Abstract
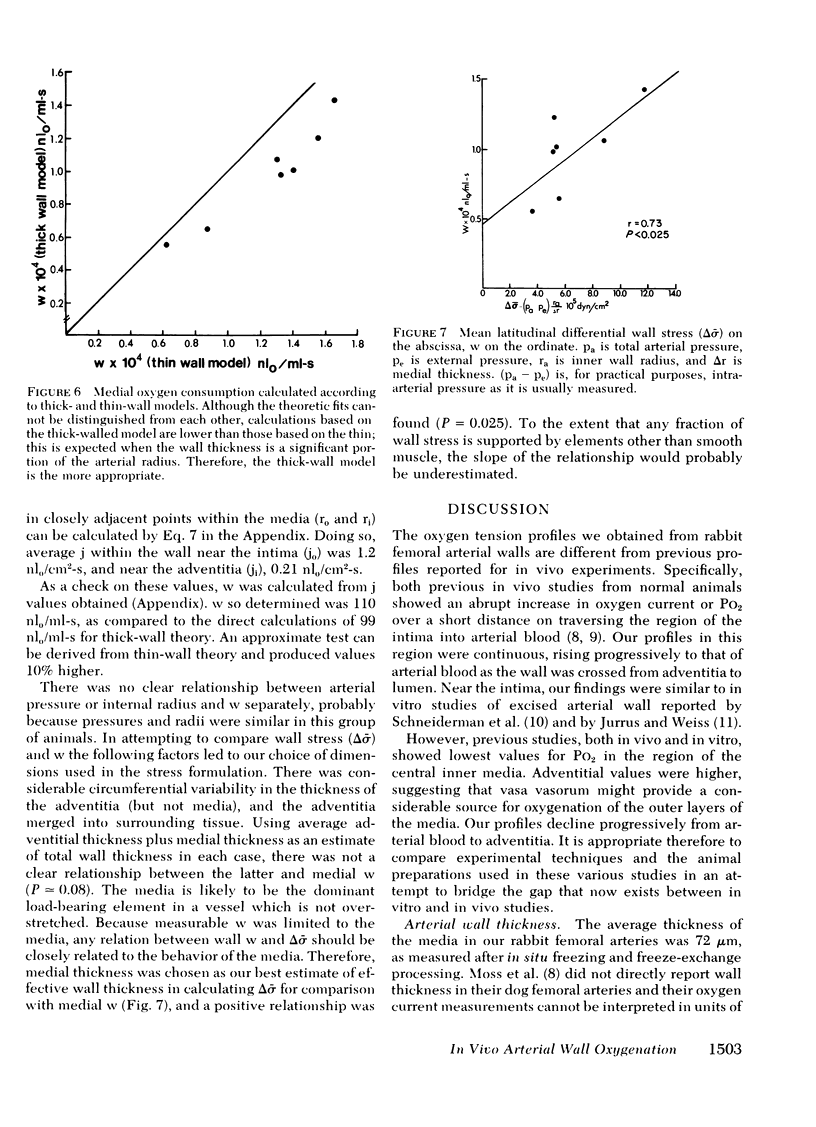
In vivo measurements of tissue oxygen tension were made at 10-micrometer intervals through functioning in situ rabbit femoral arterial walls, using inhalation anesthesia and recessed microcathodes with approximately 4-micrometer external diameters. External environment was controlled with a superfusion well at 30 torr PO2, 35 torr PCO2. Blood pressure, gas tension levels, and blood pH were held within the normal range. Radial PO2 measurements closely fit a mathematical model for unidimensional diffusion into a thick-walled artery with uniform oxygen consumption, and the distances traversed fit measured dimensions of quick-frozen in vivo sections. Using standard values of diffusion and solubility coefficients, mean calculated medial oxygen consumption was 99 nl0/ml-s. Mural oxygen consumption appeared to be related linearly to mean tangential wall stress. Differences in experimental design and technique were compared with previous in vivo and in vitro measurements of wall oxygenation, and largely account for the varying results obtained. Control of environment external to the artery, and maintenance of normally flowing blood in the lumen in vivo appeared critical to an understanding of mural oxygenation in life. If the conditions of this experiment prevailed in arteries with thicker avascular layers, PO2 could have been 20 torr at approximately 156 micrometer and 10 torr at 168 micrometer from blood (average values).
