Abstract
Although radiation-induced bystander effects have been well described over the past decade, the mechanisms of the signaling processes involved in the bystander phenomenon remain unclear. In the present study, using the Columbia University charged particle microbeam, we found that mitochondrial DNA depleted human skin fibroblasts (ρ0) showed a higher bystander mutagenic response in confluent monolayers when a fraction of the same population were irradiated with lethal doses, compared with their parental, mitochondria functional cells (ρ+). However, using mixed cultures of ρ0 and ρ+ cells and targeted only one population of cells with a lethal dose of alpha particles, a decreased bystander mutagenesis was uniformly found in non-irradiated bystander cells of both cell types, indicating that signals from one cell type can modulate expression of bystander response in another cell type. In addition, we found that Bay 11-7082, a pharmacological inhibitor of nuclear factor-κB (NF-κB) activation, and 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (c-PTIO), a scavenger of nitric oxide (NO), significantly decreased the mutation frequency in both bystander ρ0 and ρ+ cells. Furthermore, we found that NF-κB activity and its dependent proteins, cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), were lower in bystander ρ0 cells when compared with their ρ+ counterparts. Our results indicated that mitochondria play an important role in the regulation of radiation-induced bystander effects, and that mitochondria-dependent NF-κB/iNOS/NO and NF-κB/COX-2/prostaglandin-E2 (PGE2) signaling pathways are important to the process.
Keywords: Rho zero cells, Mitochondrial Function, Radiation-induced Bystander Effect, NF-κB, Nitric Oxide, Cyclooxygenase-2
Introduction
Radiation-induced bystander effect is defined as the induction of biological effects in cells that are not directly traversed by a charged particle, but are receiving signals from the irradiated cells that are in close proximity to them. Although the bystander effects have been well described over the past decade (1–4), the precise mechanisms of the process remain unclear. In sub-confluent cultures, there is evidence that reactive oxygen species (ROS), nitric oxide (NO), and cytokines such as transforming growth factor β (TGFβ) are involved in mediating the process (5–9). On the other hand, gap junction-mediated cell-cell communications have been shown to be critical for bystander effects in confluent cultures of either human or rodent origins (10–13). It is likely that a combination of pathways involving both primary and secondary signaling processes is involved in producing a bystander effect.
Using primary human fibroblasts, we showed previously that the cyclooxygenase-2 (COX-2) signaling cascade, including the activation of mitogen activated protein kinase (MARK) pathways, plays an essential role in the bystander process (14). Since COX-2 is often induced after treatment with growth factors and cytokines such as TNFα, we further demonstrated that treatment of bystander cells with anti-TNFα monoclonal antibody (mAb) partially suppressed the NF-κB and MAPK pathways. The observations that extracellularly applied antioxidant enzymes such as superoxide dismutase (15) and catalase (16) can inhibit medium-mediated bystander response suggest a role of reactive radical species in the bystander process. Since mitochondria are the main source of energy production as well as generators of free radicals in cells, especially in pathological and stressful conditions, the present studies were conducted to define the contribution of mitochondria to the bystander response using mutagenesis as an endpoint. There is recent evidence that point mutations in the mitochondrial genome are induced among either directly irradiated cells with a 5Gy dose of gamma-rays or by exposure to bystander factor(s) obtained from such cells (17). To better understand the role of mitochondria in the radiation-induced bystander effect, mitochondrial DNA depleted human skin fibroblasts (ρ0) and their parental mitochondrial functional cells (ρ+) were used in conjunction with the Columbia University charged particle microbeam and track segment irradiation system. Since TNFα and ROS may induce the inhibitor nuclear factor κB kinase (IKK)-NF-κB pathways, we further examined the link between the NF-κB signaling pathway and the bystander response. In the present study, we found that ρ0 cells were more sensitive to alpha particle induced bystander mutagenesis. In addition, NF-κB activity and both NF-κB-dependent COX-2 and iNOS expression levels were lower in bystander ρ0 cells compared with bystander ρ+ cells. Furthermore, we found that Bay 11-7082, a pharmacological inhibitor of NF-κB activation, and 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (c-PTIO), a scavenger of nitric oxide (NO), significantly decreased the mutation frequency in both bystander ρ0 and ρ+ cells. Our results indicated that mitochondria played an important role in radiation-induced bystander effect, partially via mitochondria-dependent regulation of iNOS and COX2 signaling pathways.
Materials and Methods
Cell Culture
ρ0 cells were generated by treatment of parental (ρ+) human skin fibroblasts (HSF) with 50 ng/ml ethidium bromide in growth medium supplemented with 50 μg/ml uridine as described before (18). The ρ+ cells were maintained in a 4.5g/L glucose DMEM with 4mM L-glutamine and 110mg/L sodium pyruvate supplemented with 10% fetal bovine serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin. The ρ0 cells were cultured using the same medium plus 50 μg/ml uridine (Sigma, St. Louis, MO), which provides an alternative source of energy through glycolysis to ensure optimal growth(19). Primary normal human lung fibroblasts (NHLF) in frozen vials were purchased from Cambrex/Lonza (Walkersville, MD). The NHLF cells were thawed and maintained in a fibroblast growth medium (FGM) supplied by the manufacturer (14). Cultures were maintained at 37°C in a humidified 5% CO2 incubator. The medium was changed every three days and the cells were passaged at confluence.
Microbeam Irradiation
Cell tracker orange (CTO, 1μM) stained cells were mixed with non-stained, either same or different type cells, in a 1:10 ratio. Approximately 3000 exponentially growing mixed cells in 1.5 μl volume were inoculated into each of a series of microbeam dishes coated with Cel-Tak (BD Biosciences, Bedford, MA) to enhance cell attachment as described (20, 21). The next day after plating, ~90% of the attached cells were in contact with neighboring cells. The image analysis system then located the centroid of each cell tracker orange stained cells and irradiated all of them, one at a time, with an exact number of alpha particles (90 keV/μm 3He ions) accelerated with a 5MV Singletron accelerator at the Radiological Research Accelerator Facilities (RARAF) of Columbia University. After irradiation, cells were maintained in the dishes overnight before being removed by trypsinization, and replated into culture flasks for the mutant assay as described (20, 21). Following previously described protocol, all controls were similarly stained and sham-irradiated as well.
