Abstract
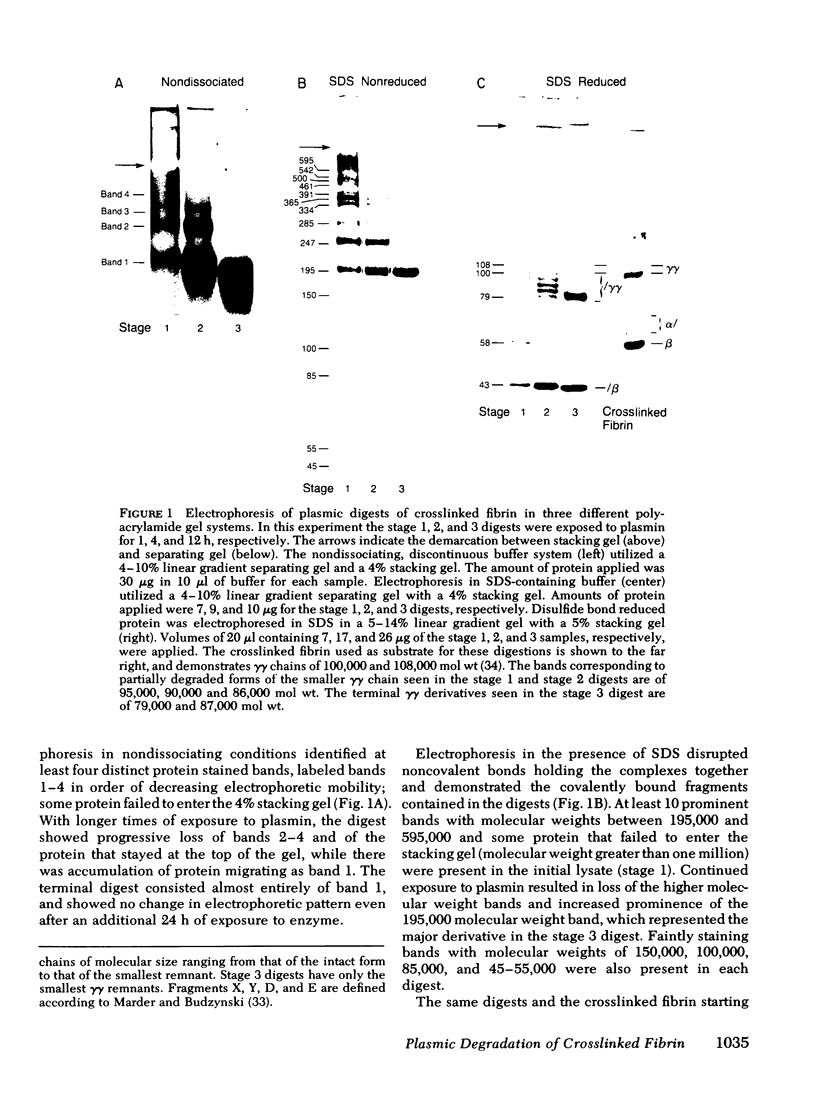
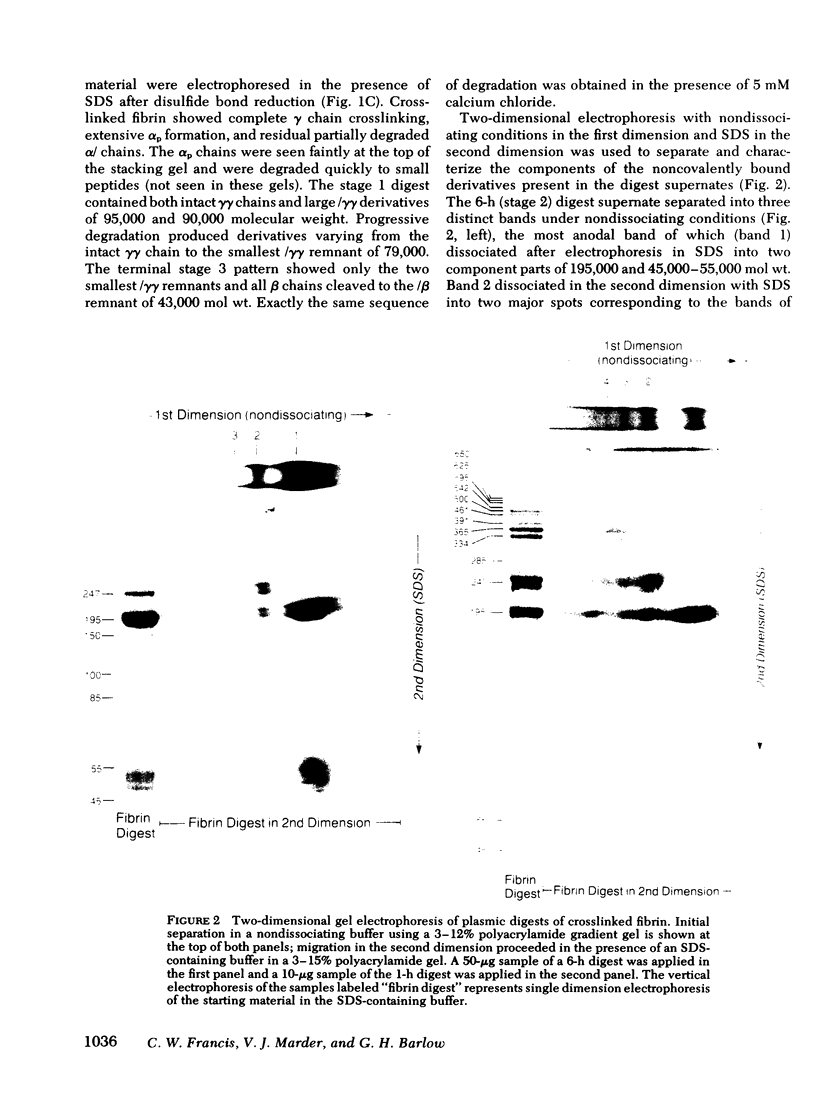
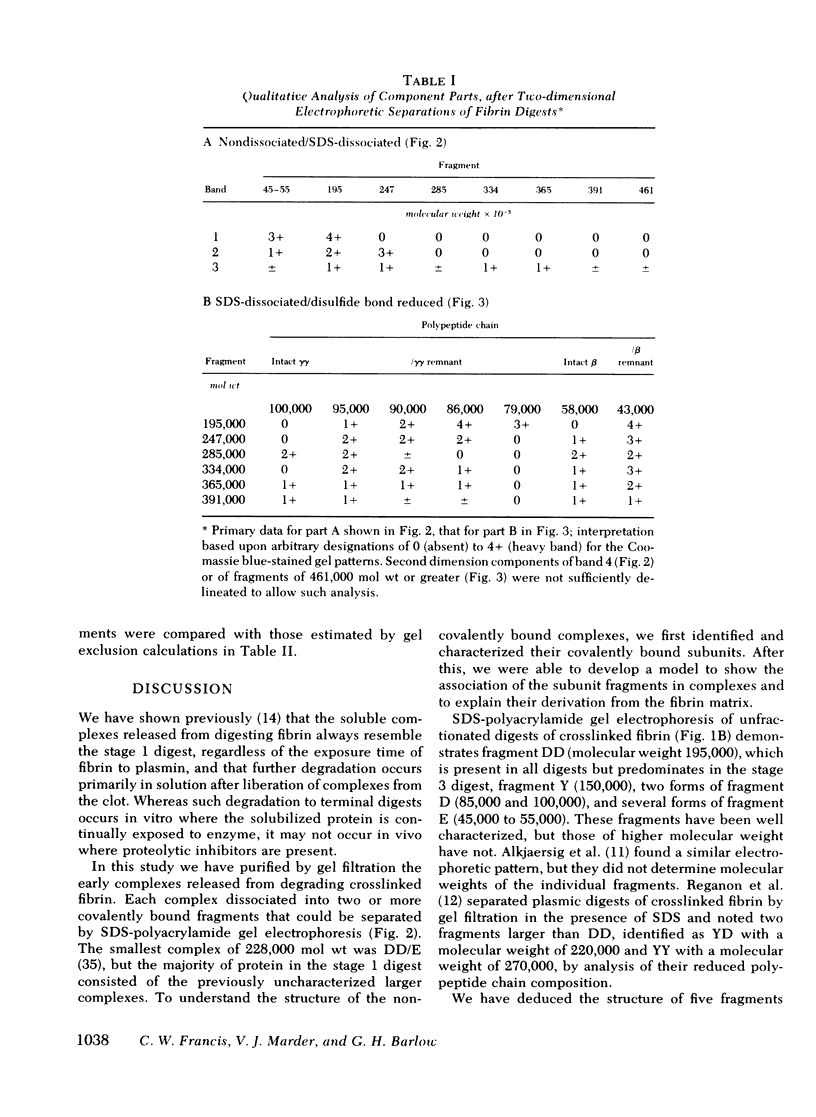
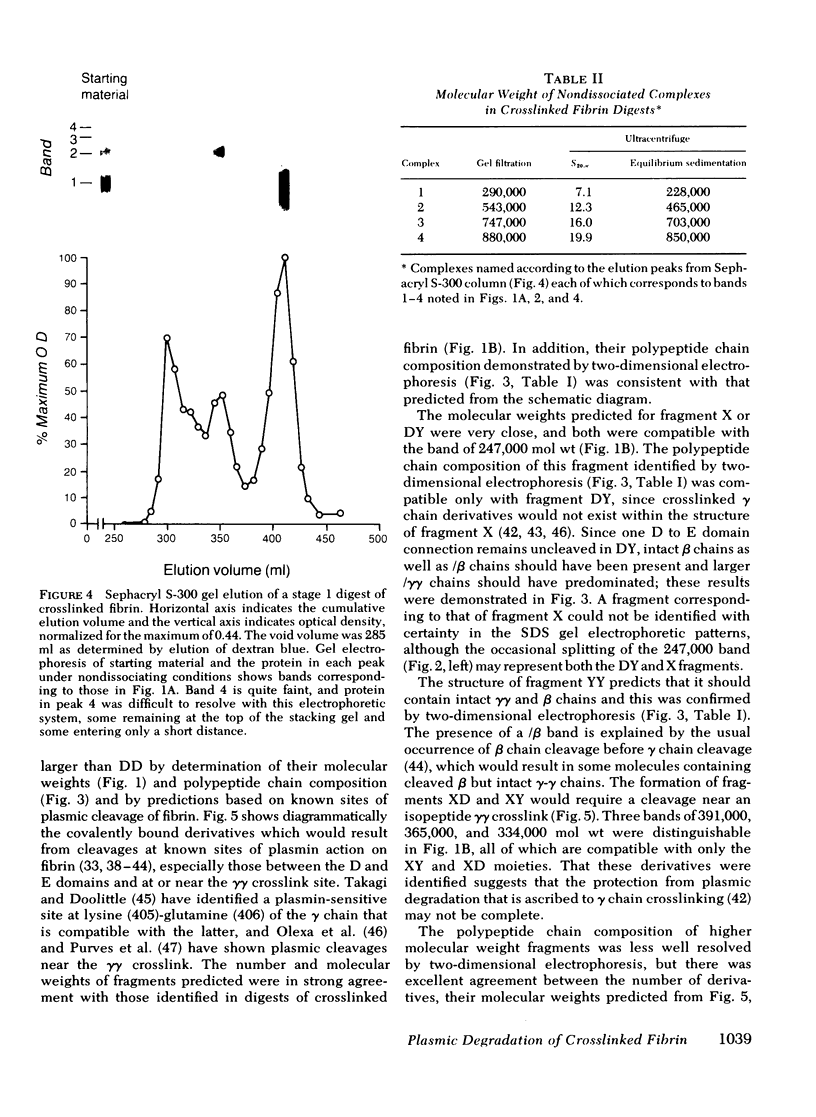
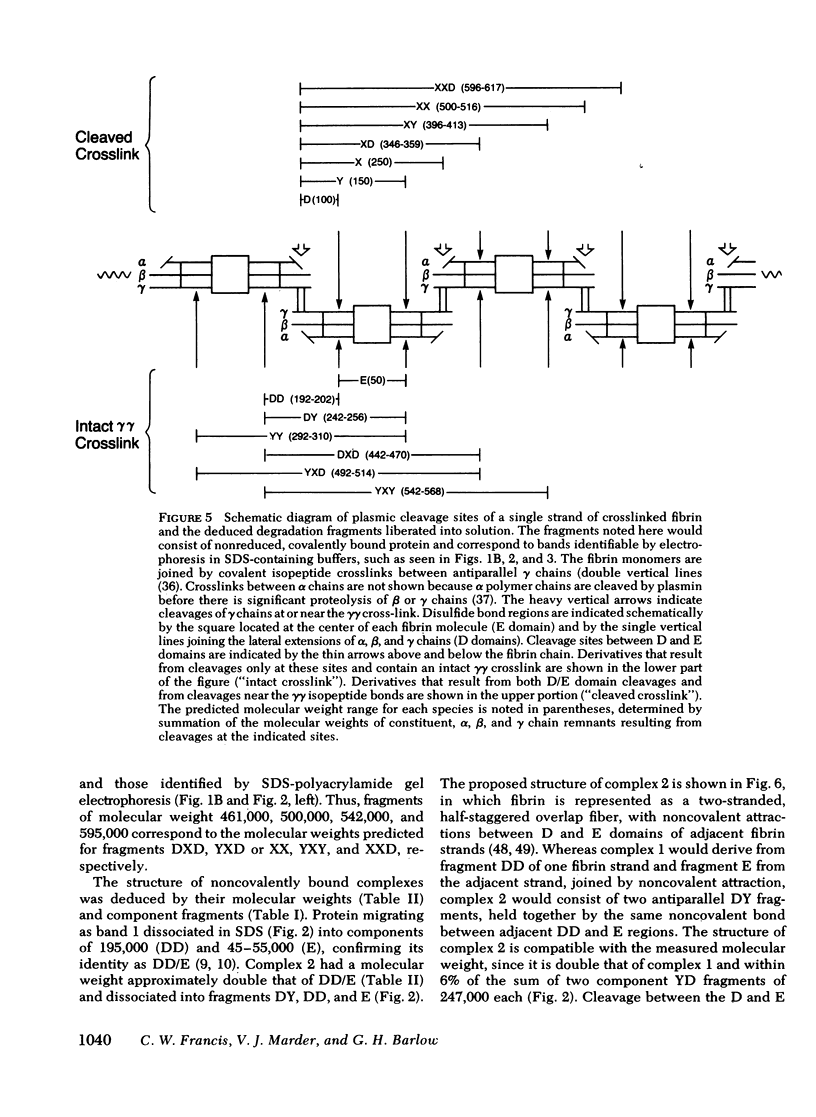
Crosslinked fibrin was digested by plasmin, and three soluble complexes larger than DD/E were purified and characterized. After gel filtration chromatography, the purified complexes were shown to have molecular weights of 465,000, 703,000, and 850,000, as determined by equilibrium sedimentation. Each of the complexes was dissociated into two or more fragments by SDS-polyacrylamide gel electrophoresis. The structure of these subunit fragments was deduced from determinations of their molecular weights and polypeptide chain composition and from known sites of plasmin cleavage of fibrin. Fragments larger than DD have been identified that contain intact γγ crosslinks as well as fragments resulting from cleavages at or near this site. The former include DY (mol wt 247,000), YY (mol wt 285,000), DXD (mol wt 461,000), and YXD (mol wt 500,000); and the latter include fragments XD (mol wt 334,000) and XY (mol wt 391,000). A schematic model was developed to explain the structure of the large noncovalently bound complexes based on their molecular weight and observed component fragments. Our scheme supports the two-stranded half-staggered overlap model as the basic unit of fibrin structure, in which each complex consists of fragments from two adjacent complementary antiparallel fibrin strands. The smallest derivative, complex 1, is the DD/E complex; complex 2 contains apposed DY and YD fragments, and complex 3 consists