Abstract
Objectives To assess the associations between both uric acid levels and hyperuricaemia, with ischaemic heart disease and blood pressure, and to explore the potentially confounding role of body mass index.
Design Mendelian randomisation analysis, using variation at specific genes (SLC2A9 (rs7442295) as an instrument for uric acid; and FTO (rs9939609), MC4R (rs17782313), and TMEM18 (rs6548238) for body mass index).
Setting Two large, prospective cohort studies in Denmark.
Participants We measured levels of uric acid and related covariables in 58 072 participants from the Copenhagen General Population Study and 10 602 from the Copenhagen City Heart Study, comprising 4890 and 2282 cases of ischaemic heart disease, respectively.
Main outcome Blood pressure and prospectively assessed ischaemic heart disease.
Results Estimates confirmed known observational associations between plasma uric acid and hyperuricaemia with risk of ischaemic heart disease and diastolic and systolic blood pressure. However, when using genotypic instruments for uric acid and hyperuricaemia, we saw no evidence for causal associations between uric acid, ischaemic heart disease, and blood pressure. We used genetic instruments to investigate body mass index as a potentially confounding factor in observational associations, and saw a causal effect on uric acid levels. Every four unit increase of body mass index saw a rise in uric acid of 0.03 mmol/L (95% confidence interval 0.02 to 0.04), and an increase in risk of hyperuricaemia of 7.5% (3.9% to 11.1%).
Conclusion By contrast with observational findings, there is no strong evidence for causal associations between uric acid and ischaemic heart disease or blood pressure. However, evidence supports a causal effect between body mass index and uric acid level and hyperuricaemia. This finding strongly suggests body mass index as a confounder in observational associations, and suggests a role for elevated body mass index or obesity in the development of uric acid related conditions.
Introduction
Uric acid is a powerful antioxidant and has been proposed to protect against cardiovascular disease and some cancers.1 In humans and great apes, the gene for urease or urate oxidase (which is expressed most in the kidney and liver2) is a non-functioning pseudogene. The absence of a functional unit disables this locus and results in uniquely high levels of serum urate, with about 5-25% of humans having impaired renal excretion and ultimately hyperuricaemia.3 The relative fitness advantages gained from the antioxidant properties of uric acid have been suggested to explain why the genetic precondition for such levels persists.4 5
Despite the expectation that the antioxidant properties of uric acid might have a protective effect against cardiovascular disease, studies have reported associations with a greater risk of ischaemic heart disease, higher blood pressure, and an adverse cardiovascular risk profile.6 7 8 9 10 11 12 13 14 These adverse effects have been confirmed in meta-analyses of prospective studies,13 15 which concluded that hyperuricaemia was associated with increases in risk for cardiovascular outcomes and blood pressure, independently of established risk factors.
A series of hypotheses has suggested why these unexpected positive associations might exist, including the upregulation of renin release and the subsequent cascade related reduction of endothelial function.16 However, in the absence of proven causal associations, a possible explanation could be reverse causality, whereby preclinical atherosclerosis before a diagnosis of ischaemic heart disease could lead to higher levels of uric acid. Confounding could also account for these associations, and it is notable that a pooled adjusted association with ischaemic heart disease was shown to be attenuated by comparison with the unadjusted association. For the incidence of ischaemic heart disease, without adjustment for risk factors of the disease, the relative risk was 1.34 (95% confidence interval 1.19 to 1.49), compared with 1.09 (1.03 to 1.16) after adjustment.16
Mendelian randomisation can account for unmeasured confounding and reverse causation by using genotypes robustly associated with the risk factor of interest as instrumental variables. This approach can be used to test and estimate the causal effect between a risk factor and an outcome.17 18 The rs7442295 single nucleotide polymorphism in the SLC2A9 gene (solute carrier family 2, facilitated glucose transporter member 9) has been found to be robustly associated with increased plasma levels of uric acid, hyperuricaemia, urate excretion, and gout in genome wide association studies.3 19 As a result, it has been proposed as an instrument for examining the causal effect of uric acid on disease outcomes.20 Hypothetically, variation at this locus divides the population into non-confounded groups assigned only by genotype that have differing levels of plasma uric acid and are not subject to reverse causation. Given the properties of these groups, the use of SLC2A9 variation as a proxy measurement in this way allows for differences in disease risk or measured phenotype across the groups to be attributed solely to the differences in average levels of uric acid attributable to genotypic variation.
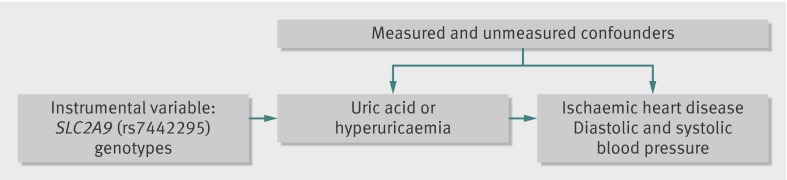
We aimed to investigate whether there was a causal effect of uric acid and hyperuricaemia on ischaemic heart disease and diastolic and systolic blood pressure using a mendelian randomisation approach (fig 1). To determine whether body mass index might be a particularly potent confounder of this association, we also examined the effect of body mass index on uric acid levels using genetic variants that have been shown to be robustly associated with body mass index.
