Abstract
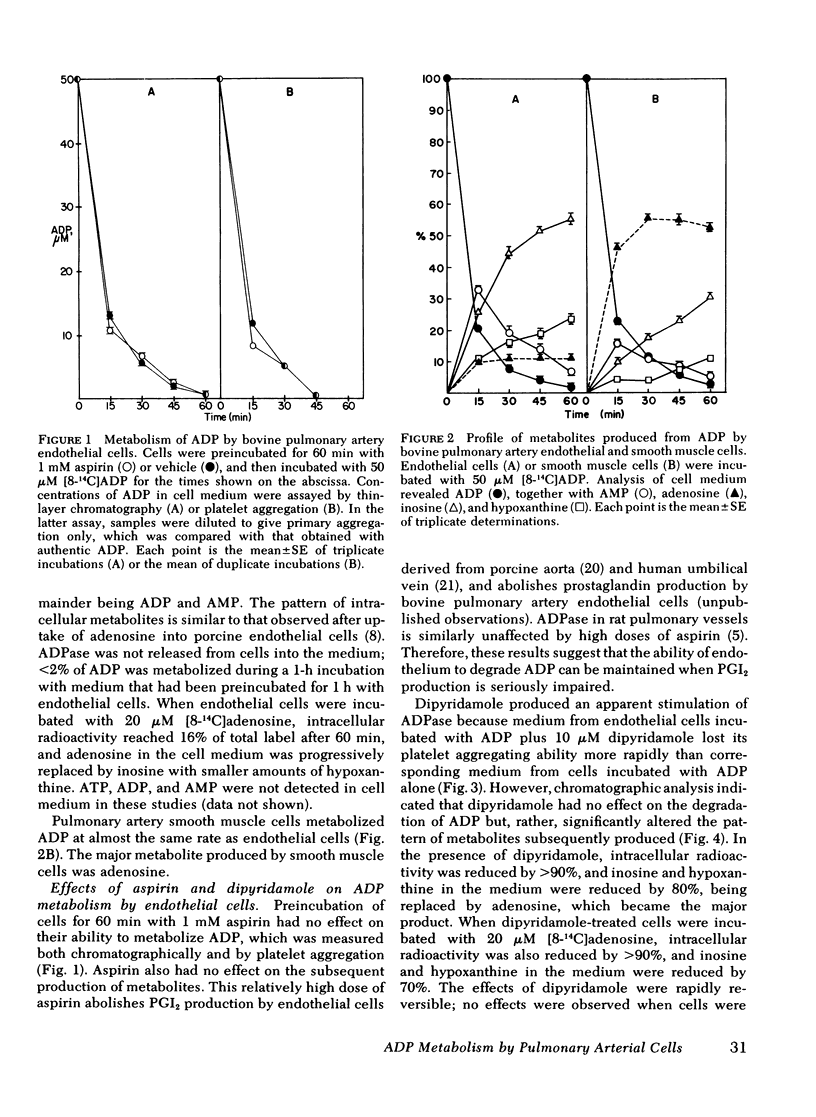
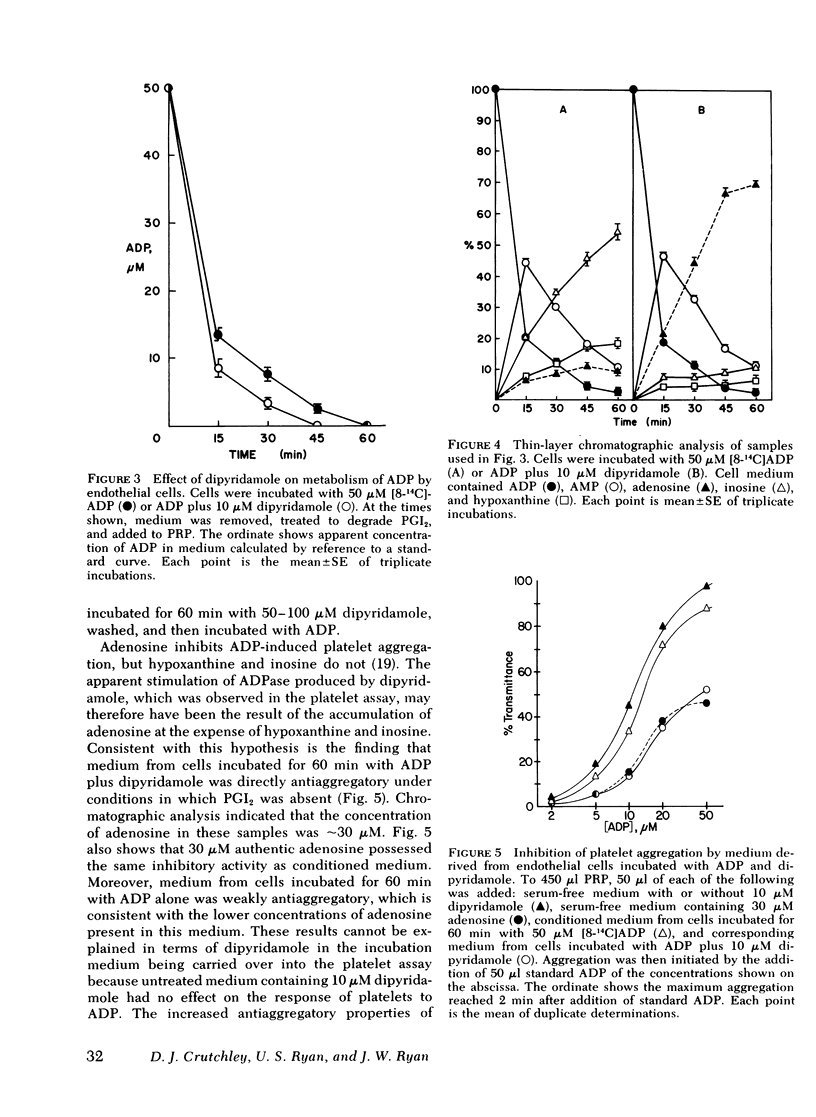
To improve understanding of the mechanisms by which ADP is degraded during passage through the pulmonary vascular bed, we examined cultured endothelial and smooth muscle cells of bovine pulmonmary artery for their abilities to metabolize [8-14C]ADP. ADP is rapidly converted to AMP and then to adenosine, hypoxanthine, and inosine. Inosine is the major metabolite produced by endothelial cells. Radioactivity (5-10%) is accumulated intracellularly primarily as ATP. Medium containing 50 micro M ADP incubated with endothelial cells rapidly loses its ability to aggregate platelets and becomes antiaggregatory under conditions in which prostacyclin is absent. The antiaggregatory activity is probably the result of accumulated adenosine. 10 micro M dipyridamole inhibits cellular uptake of radioactivity by greater than 90%, and inosine in the medium is largely replaced by adenosine. This is accompanied by increased anti-aggregatory activity of conditioned medium, which can be matched by authentic adenosine at the same concentration. 1 mM aspirin had no effect on the metabolism of ADP by endothelial cells. Our results suggest: (a) Metabolism of ADP during passage through the lung is mainly the result of endothelial ADPase. (b) ADP released from aggregating platelets can be converted to the antiaggregatory substance, adenosine. Dipyridamole may exert some of its antithrombotic actions by preventing the intracellular uptake of adenosine, thereby increasing its concentration near the site of thrombus formation. (c) The ability of the vessel wall to degrade ADP should not be compromised by the use of aspirin as an antithrombotic drug. (d) Endothelium may retain some of its antithrombogenicity when prostacyclin generation is impaired.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORN G. V., CROSS M. J. THE AGGREGATION OF BLOOD PLATELETS. J Physiol. 1963 Aug;168:178–195. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenziger N. L., Dillender M. J., Majerus P. W. Cultured human skin fibroblasts and arterial cells produce a labile platelet-inhibitory prostaglandin. Biochem Biophys Res Commun. 1977 Sep 9;78(1):294–301. doi: 10.1016/0006-291x(77)91253-0. [DOI] [PubMed] [Google Scholar]
