Abstract
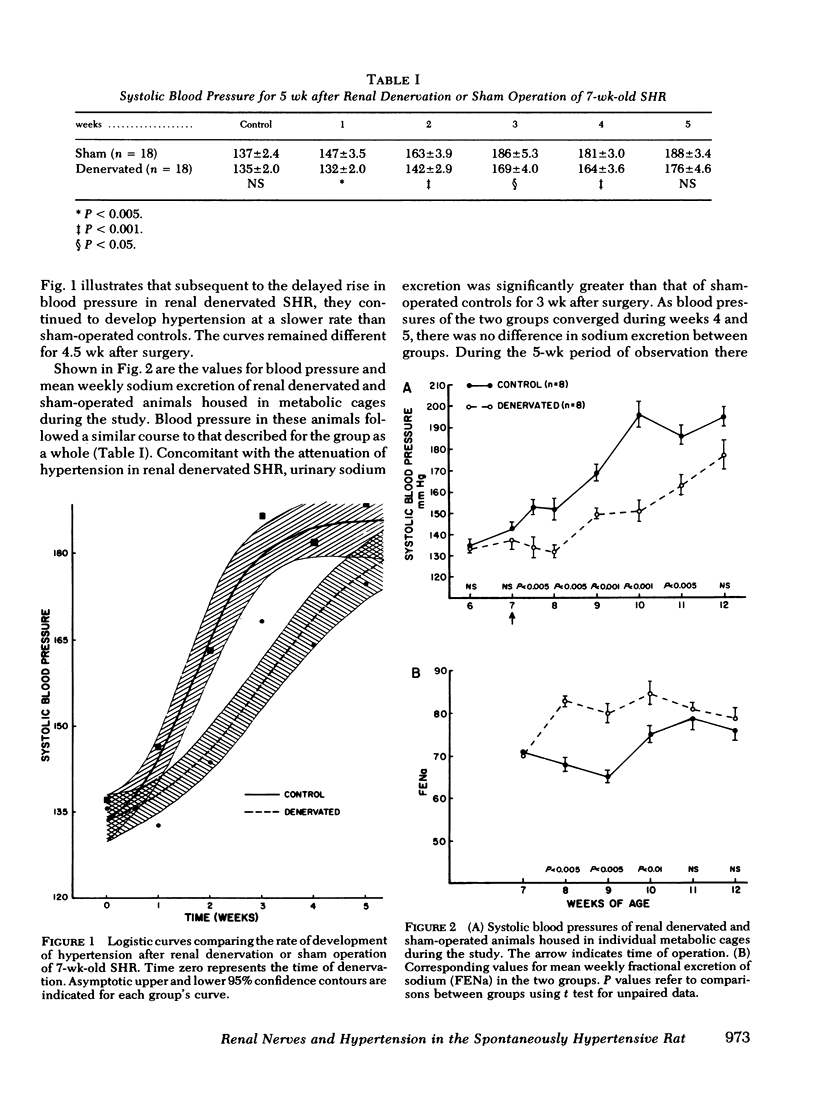
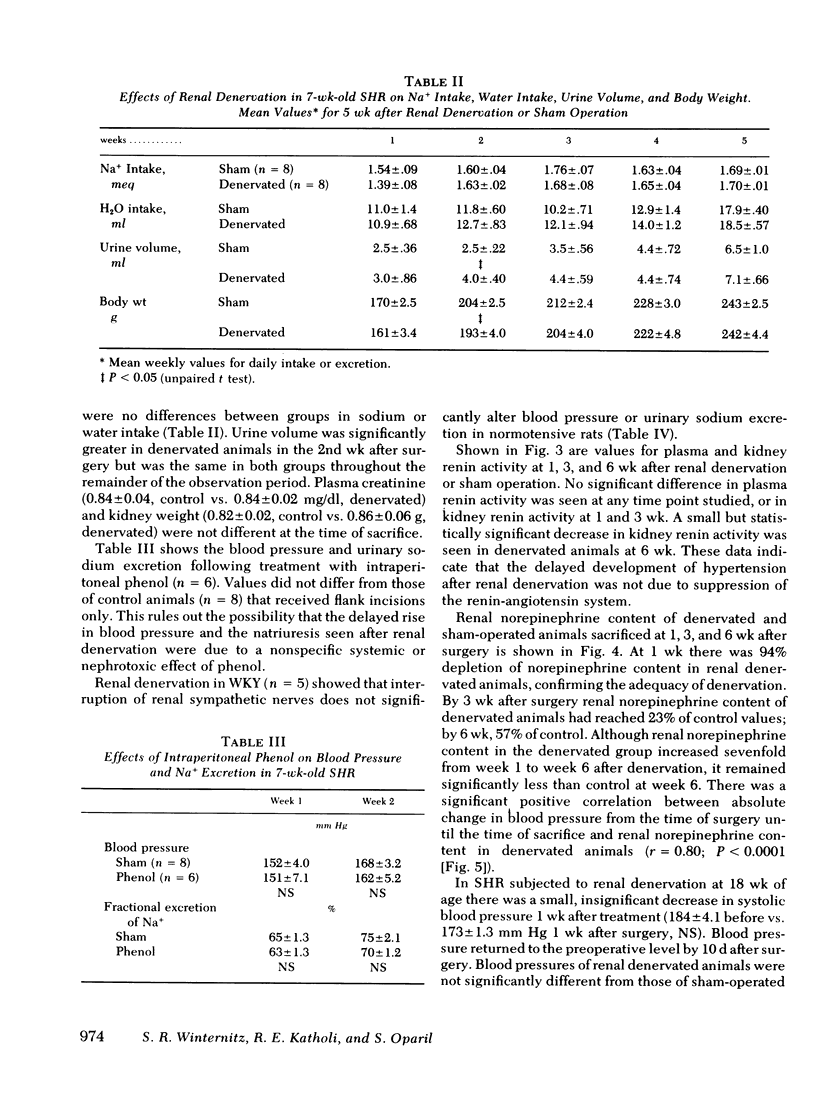
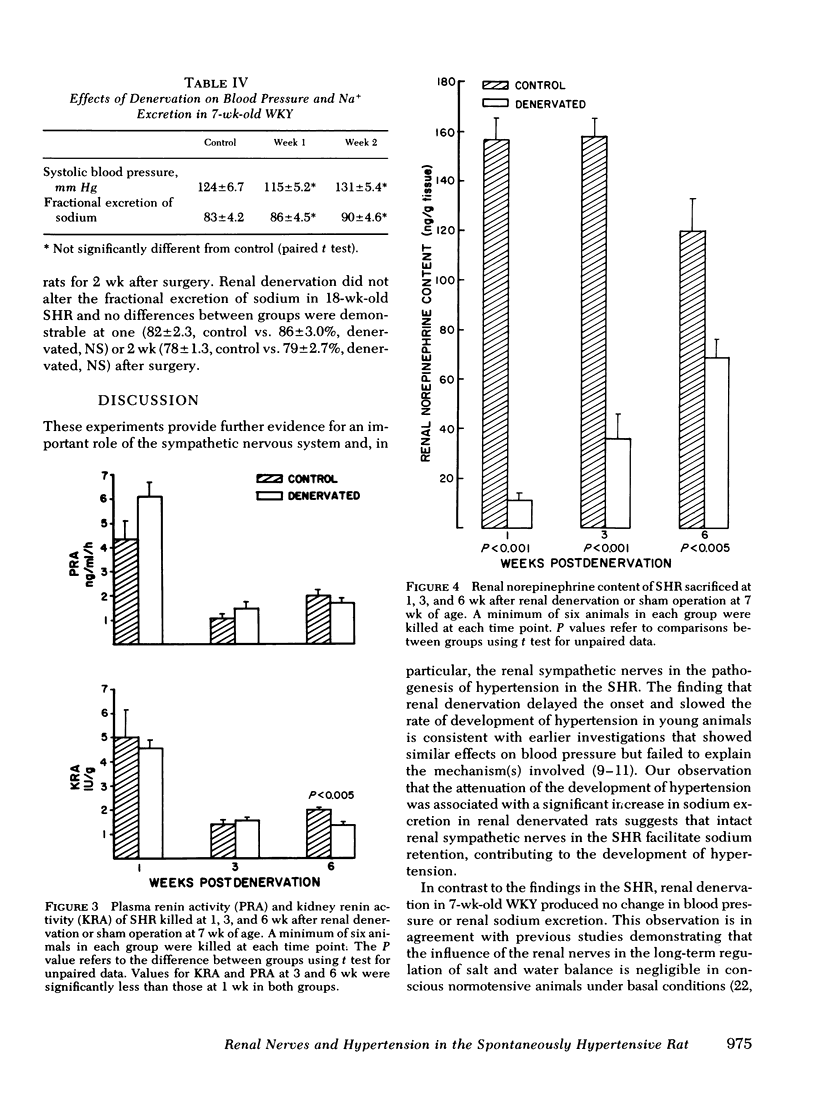
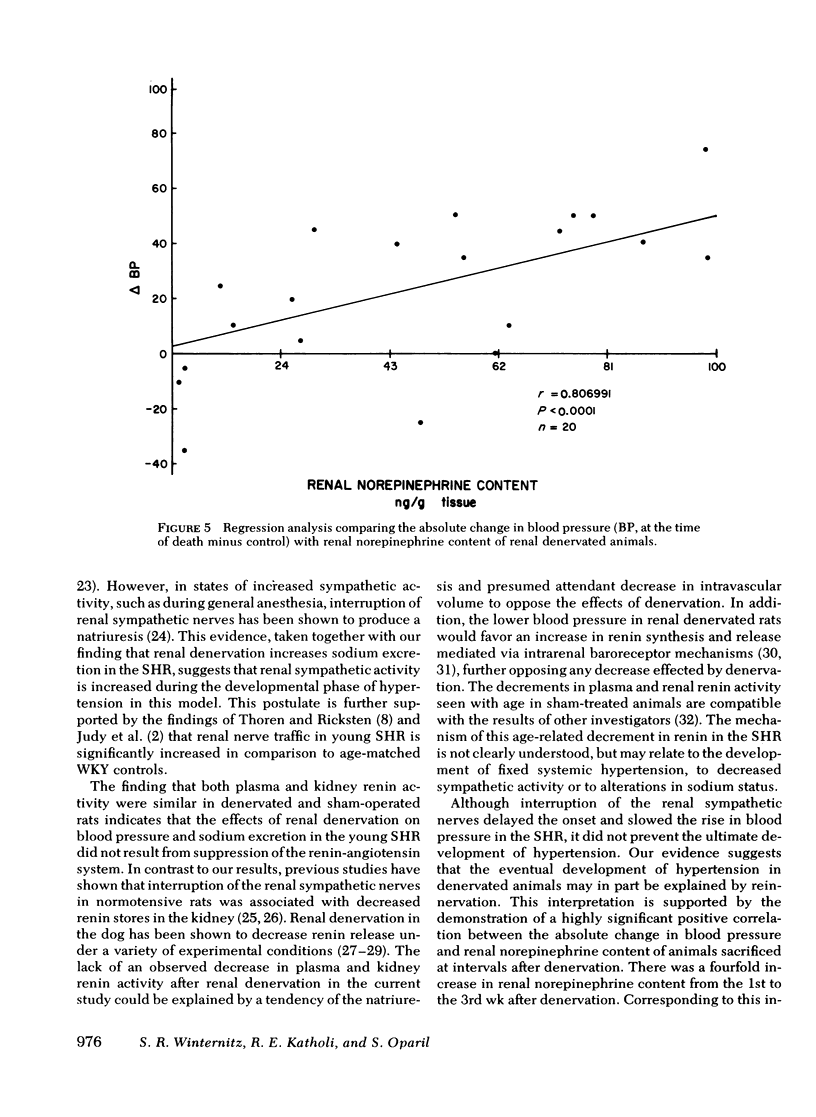
Neurogenic factors and, in particular, enhanced renal sympathetic tone, have been implicated in the pathogenesis of hypertension in the spontaneously hypertensive rat of the Okamoto strain. To examine the hypothesis that the renal sympathetic nerves contribute to the development and maintenance of hypertension by causing urinary sodium retention, 7-wk-old (early hypertensive) and 18-wk-old (established hypertensive) male spontaneously hypertensive rats were subjected to bilateral renal denervation and compared with sham-operated controls. In 7-wk-old animals renal denervation delayed the onset and slowed the rate of development of hypertension. These alterations were associated with a significantly greater fractional excretion of sodium (percentage of sodium intake excreted) during the first 3 wk after denervation. Blood pressure 2 wk after surgery was 169±3.5 (sham) vs. 150±2.4 mm Hg (denervated) (P < 0.001), corresponding to fractional sodium excretions of 65±1.3% (sham) vs. 80±2.3% (denervated) (P < 0.001). By the 5th wk after surgery, at which time an increase in renal norepinephrine content of denervated animals suggested reinnervation, blood pressures in the two groups converged (sham, 199±6.5 mm Hg vs. denervated 180±3.5 mm Hg, NS) and there was no difference in sodium excretion (sham, 77±2.5% vs. denervated 79±2.3%). Plasma and kidney renin activity of denervated animals did not differ significantly from that of sham-operated controls. In 18-wk-old rats renal denervation did not alter blood pressure or urinary sodium excretion. These data indicate that the renal