Abstract
The main objective of this study was to determine whether the principal abnormality of thyroid function observed in patients with chronic renal failure, low serum triiodothyronine (T3) concentration, causes hypothyroidism at the tissue level. A partially nephrectomized (Nx) uremic rat model was developed and the following parameters of thyroid function were assessed: serum total thyroxine (TT4), total T3 (TT3), and thyrotropin and liver T3 content, and activity of two thyroid hormone-dependent enzymes, mitochondrial α-glycerophosphate dehydrogenase (α-GPD) and cytosol malate dehydrogenase (MDH). The results were compared to those of intact control (C), thyroidectomized (Tx), and nephrectomized-thyroidectomized (NxTx) littermates.
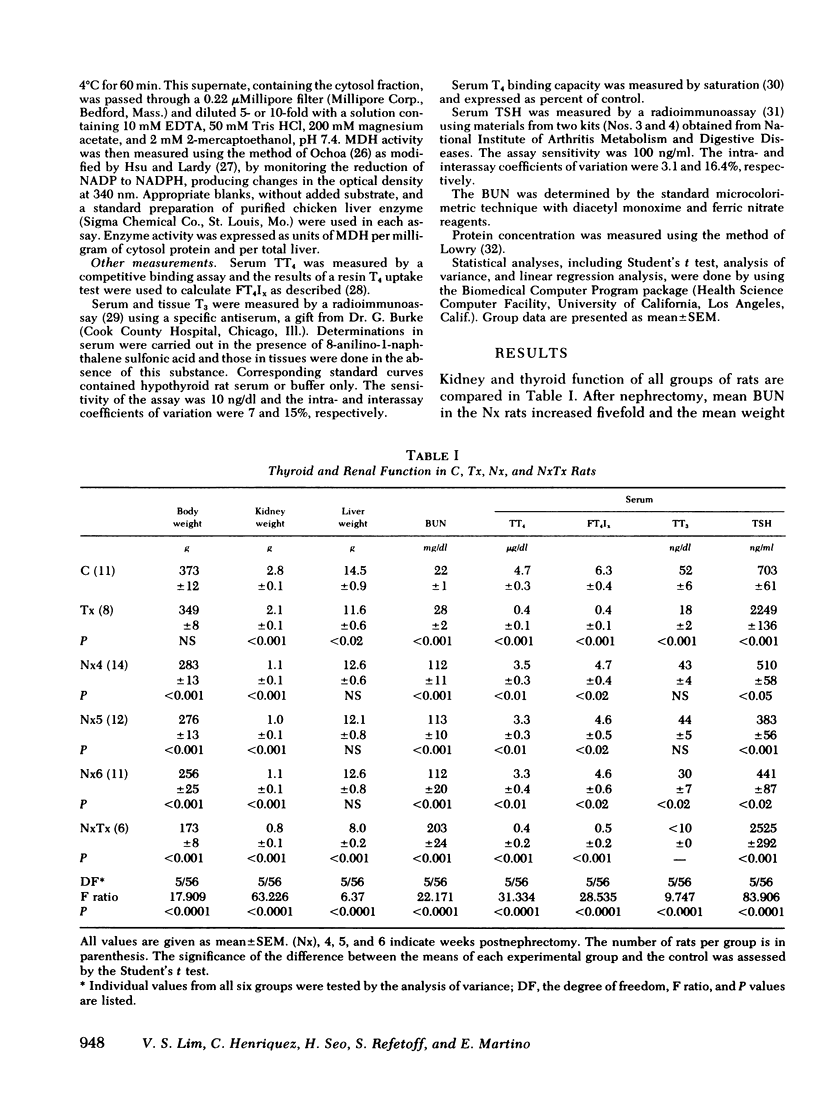
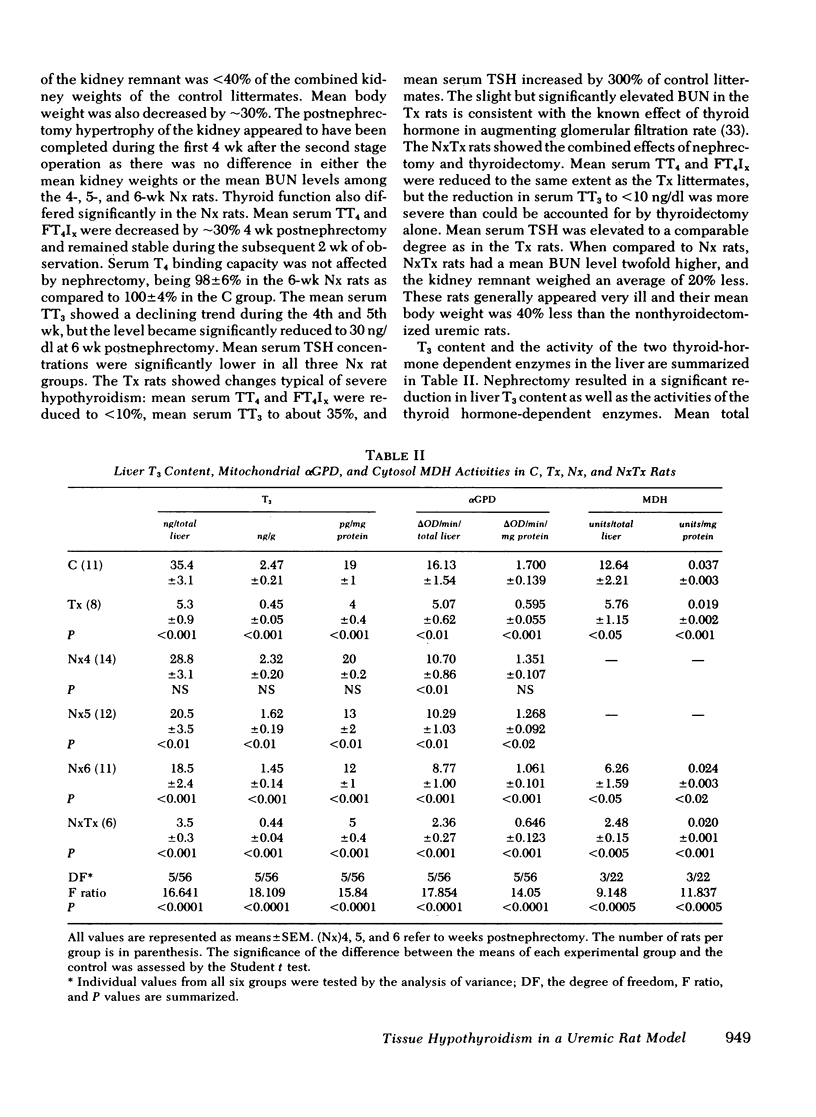
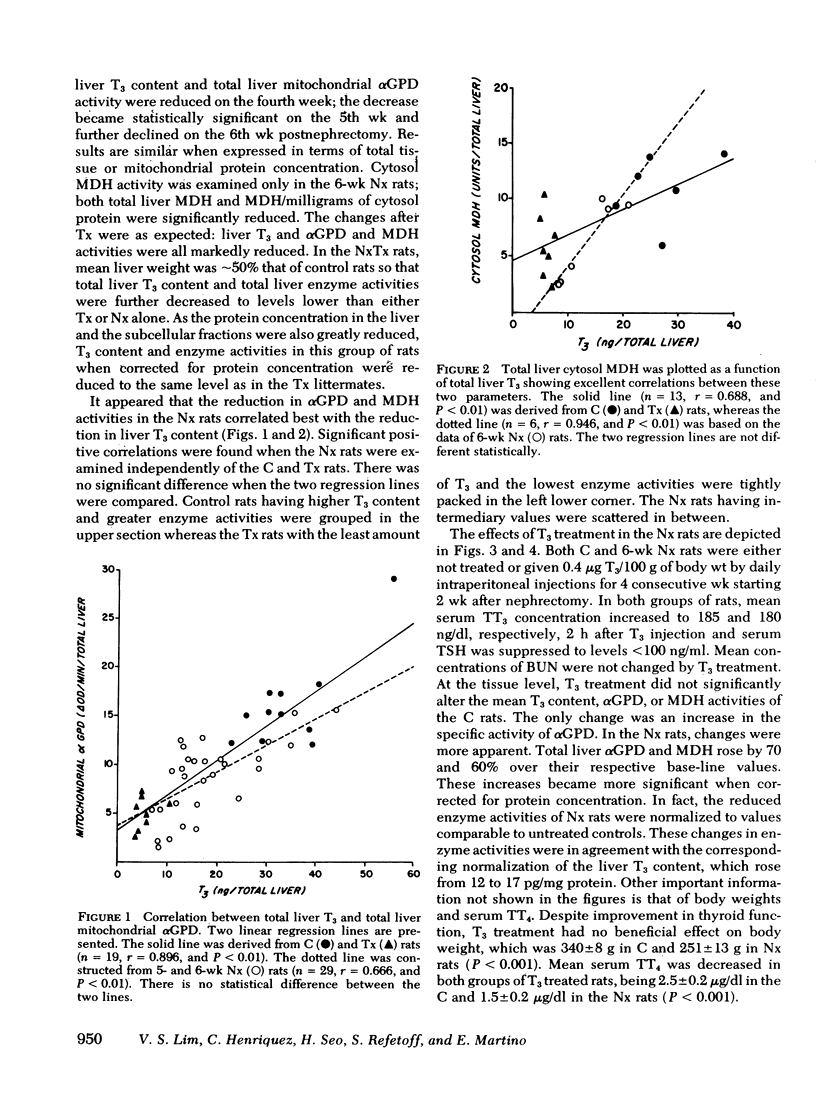
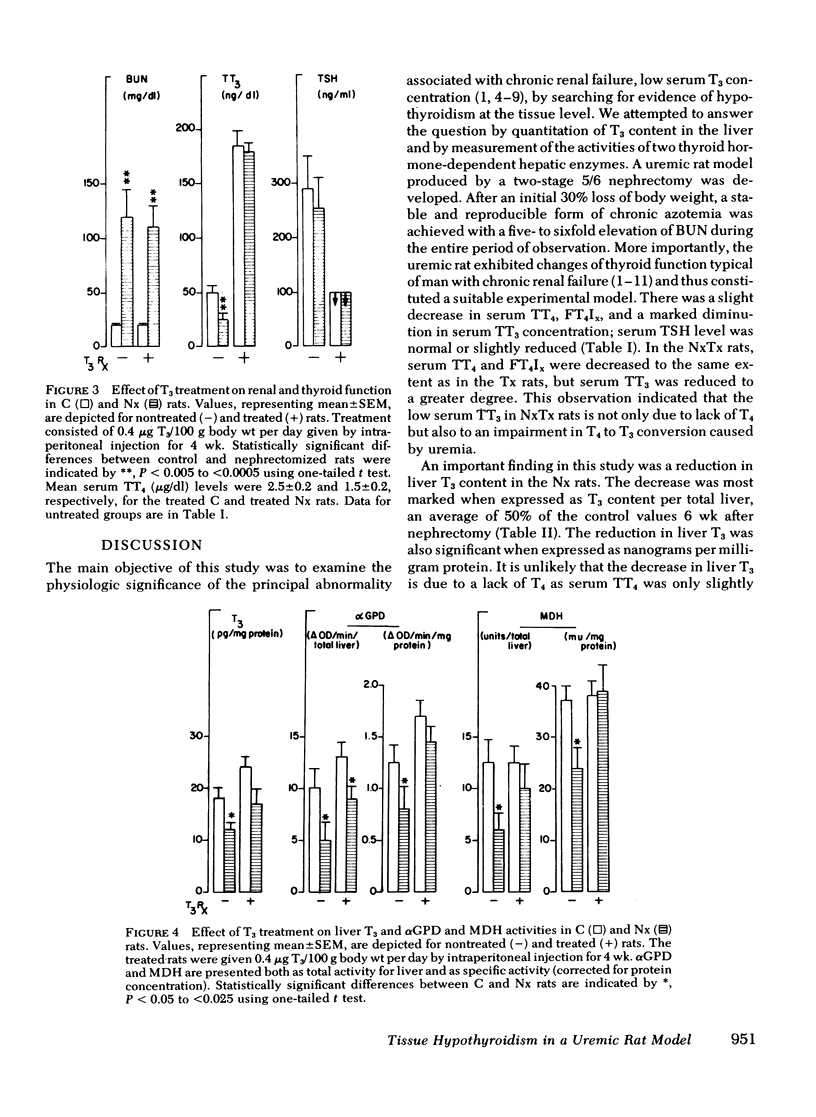
Results expressed as mean±SEM showed that Nx rats had a fivefold increase in blood urea nitrogen, (112±20 mg/dl in Nx, and 22±1 mg/dl in C) and manifested all the changes of of thyroid function observed in uremic men, including a low serum TT3 level (30±7 ng/dl in Nx and 50±6 ng/dl in C). In the liver, T3 was significantly reduced (18±2 ng/total liver in Nx and 35±3 ng/total liver in C) as well as the activities of αGPD (8.8±1.0 and 16.1±1.5 ΔOD/min per total liver in Nx and C, respectively) and MDH (6.3±1.6 and 12.6±2.2 U/total liver in Nx and C, respectively). The reduction in liver enzyme activities correlated significantly with the decrease in T3 content.
