Abstract
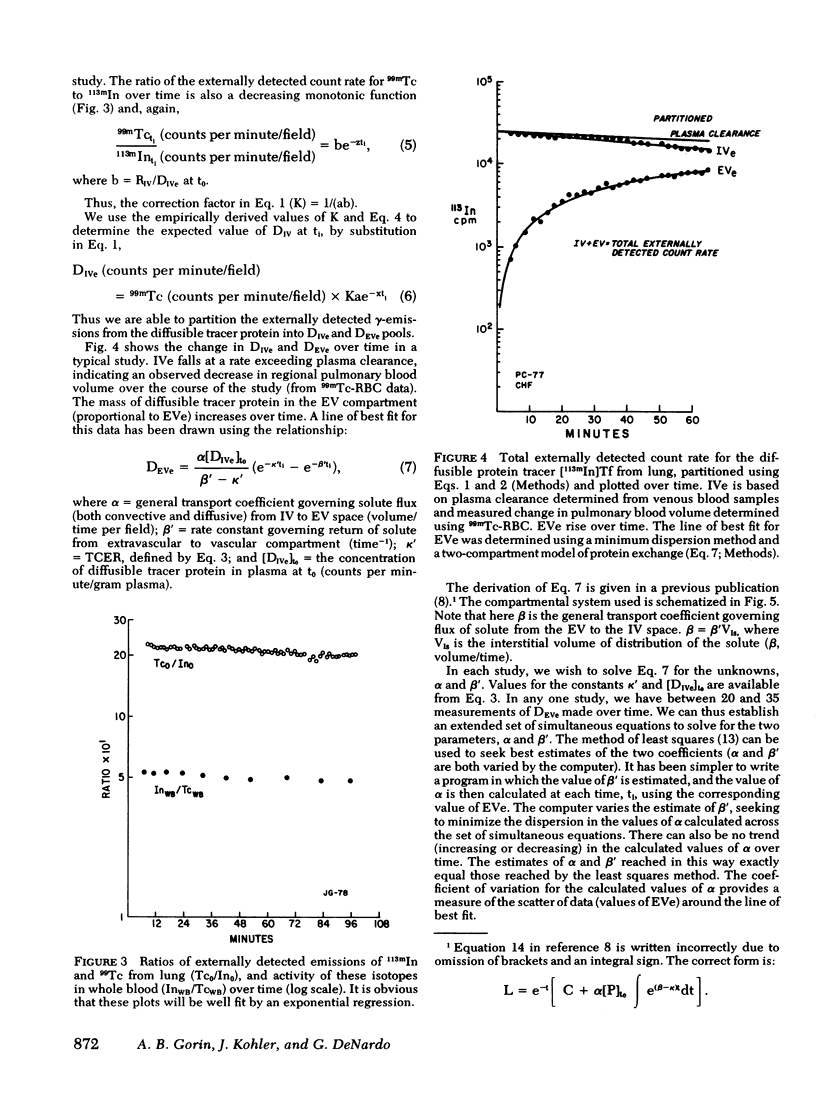
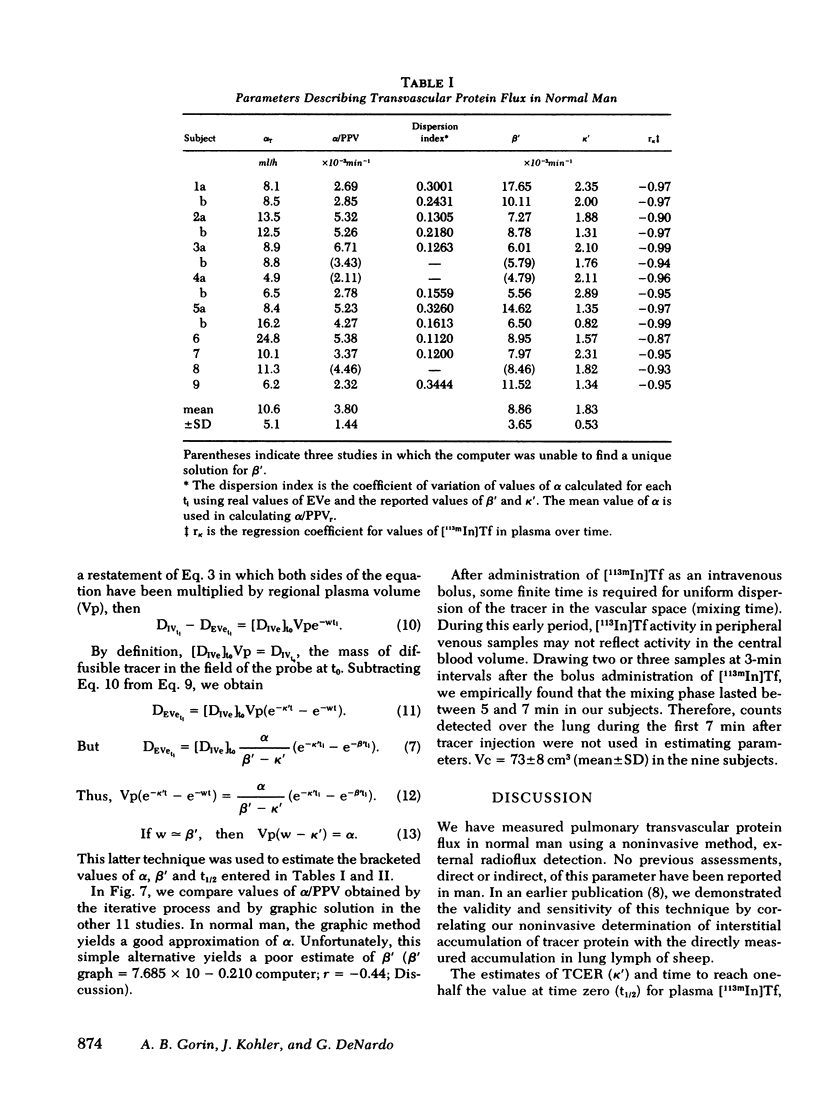
Onset of lung edema is usually associated with increase in the pulmonary transvascular flux of water and proteins. Clinical measurement of these parameters may aid in early diagnosis of pulmonary edema, and allow differentiation between "cardiogenic" and "noncardiogenic" types base on the magnitude of the detected changes. We have previously described a noninvasive method for estimating transvascular protein flux in lung (Gorin, A. B., W. J. Weidner, R. H. Demling, and N. C. Staub, 1978. Noninvasive measurement of pulmonary transvascular protein flux in sheep. J. Appl. Physiol. 45: 225-233). Using this method we measured the net transvascular flux of [113mIn]transferrin (mol wt, 76,000 in lungs of nine normal human volunteers. Plasma clearance of [113In]transferrin occurred with a T1/2 = 7.0 +/- 2.6 h (mean +/- SD). The pulmonary transvascular flux coefficient, alpha, was 2.9 +/- 1.4 X 10(-3) ml/s (mean +/- SD) in man, slightly greater than that previously measured in sheep (2.7 +/- 0.7 X 10(-3) ml/s; mean +/- SD). The pulmonary transcapillary escape rate is twofold greater than the transcapillary escape rate for the vascular bed as a whole, indicating a greater "porosity" of exchanging vessels in the lung than exists for the "average" microvessel in the body. Time taken to reach half-equilibrium concentration of tracer protein in the lung interstitium was quite short, 52 +/- 13 min (mean +/- SD). We have shown that measurement of pulmonary transvascular protein flux in man is practical. The coefficient of variation of measurements of alpha (between subjects) was 0.48, and of measurements of pulmonary transcapillary escape rates was 0.39. In animals, endothelial injury commonly results in a two- to threefold increase in transvascular protein flux. Thus, external radioflux detection should be a suitable means of quantitating lung vascular injury in human disease states.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. R., Holliday R. L., Driedger A. A., Lefcoe M., Reid B., Sibbald W. J. Documentation of pulmonary capillary permeability in the adult respiratory distress syndrome accompanying human sepsis. Am Rev Respir Dis. 1979 Jun;119(6):869–877. doi: 10.1164/arrd.1979.119.6.869. [DOI] [PubMed] [Google Scholar]
- Brigham K. L., Bowers R., Haynes J. Increased sheep lung vascular permeability caused by Escherichia coli endotoxin. Circ Res. 1979 Aug;45(2):292–297. doi: 10.1161/01.res.45.2.292. [DOI] [PubMed] [Google Scholar]