of fragments DXD and YY. Complex 4 is less well-characterized, but its intact structure is projected to consist of YXD and DXY fragments from adjacent fibrin strands. Each complex is heterogeneous in subunit composition, reflecting additional plasmin cleavages within and/or adjacent to its theoretical boundaries. Since most of the protein initially released into solution from degrading fibrin is as complexes larger than DD/E, the derivatives described in this report are likely to be major circulating degradation products of crosslinked fibrin in vivo.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkjaersig N., Davies A., Fletcher A. Fibrin and fibrinogen proteolysis products: comparison between gel filtration and SDS polyacrylamide electrophoresis analysis. Thromb Haemost. 1977 Aug 31;38(2):524–525. [PubMed] [Google Scholar]
- Budzynski A. Z., Marder V. J., Shainoff J. R. Structure of plasmic degradation products of human fibrinogen. Fibrinopeptide and polypeptide chain analysis. J Biol Chem. 1974 Apr 10;249(7):2294–2302. [PubMed] [Google Scholar]
- Chen R., Doolittle R. F. - cross-linking sites in human and bovine fibrin. Biochemistry. 1971 Nov 23;10(24):4487–4491. doi: 10.1021/bi00800a021. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Goldbaum D. M., Doolittle L. R. Designation of sequences involved in the "coiled-coil" interdomainal connections in fibrinogen: constructions of an atomic scale model. J Mol Biol. 1978 Apr 5;120(2):311–325. doi: 10.1016/0022-2836(78)90070-0. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Structural aspects of the fibrinogen to fibrin conversion. Adv Protein Chem. 1973;27:1–109. doi: 10.1016/s0065-3233(08)60446-5. [DOI] [PubMed] [Google Scholar]
