Abstract
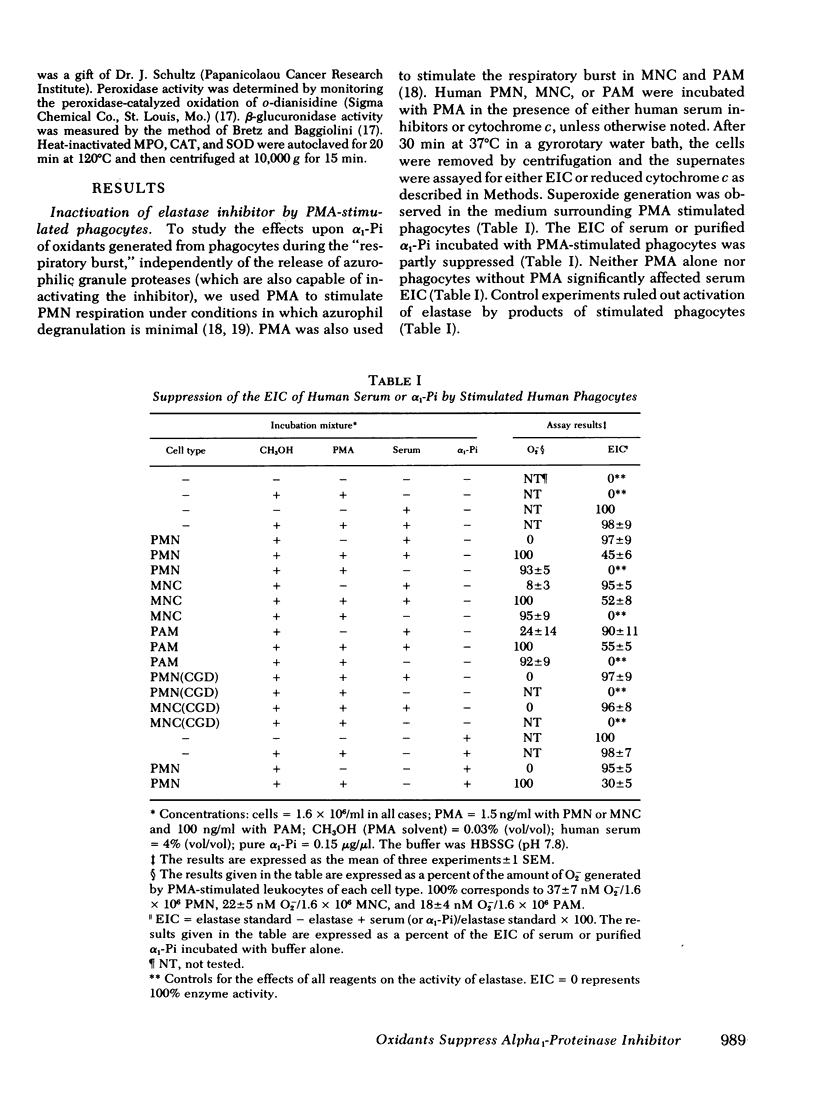
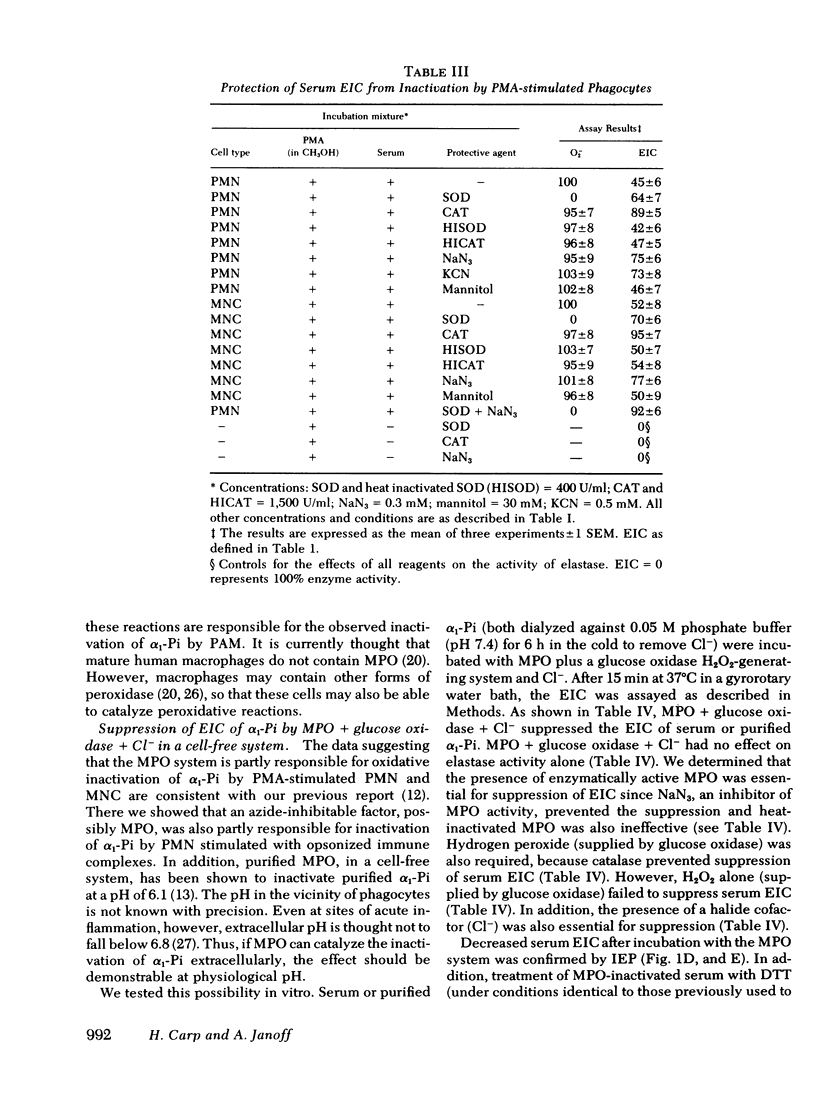
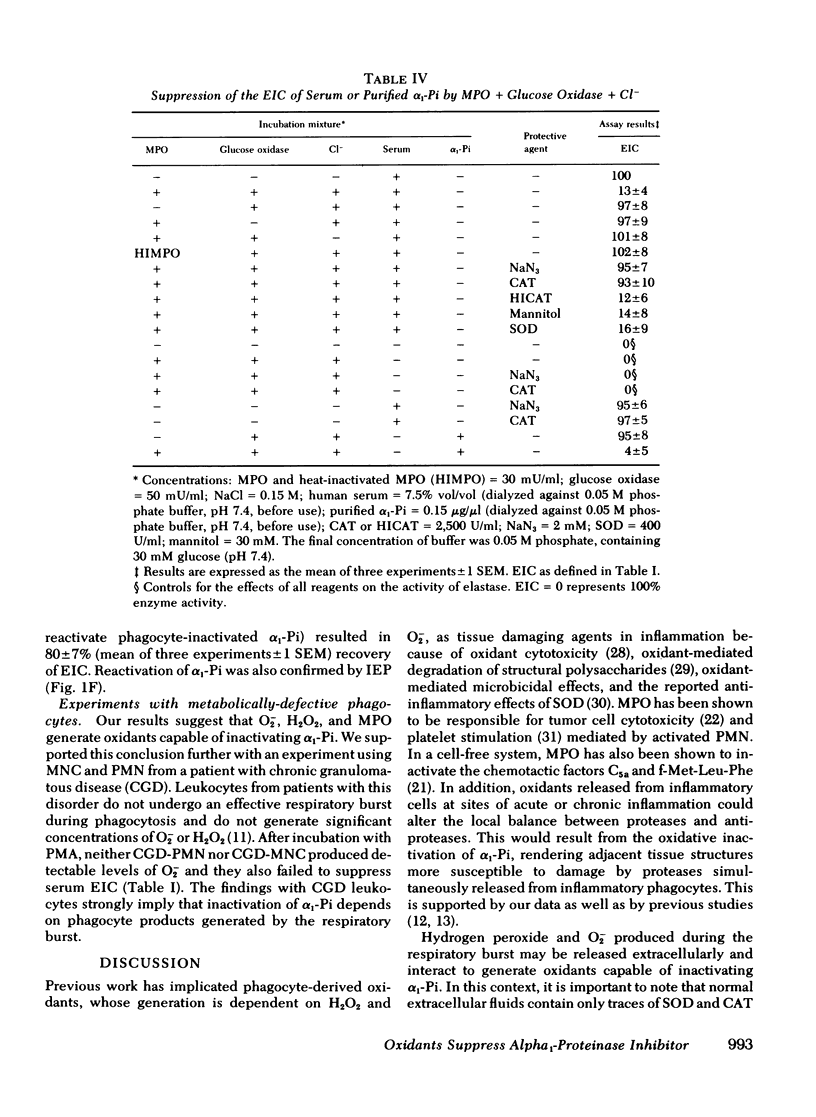
Human polymorphonuclear leukocytes, monocytes, or pulmonary alveolar macrophages, stimulated in vitro by phorbol myristate acetate (PMA), released reactive oxygen species able to suppress the elastase inhibitory capacity (EIC) of human serum. Immunoelectrophoresis using antibodies against α1-proteinase inhibitor (α1-Pi) and elastase showed that inactivation of α1-Pi was responsible for the decreased serum EIC. Treatment of phagocyte-inactivated serum with a reducing agent (dithiothreitol) resulted in significant recovery of EIC, suggesting that α1-Pi had been oxidatively inactivated. Serum EIC was partially protected by superoxide dismutase or catalase. Hydrogen peroxide alone had no effect on serum EIC. Thus, neither H2O2 nor O2− alone, but a product of the two, may have oxidatively inactivated α1-Pi. In support of the foregoing, neutrophils or monocytes from a patient with chronic granulomatous disease failed to produce detectable levels of O2− after incubation with PMA. These cells also failed to suppress serum EIC. In the case of PMA-stimulated polymorphonuclear leukocytes or monocytes, extracellular myeloperoxidase may have also played a role in α1-Pi inactivation since serum EIC was partly protected by azide, cyanide, or the depletion of extracellular chloride. Indeed, in a cell-free system consisting of purified myeloperoxidase, a glucose oxidase-H2O2-generating system, and Cl−, the EIC of human serum or purified α1-Pi could also be suppressed. Omission of any single reactant prevented this effect, as did NaN3 or catalase, suggesting that enzymatically active myeloperoxidase and H2O2 were necessary. Immunoelectrophoresis of myeloperoxidase-inactivated serum showed that, as before, inactivation of α1-Pi was responsible for the decreased EIC. Treating myeloperoxidase-inactivated serum with dithiothreitol led to significant recovery of EIC, again suggesting that oxidative inactivation of α1-Pi had occurred. Oxidative inactivation of α1-Pi in the microenvironment of inflammatory cells, at sites of acute or chronic inflammation, may allow proteases released from these cells to damage adjacent connective tissue components more readily.