- Brigham K. L., Snell J. D., Jr, Harris T. R., Marshall S., Haynes J., Bowers R. E., Perry J. Indicator dilution lung water and vascular permeability in humans. Effects of pulmonary vascular pressure. Circ Res. 1979 Apr;44(4):523–530. doi: 10.1161/01.res.44.4.523. [DOI] [PubMed] [Google Scholar]
- Brigham K. L., Woolverton W. C., Blake L. H., Staub N. C. Increased sheep lung vascular permeability caused by pseudomonas bacteremia. J Clin Invest. 1974 Oct;54(4):792–804. doi: 10.1172/JCI107819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALY W. J., GIAMMONA S. T., ROSS J. C. THE PRESSURE-VOLUME RELATIONSHIP OF THE NORMAL PULMONARY CAPILLARY BED. J Clin Invest. 1965 Jul;44:1261–1269. doi: 10.1172/JCI105232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly W. J. Pulmonary diffusing capacity for carbon monoxide and topography of perfusion during changes in alveolar pressure in man. Am Rev Respir Dis. 1969 Apr;99(4):548–553. doi: 10.1164/arrd.1969.99.4P1.548. [DOI] [PubMed] [Google Scholar]
- Erdmann A. J., 3rd, Vaughan T. R., Jr, Brigham K. L., Woolverton W. C., Staub N. C. Effect of increased vascular pressure on lung fluid balance in unanesthetized sheep. Circ Res. 1975 Sep;37(3):271–284. doi: 10.1161/01.res.37.3.271. [DOI] [PubMed] [Google Scholar]
- Georges R., Saumon G., Loiseau A. The relationship of age to pulmonary membrane conductance and capillary blood volume. Am Rev Respir Dis. 1978 Jun;117(6):1069–1078. doi: 10.1164/arrd.1978.117.6.1069. [DOI] [PubMed] [Google Scholar]
- Gorin A. B., Weidner W. J., Demling R. H., Staub N. C. Noninvasive measurement of pulmonary transvascular protein flux in sheep. J Appl Physiol Respir Environ Exerc Physiol. 1978 Aug;45(2):225–233. doi: 10.1152/jappl.1978.45.2.225. [DOI] [PubMed] [Google Scholar]
- Korubin V., Maisey M. N., McIntyre P. A. Evaluation of Technetium-labeled red cells for determination of red cell volume in man. J Nucl Med. 1972 Oct;13(10):760–762. [PubMed] [Google Scholar]
- McNamee J. E., Staub N. C. Pore models of sheep lung microvascular barrier using new data on protein tracers. Microvasc Res. 1979 Sep;18(2):229–244. doi: 10.1016/0026-2862(79)90031-1. [DOI] [PubMed] [Google Scholar]
- Nicolaysen G., Staub N. C. Time course of albumin equilibration in interstitium and lymph of normal mouse lungs. Microvasc Res. 1975 Jan;9(1):29–37. doi: 10.1016/0026-2862(75)90048-5. [DOI] [PubMed] [Google Scholar]
