Abstract
The objective of this study was to perform a comprehensive morphologic analysis of developing mouse external genitalia (ExG) and to determine specific sexual differentiation features that are responsive to androgens or estrogens. To eliminate sex steroid signaling postnatally, male and female mice were gonadectomized on the day of birth, and then injected intraperitoneally every other day with DES (200 ng/g), DHT (1 μg/g), or oil. On day-10 postnatal male and female ExG were dissected, fixed, embedded, serially sectioned and analyzed. We identified 10 sexually dimorphic anatomical features indicative of normal penile and clitoral differentiation in intact mice. Several (but not all) penile features were impaired or abolished as a result of neonatal castration. Those penile features remaining after neonatal castration were completely abolished with attendant clitoral development in androgen receptor (AR) mutant male mice (XTfm/Y and X/Y AR-null) in which AR signaling is absent both pre- and postnatally. Administration of DHT to neonatally castrated males restored development of all 10 masculine features to almost normal levels. Neonatal ovariectomy of female mice had little effect on clitoral development, whereas treatment of ovariectomized female mice with DHT induced partial masculinization of the clitoris. Administration of DES to neonatally gonadectomized male and female mice elicited a spectrum of development abnormalities. These studies demonstrate that the presence or absence of androgen prenatally specifies penile versus clitoral identity. Differentiated penile features emerge postnatally and are sensitive to and dependent upon prenatal or pre- and postnatal androgen. Emergence of differentiated clitoral features occurs postnatally in either intact or ovariectomized females. It is likely that each penile and clitoral feature has a unique time-course of hormonal dependency/sensitivity.
Keywords: External genitalia, Sex differentiation, Estrogen receptors, Androgen receptor
1. Introduction
Adult external genitalia (ExG) develop from the embryonic ambisexual genital tubercle (GT), which in mice forms over a 4-day period (embryonic day 12–16) via hormone-independent processes. Accordingly, male and female GTs are identical at 16-days of gestation, and sex differentiation begins thereafter (Suzuki et al., 2002). At birth (3 days after the end of the ambisexual stage) male GTs are slightly larger than female GTs, but sexual dimorphism is minimal as structures such as the os penis and distal fibro-cartilage are merely represented as undifferentiated mesenchymal condensations (Murakami, 1984; 1987b). Thus, actual penile and clitoral morphogenesis and differentiation mostly occurs postnatally, which is the subject of this paper.
Contemporary understanding of the morphological and molecular mechanisms of sex differentiation of mammalian ExG remains largely based on principles described by Jost over half a century ago (Jost, 1953, 1965). Jost’s theory of ExG development focuses exclusively on androgen action. Androgens acting on the ambisexual GT elicit masculinization of the ExG, whereas in the absence of androgens, female ExG develop. In this regard, androgen receptors (AR) have been detected in fetal rat and mouse ExG (see Table 2 for references), expression of AR has been shown to be androgen-dependent in fetal stages in ExG of both male and female rats (Bentvelsen et al., 1994), and signaling through AR has been shown to be critical in development of male murine ExG (Murakami, 1987b). With regard to male ExG development, androgens are involved in two different events: (1) Specification of penile identity (likely to occur prenatally), and (2) the actual morphogenesis and sex differentiation of definitive penile features, which occur postnatally. Since almost all penile features are laid down after birth, the primary research strategy used to address these issues is gonadectomy at birth. Neonatal gonadectomy has the advantage of complete removal of gonadal steroids. In contrast, prenatal hormone manipulation necessitates use of anti-hormones that may have more than one activity or may have compound-unique activity.
Table 2.
Expression of androgen receptor, estrogen receptor α and β in tissues of developing male and female external genitalia.
Current study is derived from observations of untreated wild-type 10-day postnatal male and female external genitalia.
Preputial epithelium, penile epithelium and clitoral epithelium.
The current study in mice focused exclusively with the glans clitoris, which does not contain erectile bodies. Other studies in other species and in different anatomical areas describe receptors in clitoral erectile bodies.
While the role of androgens in ExG development is well established, recent studies have demonstrated the presence of estrogen receptors alpha and beta (ERα and ERβ) and aromatase in developing rat penis (Jesmin et al., 2002, 2004). ERα and/or ERβ also have been detected in adult and fetal human penile tissue (Crescioli et al., 2003; Dietrich et al., 2004; Qiao et al., 2012), fetal mouse penis and clitoris (Agras et al., 2007), and in adult mouse clitoris (Martin-Alguacil et al., 2008). These findings suggest that penile and clitoral development may be responsive to endogenous and/or exogenous estrogens. Estrogens may affect morphogenesis and differentiation of mesenchymal, endodermal and ectodermal tissues of developing ExG or may affect their spatial organization within male and female ExG. However, the mere presence of ERα, ERβ and aromatase in developing ExG does not identify specific morphogenetic processes dependent upon or sensitive to estrogen action. To better understand estrogen action in the developing mouse ExG, the effects of pharmacologic doses of DES were examined.
This study details the actions of androgens and estrogens on morphogenesis and differentiation of ExG in order to define specific developmental features that are responsive to or dependent upon sex hormones. To accomplish this, we characterized 10 homologous sexually dimorphic features of developing penis and clitoris of 10-day intact untreated and hormonally manipulated mice, similar to those previously described in adult mice (Weiss et al., 2012; Yang et al., 2010). Analysis was carried out at 10 days postnatal, when sex differentiation of the ExG has advanced to a stage in which future adult features can be easily identified. With this protocol we sought to identify specific developmental events in ExG attributable to estrogen or androgen action. Understanding the endocrinology underlying sex differentiation of ExG will help elucidate the causes of abnormal ExG development.