- Ferguson E. W., Fretto L. J., McKee P. A. A re-examination of the cleavage of fibrinogen and fibrin by plasmin. J Biol Chem. 1975 Sep 25;250(18):7210–7218. [PubMed] [Google Scholar]
- Ferry J. D. The Mechanism of Polymerization of Fibrinogen. Proc Natl Acad Sci U S A. 1952 Jul;38(7):566–569. doi: 10.1073/pnas.38.7.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis C. W., Marder V. J., Martin S. E. Demonstration of a large molecular weight variant of the gamma chain of normal human plasma fibrinogen. J Biol Chem. 1980 Jun 25;255(12):5599–5604. [PubMed] [Google Scholar]
- Francis C. W., Marder V. J., Martin S. E. Detection of circulating crosslinked fibrin derivatives by a heat extraction-SDS gradient gel electrophoretic technique. Blood. 1979 Dec;54(6):1282–1295. [PubMed] [Google Scholar]
- Francis C. W., Marder V. J., Martin S. E. Detection of circulating crosslinked fibrin derivatives by a heat extraction-SDS gradient gel electrophoretic technique. Blood. 1979 Dec;54(6):1282–1295. [PubMed] [Google Scholar]
- Francis C. W., Marder V. J., Martin S. E. Plasmic degradation of crosslinked fibrin. I. Structural analysis of the particulate clot and identification of new macromolecular-soluble complexes. Blood. 1980 Sep;56(3):456–464. [PubMed] [Google Scholar]
- Furlan M., Beck E. A. Plasmic degradation of human fibrinogen. I. Structural characterization of degradation products. Biochim Biophys Acta. 1972 May 18;263(3):631–644. doi: 10.1016/0005-2795(72)90044-x. [DOI] [PubMed] [Google Scholar]