sympathetic nerves contribute to the development of hypertension in the spontaneously hypertensive rat in part by causing enhanced sodium retention. Once hypertension is established the renal nerves do not play a significant role in the maintenance of increased blood pressure.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNE R. M. Hemodynamics and sodium excretion of denervated kidney in anesthetized and unanesthetized dog. Am J Physiol. 1952 Oct;171(1):148–158. doi: 10.1152/ajplegacy.1952.171.1.148. [DOI] [PubMed] [Google Scholar]
- BRICKER N. S., STRAFFON R. A., MAHONEY E. P., MERRILL J. P. The functional capacity of the kidney denervated by autotransplantation in the dog. J Clin Invest. 1958 Feb;37(2):185–193. doi: 10.1172/JCI103597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher R., Ménard J., Genest J. A micromethod for measurement of renin in the plasma and kidney of rats. Can J Physiol Pharmacol. 1967 Sep;45(5):881–890. doi: 10.1139/y67-103. [DOI] [PubMed] [Google Scholar]
- DiBona G. F. Neural control of renal tubular sodium reabsorption of the dog. Fed Proc. 1978 Apr;37(5):1214–1217. [PubMed] [Google Scholar]
- Fernández B. E., Dominguez A. E., Vidal N. A., Taquini A. C., Jr Renal denervation and catecholamines of the central nervous system. Neuroendocrinology. 1974;15(6):338–345. doi: 10.1159/000122324. [DOI] [PubMed] [Google Scholar]
- Folkow B., Hallbäck M., Lundgren Y., Weiss L. Renal vascular resistance in spontaneously hypertensive rats. Acta Physiol Scand. 1971 Sep;83(1):96–105. doi: 10.1111/j.1748-1716.1971.tb05055.x. [DOI] [PubMed] [Google Scholar]
- Folkow B., Hallbäck M., Lundgren Y., Weiss L. Structurally based increase of flow resistance in spontaneously hypertensive rats. Acta Physiol Scand. 1970 Jul;79(3):373–378. doi: 10.1111/j.1748-1716.1970.tb04737.x. [DOI] [PubMed] [Google Scholar]