Track Segment Irradiation Procedure
The newly designed strip mylar dishes were used in the present studies as described (14, 22). Exponentially growing ρ0 and ρ+ cells were plated in the concentric strip mylar dishes a couple of days before irradiation to ensure a confluent state. A 50cGy dose of 4He ions (120 keV/μm) was delivered to the cells as described above. After irradiation, at selected time points, the inner and outer mylar dishes were separated and the cells from each growth surface were trypsinized and individually pooled for endpoint analysis.
Survival of Irradiated and Non-irradiated Bystander Cells
Non-irradiated bystander, irradiated, and control cells were collected after irradiation at different time points. Cultures were trypsinized, counted with a Coulter counter, and aliquots of the cells were replated into 100-mm-diameter dishes for colony formation. Representative aliquots of cells were further incubated for mutagenesis and other testings. Cultures for clonogenic survival assays were incubated for 12 days, at which time they were fixed with formaldehyde and stained with Giemsa. The number of colonies was counted to determine the surviving fraction as described (20, 21).
Cell Proliferation
Aliquots of 1 x105 ρ0 and ρ+ cells were plated in T25 flasks. On day 1, 2, 4, 6, 8, 10, and 12 after plating, 2 flasks of cells were trypsinized, then counted with a Coulter counter. The average number of cells was plotted as a cell growth curve, and the doubling time of cells was calculated as described (23).
PCR Analysis of Mitochondrial DNA
Two different pairs of primers were chosen for mitochondrial DNA detection. Primer 1 (251 bp): 1685–1709 (Sense), 1912–1936 (Antisense); primer 2 (261 bp): 3304–3324 (Sense), 3543–3565 (Antisense). PCR amplifications were performed for 30 cycles using a DNA thermal cycler model 9700 (Perkin-Elmer, Waltham, MA) in 50-μl reaction mixtures containing 0.5μg of the DNA sample. Each PCR cycle consisted of denaturation at 95°C for 30 sec, annealing at 55°C for 30 sec, and extension at 72°C for 30 sec. After the last cycle, samples were incubated at 72°C for an additional 10 min, electrophoresed on 1% agarose gels, and stained with ethidium bromide.
Oxygen consumption
Oxygen consumption in intact cells was assayed as described previously (19) Briefly, 1 × 107 wild type and ρ0 cells were suspended in 1.5 ml of DMEM lacking glucose, and oxygen concentration was assayed over 3 min at 37oC in a Hansatech (MA) Clark’s oxygen electrode unit.
Cellular Staining with Fluorescent Probes and Flow Cytometry
Cells were stained with fluorescent dyes diluted in DMEM. The following dye combinations were added for 45 minutes at 37oC in the dark. (1) 20 nM dihexyloxacarbocyanine iodide (DiOC6 for assessment of mitochondrial membrane potential (ΔΨm), Invitrogen, Eugene, OR); (2) 2 μM hydroethidine (HE for assessment of superoxide production, Invitrogen, Eugene, OR); (3) 3.5 μM 4-amino-5-methylamino-2OE, 7OE-difluorofluorescein diacetate (DAF-FM for the detecting of Nitric Oxide, Invitrogen, Eugene, OR). Flow cytometry was performed using a FACSCalibur™ (Becton Dickinson, Franklin Lakes, NJ) to analysize fluorescent signaling, PI staining of DNA and cell cycle distribution. DiOC6 and DAF-FM fluorescence were analyzed in the FL1 channel, while HE fluorescence was analyzed using the FL3 channel. Forward and side scatter data were used to gate out cellular fragments.
Quantification of Mutations at the HPRT Locus
To determine the HPRT− mutant fraction, after a seven-day expression period, 2 × 105 cells per dish were plated into twenty-five to thirty 100-mm dishes in a total of 12 ml of the growth medium, containing 100 μM 6-thioguanine (6-TG, Sigma Chemical, St. Louis, MO). The cultures were further incubated for 12 days, at which time, the cells were fixed and stained, and the number of HPRT− mutant colonies scored. Meanwhile, aliquots of 250 cells were plated in dishes containing growth medium without 6-TG for determination of plating efficiency. The mutant fraction was calculated as the number of surviving colonies divided by the total number of cells plated after correction for plating efficiency.
Micronuclei (MN) scoring
The cytokinesis block technique was used for the MN assay. Briefly, cultures were treated with 1 μg/ml cytochalasin-B for 32 hours and then fixed with 4% paraformaldehide for 20 min. Air-dried cells were covered/stained with DEPI mounting solution. MN were scored in the binucleated cells and classified according to standard criteria (24). The MN yield was calculated as the ratio of number of MN to the scored number of binucleated cells.
Western Blotting
Protein was extracted from either the control or bystander cells by lysis in extracting buffer [50 mM Tris-HCl (pH 8). 150 mM NaCl, 1% NP-40, 0.1% sodium dodecyl sulfate (SDS), 1 mM phenylmethylsulfonyl fluoride and a mixture of protease inhibitors (Roche, Indianapolis, IN). The concentration of the protein was determined by the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA). Equivalent amounts of protein (50–100 μg) were fractionated on a 10% SDS-polyacrylamide gel for 3 hours, and proteins were transferred to nitrocellulose membranes under semi-dry conditions. Mouse monoclonal anti-human COX-2 (Cayman, Ann Arbor, MI), mouse polyclonal Ab against iNOS (Cell Signaling, Denvers, MA) and monoclonal Ab against β-actin (Sigma, St. Louis, MO) were used. The secondary Abs were horseradish peroxidase (HRP)-conjugated anti-mouse or anti-rabbit IgG diluted 1:5,000 to 1 : 10, 000. After electrochemiluminescence (ECL), the band intensities were evaluated by phosphorimaging and normalized to β-actin protein level.
Electrophoretic Mobility Shift Assay
The Electrophoretic Mobility Shift Assay (EMSA) was performed for the detection of NF-κB DNA binding activity as previously described using the labeled double-strand oligonucleotide AGCTTGGGGACTTTCCAGGCG (binding site is italicized). Ubiquitous NF-Y DNA-binding activity was used as an internal control (25).
Statistical Analysis
Data were calculated as means and standard deviations. Comparisons of surviving fractions and induced mutant fractions between treated and control groups were made by the students’ t-tests. A p-value of 0.05 or less between groups was considered significant.