Fig 1 Directed acyclic graph for instrumental variable analysis using SLC2A9 (rs7442295) as an instrument for the effect of uric acid and hypericaemia on ischaemic heart disease and blood pressure
Methods
Population
Data were collected from two large cohorts, the Copenhagen General Population Study and the Copenhagen City Heart Study. The studies were approved by the Danish ethical committees and Herlev Hospital, Copenhagen University Hospital, and have been described previously.21 All participants in both studies were white and of Danish descent. The Copenhagen General Population Study is a prospective study with participants randomly selected from the Danish Civil Registration System. Eligible participants were aged 20 years and older and resident in greater Copenhagen; ongoing recruitment began in 2003. The Copenhagen City Heart Study is a prospective study of a cohort aged 20 years and older, randomly selected from the population of the city of Copenhagen followed from baseline assessment in 1976-78, with follow-up examinations in 1981-83, 1991-94, and 2001-03. The Copenhagen General Population Study and Copenhagen City Heart Study had 58 072 and 10 602 eligible participants, respectively.
Ischaemic heart disease
Information on the diagnosis of ischaemic heart disease was collected and verified from 1977 until May 2011 by reviewing all hospital admissions and diagnoses entered in the national Danish Patient Registry and all causes of death entered in the national Danish Causes of Death Registry. Ischaemic heart disease was angina pectoris or myocardial infarction (according to code 410 from ICD-8 (international classification of diseases, eighth revision) and codes I21-I22 from ICD-10), based on characteristic chest pain, electrocardiographic changes, or elevated concentrations of cardiac enzymes after changes in diagnostic criteria over time.22 We used ischaemic heart disease rather than myocardial infarction to obtain the greatest possible statistical power. Importantly, however, about 60% of all individuals with ischaemic heart disease also had a myocardial infarction. Follow-up was 100% complete—that is, no individual was lost to follow-up in either study.
Systolic and diastolic blood pressure
In both studies, systolic and diastolic blood pressure was measured by clinic assessment at baseline, as described previously.23 For participants who were receiving antihypertensive treatment, we made adjustments for systolic and diastolic blood pressure by adding a constant value of 10 mm Hg and 5 mm Hg, respectively.24
Body mass index
To exclude the influence of age and sex on our results, we standardised body mass index into age and sex adjusted z scores within each study (web table 1). One z score corresponds to a standard deviation of 4 units of body mass index; thus, for straightforward interpretation of results, estimates in z score units were converted to the scale for body mass index.
Plasma uric acid
Plasma uric acid was measured by clinic assessment at baseline, using colorimetry on a Konelab Autoanalyser (Thermo Scientific). In the Copenhagen City Heart Study, participants had up to four examinations, and measurements of plasma uric acid were available from the 1991-94 and 2001-03 examinations. Each participant’s measurement of uric acid—and hence hyperuricaemia status—that was recorded closest before their event of ischaemic heart disease was used. Hyperuricaemia was defined as plasma levels of uric acid greater than 0.4 mmol/L (or 6.72 mg/dL, which sits at the approximate limit of solubility for urate).25 Plasma uric acid was internally standardised by 5 year age groups and sex using a z score (web table 1) over the whole sample; results were therefore relative risks per one standard deviation increase in uric acid.
Genotyping
Genotyping was conducted blind to phenotypic data using an ABI PRISM 7900HT sequence detection system (Applied Biosystems) with TaqMan. Genotyping was verified by DNA sequencing in at least 30 individuals, and reruns were performed twice—resulting in successful rates of genotype collection of more than 99.96%. Genotypes of SLC2A9 (rs7442295) were coded by applying an additive genetic model based on information from a genome wide association study.19 We used specific polymorphisms (FTO (rs9939609), MC4R (rs17782313), and TMEM18 (rs6548238)) as instruments for body mass index. We selected these polymorphisms because they have the largest known common effect sizes for association with body mass index in European populations.26
Other covariables
In both studies, data were available for age, sex, smoking status, years of education, and income from questionnaires at clinic assessment at baseline. Smoking was categorised as self reported ever smoking. We grouped education according to years spent in school (<10, 10 to <13, and ≥13 years), and annual income by Danish krones (<100 000, 100 000 to 400 000, 400 000 to 600 000, and >600 000). Observational associations were estimated with and without adjustment for potential confounding factors.
Statistical analysis
For genetic variants, we investigated deviation from the Hardy-Weinberg equilibrium using a Pearson χ2 test. Cox proportional hazards regressions was used to estimate observational hazard ratios for ischaemic heart disease per standard deviation change in uric acid concentration, by hyperuricaemia status and by genotype at SLC2A9 (rs7442295). We used linear regression to estimate observational associations between blood pressure and uric acid (and therefore hyperuricaemia status), by genotype at SLC2A9 (rs7442295). Logistic regression estimated associations between genotype and binary covariates. We used ordered logistic regression to estimate associations between genotype and ordered categorical variables.
Instrumental variable analysis generated causal effects in a mendelian randomisation framework. Estimates of causal hazard ratios for risk of ischaemic heart disease were calculated by using a ratio estimator. These estimates were derived by first dividing the log of the hazard ratio for genotype-ischaemic heart disease by the genotype-exposure coefficient—the resulting value then underwent exponentiation. Standard errors for the log hazard ratios of instrumental variables were derived using the delta method.27 Instrumental variable estimation for outcome variables, diastolic blood pressure, systolic blood pressure, uric acid concentration, and hyperuricaemia were also performed using the two stage least squares estimator.28
We pooled estimates from the two studies using an inverse variance weighted, meta-analysis model implemented in the user written “metan” Stata command.29 Since there were only two studies, we used the fixed effects meta-analysis model. All analyses were performed using Stata (version 11.2).