- Gaffney P. J., Brasher M. Subunit structure of the plasmin-induced degradation products of crosslinked fibrin. Biochim Biophys Acta. 1973 Jan 25;295(1):308–313. doi: 10.1016/0005-2795(73)90098-6. [DOI] [PubMed] [Google Scholar]
- Gaffney P. J. Distinction between fibrinogen and fibrin degradation products in plasma. Clin Chim Acta. 1975 Nov 15;65(1):109–115. doi: 10.1016/0009-8981(75)90341-1. [DOI] [PubMed] [Google Scholar]
- Gaffney P. J., Dobos P. A structural aspect of human fibrinogen suggested by its plasmin degradation. FEBS Lett. 1971 Jun 2;15(1):13–16. doi: 10.1016/0014-5793(71)80067-4. [DOI] [PubMed] [Google Scholar]
- Gaffney P. J., Joe F. The lysis of crosslinked human fibrin by plasmin yields initially a single molecular complex, D dimer-E. Thromb Res. 1979;15(5-6):673–687. doi: 10.1016/0049-3848(79)90177-4. [DOI] [PubMed] [Google Scholar]
- Gaffney P. J., Lane D. A., Kakkar V. V., Brasher M. Characterisation of a soluble D dimer-E complex in crosslinked fibrin digests. Thromb Res. 1975 Jul;7(1):89–99. doi: 10.1016/0049-3848(75)90127-9. [DOI] [PubMed] [Google Scholar]
- Graeff H., Hafter R., von Hugo R. Molecular aspects of defibrination in a case of amniotic fluid embolism. Thromb Haemost. 1977 Oct 31;38(3):724–727. [PubMed] [Google Scholar]
- Hermans J. Models of fibrin. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1189–1193. doi: 10.1073/pnas.76.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudry-Clergeon G., Paturel L., Suscillon M. Identification d'un complexe (D-D)...E dans les produits de dégradation de la fibrine bovine stabilisée par le facteur XIII. Pathol Biol (Paris) 1974 Nov;22 Suppl:47–52. [PubMed] [Google Scholar]