- Best L. C., McGuire M. B., Jones P. B., Holland T. K., Martin T. J., Preston F. E., Segal D. S., Russell R. G. Mode of action of dipyridamole on human platelets. Thromb Res. 1979;16(3-4):367–379. doi: 10.1016/0049-3848(79)90084-7. [DOI] [PubMed] [Google Scholar]
- Bloom D. S., Cole A. W., Palmer T. N. The protective action of inosine on isolated arteries in hypoxia. Br J Pharmacol. 1979 Apr;65(4):587–592. doi: 10.1111/j.1476-5381.1979.tb07868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. R., Lewis G. P., Lieberman G. E., Webb H., Westwick J. ADP metabolism in vascular tissue, a possible thrombo-regulatory mechanism. Thromb Res. 1979;14(6):901–914. doi: 10.1016/0049-3848(79)90008-2. [DOI] [PubMed] [Google Scholar]
- Crutchley D. J., Eling T. E., Anderson M. W. ADPase activity of isolated perfused rat lung. Life Sci. 1978 Apr 24;22(16):1413–1420. doi: 10.1016/0024-3205(78)90635-5. [DOI] [PubMed] [Google Scholar]
- Czervionke R. L., Smith J. B., Fry G. L., Hoak J. C., Haycraft D. L. Inhibition of prostacyclin by treatment of endothelium with aspirin. Correlation with platelet adherence. J Clin Invest. 1979 May;63(5):1089–1092. doi: 10.1172/JCI109379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterle Y., Ody C., Ehrensberger A., Stalder H., Junod A. F. Metabolism and uptake of adenosine triphosphate and adenosine by porcine aortic and pulmonary endothelial cells and fibroblasts in culture. Circ Res. 1978 Jun;42(6):869–876. doi: 10.1161/01.res.42.6.869. [DOI] [PubMed] [Google Scholar]
- Dosne A. M., Legrand C., Bauvois B., Bodevin E., Caen J. P. Comparative degradation of adenylnucleotides by cultured endothelial cells and fibroblasts. Biochem Biophys Res Commun. 1978 Nov 14;85(1):183–189. doi: 10.1016/s0006-291x(78)80027-8. [DOI] [PubMed] [Google Scholar]
- FREIMAN D. G., SUYEMOTO J., WESSLER S. FREQUENCY OF PULMONARY THROMBOEMBOLISM IN MAN. N Engl J Med. 1965 Jun 17;272:1278–1280. doi: 10.1056/NEJM196506172722406. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S. Human vascular smooth muscle in culture. Growth and ultrastructure. Lab Invest. 1975 Jul;33(1):16–27. [PubMed] [Google Scholar]
- Glasgow J. G., Schade R., Pitlick F. A. Evidence that ADP hydrolysis by human cells is related to thrombogenic potential1. Thromb Res. 1978 Aug;13(2):255–266. doi: 10.1016/0049-3848(78)90013-0. [DOI] [PubMed] [Google Scholar]