Results
Characterization of mitochondrial DNA depleted (ρ0) cells and their parental (ρ+) cells
A series of experiments were conducted to characterize the differences between ρ0 cells and their parental ρ+ cells. In the presence of uridine, ρ0 cells grew very well in culture. Addition of uridine to culture medium did not affect the radiosensitivity of HSF and the dose response survival curves of ρ0 and ρ+ HSF culture were similar (data not shown). Compared with ρ+ cells, ρ0 cells showed only a slight decrease in saturation density as the culture approached confluency (Data not shown). Using two different primer sets that corresponded to different segments of the mitochondrial DNA, we detected no mitochondrial DNA band in ρ0 cells; in contrast, a clear band was detected in ρ+ cells with either of the primer pairs (Figure 1A). When the cells were stained with the mitochondrial membrane potential probe, DiOC6, ρ0 cells showed a decrease in fluorescence intensity (mean intensity 95.1 arbitrary units, A.U.) relative to control ρ+ cells (mean intensity 226 A.U. and Fig. 1B). Likewise, mitochondrial DNA depleted cells showed a decrease in intracellular superoxide content (mean intensity 8.2 A.U.) when compared with wild type cells (mean intensity 30.0 A.U. and Fig. 1C). To further confirm that ρ0 cells had compromised mitochondrial function, we showed that these cells had an oxygen consumption level that was 20 times less than wild type cells (Fig. 1D). These data clearly indicate that ρ0 cells have dysfunctional mitochondria, and that this cell line is a good model to investigate the role of mitochondria in radiation-induced bystander responses.
Figure 1.
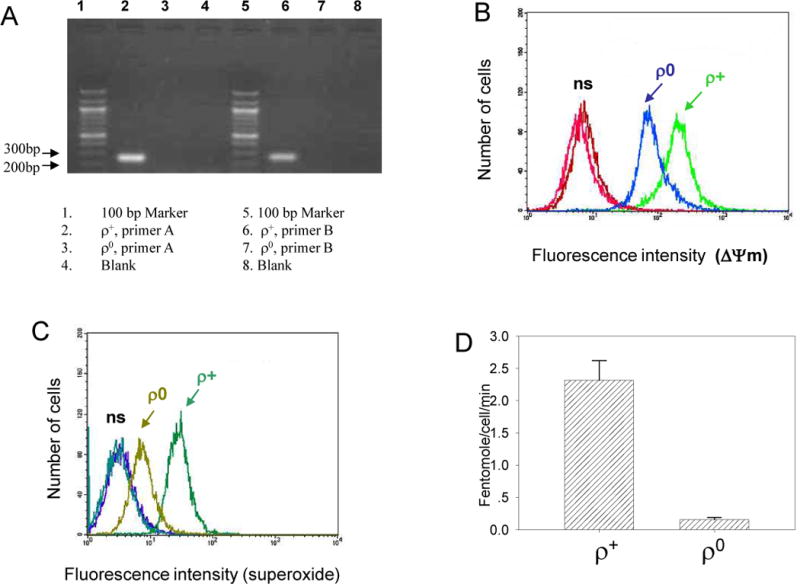
Characterization of mitochondrial DNA depleted human skin fibroblasts (ρ0) and their parental cells (ρ+). A. PCR amplification of genomic DNA from ρ0 and ρ+ cells using two different segments of mitochondrial DNA as primers. PCR products for the two primer sets: primer A (251 bp) and primer B (261 bp). B. Assessment of mitochondrial membrane potential (ΔΨm) in ρ+ and ρ0 cells using DiOC6 staining (20 nM) and flow cytometry. ρ0 cells show lower membrane potential compared with ρ+ cells. C. Assessment of superoxide production in ρ0 and ρ+ cells using hydroethidine (2μM) and flow cytometry. ρ0 cells show lower superoxide production compared with ρ+ cells. D. Rates of oxygen consumption for ρ+ and ρ0 cells using an oxygen electrode unit. Average of duplicate determinants from two or three independent clones from each line; bars represent ± SD.
Alpha-irradiation induces a higher bystander mutagenic rate in ρ0 cells than in ρ+ cells
To explore the role of mitochondria in the radiation-induced bystander effect, a microbeam was used to lethally irradiate either ρ0 or ρ+ cells with 20 alpha particles each in a mixed, confluent culture, and the bystander response was determined in the non-irradiated fraction. We found that ρ0 cells, when compared with ρ+ cells, showed a higher bystander HPRT− mutagenic response (15.2 vs 5.4 per 106 survivors, p < 0.05) in confluent monolayer when 10% of the same population were lethally irradiated (Fig. 2A, 2B). It should be noted that the background mutant fraction of ρ0 and ρ+ cells was similar being ~1.0 per 106 survivors. However, using mixed cultures of ρ0 and ρ+ cells and targeting only one population of cells with a lethal dose of alpha particles, a decreased bystander mutagenesis was uniformly found with both cell types. For example, when 10% of the CTO-stained ρ0 cells (in culture with 90% of non-stained ρ0 cells) were irradiated with 20 alpha particles, the mutant fraction among the non-hit, bystander ρ0 cells was ~15.2 per 106 survivors. However, when a similar number of ρ0 cells were irradiated among 90% of wild type cells, the resultant bystander mutant fraction was only 3.1 per 106 surviving ρ+ cells (p < 0.05). Similarly, when 10% CTO-stained ρ+ cells were lethally irradiated with alpha particles, the mutant yield was about 5.4 in 106 surviving bystander ρ+ cells, but only 1.8 per 106 surviving bystander ρ0 cells (p < 0.05). These results indicate that mitochondrial deficient cells cannot effectively communicate the bystander signals with wild type cells; or alternatively, signals from one cell type can modulate expression of bystander response in another cell type.
Figure 2.