- Grandjean B., Annat G., Vincent M., Sassard J. Influence of renal nerves on renin secretion in the conscious dog. Pflugers Arch. 1978 Feb 22;373(2):161–165. doi: 10.1007/BF00584855. [DOI] [PubMed] [Google Scholar]
- HAAS E., LAMFROM H., GOLDBLATT H. A simple method for the extraction and partial purification of renin. Arch Biochem Biophys. 1954 Feb;48(2):256–260. doi: 10.1016/0003-9861(54)90339-2. [DOI] [PubMed] [Google Scholar]
- Haber E., Koerner T., Page L. B., Kliman B., Purnode A. Application of a radioimmunoassay for angiotensin I to the physiologic measurements of plasma renin activity in normal human subjects. J Clin Endocrinol Metab. 1969 Oct;29(10):1349–1355. doi: 10.1210/jcem-29-10-1349. [DOI] [PubMed] [Google Scholar]
- Haeusler G., Finch L., Thoenen H. Central adrenergic neurones and the initiation and development of experimental hypertension. Experientia. 1972 Oct 15;28(10):1200–1203. doi: 10.1007/BF01946170. [DOI] [PubMed] [Google Scholar]
- Judy W. V., Watanabe A. M., Henry D. P., Besch H. R., Jr, Murphy W. R., Hockel G. M. Sympathetic nerve activity: role in regulation of blood pressure in the spontaenously hypertensive rat. Circ Res. 1976 Jun;38(6 Suppl 2):21–29. doi: 10.1161/01.res.38.6.21. [DOI] [PubMed] [Google Scholar]
- Kline R. L., Kelton P. M., Mercer P. F. Effect of renal denervation on the development of hypertension in spontaneously hypertensive rats. Can J Physiol Pharmacol. 1978 Oct;56(5):818–822. doi: 10.1139/y78-128. [DOI] [PubMed] [Google Scholar]
- Liard J. F. Renal denervation delays blood pressure increase in the spontaneously hypertensive rat. Experientia. 1977 Mar 15;33(3):339–340. doi: 10.1007/BF02002815. [DOI] [PubMed] [Google Scholar]