2. Materials and methods
2.1. Animal care
Animal care and study protocols were reviewed and approved by the Animal Care and Use Committee. Adult wild-type CD-1 mice (Charles River Breeding Laboratories, Wilmington, MA, USA) were housed in polycarbonate cages (20 × 25 × 47 cm3) with laboratory grade pellet bedding in the UCSF animal facility and fed LabDiet 5058 (PMI Nutrition International, PO Box 66812, St. Louis, MO 63166) whose content of phytoestrogen is incapable of eliciting vaginal cornification in ovariectomized adult mice (Buchanan et al., 1998). After mating, female mice were separated from males and were monitored until parturition.
2.2. Gonadectomy
Postnatal sex steroid deprivation was achieved by gonadectomy at birth under hypothermic anesthesia. Pups were visually sexed based upon anogenital distance and size of GTs. Males were placed supine and a vertical skin incision was made midway between the umbilicus and prepuce. The peritoneum was entered, and the testicles were extracted with forceps. Female pups were placed prone, and a mid-dorsal skin incision was made. The dorso-lateral body wall was incised and the ovaries and uterine horns were extracted with forceps. Skin incisions were closed with a 7-0 vicryl suture. Following gonadectomy, the pups were placed in an incubator at 37°C and then reintroduced to the dam. The removed gonads were examined microscopically as frozen sections to confirm their identity.
2.3. Hormonal treatments
Six gonadectomized litters were utilized (two per treatment arm). Litters were injected intraperitoneally 24 hours after surgery and every other day thereafter with DES (200 ng/g in sesame oil vehicle; for males n = 11 and for females n = 10), DHT (1 μ/g in sesame oil vehicle; for males n = 9 and for females n = 11), or oil (5 μl; for males n = 7 and for females n = 12) using separate 50 μl Hamilton syringes with 28-gage needles for each agent to prevent cross-contamination. A non-gonadectomized litter was treated with oil vehicle (5 μl) as control.
2.4. Specimen preparation and analysis
At 10-days, control and gonadectomized pups were weighed and euthanized by decapitation. The abdominal cavity was explored to confirm gonadectomy. The external genitalia were dissected, formalin fixed, paraffin embedded and serially sectioned (7 μm) for H&E and Safranin-O stains. Morphometric analysis was performed via measurement of transverse or longitudinal sections, or by counting the number of serial transverse sections containing the object of interest. Organ width was measured at mid-glans in both males and females. Our morphological analysis focused exclusively on the distal aspect of the penis and clitoris (the glans), which is the most complex area of these organs. Statistical analysis was determined using ANOVA with p < 0.05 considered statistically significant.
In addition, ExG of adult (60 to 80 day old) C57BL/6 male and female intact mice, three adult XTfm/Y mice (Jackson Labs, Bar Harbor, Maine) and three adult X/Y androgen receptor null (AR-null) mice (Lim et al., 2008) were serially sectioned and analyzed in a similar fashion as described previously (Rodriguez et al., 2011; Yang et al., 2010). XTfm/Y and X/Y AR-null mice (Lim et al., 2008) were examined because they are deficient in AR signaling both pre- and postnatally.
2.5. Immunohistochemistry
10 days ExG from untreated mice (male = 4, female = 4) were formalin fixed, paraffin embedded and serially sectioned at 7 m. Immunohistochemistry was carried out as previously (Agras et al., 2007) described utilizing the following antibodies: AR (rabbit monoclonal, diluted 1:200, GTX 62599, Genetex, Irving, CA), an ERα (mouse monoclonal—clone 1D5, diluted 1:30, Dako, Carpinteria, CA, USA) and ERβ (mouse monoclonal—clone EMR02, diluted 1:200, Leica Microsystems, Newcastle Upon Tyne, UK). Signal detection was achieved using the Vector ABC System (Vector Laboratories, Foster City, CA, USA) followed by exposure to diaminobenzidine (Sigma®). Sections exposed to all steps except the application of the primary antibodies were used as negative controls.
3. Results
3.1. Adult ExG morphology
We previously described homologous sexually dimorphic penile and clitoral features of the adult wild-type mice (Rodriguez et al., 2011; Weiss et al., 2012; Yang et al., 2010). Each morphological feature is present in the adult penis, but absent in the adult clitoris. By assigning a value of 1 for each male feature, and a value of 0 for each female feature, we have created an adult ExG differentiation index in which the penis of intact adult males scores 10 and the adult clitoris scores 0 (Table 1, Fig. 1A). This ExG scoring system provides a systematic and objective approach to compare adult wild-type ExG with various mutant mice. Using this metric, the ExG of adult XTfm/Y and X/Y AR-null mice were evaluated as a means of determining which features are androgen-dependent in animals genetically deprived of androgen signaling both prenatally and postnatally. The ExG phenotypes of both of these AR mutant male mice, as reflected in their ExG score, were almost identical to that of wild-type females (Figs. 1A and 2D), which indicates that male features can be obliterated in X/Y mice with associated clitoral development if androgen signaling is absent from embryonic as well as postnatal periods.
Table 1.