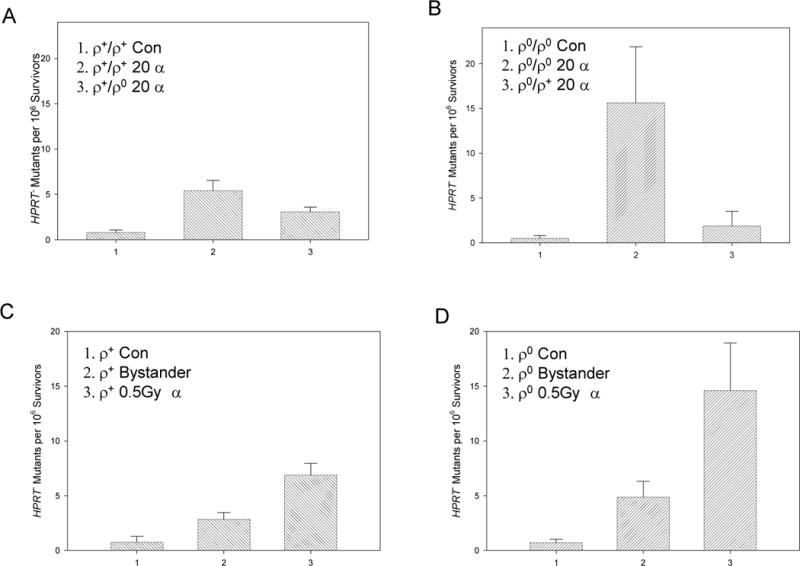
HPRT– mutation of bystander cells in mixed cultures of ρ+ and ρ0 cells using the Columbia microbeam. A. ρ+ cells were used as the bystander cells when 10% of ρ0 or ρ+ cells were irradiated with 20 alpha particles each. B. ρ0 cells were used as the bystander cells when 10% of ρ+ or ρ0 cells were irradiated with 20 alpha particles each. C.HPRT– mutation of bystander and directly irradiated ρ+ cells exposed to a 0.5Gy dose of alpha particles using the special designed strip dishes. D. Same as C with ρ0 cells. Data are pooled from 3–5 independent experiments. Bars represent ± SD.
Track segment irradiation confirms bystander mutagenesis in ρ0 and ρ+ cells
Since the microbeam can only irradiate a limited number of cells, to generate sufficient bystander cells for mechanistic studies we used the specially designed strip mylar dishes and track segment irradiation as described (14, 21). Since cells that were seeded on the thicker mylar (38μm) would not be traversed by alpha particles but would be in the vicinity of those seeded on thinner mylar (6μm) that would, we had, effectively, a pure population of bystander cells. Exposure of ρ+ cells to a dose of 0.5Gy alpha particles increased the bystander HPRT− mutant yield to a level 2.6 times higher than the background incidence. However, under similar irradiation conditions, ρ0 cells had a bystander mutant fraction that was 7.1 fold higher than non-irradiated ρ0 cells (Fig. 2C & 2D). These results are consistent with the data generated from microbeam irradiation showing that mitochondrial deficient cells have a higher mutation frequency in both directly irradiated and bystander cells. Comparing with the data generated using the microbeam, the bystander mutagenesis obtained using the broad, track segment beam for both the ρ+ and ρ0 HSFs was significantly reduced (p < 0.05).
Effect of c-PTIO in bystander mutagenesis
To determine if nitric oxide is linked to mitochondrial function in mediating the bystander response, we treated cells with 20 μM c-PTIO, a NO scavenger, 2 hours before- and maintained overnight after irradiation. As shown in figure 3A, treatment with c-PTIO significantly reduced the bystander mutagenesis in both ρ0 and ρ+ cell lines (p < 0.05). However, the effect of c-PTIO on the bystander response in ρ+ cells was more pronounced than in ρ0 cells. The induced mutation frequency was reduced from 1.90 to 0.37 per 106 survivors (5.1 fold) in wild type cells compared with a reduction from 4.19 to 2.05 per 106 survivors (2.0 fold) in ρ0 cells. These results indicated that, in addition to NO, other signaling molecules might play a role in modulating the bystander effects in mitochondrial deficient cells.
Figure 3.
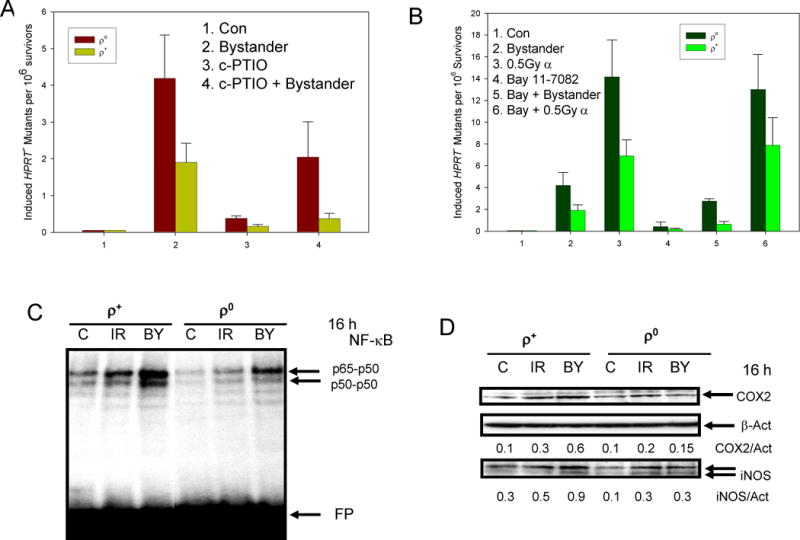
A. Effect of the nitric oxide scavenger, c-PTIO (20μM, 2 hr before and maintained overnight after irradiation) on HPRT– mutant fractions of ρ+ and ρ0 cells. B. Effect of the NF-κB inhibitor, Bay 11-7082 (1μM, 2 hr before and maintained overnight after irradiation) on HPRT– mutant fractions of ρ+ and ρ0 cells. Data are from 3–4 independent experiments. Error bars represent SD. C. Characterization of NF-κB DNA binding activities of control, bystander cells and directly irradiated (0.5 Gy dose of alpha particles) ρ+ and ρ0 cells using EMSA. FP: free labeled oligonucleotide probe. D. Western blot analyse of COX-2 and iNOS protein levels in bystander and directly irradiated (0.5Gy dose of alpha particles) ρ+ and ρ0 cells. β-Actin was used as loading controls.
Role of NF-κB in the bystander response
Expression of the iNOS gene is controlled by the transcription factor NF-κB. To define the function of NF-κB in radiation-induced bystander effects, cells were treated with 1 μM Bay 11-7082, a pharmacological inhibitor of IKK-NF-κB activation, 2 hours before irradiation, and maintained overnight after irradiation. The dose of Bay 11–7082 used was non-toxic, non-mutagenic in both ρ0 and ρ+ cell lines. Treatment of both cell types with Bay 11–7082 resulted in a significant reduction of the bystander mutagenesis (p < 0.05, Fig. 3B). The inhibition by Bay 11-7082 on radiation induced bystander effects was similar to that of c-PTIO: being more effective in ρ+ (3.0 folds, p < 0.01) than in ρ0 cells (1.5 folds, p < 0.05). Consistent with these observations, we found that the basal and inducible (both directly irradiated and bystander) levels of nuclear NF-κB DNA-binding activity were notably higher in ρ+ compare to ρ0 cells (Fig. 3C). Consequently, expression levels of NF-κB-dependent proteins such as iNOS and COX-2 were notably lower in ρ0 cells (Fig. 3D).