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Bieth J., Spiess B., Wermuth C. G. The synthesis and analytical use of a highly sensitive and convenient substrate of elastase. Biochem Med. 1974 Dec;11(4):350–357. doi: 10.1016/0006-2944(74)90134-3. [DOI] [PubMed] [Google Scholar]
- Boyum A. Separation of blood leucocytes, granulocytes and lymphocytes. Tissue Antigens. 1974;4(4):269–274. [PubMed] [Google Scholar]
- Bretz U., Baggiolini M. Biochemical and morphological characterization of azurophil and specific granules of human neutrophilic polymorphonuclear leukocytes. J Cell Biol. 1974 Oct;63(1):251–269. doi: 10.1083/jcb.63.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Janoff A. In vitro suppression of serum elastase-inhibitory capacity by reactive oxygen species generated by phagocytosing polymorphonuclear leukocytes. J Clin Invest. 1979 Apr;63(4):793–797. doi: 10.1172/JCI109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Janoff A. Possible mechanisms of emphysema in smokers. In vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis. 1978 Sep;118(3):617–621. doi: 10.1164/arrd.1978.118.3.617. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Chemotactic factor inactivation by the myeloperoxidase-hydrogen peroxide-halide system. J Clin Invest. 1979 Oct;64(4):913–920. doi: 10.1172/JCI109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. B. The effects in vivo and in vitro of oxidative damage to purified alpha1-antitrypsin and to the enzyme-inhibiting activity of plasma. Am Rev Respir Dis. 1979 Jun;119(6):953–960. doi: 10.1164/arrd.1979.119.6.953. [DOI] [PubMed] [Google Scholar]
- Fong K. L., McCay P. B., Poyer J. L., Keele B. B., Misra H. Evidence that peroxidation of lysosomal membranes is initiated by hydroxyl free radicals produced during flavin enzyme activity. J Biol Chem. 1973 Nov 25;248(22):7792–7797. [PubMed] [Google Scholar]