The changes in Tx rats were as expected, showing a profound reduction in serum hormone levels, liver T3 content, and liver enzyme activities. Serum thyrotropin was markedly elevated to 2,390±212 ng/ml as compared to 703±61 in C and 441±87 ng/ml in Nx rats. The NxTx rats showed the combined effects of nephrectomy and thyroidectomy; blood urea nitrogen was elevated to 203, and serum levels of TT4, TT3, and thyrotropin were 0.4, <10, and 2,525, respectively. Total liver T3 and αGPD and MDH were strikingly low; the corresponding values were 3.5, 2.4, and 2.5.
l-triiodothyronine replacement (0.4 μg/100 g body wt/d) for 4 wk in the Nx rats resulted in significant increases in liver enzyme activities, αGPD and MDH rose by 70 and 60% over their respective basal values without alteration in the severity of azotemia.
From these data, we conclude that the reduction of liver T3 content in the uremic rats, accompanied by a decrease in αGPD and MDH activity, indicates the presence of hypothyroidism at the tissue level. Restoration of enzyme activities toward normal levels after T3 administration provided further supporting evidence that the diminution in liver enzyme activity was causally related to tissue T3 deficiency.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bermudez F., Surks M. I., Oppenheimer J. H. High incidence of decreased serum triiodothyronine concentration in patients with nonthyroidal disease. J Clin Endocrinol Metab. 1975 Jul;41(1):27–40. doi: 10.1210/jcem-41-1-27. [DOI] [PubMed] [Google Scholar]
- Braverman L. E., Ingbar S. H., Sterling K. Conversion of thyroxine (T4) to triiodothyronine (T3) in athyreotic human subjects. J Clin Invest. 1970 May;49(5):855–864. doi: 10.1172/JCI106304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J. N., Eastman C. J., Corcoran J. M., Lazarus L. Effect of severe, chronic illness on thyroid function. Lancet. 1974 Oct 26;2(7887):971–974. doi: 10.1016/s0140-6736(74)92070-4. [DOI] [PubMed] [Google Scholar]
- Chopra I. J., Chopra U., Smith S. R., Reza M., Solomon D. H. Reciprocal changes in serum concentrations of 3,3',5-triiodothyronine (T3) in systemic illnesses. J Clin Endocrinol Metab. 1975 Dec;41(06):1043–1049. doi: 10.1210/jcem-41-6-1043. [DOI] [PubMed] [Google Scholar]
- Dandona P., Newton D., Platts M. M. Long-term haemodialysis and thyroid function. Br Med J. 1977 Jan 15;1(6054):134–136. doi: 10.1136/bmj.1.6054.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGroot L. J., Coleoni A. H., Rue P. A., Seo H., Martino E., Refetoff S. Reduced nuclear triiodothyronine receptors in starvation-induced hypothyroidism. Biochem Biophys Res Commun. 1977 Nov 7;79(1):173–178. doi: 10.1016/0006-291x(77)90076-6. [DOI] [PubMed] [Google Scholar]
- Dillmann W. H., Schwartz H. L., Oppenheimer J. H. Selective alterations in hepatic enzyme response after reduction of nuclear triiodothyronine receptor sites by partial hepatectomy and starvation. Biochem Biophys Res Commun. 1978 Jan 13;80(1):259–266. doi: 10.1016/0006-291x(78)91131-2. [DOI] [PubMed] [Google Scholar]
- Fang V. S., Refetoff S. Radioimmunoassay for serum triiodothyronine: evaluation of simple techniques to control interference from binding proteins. Clin Chem. 1974 Sep;20(9):1150–1154. [PubMed] [Google Scholar]
- García M. D., Escobar del Rey F., Morreale de Escobar G. Thyrotropin-releasing hormone and thyroid hormone interactions on thyrotropin secretion in the rat: lack of inhibiting effects of small doses of triiodo-L-thyronine in the hypothyroid rat. Endocrinology. 1976 Jan;98(1):203–213. doi: 10.1210/endo-98-1-203. [DOI] [PubMed] [Google Scholar]