Homologous features of 10-day and adult wild-type external genitalia.
| Male
|
Female
|
||||
|---|---|---|---|---|---|
| 10 days | Adult | Score | 10 days | Adult | Score |
| Large organ width (~1250 μm) | Large organ width (~2020 μm) | 1 | Small organ width (~650 μm) | Small organ width (~740 μm) | 0 |
| Defined erectile bodies | Defined erectile bodies | 1 | Diffuse erectile tissue | Diffuse erectile tissue | 0 |
| MUMP fibrocartilage | MUMP fibrocartilage | 1 | No distal fibrocartilage | No distal fibrocartilage | 0 |
| Proximal hyaline cartilage | Proximal hyaline cartilage | 1 | No proximal hyaline cartilage | No proximal hyaline cartilage | 0 |
| Immature penile spines | Mature penile spines | 1 | No epithelial spines | No epithelial spines | 0 |
| Long bone (~1500 μm) | Long bone (~3800 μm) | 1 | Miniscule bone (~100 μm) | Short bone (~580 μm) | 0 |
| Circumferential penile epithelium | Circumferential penile Epithelium | 1 | U-shaped clitoral lamina | U-shaped clitoral lamina | 0 |
| Epithelial tethering | No tethering, freely mobile | 1 | Stromal tethering | Stromal tethering, immobile | 0 |
| Urethra completely within penis | Urethra completely within penis | 1 | Urethra never completely within clitoris | Urethra never completely within Clitoris | 0 |
| Dorsal preputial cleft | Dorsal preputial cleft | 1 | Ventral preputial cleft | Ventral preputial cleft | 0 |
| Total male score* | 10 | Total female score* | 0 | ||
Score of 10 for both 10-day and adult male and a score of 0 for both 10-day and adult female.
Fig. 1.
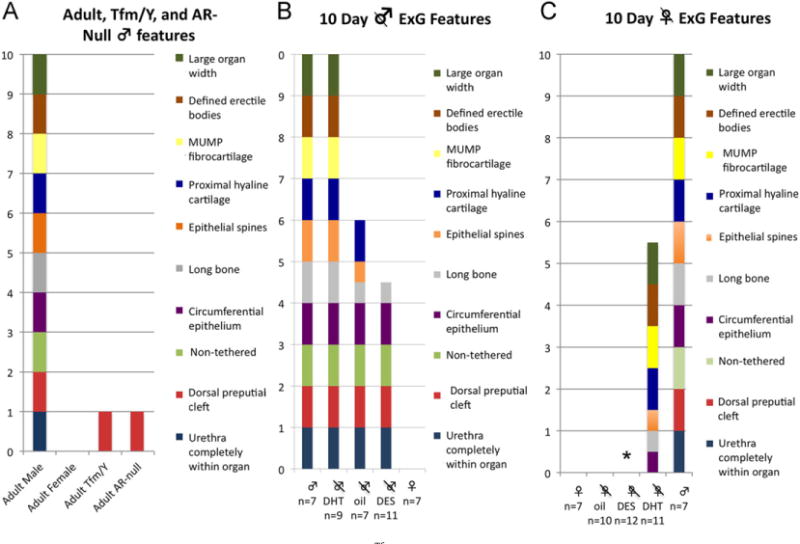
ExG Morphologic features. (A) Adult male, adult female, adult male XTfm/Y and adult X/Y androgen receptor null mice. (B and C) 10-day male and female gonadectomized treatment groups. Each wild-type male feature scores a value of 1, and each wild-type female feature a value of 0. By definition, the penis scores 10 and the clitoris scores 0. Features that were intermediate (only partly feminized or masculinized) were assigned a 1/2 point. *DES-treated ovariectomized female mice had two additional features: urethral-vaginal fistula and complete absence of os clitoris in all animals.
Fig. 2.
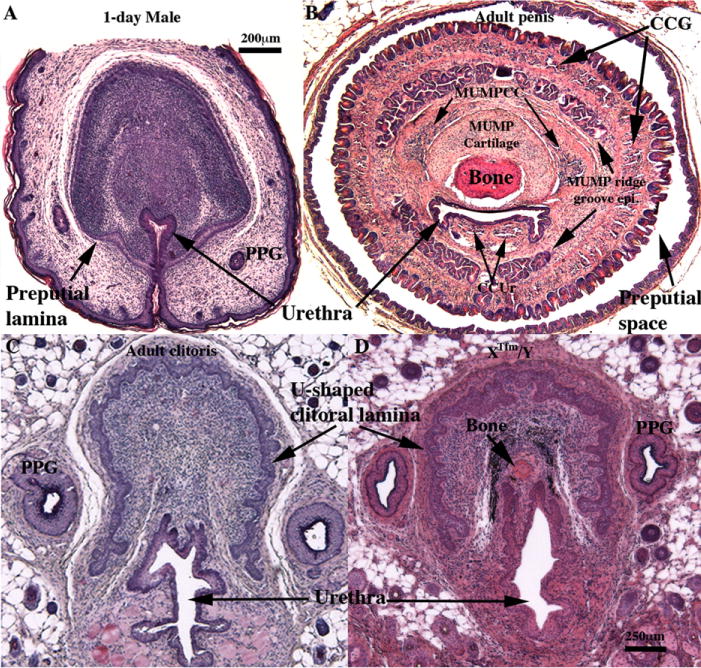
Histologic features of 1-day male ExG (A), adult wild-type penis (B), adult wild-type clitoris (C), and clitoris of Xtfm/Y male mice (D). Note that adult penile stroma (B) contains a variety of differentiated elements (bone, MUMP cartilage, three erectile bodies [corpus cavernosum glandis (CCG), MUMP corpus cavernosa (MUMPCC) and corpus cavernosum urethrae (CCUr)], urethra and MUMP ridge groove epithelium). By contrast, the mesenchyme of the day-1 male ExG (A) is devoid of these differentiated elements, and the urethra is attached to the inner aspect of the preputial lamina. PPG = ducts of preputial gland. Depicted in (C) is a transverse section of wild-type adult mouse clitoris having a U-shaped clitoral lamina, a urethra mostly below the clitoral lamina and diffuse vascular tissue. Virtually identical clitoral morphology is seen in XTfm/Y male ExG (D). Note bone in the XTfm/Y specimen (D). Scale bar for (D) applies also to (B) and (C).