Results
The 58 072 eligible participants in the Copenhagen General Population Study included 4890 (8%) with ischaemic heart disease in 141 559 person years at risk, of whom 1682 participants had incident cases. Analyses included 10 602 participants from the Copenhagen City Heart Study from the 1991-94 and 2001-03 examinations, with 2282 (16%) cases of ischaemic heart disease in 79 979 person years at risk, of which 1748 were incident cases.
The mean level of plasma uric acid was 0.30 mmol/L (standard deviation 0.09 mmol/L) in the Copenhagen General Population Study, and 0.31 mmol/L (0.09 mmol/L) in the Copenhagen City Heart Study. Following the definition of hyperuricaemia (uric acid >0.4 mmol/L), 6929 (12%) and 1569 (16%) participants were classified as having hyperuricaemia in the Copenhagen General Population Study and Copenhagen City Heart Study, respectively. Mean diastolic blood pressure was slightly lower in the Copenhagen General Population Study than in the Copenhagen City Heart Study, while mean systolic blood pressure was slightly higher in the Copenhagen General Population Study than in the Copenhagen City Heart Study (table 1).
Table 1.
Baseline characteristics of participants
| Copenhagen General Population Study (n=58 072) | Copenhagen City Heart Study (n=10 602) | |
|---|---|---|
| Ischaemic heart disease | ||
| Prevalent cases | 3208 (6) | 534 (5) |
| Incident cases | 1682 (3) | 1748 (16) |
| Total | 4890 (8) | 2282 (22) |
| Uric acid (mmol/L) | 0.30 (0.09) | 0.31 (0.09) |
| Hyperuricaemia | 6929 (12) | 1569 (16) |
| Mean blood pressure (mm Hg) | ||
| Diastolic | 84 (12) | 86 (12) |
| Systolic | 143 (23) | 140 (18) |
| Body mass index | 26 (4) | 25 (4) |
| Age (years) | 57 (14) | 60 (16) |
| Male sex | 25 921 (45) | 4652 (44) |
| Ever smoked | 34 844 (60) | 5823 (56) |
| Education (years) | ||
| <10 | 17 071 (30) | 5393 (52) |
| 10 to <13 | 30 983 (54) | 3856 (37) |
| ≥13 | 9717 (17) | 1195 (11) |
| Income (Danish krones) | ||
| <100 000 | 1130 (2) | 1884 (18) |
| 100 000 to 400 000 | 22 518 (39) | 5641 (54) |
| 400 000 to 600 000 | 22 742 (40) | 2539 (24) |
| >600 000 | 10 785 (19) | 334 (3) |
Data are mean (standard deviation) for continuous variables and number (%) of non-missing observations for each binary variable.
Observational estimates of association between uric acid, ischaemic heart disease, and blood pressure
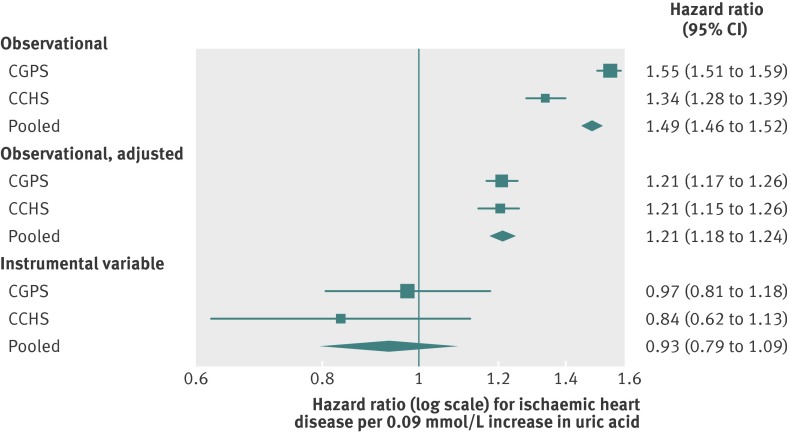
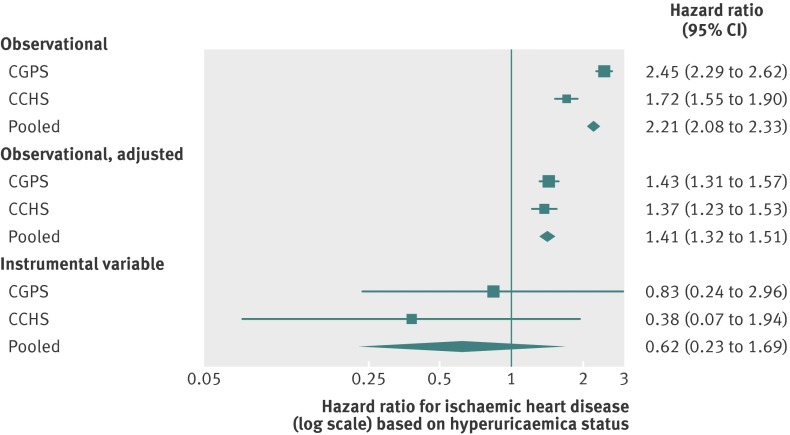
Based on observational estimates, an increase in uric acid of one standard deviation was associated with hazard ratios for ischaemic heart disease of 1.55 (95% confidence interval 1.51 to 1.59) and 1.34 (1.28 to 1.39) in the Copenhagen General Population Study and Copenhagen City Heart Study, respectively. These ratios gave a pooled estimate of 1.49 (1.46 to 1.52; fig 2). This association was attenuated after adjustment for age, sex, smoking, education, and income (pooled hazard ratio 1.21 (1.18 to 1.24)). We found similar patterns for associations of hyperuricaemia with ischaemic heart disease (estimate of pooled hazard ratio for exposed participants 2.21 (2.08 to 2.33); fig 3). This hazard ratio attenuated to 1.41 (1.32 to 1.51) on adjustment for age, sex, smoking, education, and income. In the sensitivity analysis using incident cases of ischaemic heart disease only, estimates of disease risk were attenuated but showed the same pattern across the Copenhagen General Population Study and Copenhagen City Heart Study (web fig 1).