- Gadek J. E., Fells G. A., Crystal R. G. Cigarette smoking induces functional antiprotease deficiency in the lower respiratory tract of humans. Science. 1979 Dec 14;206(4424):1315–1316. doi: 10.1126/science.316188. [DOI] [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971 Dec;107(6):1535–1546. [PubMed] [Google Scholar]
- Hoidal J. R., Repine J. E., Beall G. D., Rasp F. L., Jr, White J. G. The effect of phorbol myristate acetate on the metabolism and ultrastructure of human alveolar macrophages. Am J Pathol. 1978 Jun;91(3):469–482. [PMC free article] [PubMed] [Google Scholar]
- Janoff A., Carp H., Lee D. K., Drew R. T. Cigarette smoke inhalation decreases alpha 1-antitrypsin activity in rat lung. Science. 1979 Dec 14;206(4424):1313–1314. doi: 10.1126/science.316187. [DOI] [PubMed] [Google Scholar]
- Janoff A. Neutrophil proteases in inflammation. Annu Rev Med. 1972;23:177–190. doi: 10.1146/annurev.me.23.020172.001141. [DOI] [PubMed] [Google Scholar]
- Janoff A., Scherer J. Mediators of inflammation in leukocyte lysosomes. IX. Elastinolytic activity in granules of human polymorphonuclear leukocytes. J Exp Med. 1968 Nov 1;128(5):1137–1155. doi: 10.1084/jem.128.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D., Travis J. Structural evidence for methionine at the reactive site of human alpha-1-proteinase inhibitor. J Biol Chem. 1978 Oct 25;253(20):7142–7144. [PubMed] [Google Scholar]
- Johnson D., Travis J. The oxidative inactivation of human alpha-1-proteinase inhibitor. Further evidence for methionine at the reactive center. J Biol Chem. 1979 May 25;254(10):4022–4026. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jori G., Galiazzo G., Marzotto A., Scoffone E. Selective and reversibe photo-oxidation of the methionyl residues in lysozyme. J Biol Chem. 1968 Aug 25;243(16):4272–4278. [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Enzymatic inactivation of human alpha-1-proteinase inhibitor by neutrophil myeloperoxidase. Biochem Biophys Res Commun. 