4. 10-Day intact male ExG morphology
We have chosen 10-days postnatal as an appropriate time point to investigate sex differentiation of ExG. In mice, sex differentiation of the ExG is initiated at 16 days of gestation, after the ambisexual genital tubercle (GT) has formed. At birth it is possible to visually determine sex based upon anogenital distance and ExG size, both of which are larger in males. Thus, sex differentiation progresses between 16 days of gestation and birth. However, histological examination of male and female ExG at birth reveals organ rudiments that are undifferentiated as described previously (Murakami, 1987b) with little resemblance to the adult penis or clitoris (compare Fig. 2A, B, C). At 10-days postnatal morphology and differentiation are significantly different in male and female ExG as both organs exhibit most of the anatomical features seen in adulthood (Table 1). Accordingly, a detailed histological analysis was performed to define homologous sexually dimorphic anatomical features distinguishing 10-day mouse penis and clitoris similar to that described in adult mice (Rodriguez et al., 2011; Weiss et al., 2012; Yang et al., 2010). In 10-day hormonally manipulated mice, features that were intermediate (only partly feminized or masculinized) were assigned a 0.5 point. By this means a useful objective metric was constructed for assessing and analyzing normal and abnormal ExG development and hormonal effects on differentiation of the ExG at this age (Fig. 1B and C).
We used the human anatomic convention for ExG in which the ventral surface of the penis/clitoris is closest to the anus. As in the adult, the perineal surface elevation of 10-day males is not the penis, but rather is the prepuce. The male prepuce is bifid distally and dorsally clefted proximally. The preputial gland ducts emerge distally in the preputial cleft. Proximally the dorsal cleft fuses, forming a tubular prepuce. Deep within the prepuce lies the glans penis, circumscribed by the preputial epithelial lamina that separates the penile stroma from surrounding preputial stroma (see Fig. 3B and top two rows of images). At 10-days postnatal the glans penis is tethered to surrounding tissue by the circumferential preputial epithelial lamina, but in adulthood the glans penis is freely mobile within the preputial space following delamination of the preputial lamina (Fig. 2B). The os penis of 10-day-old intact mice is 1513 μm long on average (Figs. 3B and 5A), and the proximal end of the os penis is associated with hyaline cartilage (the growth plate) (Fig. 3C). The distal tip of the glans penis is defined by a projection called the male urogenital mating protuberance (MUMP) (Yang et al., 2010). The MUMP contains fibrocartilage (MUMP cartilage), which is bifid distally. The proximal end of the MUMP cartilage overlaps the os penis dorsally (Fig. 3B). The 10-day glans penis has three well-defined erectile bodies (corpus cavernosum glandis, MUMP corpus cavernosa and corpus cavernosa urethrae, compare Figs. 2B, 3A and B, 6A, C and E), and is surrounded by a circumferential preputial epithelial lamina containing immature penile spines (Fig. 3D). The urethra (Fig. 3A and B) lies entirely within the glans penis ventral to the MUMP and os penis. All parameters were virtually identical in each 10-day intact male examined and exhibited clear parallels with adult penile morphology (Rodriguez et al., 2011; Weiss et al., 2012).
Fig. 3.
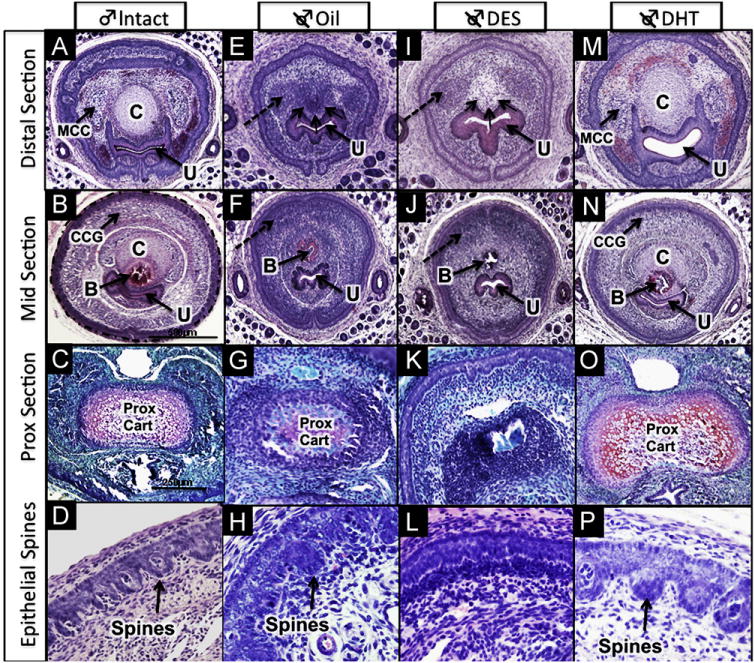
Histologic features of ExG of 10-day male mice: (A–D) intact male, (E–H) neonatally castrated + oil; (I–L) neonatally castrated + DES; (M–P) neonatally castrated + DHT. Dotted circular outline marks preputial epithelial lamina in (B) which is also seen in (A, E, F, I, J, M, and N). Well-defined erectile bodies (MUMP corpus cavernosa [MCC] and corpus cavernosum glandis [CCG]) are present in intact males (A & B) and castrated DHT-treated males (M & N). Dashed arrows in E, I, F & J highlight undifferentiated mesenchymal condensations representing erectile bodies (MUMP corpus cavernosa in [E&I] and corpus cavernosum glandis in [F&J]) seen in castrated + oil and castrated + DES specimens. Immature penile spines are most highly developed in intact males (D) and castrated DHT-treated males (P), less well developed spines are present in castrated oil-treated males (H), and penile spines are completely absent in castrated DES-treated males (L). MUMP cartilage denoted by the letter “C” is present in intact and castrated DHT-treated males, but represented as an undifferentiated mesenchymal condensation (group of three small arrows) in castrated oil-treated males (E) and castrated DES-treated males (I). Proximal hyaline cartilage (prox cart) is seen in all groups except the castrated + DES (K), although less well developed in the castrated + oil group. Other abbreviations: U = urethra; B = bone. (C, G, K, O) were stained with Safranin-O to reveal hyaline cartilage; all other images were stained with H&E.
Fig. 5.
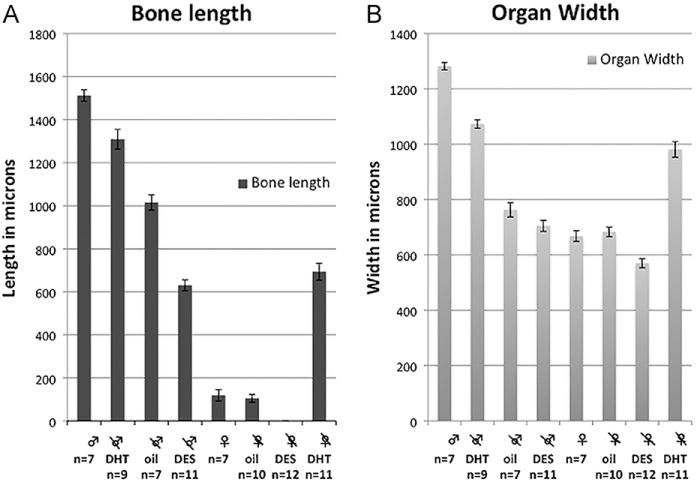
Morphometric data. (A) Bone length by treatment group (in microns). (B) Glans width by treatment group (in microns). See text for statistical analysis.
Fig. 6.
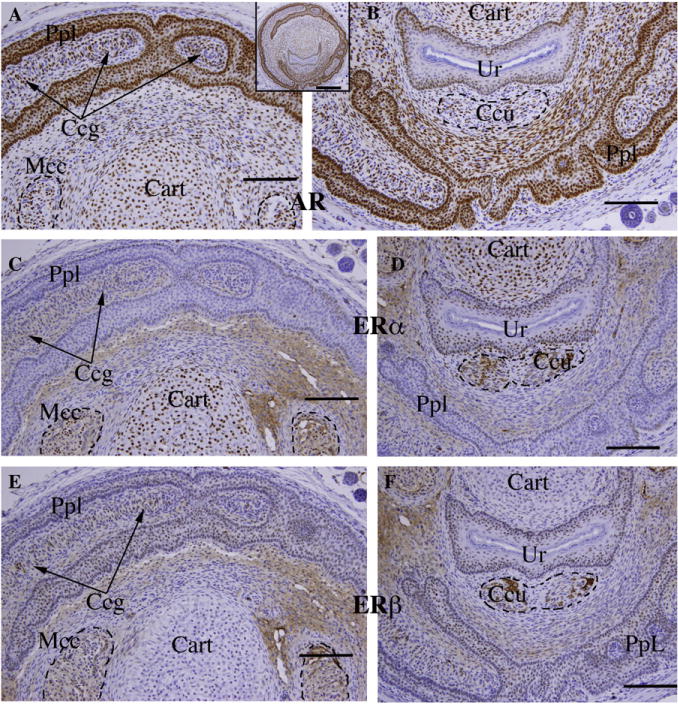
Immunohistochemical staining of transverse sections of the penis of an intact untreated 10-day male mouse. (A, B) Androgen receptor (AR), (C, D) estrogen receptor alpha (ERα) and (E, F) estrogen receptor beta (ERβ) in 3 adjacent sections. Insert is AR immunohistochemical stain. AR (A, B) is expressed in all epithelia (preputial lamina [PpL] defining the outer perimeter of the penis and its internal sub-divisions including immature spines, and in urethral epithelium [Ur]). Also AR-positive are cartilage [Cart], erectile bodies (corpus cavernosum glandis [Ccg], corpus cavernosum urethrae [Ccu] and MUMP corpus cavernosa [Mcc]), as well as mesenchyme. ERα staining (C, D) was weak to undetectable in the preputial lamina [PpL], but strongly expressed in urethral epithelium [Ur]. ERα was also detected in cartilage and in the three erectile bodies at weak to moderate levels, with little if any staining of mesenchyme. ERβ (E, F) was expressed in all epithelia (preputial lamina [PpL] and urethral epithelium [Ur]) and weakly in the erectile bodies. ERβ was undetectable in cartilage and weak in mesenchyme. Scale bar for inset = 250 μm; scale bars for A–F = 100 μm.
5. ExG morphology of 10-day males castrated at birth and hormonally treated
5.1. Neonatal castration + oil
Despite elimination of gonadal androgens as a result of neonatal castration, the poorly differentiated GT at birth (Fig. 2A) developed into a distinctly penile structure by 10 days postnatal (Fig. 3E–H). This suggests that penile identity is determined by prenatal androgens, and thus penile morphology develops despite neonatal castration. Certain features (proximal hyaline cartilage, circumferential epithelium, non-tethering, dorsal preputial cleft, and urethra completely within organ) develop independent of postnatal androgens in neonatally castrated oil-treated mice, while other penile features (organ width, defined erectile bodies, MUMP cartilage, epithelial spines and bone length) are impaired/abolished as a result of neonatal castration (Fig. 1B). Penile features strictly dependent upon postnatal androgens for development in neonatally castrated oil-treated male mice include the following: (a) Average width of the glans penis of castrated oil-treated mice was significantly smaller than that of intact controls (758 vs. 1282 μm, p < 0.0001; Fig. 5B). (b) Erectile bodies were poorly defined and appeared as undifferentiated mesenchymal condensations (Fig. 3E and F). (c) Penile spines were hypoplastic (Fig. 3H). (d) MUMP fibrocartilage was absent and represented as an undifferentiated mesenchymal condensation (Fig. 3E). (e) Average bone length was markedly decreased (1030 μm [castration + oil] vs. 1513 μm [intact + oil], p < 0.0001; Fig. 5A) and was of smaller diameter in neonatally castrated oil-treated mice (Fig. 3F).
5.2. Neonatal castration + DHT
Treatment of neonatally castrated males with DHT restored all male morphologic penile features to a condition nearly identical to that of intact 10-day males (Figs. 1B, 3M–P). On average, glans diameter was similar to intact male mice (1083 vs. 1282 μm; Fig. 5B). Erectile bodies (Fig. 3M–N) and immature penile spines (Fig. 3P) were well defined. The MUMP fibrocartilage and proximal hyaline cartilage were well developed (Fig. 3M–O), and the average bone length and diameter were nearly the size of intact male controls (length = 1270 vs. 1513 μm; Fig. 5A).
5.3. Neonatal castration + DES
In castrated DES-treated male mice, average width of the glans penis was substantially reduced relative to intact males (705 vs. 1282 μm, p < 0.001; Fig. 5B) and castrated oil-treated males, although this did not reach statistical significance (width = 705 μm [castration + DES] vs.758 μm [castration + oil], p = 0.23). Erectile bodies were poorly defined and represented as diffuse mesenchymal condensations (Figs. 1B, 3I–K). Penile epithelium was smooth and almost completely devoid of penile spines (Fig. 3K and L). The MUMP cartilage was consistently absent, and instead represented as a mesenchymal condensation (Fig. 3I). The proximal hyaline cartilage was absent in all DES-treated males (Fig. 3K). The average length of the os penis was significantly shorter than that of both intact males (630 μm vs. 1513 μm, p < 0.0001) and castrated oil-treated males (630 vs. 1030 μm, p < 0.0001 respectively; Fig. 5A). Further, the diameter of the os penis was reduced in neonatally castrated DES-treated mice (Fig. 3J).
5.4. Pre- and postnatal deprivation of androgen signaling
XTfm/Y and X/Y AR-null male mice are devoid of androgen signaling both pre- and postnatally. When examined in adulthood and scored according to our 10-point sex differentiation index, both AR mutant X/Y mice had a sex differentiation index score nearly identical to normal females (Fig. 1A) and exhibited distinct clitoral morphology (Fig. 2D). A small os clitoris was present in both AR mutant mice (Fig. 2D) similar to that seen in normal female mice. Thus, whereas postnatal androgen deprivation was compatible with penile morphology, elimination of pre- and postnatal AR signaling resulted in clitoral morphology. This suggests that the presence or absence of androgen specifies penile/clitoral identity prenatally.
6. 10-Day intact female ExG morphology
As in males, the surface elevation in the perineum of females is the prepuce, and not the clitoris, which lies deeply within the perineum. The female prepuce is bifid distally and more proximally is clefted ventrally as described previously in the adult (Rodriguez et al., 2011; Weiss et al., 2012; Yang et al., 2010). Proximally the preputial cleft closes forming the urethral meatus. The glans clitoris of intact female mice is defined by a U-shaped clitoral epithelial lamina and is devoid of spines (Fig. 4A–D). When viewed distal to proximal, the urethra is initially partially within the clitoral epithelial lamina, but courses gradually to a position ventral to the clitoral lamina (Fig. 4A–C). Therefore, the urethra is never completely circumscribed by the U-shaped clitoral lamina. At 10-days postnatal the glans clitoris has an average width of 668 μm measured at its midpoint (Fig. 5B) and contains a miniscule os clitoris (average length = 108 μm; Fig. 4B). Unlike the penis, the 10-day (and adult) clitoris lacks distal fibrocartilage and proximal hyaline cartilage. Clitoral stroma within the U-shaped epithelial lamina is in direct continuity with peri-urethral stroma. Hence the glans clitoris is tethered ventrally via its stroma and is immobile (Fig. 4A–C). Although in humans the clitoris is composed of erectile tissue, erectile tissue in the mouse clitoris is poorly defined, and not organized into discrete erectile bodies (Fig. 4A C).