Fig 2 Forest plot showing observational and instrumental variable estimates of the effect of standardised urate on ischaemic heart disease. Observational adjusted estimates adjusted for age, sex, smoking, education, and income. CGPS=Copenhagen General Population Study; CCHS=Copenhagen City Heart Study; 0.09 mmol/L change in uric acid represents one standard deviation
Fig 3 Forest plot showing observational and instrumental variable estimates of the effect of hyperuricaemia status on ischaemic heart disease. Observational adjusted estimates adjusted for age, sex, smoking, education, and income. CGPS=Copenhagen General Population Study; CCHS=Copenhagen City Heart Study; 0.09 mmol/L change in uric acid represents one standard deviation
A standard deviation increase in uric acid level was associated with an elevation in diastolic blood pressure of 2.42 mm Hg (95% confidence interval 2.32 to 2.51, pooled estimate; web fig 2A). This association was attenuated, after adjustment, to an increase of 1.56 mm Hg (1.45 to 1.67). Participants with hyperuricaemia had an average increase of 4.61 mm Hg (4.31 to 4.90) in diastolic blood pressure (pooled estimate; web fig 2B). This estimate also attenuated on adjustment (2.66 (2.36 to 2.96)). A similar pattern of observational associations was seen between uric acid and hyperuricaemia and systolic blood pressure (web fig 3).
Association between SLC2A9 (rs7442295), uric acid and hyperuricaemia, and potential confounding factors
Both the Copenhagen General Population Study and Copenhagen City Heart Study showed no strong evidence for genotypes including SLC2A9 (rs7442295) that deviated from the Hardy-Weinberg equilibrium (web table 2). The association between variation at SLC2A9 (rs7442295) and uric acid was roughly linear (web fig 4). Mean levels of uric acid showed an increase in standard deviation of 0.26 (95% confidence interval 0.24 to 0.27) and 0.25 (0.21 to 0.28) for each additional A allele in the Copenhagen General Population Study and Copenhagen City Heart Study, respectively (table 2). SLC2A9 (rs7442295) accounted for about 2% of the variance in uric acid levels. Variation at SLC2A9 (rs7442295) was associated with an increase of about 5% in the risk of hyperuricaemia in both samples.
Table 2.
Associations of SLC2A9 (rs7442295)* with exposures and outcomes
| Copenhagen General Population Study (n=58 072) | Copenhagen City Heart Study (n=10 602) | ||||||
|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | F | R2 | Hazard ratio (95% CI) | F | R2 | ||
| Exposures | |||||||
| Standardised uric acid | 0.26 (0.24 to 0.27) | 1304 | 0.022 | 0.25 (0.21 to 0.28) | 204 | 0.020 | |
| Hyperuricaemia | 0.04 (0.03 to 0.04) | 272 | 0.005 | 0.05 (0.03 to 0.06) | 52 | 0.005 | |
| Outcomes | |||||||
| Ischaemic heart disease | 0.99 (0.95 to 1.04) | — | — | 0.96 (0.89 to 1.03) | — | — | |
| Blood pressure (mm Hg) | |||||||
| Diastolic | 0.11 (−0.07 to 0.29) | 1 | <0.0001 | 0.40 (0.00 to 0.80) | 4 | 0.0004 | |
| Systolic | 0.17 (−0.17 to 0.51) | 1 | <0.0001 | 0.12 (−0.50 to 0.73) | 0.1 | <0.0001 | |
| Body mass index | −0.01 (−0.07 to 0.05) | 0.1 | <0.0001 | −0.07 (−0.20 to 0.06) | 1 | 0.0001 | |
Data are hazard ratio (95% confidence interval), F statistic from analysis of variance F test, and R2 coefficient of determination.
*Genotypes under an additive model.
In the Copenhagen General Population Study and Copenhagen City Heart Study, SLC2A9 (rs7442295) was not associated with potential confounders: age, sex, body mass index, smoking, education, and income (web table 2). By contrast, ischaemic heart disease, systolic blood pressure, and diastolic blood pressure showed strong associations with these potential confounders (web table 3). Uric acid and hyperuricaemia exposures also showed associations with these potential confounders (web table 4).
Association of SLC2A9 (rs7442295) with health outcomes
Variation at SLC2A9 (rs7442295) was not associated with ischaemic heart disease in either the Copenhagen General Population Study (hazard ratio 0.99 (95% confidence interval 0.95 to 1.04)) or the Copenhagen City Heart Study (0.96 (0.89 to 1.03)). Similarly, we did not find any associations between SLC2A9 (rs7442295) and either diastolic or systolic blood pressure (table 2).