- Gardner D. F., Kaplan M. M., Stanley C. A., Utiger R. D. Effect of tri-iodothyronine replacement on the metabolic and pituitary responses to starvation. N Engl J Med. 1979 Mar 15;300(11):579–584. doi: 10.1056/NEJM197903153001102. [DOI] [PubMed] [Google Scholar]
- Hershman J. M., Krugman L. G., Kopple J. D., Reed A. W., Azukizawa M., Shinaberger J. H. Thyroid function in patients undergoing maintenance hemodialysis: unexplained low serum thyroxine concentration. Metabolism. 1978 Jul;27(7):755–759. doi: 10.1016/0026-0495(78)90209-3. [DOI] [PubMed] [Google Scholar]
- Joasoo A., Murray I. P., Parkin J., Robertson M. R., Jeremy D. Abnormalities of in vitro thyroid function tests in renal disease. Q J Med. 1974 Apr;43(170):245–261. [PubMed] [Google Scholar]
- LEE Y. P., LARDY H. A. INFLUENCE OF THYROID HORMONES ON L-ALPHA-GLYCEROPHOSPHATE DEHYDROGENASES AND OTHER DEHYDROGENASES IN VARIOUS ORGANS OF THE RAT. J Biol Chem. 1965 Mar;240:1427–1436. [PubMed] [Google Scholar]
- Lim V. S., Fang V. S. Gonadal dysfunction in uremic men. A study of the hypothalamo-pituitary-testicular axis before and after renal transplantation. Am J Med. 1975 May;58(5):655–662. doi: 10.1016/0002-9343(75)90501-x. [DOI] [PubMed] [Google Scholar]
- Lim V. S., Fang V. S., Katz A. I., Refetoff S. Thyroid dysfunction in chronic renal failure. A study of the pituitary-thyroid axis and peripheral turnover kinetics of thyroxine and triiodothyronine. J Clin Invest. 1977 Sep;60(3):522–534. doi: 10.1172/JCI108804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim V. S., Henriquez C., Sievertsen G., Frohman L. A. Ovarian function in chronic renal failure: evidence suggesting hypothalamic anovulation. Ann Intern Med. 1980 Jul;93(1):21–27. doi: 10.7326/0003-4819-93-1-21. [DOI] [PubMed] [Google Scholar]
- Lim V. S., Kathpalia S. C., Frohman L. A. Hyperprolactinemia and impaired pituitary response to suppression and stimulation in chronic renal failure: reversal after transplantation. J Clin Endocrinol Metab. 1979 Jan;48(1):101–107. doi: 10.1210/jcem-48-1-101. [DOI] [PubMed] [Google Scholar]
- Michael U. F., Barenberg R. L., Chavez R., Vaamonde C. A., Papper S. Renal handling of sodium and water in the hypothyroid rat. Clearance and micropuncture studies. J Clin Invest. 1972 Jun;51(6):1405–1412. doi: 10.1172/JCI106936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejad I., Bollinger J., Mitnick M. A., Sullivan P., Reichlin S. Measurement of plasma and tissue triiodothyronine concentration in the rat by radioimmunoassay. Endocrinology. 1975 Mar;96(3):773–780. doi: 10.1210/endo-96-3-773. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Silva E., Schwartz H. L., Surks M. I. Stimulation of hepatic mitochondrial alpha-glycerophosphate dehydrogenase and malic enzyme by L-triiodothyronine. Characteristics of the response with specific nuclear thyroid hormone binding sites fully saturated. J Clin Invest. 1977 Mar;59(3):517–527. doi: 10.1172/JCI108667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUEGAMER W. R., WESTERFELD W. W., RICHERT D. A. ALPHA-GLYCEROPHOSPHATE DEHYDROGENASE RESPONSE TO THYROXINE IN THYROIDECTOMIZED, THIOURACIL-FED AND TEMPERATURE-ADAPTED RATS. Endocrinology. 1964 Dec;75:908–916. doi: 10.1210/endo-75-6-908. [DOI] [PubMed] [Google Scholar]
- Ramirez G., O'Neill W., Jr, Jubiz W., Bloomer H. A. Thyroid dysfunction in uremia: evidence for thyroid and hypophyseal abnormalities. Ann Intern Med. 1976 Jun;84(6):672–676. doi: 10.7326/0003-4819-84-6-672. [DOI] [PubMed] [Google Scholar]