- Gordon J. L., Pearson J. D. Effects of sulphinpyrazone and aspirin on prostaglandin I2 (prostacyclin) synthesis by endothelial cells. Br J Pharmacol. 1978 Dec;64(4):481–483. doi: 10.1111/j.1476-5381.1978.tb17308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Korbut R., Ocetkiewicz A. Generation of prostacyclin by lungs in vivo and its release into the arterial circulation. Nature. 1978 Jun 29;273(5665):765–767. doi: 10.1038/273765a0. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J. Prostacyclin as a circulatory hormone. Biochem Pharmacol. 1979 Nov 1;28(21):3161–3166. doi: 10.1016/0006-2952(79)90055-8. [DOI] [PubMed] [Google Scholar]
- Habliston D. L., Whitaker C., Hart M. A., Ryan U. S., Ryan J. W. Isolation and culture of endothelial cells from the lungs of small animals. Am Rev Respir Dis. 1979 Jun;119(6):853–868. doi: 10.1164/arrd.1979.119.6.853. [DOI] [PubMed] [Google Scholar]
- Kolassa N., Pfleger K., Träm M. Species differences in action and elimination of adenosine after dipyridamole and hexobendine. Eur J Pharmacol. 1971;13(3):320–325. doi: 10.1016/0014-2999(71)90221-4. [DOI] [PubMed] [Google Scholar]
- Kübler W., Spieckermann P. G., Bretschneider H. J. Influence of dipyridamol (Persantin) on myocardial adenosine metabolism. J Mol Cell Cardiol. 1970 Mar;1(1):23–38. doi: 10.1016/0022-2828(70)90026-x. [DOI] [PubMed] [Google Scholar]
- Lieberman G. E., Lewis G. P., Peters T. J. A membrane-bound enzyme in rabbit aorta capable of inhibiting adenosine-diphosphate-induced platelet aggregation. Lancet. 1977 Aug 13;2(8033):330–332. doi: 10.1016/s0140-6736(77)91488-x. [DOI] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Moncada S., Korbut R., Bunting S., Vane J. R. Prostacyclin is a circulating hormone. Nature. 1978 Jun 29;273(5665):767–768. doi: 10.1038/273767a0. [DOI] [PubMed] [Google Scholar]
- Moncada S., Korbut R. Dipyridamole and other phosphodiesterase inhibitors act as antithrombotic agents by potentiating endogenous prostacyclin. Lancet. 1978 Jun 17;1(8077):1286–1289. doi: 10.1016/s0140-6736(78)91269-2. [DOI] [PubMed] [Google Scholar]
- Norman G. A., Follett M. J., Hector D. A. Quantitative thin-layer chromatography of ATP and the products of its degradation in meat tissue. J Chromatogr. 1974 Mar 13;90(1):105–111. doi: 10.1016/s0021-9673(01)94779-x. [DOI] [PubMed] [Google Scholar]