Instrumental variable estimates of the causal effect of uric acid and hyperuricaemia on ischaemic heart disease, diastolic blood pressure, and systolic blood pressure
Instrumental variable analysis, when pooled, gave an estimate of the causal hazard ratio for ischaemic heart disease of 0.93 (95% confidence interval 0.79 to 1.09) per standard deviation increase in uric acid (fig 2). The corresponding estimate of the effect of hyperuricaemia status on risk of ischaemic heart disease was 0.62 (0.23 to 1.69; fig 3). In the sensitivity analysis using incident cases of ischaemic heart disease only, we found weaker hazard ratios per standard deviation increase in uric acid and hyperuricaemia (0.87 (0.69 to 1.09) and 0.42 (0.10 to 1.68), respectively; web fig 1).
Instrumental variable estimates of the causal effect of uric acid on diastolic blood pressure failed to show convincing evidence of an effect and had wide confidence intervals. A standard deviation increase in uric acid was estimated to increase diastolic blood pressure by 0.63 mm Hg (95% confidence interval −0.04 to 1.29). This point estimate was largely driven by the association in Copenhagen City Heart Study, and was weaker than the adjusted observational estimate (1.56 mm Hg (1.45 to 1.67); web fig 2A). Similarly, owing to a stronger effect in the Copenhagen City Heart Study, the instrumental variable estimate showed an increase of 4.56 mm Hg (0.01 to 9.12) in diastolic blood pressure in patients with hyperuricaemia (web fig 2B).
We saw no evidence of a causal effect of uric acid or hyperuricaemia on systolic blood pressure (web fig 3). The instrumental variable estimate of the causal effect of uric acid on systolic blood pressure, when pooled, was 0.65 mm Hg (95% confidence interval −0.54 to 1.85) per standard deviation increase in uric acid. In the Copenhagen General Population Study, the instrumental variable estimate of the causal effect of hyperuricaemia on systolic blood pressure was similar to the observational estimates. However, this instrumental variable estimate was much smaller in the Copenhagen City Heart Study, producing a pooled estimate of 4.31 (−3.72 to 12.33).
Mendelian randomisation analysis of the causal effect of body mass index on uric acid and hyperuricaemia
Observational estimates showed an association of body mass index, a potential confounding factor, with both standardised uric acid and hyperuricaemia. This association remained on adjustment for potential confounding factors. For example, a standard deviation elevation in uric acid was associated with a unit increase of 1.06 (95% confidence interval 1.03 to 1.09) in body mass index, which increased to 1.43 (1.40 to 1.47) on adjustment for the other potential confounders (web fig 5). Furthermore, when assessing the observational relations between uric acid and hyperuricaemia with risk of ischaemic heart disease, diastolic blood pressure, and systolic blood pressure, inclusion of body mass index as an additional covariable resulted in the attenuation of association. Specifically, associations for a standard deviation elevation in uric acid were reduced to a hazard ratio for ischaemic heart disease of 1.16 (1.12 to 1.19), a change of 0.60 mm Hg (0.49 to 0.71) in diastolic blood pressure, and a change of 1.37 mm Hg (1.18 to 1.56) in systolic blood pressure. Hyperuricaemia was associated with a hazard ratio for ischaemic heart disease of 1.30 (1.21 to 1.39), a change of 1.03 mm Hg (0.74 to 1.33) in diastolic blood pressure, and a change of 2.41 mm Hg (1.91 to 2.91) in systolic blood pressure (pooled estimates) after accounting for body mass index (fig 2 and 3; web figs 2 and 3).
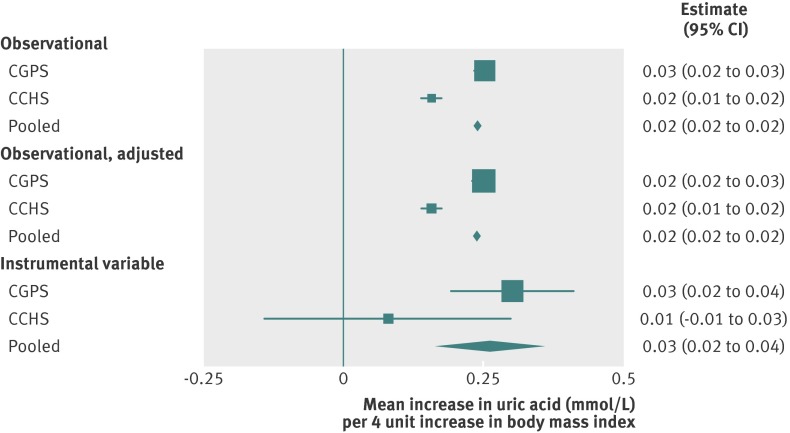
In a mendelian randomisation analysis, we reciprocally30 assessed whether uric acid had a causal effect on body mass index and whether body mass index had a causal effect on uric acid. Evidence of a causal association between body mass index and uric acid and hyperuricaemia was only found in the direction implicating body mass index as a causal factor. Instrumental variable estimates of the effect of body mass index on uric acid and hyperuricaemia indicated that a standard deviation increase in body mass index (that is, 4 units) causes an increase of 0.03 mmol/L (95% confidence interval 0.02 to 0.04) in uric acid and a 7.5% increase (3.9% to 11.1%) in risk of hyperuricaemia (figs 4 and 5). By contrast, instrumental variable estimates of the effect of either uric acid or hyperuricaemia status on body mass index, which used genotypes with SLC2A9 (rs7442295) as instruments for these measures, did not suggest a causal effect on body mass index (web fig 5).