- Ramírez G., Jubiz W., Gutch C. F., Bloomer H. A., Siegler R., Kolff W. J. Thyroid abnormalities in renal failure. A study of 53 patients on chronic hemodialysis. Ann Intern Med. 1973 Oct;79(4):500–504. doi: 10.7326/0003-4819-79-4-500. [DOI] [PubMed] [Google Scholar]
- Refetoff S., Hagen S. R., Selenkow H. A. Estimation of the T 4 binding capacity of serum TBG and TBPA by a single T 4 load ion exchange resin method. J Nucl Med. 1972 Jan;13(1):2–12. [PubMed] [Google Scholar]
- Reichlin S., Bollinger J., Nejad I., Sullivan P. Tissue thyroid hormone concentration of rat and man determined by radiommunoassay: biologic significance. Mt Sinai J Med. 1973 May-Jun;40(3):502–510. [PubMed] [Google Scholar]
- Reichlin S., Martin J. B., Boshans R. L., Schalch D. S., Pierce J. G., Bollinger J. Measurement of TSH in plasma and pituitary of the rat by a radioimmunoassay utilizing bovine TSH: effect of thyroidectomy or thyroxine administration on plasma TSH levels. Endocrinology. 1970 Nov;87(5):1022–1031. doi: 10.1210/endo-87-5-1022. [DOI] [PubMed] [Google Scholar]
- Robin N. I., Hagen S. R., Collaço F., Refetoff S., Selenkow H. A. Serum tests for measurement of thyroid function. Hormones. 1971;2(5):266–279. doi: 10.1159/000178240. [DOI] [PubMed] [Google Scholar]
- Schwartz H. L., Forciea M. A., Mariash C. N., Oppenheimer J. H. Age-related reduction in response of hepatic enzymes to 3,5,3'-triiodothyronine administration. Endocrinology. 1979 Jul;105(1):41–46. doi: 10.1210/endo-105-1-41. [DOI] [PubMed] [Google Scholar]
- Sewell D. L., Wostmann B. S. Thyroid function and related hepatic enzymes in the germfree rat. Metabolism. 1975 Jun;24(6):695–701. doi: 10.1016/0026-0495(75)90037-2. [DOI] [PubMed] [Google Scholar]
- Sievertsen G. D., Lim V. S., Nakawatase C., Frohman L. A. Metabolic clearance and secretion rates of human prolactin in normal subjects and in patients with chronic renal failure. J Clin Endocrinol Metab. 1980 May;50(5):846–852. doi: 10.1210/jcem-50-5-846. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Dick T. E., Larsen P. R. The contribution of local tissue thyroxine monodeiodination to the nuclear 3,5,3'-triiodothyronine in pituitary, liver, and kidney of euthyroid rats. Endocrinology. 1978 Oct;103(4):1196–1207. doi: 10.1210/endo-103-4-1196. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Pituitary nuclear 3,5,3'-triiodothyronine and thyrotropin secretion: an explanation for the effect of thyroxine. Science. 1977 Nov 11;198(4317):617–620. doi: 10.1126/science.199941. [DOI] [PubMed] [Google Scholar]
- Silverberg D. S., Ulan R. A., Fawcett D. M., Dossetor J. B., Grace M., Bettcher K. Effects of chronic hemodialysis on thyroid function in chronic renal failure. Can Med Assoc J. 1973 Aug 18;109(4):282–286. [PMC free article] [PubMed] [Google Scholar]
- Spector D. A., Davis P. J., Helderman J. H., Bell B., Utiger R. D. Thyroid function and metabolic state in chronic renal failure. Ann Intern Med. 1976 Dec;85(6):724–730. doi: 10.7326/0003-4819-85-6-724. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Oppenheimer J. H. Concentration of L-thyroxine and L-triiodothyronine specifically bound to nuclear receptors in rat liver and kidney. Quantitative evidence favoring a major role of T3 in thyroid hormone action. J Clin Invest. 1977 Sep;60(3):555–562. doi: 10.1172/JCI108807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimpfheimer C., Saville E., Voirol M. J., Danforth E., Jr, Burger A. G. Starvation-induced decreased sensitivity of resting metabolic rate to triiodothyronine. Science. 1979 Sep 21;205(4412):1272–1273. doi: 10.1126/science.224460. [DOI] [PubMed] [Google Scholar]