We previously reported that the COX-2/PGE2 signaling pathway, which is a hallmark of inflammation and ROS production, was critically linked to radiation bystander phenomenon in normal human fibroblasts (14). In the present study, 3 and 6 fold increases in COX-2 expression level were found in directly irradiated and bystander ρ+ cells, respectively (Fig. 3D). However, COX-2 expression increased only slightly in directly irradiated and bystander ρ0 cells (Fig. 3D). Taken together, these results indicated that inducible (but not basal) expression of COX-2, which was substantially lower in mitochondria-deficient cells, plays a critical role in regulating mechanisms of bystander effects, which is consistent with our previous findings (14). However, these results also pointed out that other mechanisms of radiation-induced bystander effect might be different in mitochondrial deficient cells. Furthermore, we can not exclude that signaling pathways, other than the COX-2/PGE2 and iNOS/NO pathways, might be activated by NF-κB and can induce bystander mutagenesis.
Role of NF-κB in alpha particle-induced bystander effects in normal human lung fibroblasts (NHLF)
Since NF-κB is an important transcription factor for many signaling genes including COX-2, we used NHLF cultures, which were used previously to document COX-2 activities in bystander signaling(14), to exam the role of NF-κB in the bystander response. Alpha particle irradiation upregulated NF-κB binding activity in both directly irradiated and bystander cells, while Bay 11-7082, a pharmacological inhibitor of the IKK/NF-κB, efficiently suppressed this up-regulation and also reduced levels below basal amount (Fig. 4A). This inhibitor of NF-κB activity also efficiently down-regulated COX-2 and iNOS-expression levels in both directly irradiated and bystander fibroblasts (Fig. 4B). Micronuclei formation was readily detected in directly irradiated and bystander cells, while both Bay 11-7082 (Fig. 4C) and c-PTIO (Fig. 4D) effectively decreased this level in bystander cells (p < 0.05).
Figure 4.
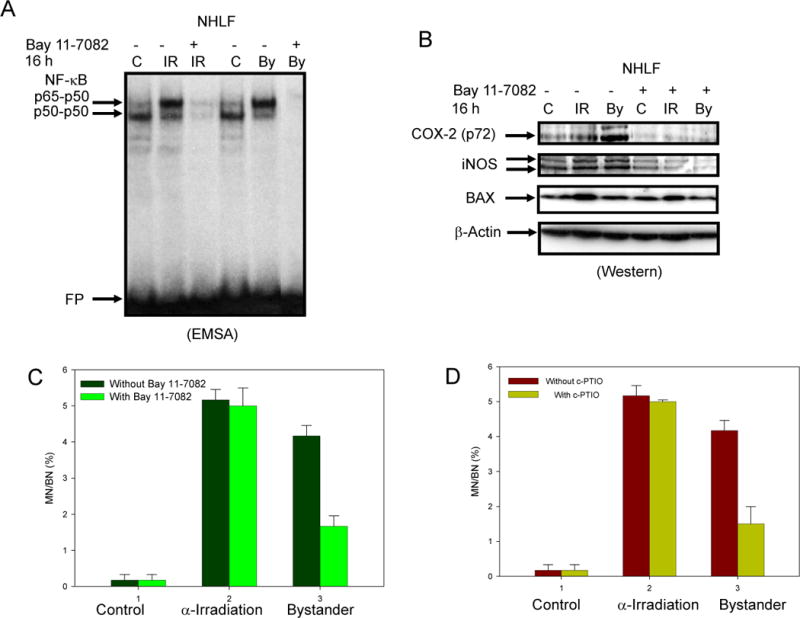
Effects of NF-κB inhibition on NF-κB-dependent COX-2 and iNOS protein levels, and micronuclei formation in bystander and directly irradiated normal human lung fibroblasts (NHLF). Cells were treated by 0.5Gy alpha particles in special designed strip dishes with or without Bay 11-7082 (1 μM). A. EMSA was performed with nuclear extracts of directly irradiated and bystander NHLF 16 h after irradiation. Two NF-κB DNA-binding complexes, p65-p50 and p50-p50, are indicated. FP: free labeled oligonucleotide probe. B. Western blot analysis of COX-2, iNOS, BAX and β-actin levels in NHLF after indicated treatment. C and D. Effect of Bay 11-7082 and c-PTIO (1 μM and 20 μM, respectively) on micronuclei formation in irradiated and bystander cells. Data are pooled from three independent experiments. Bar represents ± SD.
Effects of cytokines on the bystander effects
TNFα might be an excellent candidate in mediating bystander effects in NHLF. Exogenous TNFα in concert with IL1β directly controls COX-2 expression in NHLF (Fig. 5A). Both TNFα and IL1β could be induced following α-irradiation of NHLF. The inhibitory mAb against TNFα, which was introduced into the cell media, substantially decreased levels of NF-κB (Fig 5B) and JNK (data not shown) that was accompanied by a well pronounced decrease in the COX-2 expression level in both irradiated and, especially, in bystander NHLF (Fig. 5C). Simultaneously, the negative effect of anti-TNF mAb on ERK activity (phospho-ERK protein level) was relatively modest (data not shown). As an additional physiological test, we determined clonogenic survival of NHLF during a partial suppression of TNF levels by the inhibitory effect of anti-TNF mAb. We found a significant increase in cell survival of bystander cells treated with anti-TNF mAb (p < 0.05, Fig. 5D).
Figure 5.