Fig 4 Forest plot of observational and instrumental variable estimates of a 4 unit increase in body mass index on standardised urate converted to mmol/L. Observational adjusted estimates adjusted for age, sex, smoking, education, and income. CGPS=Copenhagen General Population Study; CCHS=Copenhagen City Heart Study
Fig 5 Forest plot of observational and instrumental variable estimates of a 4 unit increase in body mass index on hyperuricaemia status. Observational adjusted estimates adjusted for age, sex, smoking, education, and income. CGPS=Copenhagen General Population Study; CCHS=Copenhagen City Heart Study
Discussion
Using data from two large prospective cohort studies, we found no evidence of a causal effect of uric acid or hyperuricaemia on the risk of ischaemic heart disease. Unadjusted observational analyses found an increase of 34-55% in risk of ischaemic heart disease per standard deviation increase in uric acid. These estimates attenuated slightly on adjustment for established cardiovascular risk factors. The same trend was present in our estimates of the association of uric acid and hyperuricaemia on systolic blood pressure. However, mendelian randomisation estimates showed no evidence of an effect of elevated uric acid (and hyperuricaemia) on the risk of ischaemic heart disease or elevated blood pressure.
In view of the weak evidence supporting causal associations, further explanation for known observational associations was sought through the analysis of body mass index as a confounding factor. Body mass index is an established risk factor for both blood pressure and ischaemic heart disease observationally,31 32 and there is causal evidence pertaining to the effect of body mass index on ischaemic heart disease, dystolic blood pressure, and systolic blood pressure.33 34 It was anticipated and subsequently shown that body mass index exerts a causal and independent effect on levels of uric acid and the risk of hyperuricaemia. Indeed, these instrumental variable estimates fall into the context of a broader literature on the observational association between elevated body mass index and uric acid levels, including that using a mendelian randomisation approach.35 36 37
Comparison with other studies
The mendelian randomisation estimates presented here broadly agree with several recent studies. Stark and colleagues found that SLC2A9 (rs7442295) was not directly associated with coronary artery disease in a case-control study.38 Furthermore, plasma uric acid was not associated with coronary heart disease or ischaemic heart disease in two large cohort studies.39 40 More recent evidence using SLC2A9 variation as an instrument for plasma uric acid failed to find evidence of a causal effect of uric acid on metabolic syndrome,41 and further direct analyses have found that SLC2A9 variants are unlikely to be associated with blood pressure.42 Use of a genetic score for hyperuricaemia in a mendelian randomisation approach investigating its association with gout, blood pressure, glucose, chronic kidney disease, and coronary heart disease also revealed no evidence of causal effects.43 Despite these findings, another SLC2A9 locus (rs16890979) has been associated with blood pressure,44 suggesting that there is a need to undertake further analyses concerning the possible role of this genomic region and transport system.
Strengths and limitations of the study
Analyses were undertaken within large, ethnically homogeneous, clinically assessed case series with access to control sets of comparable quality. The nature and size of the existing sample place it particularly well for the undertaking of mendelian randomisation experiments. However, the potential for the complicating effects of linkage disequilibrium, pleiotropy, or the nature of genotypic effect are difficult to account for.45 But in favour of the use of SLC2A9 variation as an instrument for uric acid levels, this approach does encode for a high capacity sugar transporter, and functional analyses have shown it to have a preferential action on urate.3 Yet despite this advantage, causal variants for the reliable effects on uric acid levels have yet to be fully established, and the role of differential isoforms of this gene in the transport of sugars overall could be complex.
Another potential limitation was that we did not have data available to identify participants receiving uric acid lowering drugs at the time of measurement. Although this will have been a small fraction of our total sample size and unlikely to have materially altered our main results, it may have led to underestimates of both observational and instrumental variable estimates.
Conclusions and clinical implications of results
Overall, mendelian randomisation estimates found no evidence of causal effects of either uric acid or hyperuricaemia on risk of either ischaemic heart disease or raised blood pressure. Our Mendelian randomisation results alone suggest that uric acid is of limited clinical interest in ischaemic heart disease or blood pressure. However, there is strong evidence for an effect of body mass index on both uric acid and hyperuricaemia—indicating that body mass index is an important confounding factor to observational association analyses of uric acid and hyperuricaemia. This finding contrasts the notion of body mass index operating as a mediator or being on the causal pathway from uric acid to vascular outcomes. In this case, one would expect to find evidence for a causal association both for body mass index and uric acid level, as opposed to just one of these risk factors.
This study clearly shows the value of mendelian randomisation to help dissect complex networks of risk factor association by using independent proxies or instrumental variables for risk factors of interest. Furthermore, our findings suggest that interventions to reduce body mass index could help improve management of gout and related conditions such as urolithiasis.