- Mogil R. A., Itskovitz H. D., Russell J. H., Murphy J. J. Renal innervation and renin activity in salt metabolism and hypertension. Am J Physiol. 1969 Apr;216(4):693–697. doi: 10.1152/ajplegacy.1969.216.4.693. [DOI] [PubMed] [Google Scholar]
- Nadeau R. A., de Champlain J., Tremblay G. M. Supersensitivity of the isolated rat heart after chemical sympathectomy with 6-hydroxydopamine. Can J Physiol Pharmacol. 1971 Jan;49(1):36–44. doi: 10.1139/y71-005. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Nosaka S., Yamori Y., Matsumoto M. Participation of neural factor in the pathogenesis of hypertension in the spontaneously hypertensive rat. Jpn Heart J. 1967 Mar;8(2):168–180. doi: 10.1536/ihj.8.168. [DOI] [PubMed] [Google Scholar]
- Oparil S., Erinoff L., Cutilletta A. Catecholamines, blood pressure, renin and myocardial function in the spontaneously hypertensive rat. Clin Sci Mol Med Suppl. 1976 Dec;3:445s–459s. [PubMed] [Google Scholar]
- Peuler J. D., Johnson G. A. Simultaneous single isotope radioenzymatic assay of plasma norepinephrine, epinephrine and dopamine. Life Sci. 1977 Sep 1;21(5):625–636. doi: 10.1016/0024-3205(77)90070-4. [DOI] [PubMed] [Google Scholar]
- Prosnitz E. H., DiBona G. F. Effect of decreased renal sympathetic nerve activity on renal tubular sodium reabsorption. Am J Physiol. 1978 Dec;235(6):F557–F563. doi: 10.1152/ajprenal.1978.235.6.F557. [DOI] [PubMed] [Google Scholar]
- Saavedra J. M., Grobecker H., Axelrod J. Changes in central catecholaminergic neurons in the spontaneously (genetic) hypertensive rat. Circ Res. 1978 Apr;42(4):529–534. doi: 10.1161/01.res.42.4.529. [DOI] [PubMed] [Google Scholar]