- Johnson A. J., Kline D. L., Alkjaersig N. Assay methods and standard preparations for plasmin, plasminogen and urokinase in purified systems, 1967-1968. Thromb Diath Haemorrh. 1969 Apr 30;21(2):259–272. [PubMed] [Google Scholar]
- Krakow W., Endres G. F., Siegel B. M., Scheraga H. A. An electron microscopic investigation of the polymerization of bovine fibrin monomer. J Mol Biol. 1972 Oct 28;71(1):95–103. doi: 10.1016/0022-2836(72)90403-2. [DOI] [PubMed] [Google Scholar]
- Kudryk B. J., Collen D., Woods K. R., Blombäck B. Evidence for localization of polymerization sites in fibrinogen. J Biol Chem. 1974 May 25;249(10):3322–3325. [PubMed] [Google Scholar]
- Marder V. J., Budzynski A. Z., Barlow G. H. Comparison of the physicochemical properties of fragment D derivatives of fibrinogen and fragment D-D of cross-linked fibrin. Biochim Biophys Acta. 1976 Mar 18;427(1):1–14. doi: 10.1016/0005-2795(76)90279-8. [DOI] [PubMed] [Google Scholar]
- Marder V. J., Budzynski A. Z. Data for defining fibrinogen and its plasmic degradation products. Thromb Diath Haemorrh. 1975 Apr 30;33(2):199–207. [PubMed] [Google Scholar]
- Marder V. J., Shulman N. R., Carroll W. R. High molecular weight derivatives of human fibrinogen produced by plasmin. I. Physicochemical and immunological characterization. J Biol Chem. 1969 Apr 25;244(8):2111–2119. [PubMed] [Google Scholar]
- Marder V. J., Shulman N. R., Carroll W. R. The importance of intermediate degradation products of fibrinogen in fibrinolytic hemorrhage. Trans Assoc Am Physicians. 1967;80:156–167. [PubMed] [Google Scholar]