Full text
PDF

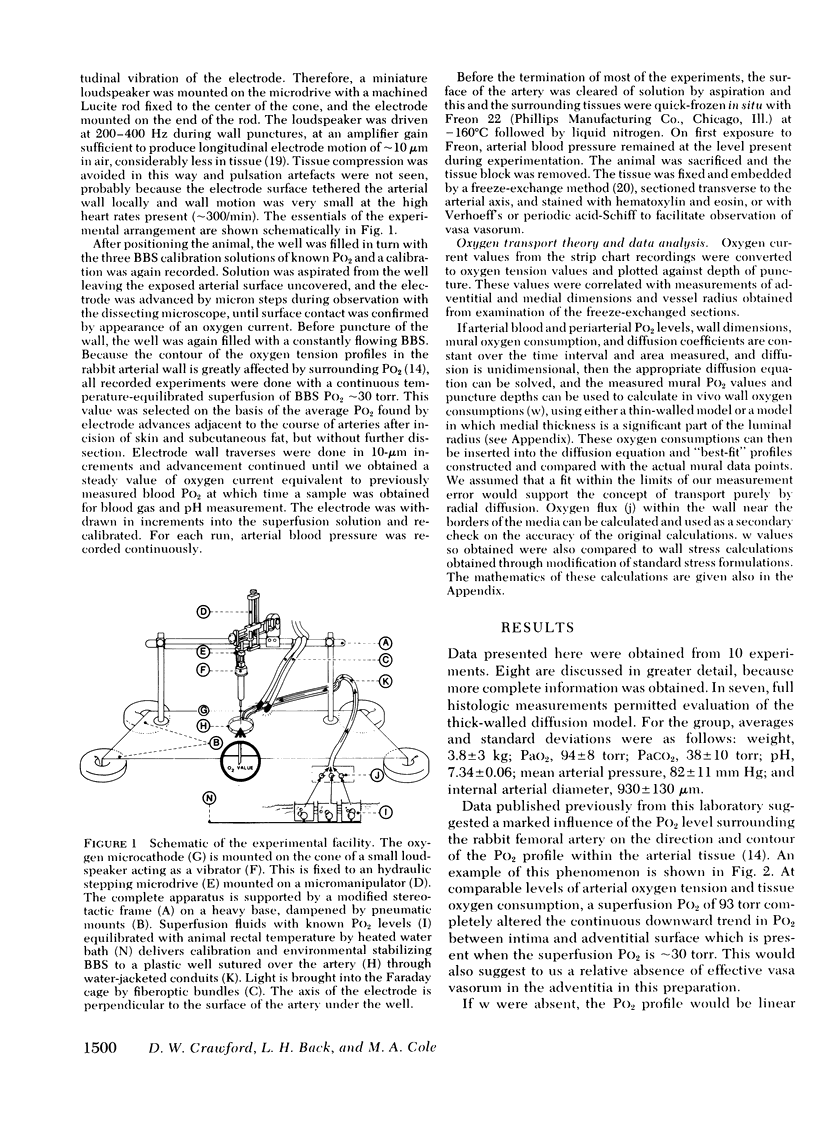
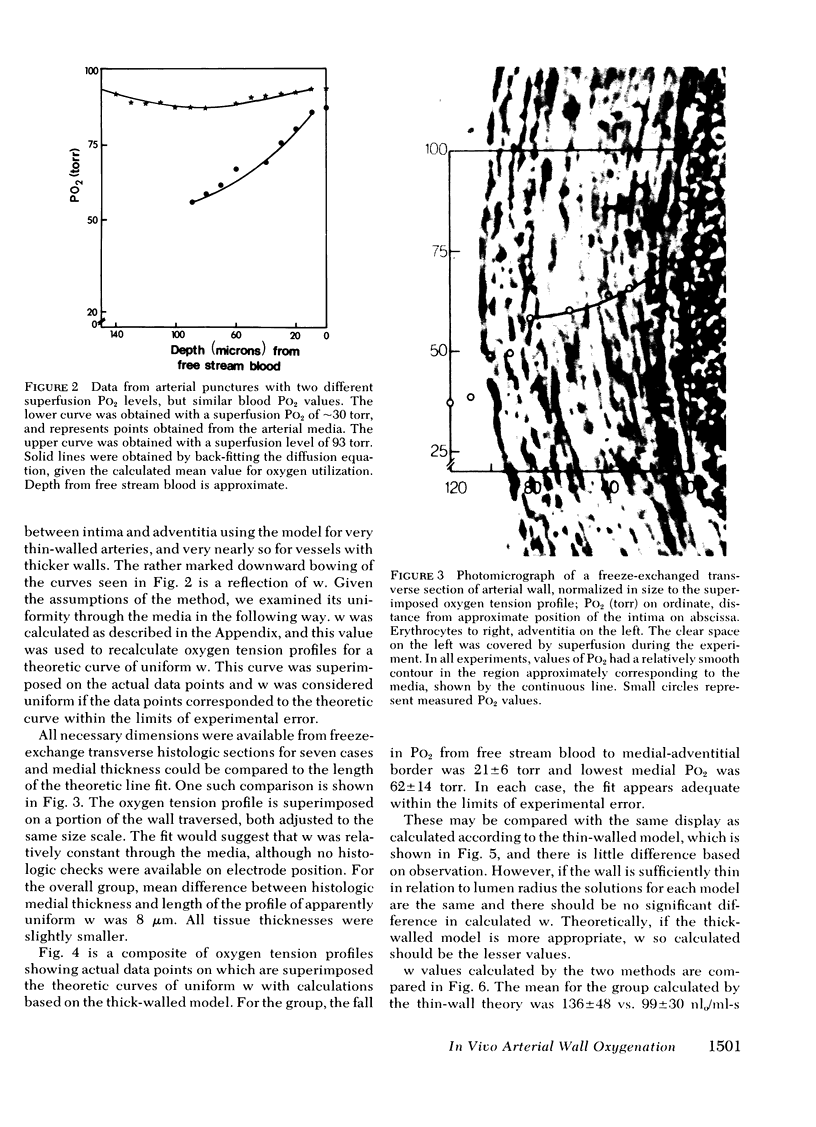
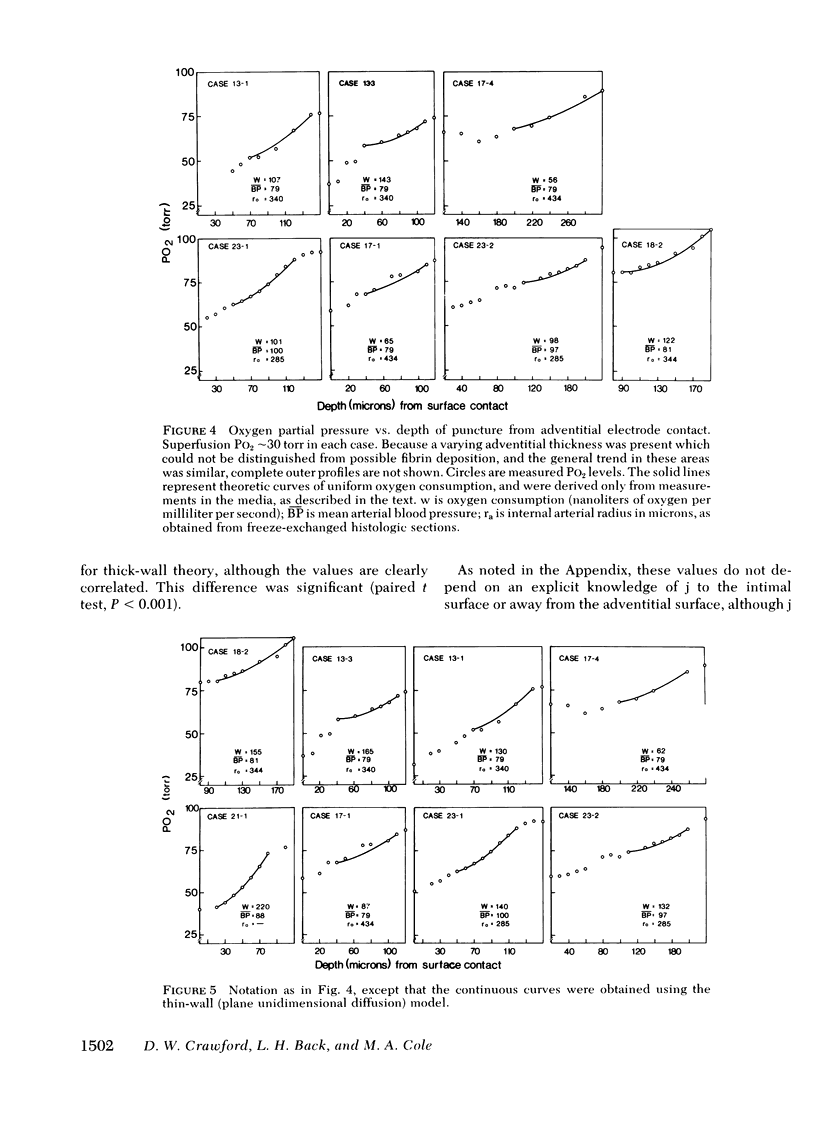





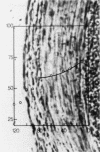
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Bayliss O. B. The relationship between diffuse intimal thickening, medial enzyme failure and intimal lipid deposition in various human arteries. J Atheroscler Res. 1969 Nov-Dec;10(3):327–339. doi: 10.1016/s0368-1319(69)80036-0. [DOI] [PubMed] [Google Scholar]
- Bates M. L., Feingold A., Gold M. I. The effects of anesthetics on an in-vivo oxygen electrode. Am J Clin Pathol. 1975 Oct;64(4):448–451. doi: 10.1093/ajcp/64.4.448. [DOI] [PubMed] [Google Scholar]
- Colthart C., Roach M. R. The effect of temperature on the oxygen consumption of isolated human umbilical arteries. Can J Physiol Pharmacol. 1970 Jun;48(6):377–381. doi: 10.1139/y70-060. [DOI] [PubMed] [Google Scholar]
- Duling B. R., Staples E. A comparison of the performance of microvessels in the hamster cheek pouch during exposure to tris and bicarbonate buffers. Microvasc Res. 1974 Mar;7(2):277–279. doi: 10.1016/0026-2862(74)90012-0. [DOI] [PubMed] [Google Scholar]
- Gore R. W., Whalen W. J. Relations among tissue PO2, QO2, and resting heat production of frog sartorius muscle. Am J Physiol. 1968 Feb;214(2):277–286. doi: 10.1152/ajplegacy.1968.214.2.277. [DOI] [PubMed] [Google Scholar]