1979 May 28;88(2):402–409. doi: 10.1016/0006-291x(79)92062-x. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974 Aug 9;185(4150):529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Menkin V. Studies on Inflammation: X. The Cytological Picture of an Inflammatory Exudate in Relation to its Hydrogen Ion Concentration. Am J Pathol. 1934 Mar;10(2):193–210. [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., White J. G., Clawson C. C., Holmes B. M. The influence of phorbol myristate acetate on oxygen consumption by polymorphonuclear leukocytes. J Lab Clin Med. 1974 Jun;83(6):911–920. [PubMed] [Google Scholar]
- Romeo D., Cramer R., Marzi T., Soranzo M. R., Zabucchi G., Rossi F. Peroxidase activity of alveolar and peritoneal macrophages. J Reticuloendothel Soc. 1973 May;13(5):399–409. [PubMed] [Google Scholar]
- Salin M. L., McCord J. M. Free radicals and inflammation. Protection of phagocytosine leukocytes by superoxide dismutase. J Clin Invest. 1975 Nov;56(5):1319–1323. doi: 10.1172/JCI108208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W., Havemann K. Isolation of elastase-like and chymotrypsin-like neutral proteases from human granulocytes. Hoppe Seylers Z Physiol Chem. 1974 Sep;355(9):1077–1082. doi: 10.1515/bchm2.1974.355.2.1077. [DOI] [PubMed] [Google Scholar]
- Segal A. W., Jones O. T. Reduction and subsequent oxidation of a cytochrome b of human neutrophils after stimulation with phorbol myristate acetate. Biochem Biophys Res Commun. 1979 May 14;88(1):130–134. doi: 10.1016/0006-291x(79)91706-6. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Rustagi P. K., LoBuglio A. F. Human granulocyte generation of hydroxyl radical. J Exp Med. 1978 Feb 1;147(2):316–323. doi: 10.1084/jem.147.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R., Lin H. S., Kuhn C., 3rd Elastase secretion by peritoneal exudative and alveolar macrophages. J Exp Med. 1977 Sep 1;146(3):802–808. doi: 10.1084/jem.146.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]