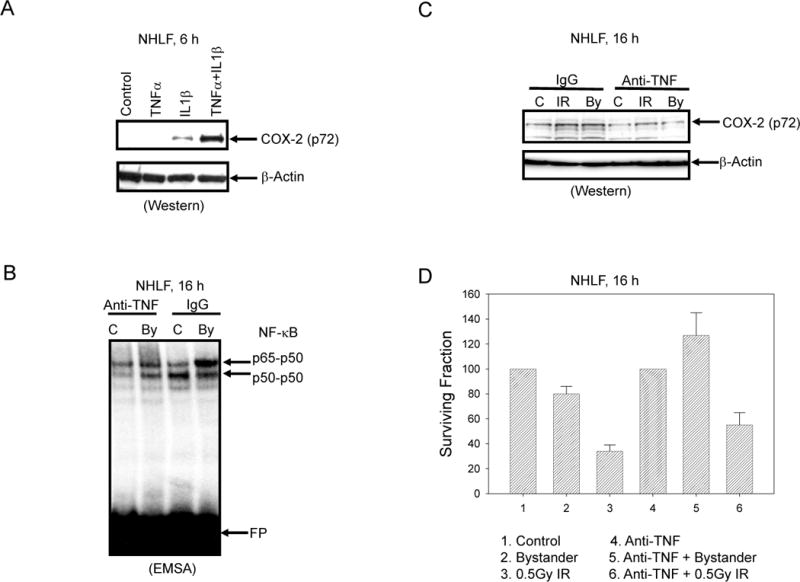
Effects of TNFα in the bystander effects. A. Combined treatment of NHLF with TNFα (20 ng/ml) and IL1β (2 ng/ml) induced COX-2 expression as determined by Western blot analysis. B. EMSA of NF-κB DNA-binding activity in control and bystander cells, with or without anti-TNF mAb (5 μg/ml) in the medium. C. Effects of treatment with the inhibitory anti-TNF mAb (5 μg/ml) on COX-2 levels in control, α-irradiated and bystander cells. D. Clonogenic survival assay of NHLF after indicated treatment with and without inhibitory anti-TNF mAb (5 μg/ml). Data are pooled from three independent experiments. Bar represents ± SD.
Discussion
Although radiation-induced bystander effects have attracted much attention in the past decade, the mechanisms of the process remain unclear. It is likely that cellular signaling pathways, for example, membrane ligands and the downstream MAPK cascade provide a common link between medium-mediated and gap-junction dependent bystander phenomena. Previous studies by Mitchell et al. (2004) have shown that when 10% of cells are exposed to alpha particles, a significantly greater number of cells are inactivated when irradiated at high density (> 90% in contact with neighbors) than at low density (< 10% in contact). In addition, the bystander oncogenic transformation frequency is significantly higher in high-density cultures (26). These results suggest that when cells are traversed by alpha particles, the transmission of a bystander signal through cell-to-cell contact results in a higher response compared to those mediated by soluble factors. In the present study, we observed a similar density-dependent bystander response. In microbeam irradiation, a higher bystander mutagenesis was found because cells had more cell-cell contact compared with cells plated in specially designed strip dishes.
Nitric oxide has been postulated to be a potential signaling molecule in radiation-induced bystander effects (27–29). Shao et al (2003) reported that when only 1% of cell nuclei were individually targeted with a single helium ion, approximately 40% of the cells showed an increase in fluorescence intensity of the NO-sensitive dye, 4-amino-5-methylamino-2′, 7′-difluorofluorescein (DAF-FM). Moreover, it was found that when only 1 cell in a population of approximately 1,200 cells was targeted with one or five ions, the incidence of micronuclei increased by 20%, and concurrent treatment with c-PTIO significantly reduced the bystander effect (30). In the present study, we found that the NO scavenger had a similar suppressive effect on bystander mutagenesis of both ρ+ and ρo cells, though the effects were more pronounced in the former. Since mitochondria are the main source of reactive radical species in cells, the basal level of free radicals in ρo cells is lower than in mitochondria functional cells (Fig. 1B, C, and D). There is evidence that in ρo cells with a complete shut down of the electron transport chain, the decreased level of reactive oxygen species resulted in a down-regulation of the manganese superoxide dismutase, glutathione, and glutathione peroxidase, an important intracellular antioxidant pool (31). As such, compared with wild type cells, ρo cells are more susceptible to oxidative stress and expressed in a higher bystander mutagenesis as shown in the present studies. It is likely that one or more other signaling molecules, in addition to NO, are involved in radiation induced bystander effects in mitochondrial deficient cells. This could explain the difference in bystander signaling response in mixed cultures of ρ+ and ρ0 cells. Alternatively, ρ0 cells, by virtue of their reduced apoptotic response (Ivanov and Hei, unpublished observation), may accumulate a higher mutant fraction. This will be consistent with the higher bystander response seen in these cells. It is also possible that signal from one cell line can modulate expression of bystander response in another cell type (32) and is consistent with the findings with the mixed cell population (Figure 2A and B).
Elevated levels of NO have been detected in a variety of pathophysiological processes, including inflammation and carcinogenesis (33, 34). In our previous study, we found that COX-2 is critically linked to the radiation-induced bystander effect in normal human fibroblasts (14). There is evidence that NO can induce expression of COX-2 in mouse skin and human cultured airway epithelial cells, and that the NF-κB pathway is involved in the process (35, 36). Our findings that Bay 11-7082, a specific IKK/NF-κB inhibitor, could eliminate bystander mutagenesis in both wild type and ρ0 cells, highlight the important role of this transcription factor in the bystander phenomenon (Fig. 3B). NF-κB directly controls gene expression of COX-2 and iNOS. As proposed in our working model, cytokines of the TNFα super-family might be excellent candidates in mediating bystander effects (Fig. 6). Ionizing radiation is a strong inducer of the ATM-IKK-NF-κB signaling pathway (37), which is further involved in the up-regulation of TNFα gene expression (38). Secreted or membrane forms of TNFα could induce bystander effects in non-irradiated cells via activation of COX-2 gene expression, as we observed in the present study. Inhibitory mAb against TNF partially suppressed NF-κB activation and the subsequent COX-2 up-regulation in both directly irradiated and bystander cells (Fig. 5). Taken together, our data demonstrated a connection between up-regulation of NF-κB activation and mitochondrial function in mediating the bystander phenomenon. Indeed, NF-κB-dependent functions are characteristic for both ρ+ and ρ0 cells, although in the last case, levels of inducible NF-κB activity were substantially lower (Fig. 3C). As expected, in cells with functional mitochondria, up-regulation of COX-2 was detected in both directly irradiated and bystander cells (Fig. 3D). This increase in COX-2 expression levels was almost completely blocked in the presence of Bay 11-7082 (Fig. 4B). It indicates that the NF-κB/COX-2/PGE2 and NF-κB/iNOS/NO pathways are critical to the radiation induced bystander effect in mitochondrial functional cells. However, in ρ0 cells, the contribution of COX-2 to the bystander process is less pronounced, while NF-κB/iNOS/NO pathway actively operates, although at lower level compared to normal cells.