- O'Grady J., Moncada S. Aspirin: A paradoxical effect on bleeding-time. Lancet. 1978 Oct 7;2(8093):780–780. doi: 10.1016/s0140-6736(78)92661-2. [DOI] [PubMed] [Google Scholar]
- Pearson J. D., Carleton J. S., Hutchings A., Gordon J. L. Uptake and metabolism of adenosine by pig aortic endothelial and smooth-muscle cells in culture. Biochem J. 1978 Feb 15;170(2):265–271. doi: 10.1042/bj1700265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J. D., Gordon J. L. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature. 1979 Oct 4;281(5730):384–386. doi: 10.1038/281384a0. [DOI] [PubMed] [Google Scholar]
- Rajah S. M., Crow M. J., Penny A. F., Ahmad R., Watson D. A. The effect of dipyridamole on platelet function: correlation with blood levels in man. Br J Clin Pharmacol. 1977 Apr;4(2):129–133. doi: 10.1111/j.1365-2125.1977.tb00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio V. R., Wiedmeier T., Berne R. M. Nucleoside phosphorylase: localization and role in the myocardial distribution of purines. Am J Physiol. 1972 Mar;222(3):550–555. doi: 10.1152/ajplegacy.1972.222.3.550. [DOI] [PubMed] [Google Scholar]
- Ryan J. W., Chung A., Martin L. C., Ryan U. S. New substrates for the radioassay of angiotensin converting enzyme of endothelial cells in culture. Tissue Cell. 1978;10(3):555–562. doi: 10.1016/s0040-8166(16)30348-2. [DOI] [PubMed] [Google Scholar]
- Ryan J. W., Smith U. Metabolism of adenosine 5'-monophosphate during circulation through the lungs. Trans Assoc Am Physicians. 1971;84:297–306. [PubMed] [Google Scholar]
- Ryan U. S., Clements E., Habliston D., Ryan J. W. Isolation and culture of pulmonary artery endothelial cells. Tissue Cell. 1978;10(3):535–554. doi: 10.1016/s0040-8166(16)30347-0. [DOI] [PubMed] [Google Scholar]
- Schrader J., Berne R. M., Rubio R. Uptake and metabolism of adenosine by human erythrocyte ghosts. Am J Physiol. 1972 Jul;223(1):159–166. doi: 10.1152/ajplegacy.1972.223.1.159. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Creveling C. R., Daly J. Stimulated formation of adenosine 3',5'-cyclic phosphate in cerebral cortex: synergism between electrical activity and biogenic amines. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1033–1040. doi: 10.1073/pnas.65.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]