What is already known on this topic
Uric acid has been suggested to protect against cardiovascular disease
Observational studies have suggested that increased levels of uric acid is associated with ischaemic heart disease and blood pressure; but owing to confounding, bias, and reverse causation, such associations can be difficult to interpret
The SLC2A9 gene has been reliably associated with circulating levels of uric acid, and has been proposed as an instrument to investigate causal associations with blood pressure and ischaemic heart disease
What this study adds
Genetic variation at the SLC2A9 gene shows little evidence of a causal association between increased levels of uric acid, raised blood pressure, and risk of ischaemic heart disease
However, causal analysis of body mass index shows strong evidence of an effect of body mass index on uric acid levels, suggesting considerable confounding in observational associations
Mendelian randomisation analysis suggests that uric acid is of limited clinical interest in ischaemic heart disease or blood pressure. But interventions to reduce body mass index could help improve the management of gout and related conditions such as urolithiasis
We thank Dorthe Uldall Andersen for her excellent genotyping, and the staff and participants of the Copenhagen General Population Study and Copenhagen City Heart Study for their important contributions.
Contributors: NJT, GDS, and BGN conceived and coordinated the investigation. NJT and TMP wrote the manuscript. MB was responsible for the preparation of data and TMP, BGN, MB, AT-H, GDS, DAL, and NJT undertook revisions and contributed intellectually to the development of this paper.
Funding: NJT, DAL, and GDS work in the UK Medical Research Council Integrative Epidemiology Unit at the University of Bristol. TMP also received support from a UK Medical Research Council grant (G0601625). The Copenhagen General Population Study and Copenhagen City Heart Study are supported by the Danish Heart Foundation, Danish Medical Research Council, Copenhagen County Foundation, and Herlev Hospital, Copenhagen University Hospital. All researchers operate independently of the funding bodies noted in this disclosure.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; NJT, GDS, and DAL are supported by UK Medical Research Council funding; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: The studies were approved by the Danish ethical committees and Herlev Hospital, Copenhagen University Hospital (100.2039/91 and 01- 144/01, Copenhagen and Frederiksberg committee).
Data sharing: Additional data regarding technical details, statistical code, and derivative data is available from the principal investigator at boerge.nordestgaard@regionh.dk. Data access for further analyses is possible through direct collaborative agreement or through locally managed access arranged through the study’s principal investigator.
Cite this as: BMJ 2013;347:f4262
Web Extra. Extra material supplied by the author
Web appendix: Supplementary material
References
- 1.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A 1981;78:6858-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phay JE, Hussain HB, Moley JF. Cloning and expression analysis of a novel member of the facilitative glucose transporter family, SLC2A9 (GLUT9). Genomics 2000;66:217-20. [DOI] [PubMed] [Google Scholar]
- 3.Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, Palmer CNA, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet 2008;40:437-42. [DOI] [PubMed] [Google Scholar]
- 4.Wu X, Muzny DM, Chi Lee C, Thomas Caskey C. Two independent mutational events in the loss of urate oxidase during hominoid evolution. J Mol Evol 1992;34:78-84. [DOI] [PubMed] [Google Scholar]
- 5.Nesse RM, Williams GC. Evolution and healing: the new science of Darwinian medicine. Phoenix, 1996.
- 6.Liese AD, Hense HW, Lowel H, Doring A, Tietze M, Keil U. Association of serum uric acid with all-cause and cardiovascular disease mortality and incident myocardial infarction in the MONICA Augsburg cohort. Epidemiology 1999;10:391-7. [DOI] [PubMed] [Google Scholar]
- 7.Alderman M, Aiyer KJV. Uric acid: role in cardiovascular disease and effects of losartan. Curr Med Res Opin 2004;20:369-79. [DOI] [PubMed] [Google Scholar]
- 8.Sundstrom J, Sullivan L, D’Agostino RB, Levy D, Kannel WB, Vasan RS. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension 2005;45:28-33. [DOI] [PubMed] [Google Scholar]
- 9.Perlstein TS, Gumieniak O, Williams GH, Sparrow D, Vokonas PS, Gaziano M, et al. Uric acid and the development of hypertension. Hypertension 2006;48:1031-6. [DOI] [PubMed] [Google Scholar]
- 10.Bos MJ, Koudstaal PJ, Hofman A, Witteman JCM, Breteler MMB. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke 2006;37:1503-7. [DOI] [PubMed] [Google Scholar]
- 11.Deveci OS, Kabakci G, Okutucu S, Tulumen E, Aksoy H, Kaya EB, et al. The association between serum uric acid level and coronary artery disease. Int J Clin Pract 2010;64:900-7. [DOI] [PubMed] [Google Scholar]
- 12.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality: the NHANES I epidemiologic follow-up study, 1971-1992. JAMA 2000;283:2404-10. [DOI] [PubMed] [Google Scholar]
- 13.Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and risk of stroke: a systematic review and meta-analysis. Arthritis Care Res 2009;61:885-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meisinger C, Koenig W, Baumert J, Doring A. Uric acid levels are associated with all-cause and cardiovascular disease mortality independent of systemic inflammation in men from the general population: the MONICA/KORA cohort study. Arterioscler Thromb Vasc Biol 2008;28:1186-92. [DOI] [PubMed] [Google Scholar]
- 15.Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res 2010;62:170-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension 2003;41:1183-90. [DOI] [PubMed] [Google Scholar]