- Parving H. H., Rossing N., Nielsen S. L., Lassen N. A. Increased transcapillary escape rate of albumin, IgG, and IgM after plasma volume expansion. Am J Physiol. 1974 Aug;227(2):245–250. doi: 10.1152/ajplegacy.1974.227.2.245. [DOI] [PubMed] [Google Scholar]
- Prichard J. S., Lee G. J. Measurement of water distribution and transcapillary solute flux in dog lung by external radioactivity counting. Clin Sci (Lond) 1979 Aug;57(2):145–154. doi: 10.1042/cs0570145. [DOI] [PubMed] [Google Scholar]
- ROUGHTON F. J., FORSTER R. E. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol. 1957 Sep;11(2):290–302. doi: 10.1152/jappl.1957.11.2.290. [DOI] [PubMed] [Google Scholar]
- Renkin E. M. The microcirculatory society Eugene M. Landis award lecture. Transport pathways through capillary endothelium. Microvasc Res. 1978 Jan;15(1):123–135. doi: 10.1016/0026-2862(78)90013-4. [DOI] [PubMed] [Google Scholar]
- Staub N. C. Pulmonary edema. Physiol Rev. 1974 Jul;54(3):678–811. doi: 10.1152/physrev.1974.54.3.678. [DOI] [PubMed] [Google Scholar]
- Studer R. K., Morgan J., Penkoske M., Potchen E. J. Regional vascular volume and extravascular accumulation of labeled protein during plasma volume expansion. Am J Physiol. 1973 Mar;224(3):699–704. doi: 10.1152/ajplegacy.1973.224.3.699. [DOI] [PubMed] [Google Scholar]
- Studer R., Potchen J. The radioisotopic assessment of regional microvascular permeability to macromolecules. Microvasc Res. 1971 Jan;3(1):35–48. doi: 10.1016/0026-2862(71)90005-7. [DOI] [PubMed] [Google Scholar]
- Vreim C. E., Staub N. C. Indirect and direct capillary blood volume in anesthetized open-thorax cats. J Appl Physiol. 1973 Apr;34(4):452–459. doi: 10.1152/jappl.1973.34.4.452. [DOI] [PubMed] [Google Scholar]
- Vreim C. E., Staub N. C. Protein composition of lung fluids in acute alloxan edema in dogs. Am J Physiol. 1976 Feb;230(2):376–379. doi: 10.1152/ajplegacy.1976.230.2.376. [DOI] [PubMed] [Google Scholar]
- Wittmers L. E., Jr, Bartlett M., Johnson J. A. Estimation of the capillary permeability coefficients of inulin in various tissues of the rabbit. Microvasc Res. 1976 Jan;11(1):67–78. doi: 10.1016/0026-2862(76)90078-9. [DOI] [PubMed] [Google Scholar]
- Wochner R. D., Adatepe M., Van Amburg A., Potchen E. J. A new method for estimation of plasma volume with the use of the distribution space of indium-113m-transferrin. J Lab Clin Med. 1970 May;75(5):711–720. [PubMed] [Google Scholar]
- van der Merwe E. J., Lotter M. G., van Heerden P. D., Slabber C. F., Bester J. Absorbed dose calculations for 113mIn placental scanning. J Nucl Med. 1970 Jan;11(1):31–35. [PubMed] [Google Scholar]