- Hugod C., Hawkins L. H., Kjeldsen K., Thomsen H. K., Astrup P. Effect of carbon monoxide exposure on aortic and coronary intimal morphology in the rabbit. A revaluation. Atherosclerosis. 1978 Aug;30(4):333–342. doi: 10.1016/0021-9150(78)90126-0. [DOI] [PubMed] [Google Scholar]
- Jurrus E. R., Weiss H. S. In vitro tissue oxygen tensions in the rabbit aortic arch. Atherosclerosis. 1977 Nov;28(3):223–232. doi: 10.1016/0021-9150(77)90172-1. [DOI] [PubMed] [Google Scholar]
- KIRK J. E., EFFERSØE P. G., CHIANG S. P. The rate of respiration and glycolysis by human and dog aortic tissue. J Gerontol. 1954 Jan;9(1):10–53. doi: 10.1093/geronj/9.1.10. [DOI] [PubMed] [Google Scholar]
- KIRK J. E., LAURSEN T. J. Diffusion coefficients of various solutes for human aortic tissue, with special reference to variation to tissue permeability with age. J Gerontol. 1955 Jul;10(3):288–302. doi: 10.1093/geronj/10.3.288. [DOI] [PubMed] [Google Scholar]
- Kjeldsen K., Wanstrup J., Astrup P. Enhancing influence of arterial hypoxia on the development of atheromatosis in cholesterol-fed rabbits. J Atheroscler Res. 1968 Sep-Oct;8(5):835–845. doi: 10.1016/s0368-1319(68)80046-8. [DOI] [PubMed] [Google Scholar]
- Kosan R. L., Burton A. C. Oxygen consumption of arterial smooth muscle as a function of active tone and passive stretch. Circ Res. 1966 Jan;18(1):79–88. doi: 10.1161/01.res.18.1.79. [DOI] [PubMed] [Google Scholar]