- Margolis J., Kenrick K. C. Polyacrylamide gel-electrophoresis across a molecular sieve gradient. Nature. 1967 Jun 24;214(5095):1334–1336. doi: 10.1038/2141334a0. [DOI] [PubMed] [Google Scholar]
- Martin S. E., Marder V. J., Francis C. W., Loftus L. S., Barlow G. H. Enzymatic degradation of the factor-VIII-von-Willebrand protein: a unique tryptic fragment with ristocetin cofactor activity. Blood. 1980 May;55(5):848–858. [PubMed] [Google Scholar]
- Mills D. A. A molecular model for the proteolysis of human fibrinogen by plasmin. Biochim Biophys Acta. 1972 May 18;263(3):619–630. doi: 10.1016/0005-2795(72)90043-8. [DOI] [PubMed] [Google Scholar]
- Mills D., Karpatkin S. Heterogeneity of human fibrinogen: possible relation to proteolysis by thrombin and plasmin as studies by SDS-polyacrylamide gel electrophoresis. Biochem Biophys Res Commun. 1970 Jul 13;40(1):206–211. doi: 10.1016/0006-291x(70)91067-3. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Olexa S. A., Budzynski A. Z., Marder V. J. Modification of high molecular weight plasmic degradation products of human crosslinked fibrin. Biochim Biophys Acta. 1979 Jan 25;576(1):39–50. doi: 10.1016/0005-2795(79)90482-3. [DOI] [PubMed] [Google Scholar]
- Pizzo S. V., Schwartz M. L., Hill R. L., McKee P. A. The effect of plasmin on the subunit structure of human fibrin. J Biol Chem. 1973 Jul 10;248(13):4574–4583. [PubMed] [Google Scholar]
- Pizzo S. V., Schwartz M. L., Hill R. L., McKee P. A. The effect of plasmin on the subunit structure of human fibrinogen. J Biol Chem. 1972 Feb 10;247(3):636–645. [PubMed] [Google Scholar]