Figure 6.
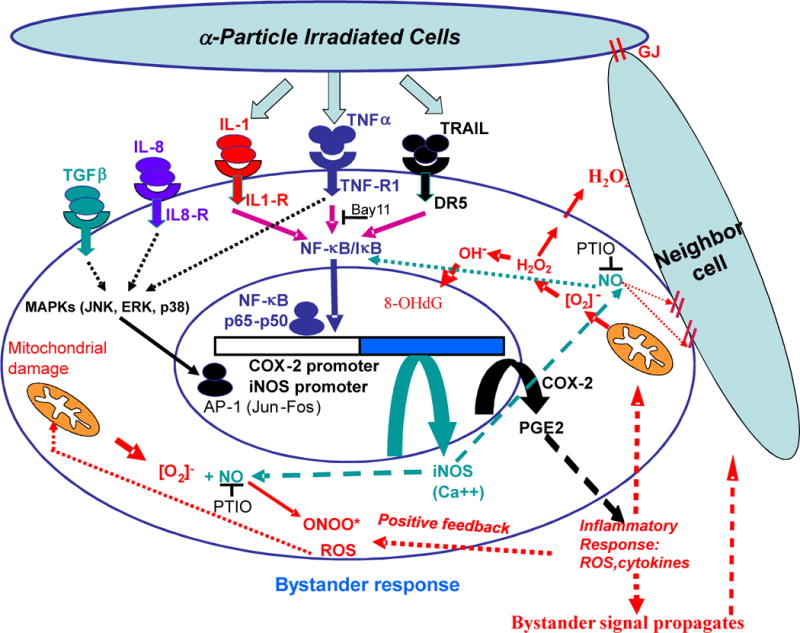
A unifying model of the signaling pathways involved in radiation-induced bystander effects. Secreted or membrane forms of cytokines such as TNFα activate IKK-mediated phosphorylation of IκB, which releases NF-κB, that enters the nucleus and acts as a transcription factor for COX-2 and iNOS. TNFα also activates MAPK pathways (ERK, JNK and p38) that via AP-1 transcription factor additionally up-regulates expression of COX-2 (14) and iNOS, which stimulate the production of nitric oxide. Mitochondrial damage facilities the production of hydrogen peroxide that migrates freely across plasma membrane and is subjected to antioxidant removal. Activation of COX-2 provides a continuous supply of reactive radicals and cytokines for the propagation of the bystander signals either through gap junction or medium.
Acknowledgments
The authors thank Dr. Howard Lieberman for critical reading of the manuscript. This research was supported by funding from the National Institutes of Health Grants CA 49062, ES 12888 and National Cancer Institute Research Resource Grant 11623.
References
- 1.Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat Res. 2003;159:567–80. doi: 10.1667/0033-7587(2003)159[0567:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 2.Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: II. Radiation-induced genomic instability and bystander effects in vivo, clastogenic factors and transgenerational effects. Radiat Res. 2003;159:581–96. doi: 10.1667/0033-7587(2003)159[0581:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Hei TK, Persaud R, Zhou H, Suzuki M. Genotoxicity in the eyes of bystander cells. Mutat Res. 2004;568:111–120. doi: 10.1016/j.mrfmmm.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Mothersill C, Seymour CB. Radiation-induced bystander effects--implications for cancer. Nat Rev Cancer. 2004;4:158–64. doi: 10.1038/nrc1277. [DOI] [PubMed] [Google Scholar]
- 5.Hickman AW, Jaramillo RJ, Lechner JF, Johnson NF. Alpha-particle-induced p53 protein expression in a rat lung epithelial cell strain. Cancer Res. 1994;54:5797–800. [PubMed] [Google Scholar]
- 6.Deshpande A, Goodwin EH, Bailey SM, Marrone BL, Lehnert BE. Alpha-particle-induced sister chromatid exchange in normal human lung fibroblasts: evidence for an extranuclear target. Radiat Res. 1996;145:260–7. [PubMed] [Google Scholar]
- 7.Narayanan PK, Goodwin EH, Lehnert BE. α Particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res. 1997;57:3963–71. [PubMed] [Google Scholar]
- 8.Mothersill C, Seymour CB. Cell-cell contact during gamma irradiation is not required to induce a bystander effect in normal human keratinocytes: evidence for release during irradiation of a signal controlling survival into the medium. Radiat Res. 1998;149:256–62. [PubMed] [Google Scholar]
- 9.Iyer R, Lehnert BE. Factors underlying the cell growth-related bystander responses to alpha particles. Cancer Res. 2000;60:1290–8. [PubMed] [Google Scholar]
- 10.Azzam EI, de Toledo SM, Gooding T, Little JB. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluencies of alpha particles. Radiat Res. 1998;150:497–504. [PubMed] [Google Scholar]
- 11.Azzam EI, de Toledo SM, Little JB. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha -particle irradiated to nonirradiated cells. Proc Natl Acad Sci (USA) 2001;98:473–8. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou H, Randers-Pehrson G, Waldren CA, Vannais D, Hall EJ, Hei TK. Induction of a bystander mutagenic effect of alpha particles in mammalian cells. Proc Natl Acad Sci (USA) 2000;97:2099–104. doi: 10.1073/pnas.030420797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou H, Suzuki M, Randers-Pehrson G, Vannais D, Waldren CA, Cheng G, Trosko JE, Hei TK. Radiation risk to low fluences of α particles may be greater than we thought. Proc Natl Acad Sci (USA) 2001;98:14410–5. doi: 10.1073/pnas.251524798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou H, Ivanov VN, Gillespie J, Geard CR, Amundson SA, Brenner DJ, Yu Z, Lieberman HB, Hei TK. Mechanism of radiation-induced bystander effect: role of the cyclooxygenase-2 signaling pathway. Proc Natl Acad Sci U S A. 2005;102:14641–6. doi: 10.1073/pnas.0505473102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang H, Asaad N, Held KD. Medium-mediated intercellular communication is involved in bystander responses of X-ray-irradiated normal human fibroblasts. Oncogene. 2005;24:2096–103. doi: 10.1038/sj.onc.1208439. [DOI] [PubMed] [Google Scholar]
- 16.Lyng FM, Maguire P, McClean B, Seymour C, Mothersill C. The involvement of calcium and MAP kinase signaling pathways in the production of radiation-induced bystander effects. Radiat Res. 2006;165:400–9. doi: 10.1667/rr3527.1. [DOI] [PubMed] [Google Scholar]