- Morrison A. D., Berwick L., Orci L., Winegrad A. I. Morphology and metabolism of an aortic intima-media preparation in which an intact endothelium is preserved. J Clin Invest. 1976 Mar;57(3):650–660. doi: 10.1172/JCI108321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss A. J., Samuelson P., Angell C., Minken S. L. Polarographic evaluation of transmural oxygen availabitlity in intact muscular arteries. J Atheroscler Res. 1968 Sep-Oct;8(5):803–810. doi: 10.1016/s0368-1319(68)80042-0. [DOI] [PubMed] [Google Scholar]
- Niinikoski J., Heughan C., Hunt T. K. Oxygen tensions in the aortic wall of normal rabbits. Atherosclerosis. 1973 May-Jun;17(3):353–359. doi: 10.1016/0021-9150(73)90026-9. [DOI] [PubMed] [Google Scholar]
- Paul R. J., Peterson J. W., Caplan S. R. Oxygen consumption rate in vascular smooth muscle: relation to isometric tension. Biochim Biophys Acta. 1973 May 30;305(2):474–480. doi: 10.1016/0005-2728(73)90192-8. [DOI] [PubMed] [Google Scholar]
- Schneiderman G., Goldstick T. K. Oxygen electrode design criteria and performance characteristics: recessed cathode. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jul;45(1):145–154. doi: 10.1152/jappl.1978.45.1.145. [DOI] [PubMed] [Google Scholar]
- Severinghaus J. W. Blood gas calculator. J Appl Physiol. 1966 May;21(3):1108–1116. doi: 10.1152/jappl.1966.21.3.1108. [DOI] [PubMed] [Google Scholar]