- Pizzo S. V., Taylor L. M., Jr, Schwartz M. L., Hill R. L., McKee P. A. Subunit structure of fragment D from fibrinogen and cross-linked fibrin. J Biol Chem. 1973 Jul 10;248(13):4584–4590. [PubMed] [Google Scholar]
- Plow E. F., Edgington T. S. Discriminating neoantigenic differences between fibrinogen and fibrin derivatives. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1169–1173. doi: 10.1073/pnas.70.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves L. R., Lindsey G. G., Brown G., Franks J. Stabilization of the plasmin digestion products of fibrinogen and fibrin by calcium ions. Thromb Res. 1978 Mar;12(3):473–484. doi: 10.1016/0049-3848(78)90318-3. [DOI] [PubMed] [Google Scholar]
- Regañon E., Vila V., Aznar J. Identification of high molecular weight derivatives of plasmic digests of cross-linked human fibrin. Thromb Haemost. 1978 Oct 31;40(2):368–376. [PubMed] [Google Scholar]
- Schachman H. K., Edelstein S. J. Ultracentrifuge studies with absorption optics. IV. Molecular weight determinations at the microgram level. Biochemistry. 1966 Aug;5(8):2681–2705. doi: 10.1021/bi00872a029. [DOI] [PubMed] [Google Scholar]
- Takagi T., Doolittle R. F. Amino acid sequence of the carboxy-terminal cyanogen bromide peptide of the human fibrinogen beta-chain: homology with the corresponding gamma-chain peptide and presence in fragment D. Biochim Biophys Acta. 1975 Apr 29;386(2):617–622. doi: 10.1016/0005-2795(75)90306-2. [DOI] [PubMed] [Google Scholar]
- Takagi T., Doolittle R. F. Amino acid sequence studies on plasmin-derived fragments of human fibrinogen: amino-terminal sequences of intermediate and terminal fragments. Biochemistry. 1975 Mar 11;14(5):940–946. doi: 10.1021/bi00676a010. [DOI] [PubMed] [Google Scholar]