- 17.Davey Smith G, Ebrahim S. ‘Mendelian Randomisation’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 2003;32:1-22. [DOI] [PubMed] [Google Scholar]
- 18.Burgess S, Butterworth A, Malarstig A, Thompson SG. Use of Mendelian randomisation to assess potential benefit of clinical intervention. BMJ 2012;345:e7325. [DOI] [PubMed] [Google Scholar]
- 19.Wallace C, Newhouse SJ, Braund P, Zhang F, Tobin M, Falchi M, et al. Genome-wide association study identifies genes for biomarkers of cardiovascular disease: serum urate and dyslipidemia. Am J Hum Genet 2008;82:139-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright AF, Rudan I, Hastie ND, Campbell H. A ‘complexity’ of urate transporters. Kidney Int 2010;78:446-52. [DOI] [PubMed] [Google Scholar]
- 21.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007;298:299-308. [DOI] [PubMed] [Google Scholar]
- 22.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol 2007;50:2173-95. [DOI] [PubMed] [Google Scholar]
- 23.Sethi AA, Nordestgaard BG, Agerholm-Larsen B, Frandsen E, Jensen G, Tybjaerg-Hansen A. Angiotensinogen polymorphisms and elevated blood pressure in the general population: the Copenhagen City Heart Study. Hypertension 2001;37:875-81. [DOI] [PubMed] [Google Scholar]
- 24.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med 2005;24:2911-35. [DOI] [PubMed] [Google Scholar]
- 25.Becker MA, Schumacher HR Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 2005;353:2450-61. [DOI] [PubMed] [Google Scholar]
- 26.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 2010;42:937-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas DC, Lawlor DA, Thompson JR. Re: Estimation of bias in nongenetic observational studies using “Mendelian triangulation” by Bautista et al. Ann Epidemiol 2007;17:511-3. [DOI] [PubMed] [Google Scholar]
- 28.Angrist JD, Imbens GW. Two-stage least squares estimation of average causal effects in models with variable treatment intensity. J Am Stat Soc 1995;90:431-42. [Google Scholar]
- 29.Harris R. metan: fixed- and random-effects meta-analysis. Stata J 2008;8:3-28. [Google Scholar]
- 30.Timpson NJ, Nordestgaard BG, Harbord RM, Zacho J, Frayling TM, Tybjarg-Hansen A, et al. C-reactive protein levels and body mass index: elucidating direction of causation through reciprocal Mendelian randomization. Int J Obes 2011;35:300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prospective Studies Collaboration, Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng W, McLerran DF, Rolland B, Zhang X, Inoue M, Matsuo K, et al. Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med 2011;364:719-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Timpson NJ, Harbord R, Davey Smith G, Zacho J, Tybjaerg-Hansen A, Nordestgaard BG. Does greater adiposity increase blood pressure and Hypertension risk? Mendelian randomization using the FTO/MC4R genotype. Hypertension 2009;54:84-90. [DOI] [PubMed] [Google Scholar]
- 34.Nordestgaard BG, Palmer TM, Benn M, Zacho J, Tybjaerg-Hansen A, Davey Smith G, et al. The effect of elevated body mass index on ischemic heart disease risk: causal estimates from a Mendelian randomisation approach. PLoS Med 2012;9:e1001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brandstatter A, Kiechl S, Kollerits B, Hunt SC, Heid IM, Coassin S, et al. Sex-specific association of the putative fructose transporter slc2a9 variants with uric acid levels is modified by BMI. Diabetes Care 2008;31:1662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyngdoh T, Vuistiner P, Marques-Vidal P, Rousson V, Waeber Gr, Vollenweider P, et al. Serum uric acid and adiposity: deciphering causality using a bidirectional Mendelian randomization approach. PLoS One 2012;7:e39321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishizaka N, Ishizaka Y, Toda A, Tani M, Koike K, Yamakado M, et al. Changes in waist circumference and body mass index in relation to changes in serum uric acid in Japanese individuals. J Rheumatol 2010;37:410-6. [DOI] [PubMed] [Google Scholar]
- 38.Stark K, Reinhard W, Grassl M, Erdmann J, Schunkert H, Illig T, et al. Common polymorphisms influencing serum uric acid levels contribute to susceptibility to gout, but not to coronary artery disease. PLoS One 2009;4:e7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med 1999;131:7-13. [DOI] [PubMed] [Google Scholar]
- 40.Strasak A, Ruttmann E, Brant L, Kelleher C, Klenk J, Concin H, et al. Serum uric acid and risk of cardiovascular mortality: a prospective long-term study of 83 683 Austrian men. Clin Chem 2008;54:273-84. [DOI] [PubMed] [Google Scholar]
- 41.McKeigue PM, Campbell H, Wild S, Vitart V, Hayward C, Rudan I, et al. Bayesian methods for instrumental variable analysis with genetic instruments (“Mendelian randomization”): example with urate transporter SLC2A9 as instrumental variable for effect of urate levels on metabolic syndrome. Int J Epidemiol 2010;39:907-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caulfield MJ, Munroe PB, O’Neill D, Witkowska K, Charchar FJ, Doblado M, et al. SLC2A9 is a high-capacity urate transporter in humans. PLoS Med 2008;5:e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Q, Kottgen A, Dehghan A, Smith AV, Glazer NL, Chen MH, et al. Multiple genetic loci influence serum urate levels and their relationship with gout and cardiovascular disease risk factors. Circ Cardiovasc Genet 2010;3:523-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parsa A, Brown E, Weir MR, Fink JC, Shuldiner AR, Mitchell BD, et al. Genotype-based changes in serum uric acid affect blood pressure. Kidney Int 2012;81:502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davey Smith G, Ebrahim S. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol 2004;33:30-42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary material