Abstract
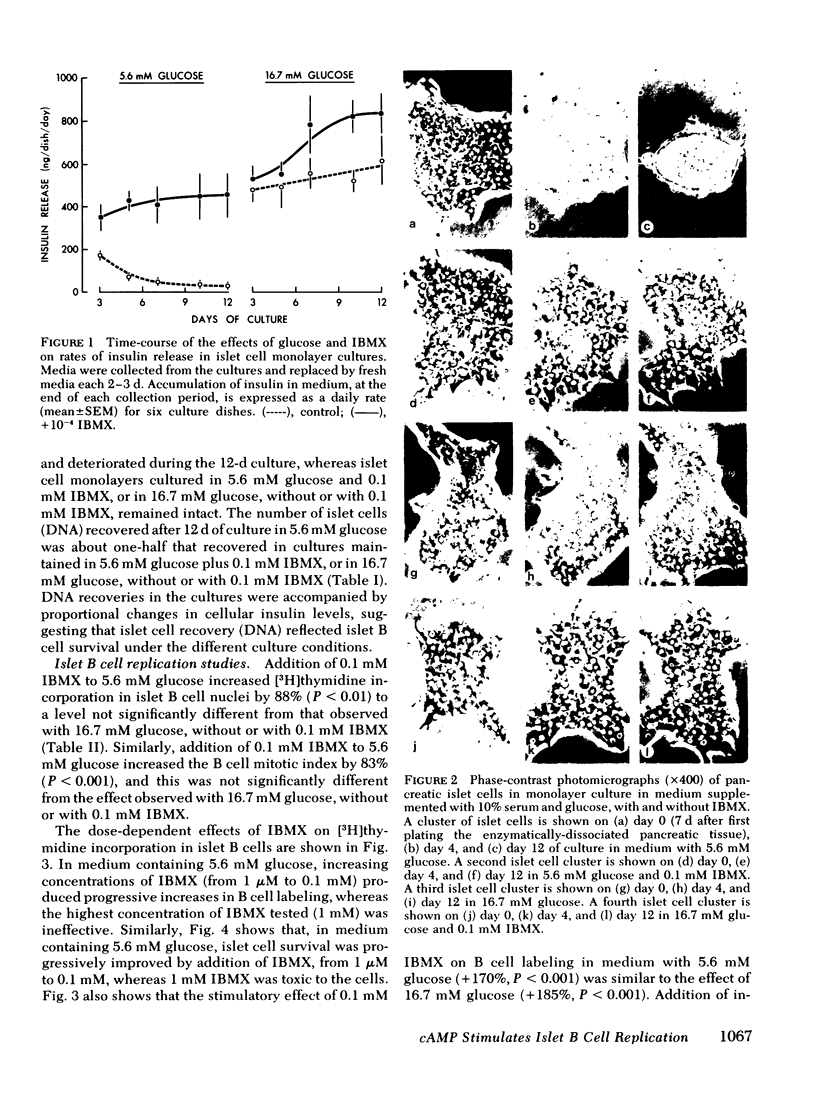
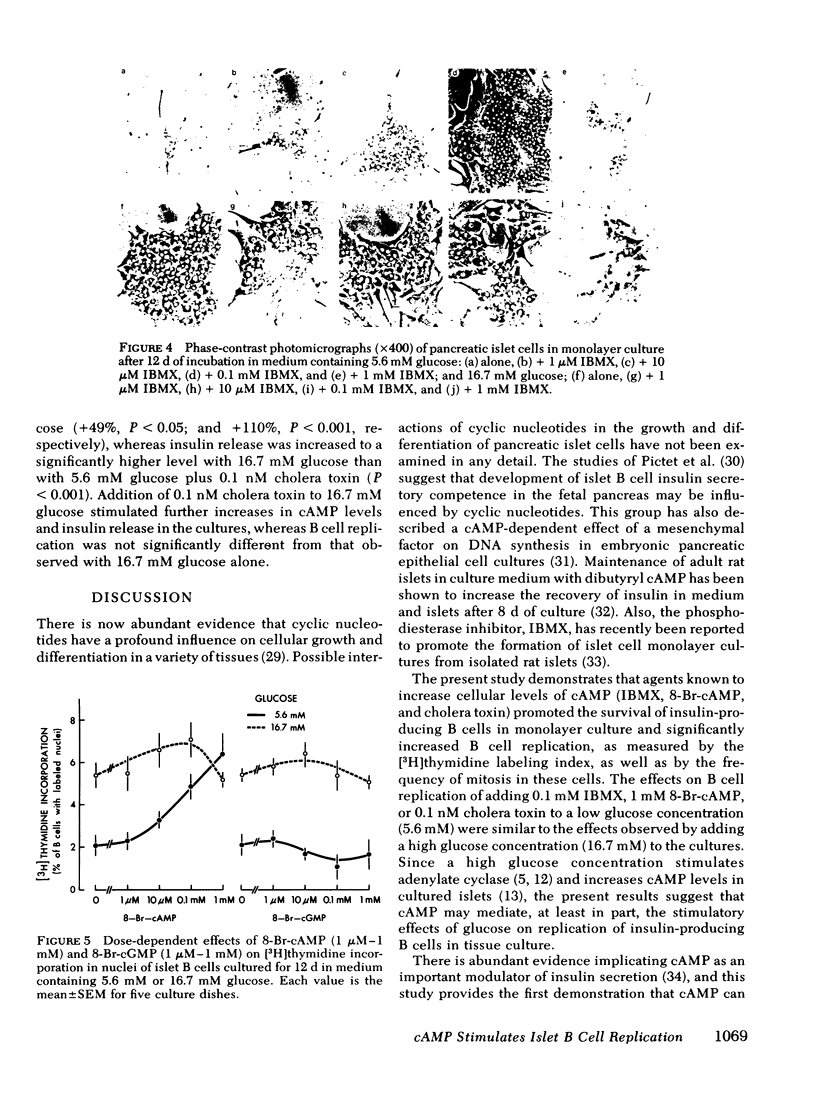
A possible role for cyclic adenosine 3′,5′-monophosphate (cAMP) in islet B cell replication was examined in neonatal rat pancreatic monolayer cultures. Islet cells deteriorated and insulin release decreased during 12 d of culture in medium with 5.6 mM glucose, whereas the cells survived and insulin release increased during culture in medium with 5.6 mM glucose plus the phosphodiesterase inhibitor, 3-isobutyl-1-methylxanthine (IBMX, 0.1 mM), or in medium with 16.7 mM glucose with or without IBMX. IBMX also increased the mitotic index and stimulated dose-dependent increases in [3H]thymidine incorporation in nuclei of islet B cells in aldehydethionine stained radioautographs; maximal stimulation of B cell replication occurred with addition of 0.1 mM IBMX to 5.6 mM glucose (+170%, P < 0.001), and this increase was similar to that observed with 16.7 mM glucose (+185%, P < 0.001). Also, 8-bromo-adenosine-3′,5-monophosphate, but not 8-bromo-guanosine-3′,5′-monophosphate produced dose-dependent increases in islet B cell replication in medium with 5.6 mM glucose. Measurement of cAMP levels in the cultures revealed dissociations between effects on B cell replication and insulin release. Thus, addition of 0.1 mM IBMX, or 0.1 nM cholera toxin, to 5.6 mM glucose produced slightly greater increases in cAMP levels and B cell replication than did 16.7 mM glucose, whereas insulin release was increased significantly more with 16.7 mM glucose. Also, addition of 0.1 mM IBMX, or 0.1 nM cholera toxin, to 16.7 mM glucose stimulated further increases in cAMP levels and insulin release in the cultures, but no further increases in B cell replication. We conclude that (a) cAMP stimulates islet B cell replication, (b) cAMP may mediate the effects of glucose on B cell replication, and (c) mechanisms regulating B cell replication may be more sensitive to cAMP and/or different from those regulating insulin secretion.
Full text
PDF

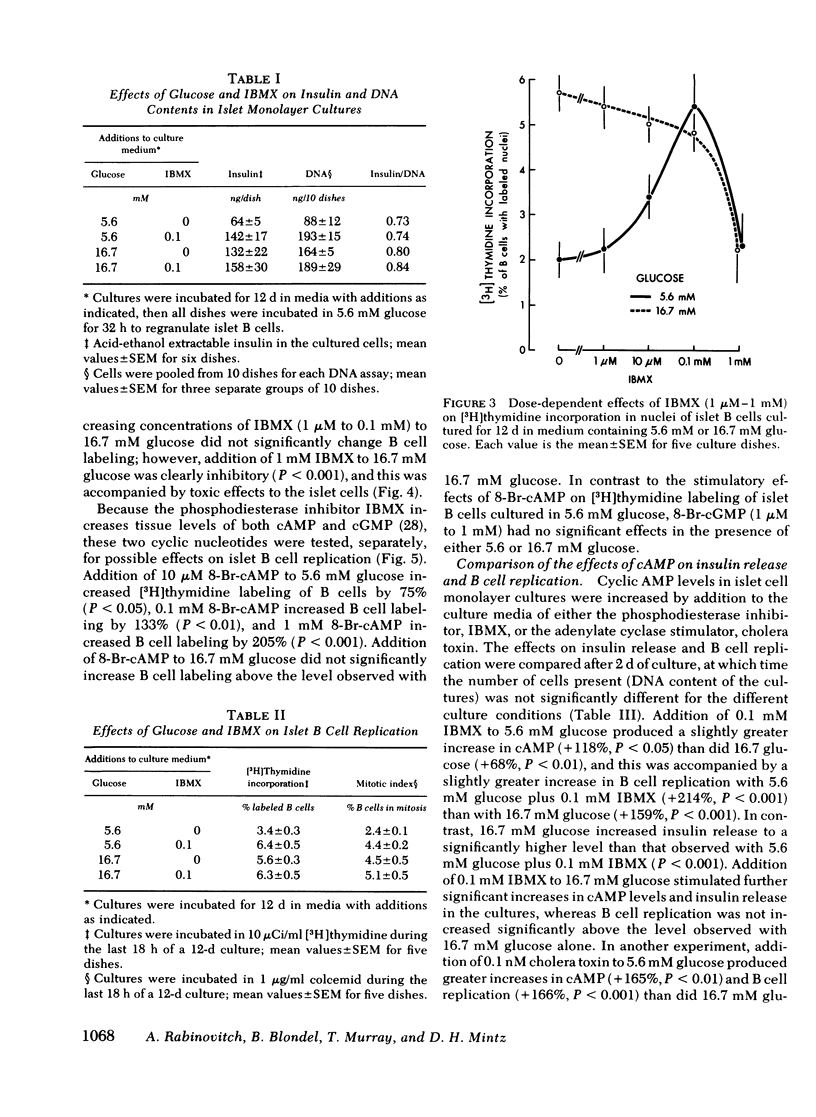
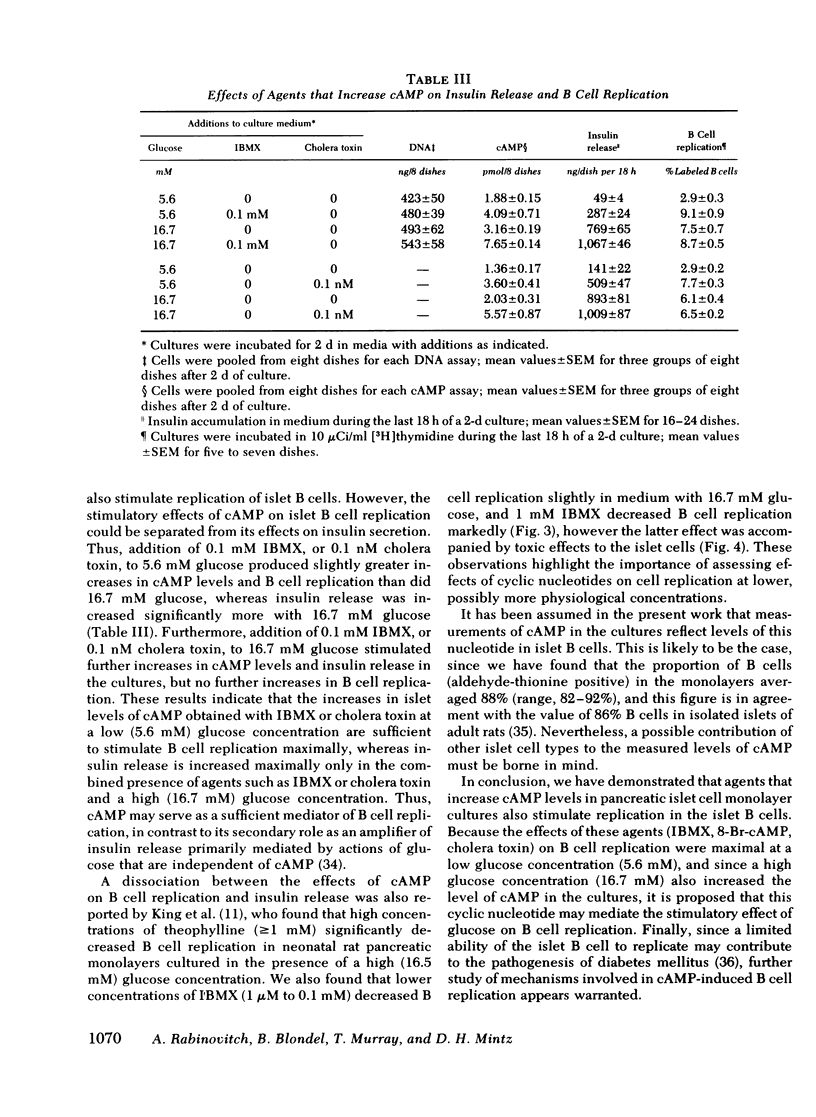

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. Synthesis of DNA in isolated pancreatic islets maintained in tissue culture. Endocrinology. 1975 Apr;96(4):1051–1054. doi: 10.1210/endo-96-4-1051. [DOI] [PubMed] [Google Scholar]
- Andersson A., Hellerström C. Metabolic characteristics of isolated pancreatic islets in tissue culture. Diabetes. 1972;21(2 Suppl):546–554. doi: 10.2337/diab.21.2.s546. [DOI] [PubMed] [Google Scholar]
- Andersson A. Long-term effects of glucose on insulin release and glucose oxidation by mouse pancreatic islets maintained in tissue culture. Biochem J. 1974 Jun;140(3):377–382. doi: 10.1042/bj1400377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson A., Westman J., Hellerström C. Effects of glucose on the ultrastructure and insulin biosynthesis of isolated mouse pancreatic islets maintained in tissue culture. Diabetologia. 1974 Dec;10(6):743–753. doi: 10.1007/BF01219536. [DOI] [PubMed] [Google Scholar]
- Buitrago A., Gylfe E., Hellman B., Idahl L. A., Johansson M. Function of microdissected pancreatic islets cultured in a chemically defined medium. I. Insulin content and release. Diabetologia. 1975 Dec;11(6):535–540. doi: 10.1007/BF01222103. [DOI] [PubMed] [Google Scholar]
- CARO L. G., VAN TUBERGEN R. P., KOLB J. A. High-resolution autoradiography. I. Methods. J Cell Biol. 1962 Nov;15:173–188. doi: 10.1083/jcb.15.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chick W. L. Beta cell replication in rat pancreatic monolayer cultures. Effects of glucose, tolbutamide, glucocorticoid, growth hormone and glucagon. Diabetes. 1973 Sep;22(9):687–693. doi: 10.2337/diab.22.9.687. [DOI] [PubMed] [Google Scholar]
- Chick W. L., Lauris V., Flewelling J. H., Andrews K. A., Woodruff J. M. Effects of glucose on beta cells in pancreatic monolayer cultures. Endocrinology. 1973 Jan;92(1):212–218. doi: 10.1210/endo-92-1-212. [DOI] [PubMed] [Google Scholar]
- Chick W. L., Like A. A., Lauris V. Pancreatic beta cell culture: preparation of purified monolayers. Endocrinology. 1975 Mar;96(3):637–643. doi: 10.1210/endo-96-3-637. [DOI] [PubMed] [Google Scholar]
- Filosa S., Pictet R., Rutter W. Positive control of cyclic AMP on mesenchymal factor controlled DNA synthesis in embryonic pancreas. Nature. 1975 Oct 23;257(5528):702–705. doi: 10.1038/257702a0. [DOI] [PubMed] [Google Scholar]
- Frandsen E. K., Krishna G. A simple ultrasensitive method for the assay of cyclic AMP and cyclic GMP in tissues. Life Sci. 1976 Mar 1;18(5):529–541. doi: 10.1016/0024-3205(76)90331-3. [DOI] [PubMed] [Google Scholar]
- Frankel B. J., Gylfe E., Hellman B., Idahl L. A. Maintenance of insulin release from pancreatic islets stored in the cold for up to 5 weeks. J Clin Invest. 1976 Jan;57(1):47–52. doi: 10.1172/JCI108267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. L. Role of cyclic nucleotides in cell growth and differentiation. Physiol Rev. 1976 Oct;56(4):652–708. doi: 10.1152/physrev.1976.56.4.652. [DOI] [PubMed] [Google Scholar]
- Gepts W., De Mey J. Islet cell survival determined by morphology. An immunocytochemical study of the islets of Langerhans in juvenile diabetes mellitus. Diabetes. 1978;27 (Suppl 1):251–261. doi: 10.2337/diab.27.1.s251. [DOI] [PubMed] [Google Scholar]
- Green H. Cyclic AMP in relation to proliferation of the epidermal cell: a new view. Cell. 1978 Nov;15(3):801–811. doi: 10.1016/0092-8674(78)90265-9. [DOI] [PubMed] [Google Scholar]
- Green I. C., Taylor K. W. Effects of pregnancy in the rat on the size and insulin secretory response of the islets of Langerhans. J Endocrinol. 1972 Aug;54(2):317–325. doi: 10.1677/joe.0.0540317. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Howell S. L., Green I. C., Montague W. A possible role of adenylate cyclase in the long-tern dietary regulation of insulin secretion from rat islets of Langerhans. Biochem J. 1973 Oct;136(2):343–349. doi: 10.1042/bj1360343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- King D. L., Chick W. L. Pancreatic beta cell replication: effects of hexose sugars. Endocrinology. 1976 Oct;99(4):1003–1009. doi: 10.1210/endo-99-4-1003. [DOI] [PubMed] [Google Scholar]
- King D. L., Kitchen K. C., Chick W. L. Pancreatic beta-cell replication: relation to insulin secretion. Endocrinology. 1978 Oct;103(4):1321–1327. doi: 10.1210/endo-103-4-1321. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Finke E. H., Conant S., Naber S. Long-term perfusion of isolated rats islets in vitro. Diabetes. 1976 Jun;25(6):484–493. doi: 10.2337/diab.25.6.484. [DOI] [PubMed] [Google Scholar]
- Lambert A. E., Blondel B., Kanazawa Y., Orci L., Renold A. E. Monolayer cell culture of neonatal rat pancreas: light microscopy and evidence for immunoreactive insulin synthesis and release. Endocrinology. 1972 Jan;90(1):239–248. doi: 10.1210/endo-90-1-239. [DOI] [PubMed] [Google Scholar]
