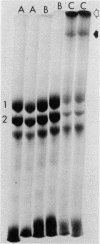
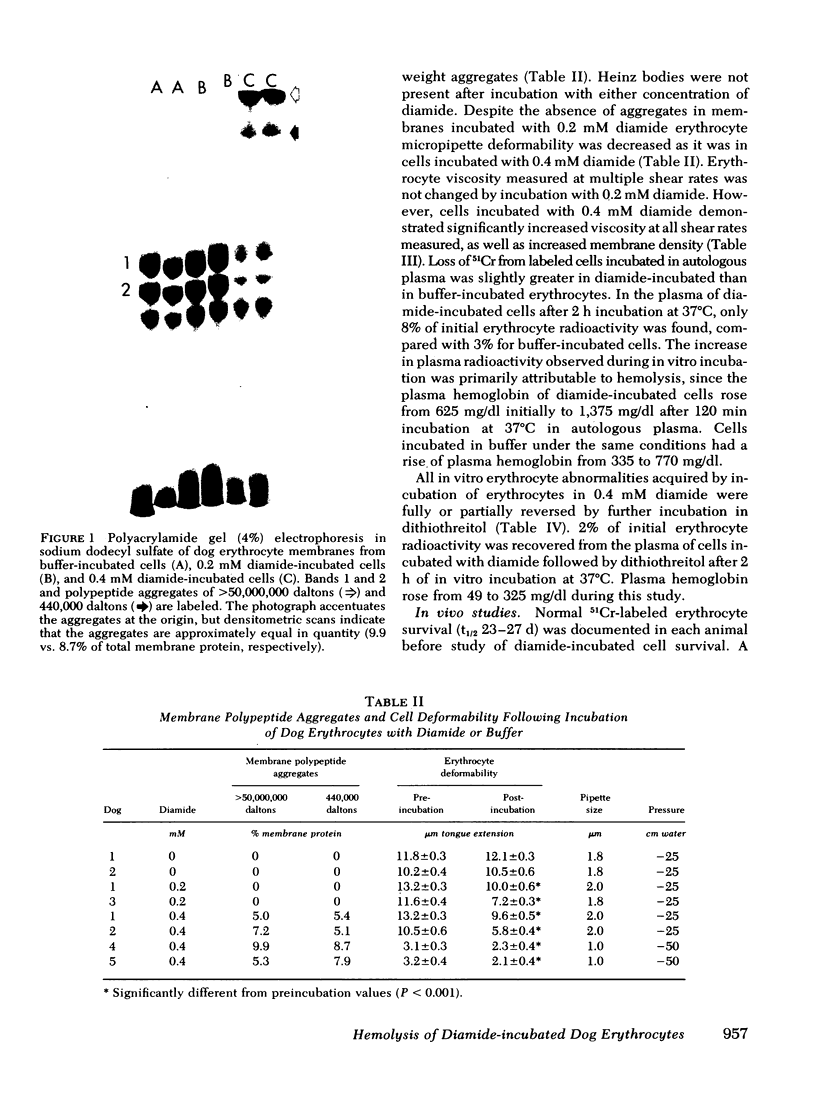
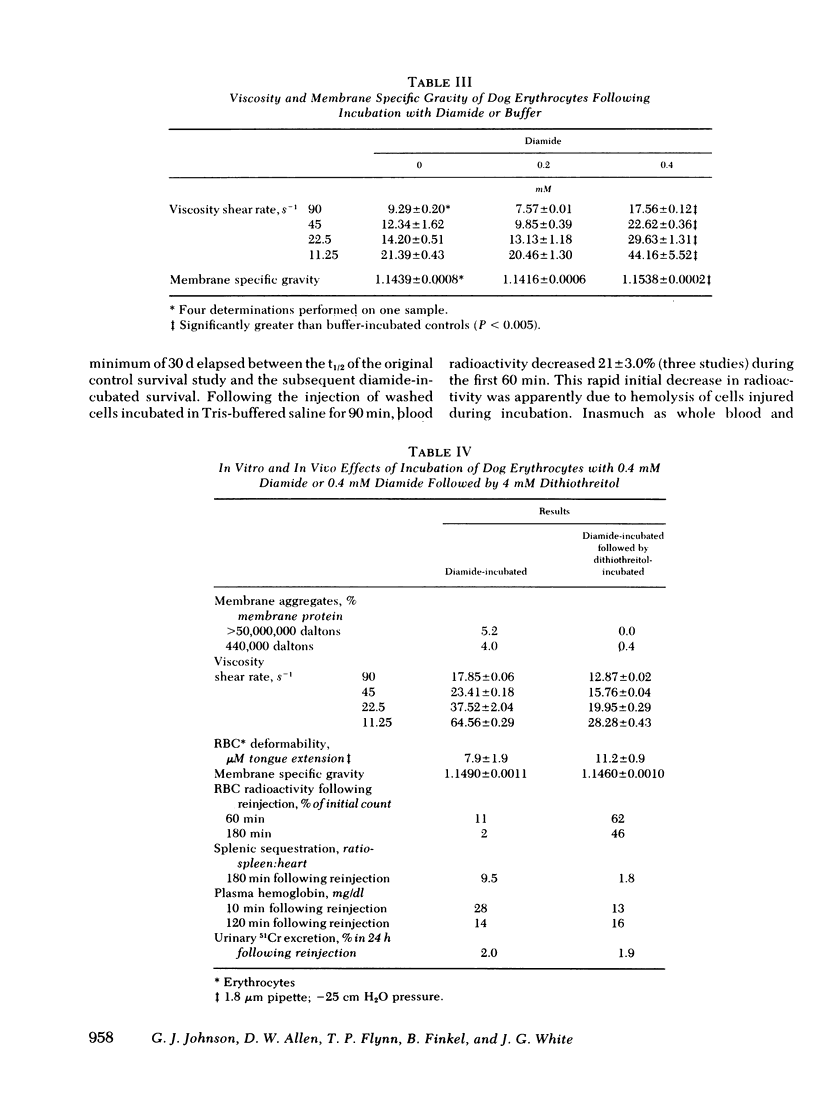
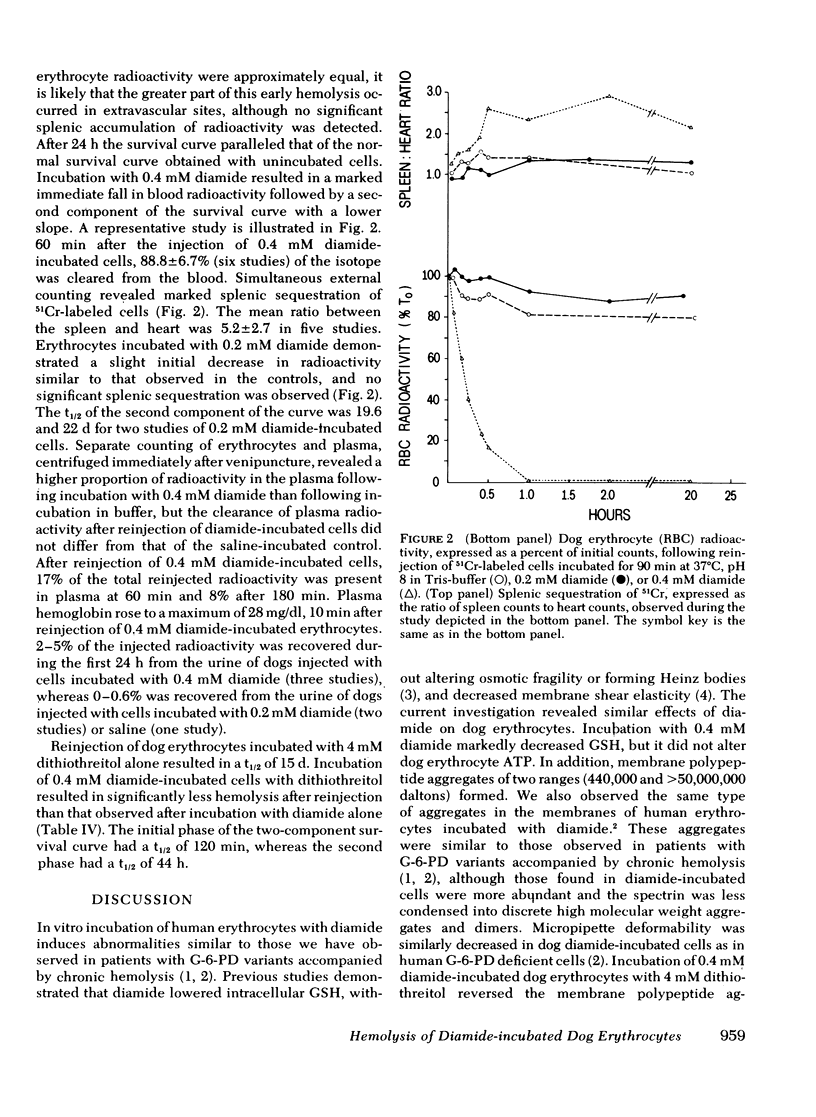
Abstract
Erythrocytes from patients with chronic hemolytic variants of glucose-6-phosphate dehydrogenase (G-6-PD) deficiency have structural membrane protein abnormalities accompanied by decreased cell membrane deformability which we postulate represent the consequences of oxidant-induced membrane injury. To evaluate the pathophysiologic significance of oxidant-induced membrane injury, we studied the in vitro and in vivo effects of the thiol-oxidizing agent, diamide, on dog erythrocytes. In vitro incubation of dog erythrocytes with 0.4 mM diamide in Tris-buffered saline for 90 min at 37 degrees C resulted in depletion of GSH, formation of membrane polypeptide aggregates (440,000 and > 50,000,000 daltons) and decreased cell micropipette deformability, abnormalities similar to those observed in the erythrocytes of patients with chronic hemolytic variants of G-6-PD deficiency. In addition, diamide-incubated cells had increased viscosity and increased membrane specific gravity, but no change in ATP. Reinjection of 51Cr-labeled, diamide-incubated cells was followed by markedly shortened in vivo survival and splenic sequestration. Further incubation of diamide-incubated cells in 4 mM dithiothreitol reversed the