- Sobin S. S., Bernick S., Tremer H. M., Rosenquist T. H., Lindal R., Fung Y. C. The fibroprotein network of the pulmonary interalveolar wall. Chest. 1974 Apr;65(Suppl):4S–5S. doi: 10.1378/chest.65.4_supplement.4s. [DOI] [PubMed] [Google Scholar]
- Sobin S. S., Rosenquist T. H. Determintion of dimensions and geometry in fixed specimens. Microvasc Res. 1973 May;5(3):271–284. doi: 10.1016/0026-2862(73)90038-1. [DOI] [PubMed] [Google Scholar]
- Whalen W. J. Intracellular PO2 in heart and skeletal muscle. Physiologist. 1971 May;14(2):69–82. [PubMed] [Google Scholar]
- Whalen W. J., Nair P., Ganfield R. A. Measurements of oxygen tension in tissues with a micro oxygen electrode. Microvasc Res. 1973 May;5(3):254–262. doi: 10.1016/0026-2862(73)90035-6. [DOI] [PubMed] [Google Scholar]
- Whalen W. J., Riley J., Nair P. A microelectrode for measuring intracellular PO2. J Appl Physiol. 1967 Nov;23(5):798–801. doi: 10.1152/jappl.1967.23.5.798. [DOI] [PubMed] [Google Scholar]
- Whalen W. J., Savoca J., Nair P. Oxygen tension measurements in carotid body of the cat. Am J Physiol. 1973 Oct;225(4):986–991. doi: 10.1152/ajplegacy.1973.225.4.986. [DOI] [PubMed] [Google Scholar]
- Wolinsky H., Glagov S. Nature of species differences in the medial distribution of aortic vasa vasorum in mammals. Circ Res. 1967 Apr;20(4):409–421. doi: 10.1161/01.res.20.4.409. [DOI] [PubMed] [Google Scholar]