- 17.Murphy JE, Nugent S, Seymour C, Mothersill C. Mitochondrial DNA point mutations and a novel deletion induced by direct low-LET radiation and by medium from irradiated cells. Mutat Res. 2005;585:127–36. doi: 10.1016/j.mrgentox.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Liu SX, Davidson MM, Tang X, Walker WF, Athar M, Ivanov V, Hei TK. Mitochondrial damage mediates genotoxicity of arsenic in mammalian cells. Cancer Res. 2005;65:3236–42. doi: 10.1158/0008-5472.CAN-05-0424. [DOI] [PubMed] [Google Scholar]
- 19.Partridge MA, Huang SX, Hernandez-Rosa E, Davidson MM, Hei TK. Arsenic induced mitochondrial DNA damage and altered mitochondrial oxidative function: implications for genotoxic mechanisms in mammalian cells. Cancer Res. 2007;67:5239–47. doi: 10.1158/0008-5472.CAN-07-0074. [DOI] [PubMed] [Google Scholar]
- 20.Hei TK, Wu LJ, Liu SX, Vannais D, Waldren CA. Mutagenic effects of a single and an exact number of alpha particles in mammalian cells. Proc Natl Acad Sci (USA) 1997;94:3765–70. doi: 10.1073/pnas.94.8.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu L, Randers-Pehrson G, Xu A, Waldren CA, Geard CR, Yu Z, Hei TK. Targeted cytoplasmic irradiation with alpha particles induces mutations in mammalian cells. Proc Natl Acad Sci (USA) 1999;96:4959–64. doi: 10.1073/pnas.96.9.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu A, Zhou H, Leloup C, Marino SA, Geard CR, Hei TK, Lieberman HB. Differential impact of mouse Rad9 deletion on ionizing radiation-induced bystander effects. Radiation Res. 2005;164:655–61. doi: 10.1667/rr3458.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou H, Calaf G, Hei TK. Malignant Transformation of Human Bronchial Epithelial Cells with Tobacco-Specific Nitrosamine, 4-Methynitrosamine-1-3-Pyridyl-1-Butanone (NNK) Int J Cancer. 2003;106:821–6. doi: 10.1002/ijc.11319. [DOI] [PubMed] [Google Scholar]
- 24.Albertini RJ, Anderson D, Douglas GR, Hagmar L, Hemminki K, Merlo F, Natarajan AT, Norppa H, Shuker DEG, Tice R, Waters MD. Aitio A. IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. International Programme on Chemical Safety Mutat Res. 2000;463:111–172. doi: 10.1016/s1383-5742(00)00049-1. [DOI] [PubMed] [Google Scholar]
- 25.Ivanov V, Fleming TJ, Malek TR. Regulation of nuclear factor-kappa B and activator protein-1 activities after stimulation of T cells via glycosylphosphatidylinositol-anchored Ly-6A/E. J Immunol. 1994;153:2394–406. [PubMed] [Google Scholar]
- 26.Mitchell SA, Randers-Pehrson G, Brenner DJ, Hall EJ. The bystander response in C3H 10T1/2 cells: the influence of cell-to-cell contact. Radiat Res. 2004;161:397–401. doi: 10.1667/rr3137. [DOI] [PubMed] [Google Scholar]
- 27.Sokolov MV, Smilenov LB, Hall EJ, Panyutin IG, Bonner WM, Sedelnikova OA. Ionizing radiation induces DNA double-strand breaks in bystander primary human fibroblasts. Oncogene. 2005;24:7257–65. doi: 10.1038/sj.onc.1208886. [DOI] [PubMed] [Google Scholar]
- 28.Shao C, Folkard M, Michael BD, Prise KM. Targeted cytoplasmic irradiation induces bystander responses. Proc Natl Acad Sci (USA) 2004;101:13495–500. doi: 10.1073/pnas.0404930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Müller WE, Ushijima H, Batel R, Krasko A, Borejko A, Müller IM, Schröder H. Novel mechanism for the radiation-induced bystander effect: Nitric oxide and ethylene determine the response in sponge cells. Mutat Res. 2006;597:62–72. doi: 10.1016/j.mrfmmm.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Shao C, Stewart V, Folkard M, Michael BD, Prise KM. Nitric Oxide-Mediated Signaling in the Bystander Response of Individually Targeted Glioma Cells. Cancer Res. 2003;63:8437–42. [PubMed] [Google Scholar]
- 31.Isaac AO, Dukhande VV, Lai JC. Metabolic and Antioxidant System Alterations in an Astrocytoma Cell Line Challenged with Mitochondrial DNA Deletion. Neurochem Res. 2007 Jun 12; doi: 10.1007/s11064-007-9380-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Mothersill C, Seymour C. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells. Int J Radiat Biol. 1997;71:421–7. doi: 10.1080/095530097144030. [DOI] [PubMed] [Google Scholar]
- 33.Czapski GA, Cakala M, Chalimoniuk M, Gajkowska B, Strosznajder JB. Role of nitric oxide in the brain during lipopolysaccharide-evoked systemic inflammation. J Neuroscience Research. 2007 Apr 26; doi: 10.1002/jnr.21294. [DOI] [PubMed] [Google Scholar]
- 34.Seril DN, Liao J, Yang G-Y. Colorectal carcinoma development in inducible nitric oxide synthase-deficient mice with dextran sulfate sodium-induced ulcerative colitis. Mol Carcinog. 2007;46:341–53. doi: 10.1002/mc.20282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chun K-S, Cha H-H, Shin J-W, Na H-K, Park K-K, Chung W-Y, Surh Y-J. Nitric oxide induces expression of cyclooxygenase-2 in mouse skin through activation of NF-B. Carcinogenesis. 2004;25:445–454. doi: 10.1093/carcin/bgh021. [DOI] [PubMed] [Google Scholar]
- 36.Watkins DN, Garlepp M, Thompson PJ. Regulation of the inducible cyclo-oxygenase pathway in human cultured airway epithelial (A549) cells by nitric oxide. British J Pharmacology. 1997;121:1482–8. doi: 10.1038/sj.bjp.0701283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;357:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 38.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]