membrane polypeptide aggregates, normalized micropipette deformability, decreased cell viscosity, prolonged in vivi survival, and decreased splenic sequestration. These studied demonstrate that diamide induces a partially reversible erythrocyte lesion which is a useful model of oxidant-induced membrane injury. They suggest that oxidant-induced erythrocyte membrane injury plays an important role in the pathophysiology of chronic hemolysis which accompanies some G-6-PD variants.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. W., Cadman S., McCann S. R., Finkel B. Increased membrane binding of erythrocyte catalase in hereditary spherocytosis and in metabolically stressed normal cells. Blood. 1977 Jan;49(1):113–123. [PubMed] [Google Scholar]
- Allen D. W., Johnson G. J., Cadman S., Kaplan M. E. Membrane polypeptide aggregates in glucose 6-phosphate dehydrogenase-deficient and in vitro aged red blood cells. J Lab Clin Med. 1978 Feb;91(2):321–327. [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Dreher K. L., Eaton J. W., Kuettner J. F., Breslawec K. P., Blackshear P. L., White J. G. Retention of water and potassium by erythrocytes prevents calcium-induced membrane rigidity. Am J Pathol. 1978 Jul;92(1):215–225. [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fischer T. M., Haest C. W., Stöhr M., Kamp D., Deuticke B. Selective alteration of erythrocyte deformabiliby by SH-reagents: evidence for an involvement of spectrin in membrane shear elasticity. Biochim Biophys Acta. 1978 Jul 4;510(2):270–282. doi: 10.1016/0005-2736(78)90027-5. [DOI] [PubMed] [Google Scholar]
- Johnson G. J., Allen D. W., Cadman S., Fairbanks V. F., White J. G., Lampkin B. C., Kaplan M. E. Red-cell-membrane polypeptide aggregates in glucose-6-phosphate dehydrogenase mutants with chronic hemolytic disease. A clue to the mechanism of hemolysis. N Engl J Med. 1979 Sep 6;301(10):522–527. doi: 10.1056/NEJM197909063011004. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M., Wertheim B., Correa W. S. Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem Biophys Res Commun. 1969 Nov 6;37(4):593–596. doi: 10.1016/0006-291x(69)90850-x. [DOI] [PubMed] [Google Scholar]
- MOLLISON P. L., VEALL N. The use of the isotope 51Cr as a label for red cells. Br J Haematol. 1955 Jan;1(1):62–74. doi: 10.1111/j.1365-2141.1955.tb05489.x. [DOI] [PubMed] [Google Scholar]
- Pinteric L., Manery J. F., Chaudry I. H., Madapallimattam G. The effect of EDTA, cations, and various buffers on the morphology of erythrocyte membranes: an electron-microscopic study. Blood. 1975 May;45(5):709–724. [PubMed] [Google Scholar]
- Rifkind R. A. Heinz body anemia: an ultrastructural study. II. Red cell sequestration and destruction. Blood. 1965 Oct;26(4):433–448. [PubMed] [Google Scholar]