Fig. 4.
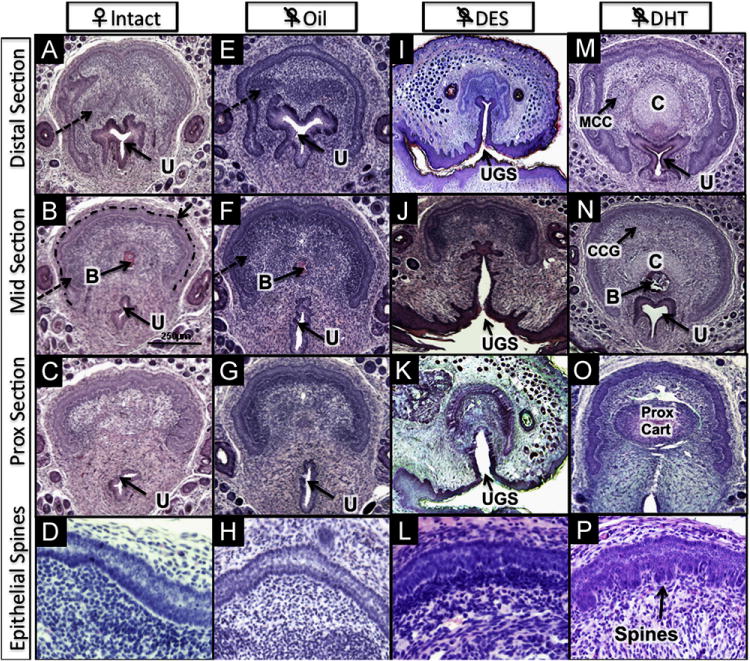
Histologic features of the ExG of 10-day female mice: (A–D) intact, (E–H) neonatally ovariectomized + oil; (I–L) neonatally ovariectomized + DES; (M–P) neonatally ovariectomized + DHT. Note U-shaped clitoral lamina in all treatment groups (intact [A], oil [E], DES [I] and DHT [O]). MUMP cartilage denoted by (C) and proximal hyaline cartilage (Prox Cart) is induced by DHT in (M, N & O) and is absent in all other groups. MUMP corpus cavernosa (MCC) in (M) and corpus cavernosum glandis (CCG) in (N) are induced by DHT, but are represented as undifferentiated mesenchymal condensations (dashed arrows) in intact (A&B) and in ovariectomized + oil (E) mice. Immature spines are induced in the preputial lamina by DHT (P) and are absent in all other groups. All ovariectomized DES-treated mice have persistent urogenital sinus (UGS), also known as urethral–vaginal fistula (I, J, K). Other abbreviations: U = urethra; B = bone; Dotted line marks clitoral epithelial lamina in (B).
7. ExG morphology of 10-day females castrated at birth and hormonally treated
7.1. Neonatal ovariectomy + oil
Females deprived of ovarian steroids as a result of neonatal ovariectomy exhibited normal female ExG development (Fig. 1C). The average width of the glans clitoris was similar in size to that of intact female controls (680 vs. 668 μm, p = 0.69; Fig. 5B). Erectile bodies were poorly defined as in intact females (Fig. 4E–G). The epithelial lamina defining the clitoris was U-shaped (Fig. 4E–G). As in intact controls, the distal fibrocartilage and proximal hyaline cartilage were absent. A minuscule os clitoris (Fig. 4F) was seen as in intact controls (bone length = 98 vs. 108 μm p = 0.73; Fig. 5A). The urethral meatus was in its normal location, with no evidence of malformation (Fig. 4E–G).
7.2. Neonatal ovariectomy + DHT
Treatment with DHT from birth to day 10 induced profound masculinization of the female ExG even though overall morphology remained clitoral (Fig. 1C). The average width of the glans clitoris was significantly larger than the intact female control (981 vs. 668 μm, p < 0.0001; Fig. 5B), and approached the size of a normal 10-day penis. Erectile bodies were sharply defined (Fig. 4M). The epithelial lamina was C-shaped, almost completely encircling the hypertrophied clitoris (Fig. 4M and N) even though the U-shaped clitoral lamina was retained proximally (Fig. 4O), and was adorned with immature penile-like spines in DHT-treated females (Fig. 4P). The os clitoris had robust proximal hyaline cartilage similar to intact males (Fig. 4O). The os clitoris (Fig. 4N) was over six times longer than in untreated intact female controls (691 vs. 108 μm, p < 0.0001; Fig. 5A). The distal MUMP cartilage, always lacking in normal females, was well developed (compare Fig. 3A [intact male] with Fig. 4M and N).
7.3. Neonatal ovariectomy + DES
Treatment of neonatally ovariectomized females with pharmacologic doses of DES elicited teratogenic alterations in several structures (Fig. 1C). The average width of the glans clitoris (Fig. 5B) was reduced relative to that of intact females (564 vs. 668 μm, p < 0.001) and gonadectomized oil-treated females (564 μm vs. 680 μm, p < 0.001). Erectile bodies remained poorly defined (Fig. 4I–K). The epithelial lamina surrounding the clitoris was U-shaped (Fig. 4I–K) and devoid of spines (Fig. 4L). Distal fibrocartilage and proximal hyaline cartilage were absent. Notably, the os clitoris was also consistently absent, and a common urogenital sinus or urethral-vaginal fistula was present in 100% of gonadectomized DES-treated mice (Fig. 4I–K).