- Ohgawara H., Carroll R., Hofmann C., Takahashi C., Kikuchi M., Labrecque A., Hirata Y., Steiner D. F. Promotion of monolayer formation in cultured whole pancreatic islets by 3-isobutyl-1-methylxanthine. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1897–1900. doi: 10.1073/pnas.75.4.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAGET G. E. Aldehyde-thionin: a stain having similar properties to aldehydefuchsin. Stain Technol. 1959 Jul;34(4):223–226. doi: 10.3109/10520295909114679. [DOI] [PubMed] [Google Scholar]
- Pawelek J., Halaban R., Christie G. Melanoma cells which require cyclic AMP for growth. Nature. 1975 Dec 11;258(5535):539–540. doi: 10.1038/258539a0. [DOI] [PubMed] [Google Scholar]
- Pictet R. L., Clark W. R., Williams R. H., Rutter W. J. An ultrastructural analysis of the developing embryonic pancreas. Dev Biol. 1972 Dec;29(4):436–467. doi: 10.1016/0012-1606(72)90083-8. [DOI] [PubMed] [Google Scholar]
- Rabinovitch A., Cuendet G. S., Sharp G. W., Renold A. E., Mintz D. H. Relation of insulin release to cyclic AMP content in rat pancreatic islets maintained in tissue culture. Diabetes. 1978 Jul;27(7):766–773. doi: 10.2337/diab.27.7.766. [DOI] [PubMed] [Google Scholar]
- Reaven E. P., Gold G., Reaven G. M. Effect of age on glucose-stimulated insulin release by the beta-cell of the rat. J Clin Invest. 1979 Aug;64(2):591–599. doi: 10.1172/JCI109498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz G., Hardman J. G., Schultz K., Davis J. W., Sutherland E. W. A new enzymatic assay for guanosine 3':5'-cyclic monophosphate and its application to the ductus deferens of the rat. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1721–1725. doi: 10.1073/pnas.70.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D. A., Fisher A. M. THE INSULIN AND THE ZINC CONTENT OF NORMAL AND DIABETIC PANCREAS. J Clin Invest. 1938 Nov;17(6):725–728. doi: 10.1172/JCI101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. W. The adenylate cyclase-cyclic AMP system in islets of Langerhans and its role in the control of insulin release. Diabetologia. 1979 May;16(5):287–296. doi: 10.1007/BF01223617. [DOI] [PubMed] [Google Scholar]
- Short J., Tsukada K., Rudert W. A., Lieberman I. Cyclic adenosine 3':5'-monophosphate and the induction of deoxyribonucleic acid synthesis in liver. J Biol Chem. 1975 May 25;250(10):3602–3606. [PubMed] [Google Scholar]
- Worton R. G., Duff C. Karyotyping. Methods Enzymol. 1979;58:322–344. doi: 10.1016/s0076-6879(79)58148-8. [DOI] [PubMed] [Google Scholar]
- Ziegler B., Ziegler M., Mehling R. Effect of dibutyryl cyclic AMP on glucagon and insulin storage and secretion in organ culture of rat islets. Experientia. 1975 May 15;31(5):610–612. doi: 10.1007/BF01932487. [DOI] [PubMed] [Google Scholar]