- Schrier R. W. Effects of adrenergic nervous system and catecholamines on systemic and renal hemodynamics, sodium and water excretion and renin secretion. Kidney Int. 1974 Nov;6(5):291–306. doi: 10.1038/ki.1974.115. [DOI] [PubMed] [Google Scholar]
- TAQUINI A. C., BLAQUIER P., TAQUINI A. C., Jr ON THE PRODUCTION AND ROLE OF RENIN. Can Med Assoc J. 1964 Jan 25;90:210–213. [PMC free article] [PubMed] [Google Scholar]
- TOBIAN L., TOMBOULIAN A., JANECEK J. The effect of high perfusion pressures on the granulation of juxtaglomerular cells in an isolated kidney. J Clin Invest. 1959 Apr;38(4):605–610. doi: 10.1172/JCI103838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames M. D., DiBona G. F. Renal nerves modulate the secretion of renin mediated by nonneural mechanisms. Circ Res. 1979 May;44(5):645–652. doi: 10.1161/01.res.44.5.645. [DOI] [PubMed] [Google Scholar]
- Thorén P., Ricksten S. E. Recordings of renal and splanchnic sympathetic nervous activity in normotensive and spontaneously hypertensive rats. Clin Sci (Lond) 1979 Dec;57 (Suppl 5):197s–199s. doi: 10.1042/cs057197s. [DOI] [PubMed] [Google Scholar]
- Vander A. J. Control of renin release. Physiol Rev. 1967 Jul;47(3):359–382. doi: 10.1152/physrev.1967.47.3.359. [DOI] [PubMed] [Google Scholar]
- Warshaw D. M., Mulvany M. J., Halpern W. Mechanical and morphological properties of arterial resistance vessels in young and old spontaneously hypertensive rats. Circ Res. 1979 Aug;45(2):250–259. doi: 10.1161/01.res.45.2.250. [DOI] [PubMed] [Google Scholar]
- Wijnen H. J., Versteeg D. H., Palkovits M., De Jong W. Increased adrenaline content of individual nuclei of the hypothalamus and the medulla oblongata of genetically hypertensive ralamus and the medulla oblongata of genetically hypertensive rats. Brain Res. 1977 Oct 21;135(1):180–185. doi: 10.1016/0006-8993(77)91064-2. [DOI] [PubMed] [Google Scholar]


