samama MM, Weitz JI. Parenteral anticoagulants: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (8th Edition) 2008;133:141S–159S. doi: 10.1378/chest.08-0689. [DOI] [PubMed] [Google Scholar]
- 62.Wells PS, Kovacs MJ, Bormanis J, et al. Expanding eligibility for outpatient treatment of deep venous thrombosis and pulmonary embolism with low-molecular-weight heparin: a comparison of patient self-injection with homecare injection. Arch Intern Med. 1998;158:1809–1812. doi: 10.1001/archinte.158.16.1809. [DOI] [PubMed] [Google Scholar]
- 63.Engman CA, Zacharski LR. Low molecular weight heparins as extended prophylaxis against recurrent thrombosis in cancer patients. J Natl Compr Canc Netw. 2008;6:637–645. doi: 10.6004/jnccn.2008.0050. [DOI] [PubMed] [Google Scholar]
- 64.American Society of Clinical Oncology 2007 Clinical Practice Guideline Recommendations for Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer. J Oncol Practice. 2007;3:326–329. doi: 10.1200/JOP.0768502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lyman GH, Kuderer NM. Improving outcomes with prophylactic anticoagulation in patients with cancer: Lessons from the American Society of Clinical Oncology Guidelines. In: Khorana AA, Francis CW, editors. Cancer-associated thrombosis: new findings in translational science, prevention and treatment. Informa Healthcare USA, Inc.; New York, NY: 2008. pp. 255–272. [Google Scholar]
- 66.Lyman GH. ASCO Clinical Practice Guidelines and Beyond. J Oncol Pract. 2007;3:330–331. doi: 10.1200/JOP.0761501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bergqvist D, Burmark US, Flordal PA, et al. Low molecular weight heparin started before surgery as prophylaxis against deep vein thrombosis: 2500 versus 5000 XaI units in 2070 patients. Br J Surg. 1995;82:496–501. doi: 10.1002/bjs.1800820421. [DOI] [PubMed] [Google Scholar]
- 68.Gardlund B. Randomised, controlled trial of low-dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases: The Heparin Prophylaxis Study Group. Lancet. 1996;347:1357–1361. doi: 10.1016/s0140-6736(96)91009-0. [DOI] [PubMed] [Google Scholar]
- 69.Mismetti P, Laporte-Simitsidis S, Tardy B, et al. Prevention of venous thromboembolism in internal medicine with unfractionated or low-molecular weight heparins: A meta-analysis of randomized clinical trials. Thromb Haemost. 2000;83:14–19. [PubMed] [Google Scholar]