7.4. Immunohistochemistry ExG of 10-day-old mice
Given the response to exogenous DHT and DES described above, immunohistochemistry was carried out on untreated 10-day male and female ExG. In the 10-day penis androgen receptor (AR) was prominently expressed in epithelium of the preputial lamina and its subdivisions, and also in the urethral epithelium, MUMP cartilage, penile erectile bodies (corpus cavernosum glandis, MUMP corpus cavernosa, corpus cavernosum urethrae) and in mesenchymal cells throughout the developing penis (Fig. 6A, B). AR was also detected in the rudimentary penile spines, os penis and proximal hyaline cartilage (not illustrated). Estrogen receptor alpha (ERα) had a much more restricted distribution being prominently expressed in MUMP cartilage (Fig. 6C) and urethral epithelium (Fig. 6D), with weak expression in the erectile bodies (Fig. 6C, D). ERα was also weakly expressed in the proximal hyaline cartilage and bone (not illustrated) and rarely if at all in the preputial lamina (Fig. 6C, D). Estrogen receptor beta (ERβ was detected in epithelial cells of the preputial lamina, its subdivisions and rudimentary penile spines (Fig. 6E, F), urethral epithelium (Fig. 6F), and very weakly expressed in erectile bodies (Fig. 6E, F) and in the proximal hyaline cartilage and os penis (not illustrated). The pattern of expression of AR, ERα, ERβ corresponded closely with the response of penile structures to exogenous DHT and DES. In 10-day female external genitalia AR, ERα and ERβ were expressed in homologous structures/areas (Table 2). One area needing special attention in the female was the expression of both ERα (Fig. 7) and ERβ (not illustrated) in the central epithelium surrounding the distal-most portion of the urethral lumen, in the mesenchymal cells surrounding the central epithelium and in clitoral mesenchyme (Fig. 7).
Fig. 7.
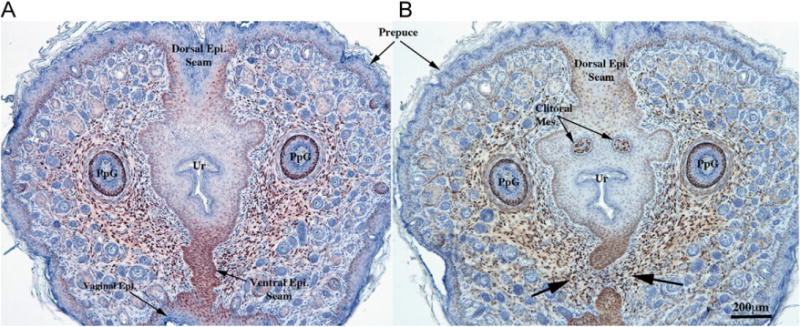
Estrogen receptor alpha immunohistochemical staining of intact untreated 10-day female mice. (A) is in the region where the preputial groove has just closed to form the urethra, initially surrounded by central epithelium attached dorsally and ventrally to preputial skin via epithelial seams (Dorsal Epi. Seam and Ventral Epi. Seam). Note strong ERα staining in the ventral epithelial seam and generally throughout all epithelia including skin and certain elements of hair follicles, in mesenchyme surrounding the epithelium and in the preputial gland ducts (PpG). In (B), a slight more proximal section, the ventral epithelial seam has disappeared with mesenchymal confluence (arrows) across the ventral midline. Note strong ERα staining in the remains of the ventral epithelial seam and the mesenchyme associated with this ventral epithelium, and in the distal clitoral mesenchyme (clitoral mes.).
8. Discussion
The currently held concept of sexual differentiation of mammalian ExG is that masculine development is an androgen-dependent process. During prenatal development the presence or absence of androgens determine penile/clitoral identity and secondly androgens elicit development/differentiation of penile features, which are expressed postnatally. In the present study hormone action was impaired pre- and postnatally in XTfm/Y and X/Y AR-null male mice or postnatally only as a result of castration at birth. To better characterize the hormonal effects on the glans penis and clitoris we identified 10 sexually dimorphic features in 10-day and adult mice (Table 1). Since male and female ExG of mice are profoundly undifferentiated at birth, by specifically eliminating the effect of endogenous sex hormones via gonadectomy at birth, and subsequently implementing hormonal manipulations, we identified specific morphogenetic features in ExG development responsive to androgen and estrogen. Being able to attribute specific effects to either sex hormone, or to identify hormone-independent mechanisms, will permit a rational approach to understanding the causes of abnormal ExG development.
Following neonatal castration, development of several penile features was impaired/eliminated, but overall morphology remained distinctly penile, suggesting that penile identity is specified prenatally by androgens. This conclusion is confirmed by observations of XTfm/Y and X/Y AR-null male mice, which both exhibit clitoral development as a result of androgen resistance beginning prenatally. In castrated oil-treated male mice, several features (penile organ width, defined erectile bodies, MUMP cartilage, epithelial spines and bone length) were impaired, suggesting that these features are dependent upon postnatal androgen. In contrast, other penile features (proximal hyaline cartilage, circumferential epithelium, non-tethering, dorsal preputial cleft, and urethra completely within organ) were preserved in neonatally castrated oil-treated mice suggesting that these features are either androgen-independent or triggered by prenatal androgens and not requiring postnatal androgens for expression. The later interpretation is supported insofar as all of these features are eliminated in XTfm/Y and X/Y AR-null male mice devoid of both pre- and postnatal AR signaling. Furthermore, postnatal induction of penile features in females by DHT (without requiring simultaneous estrogen signaling) is further supported by the observations that seven of 10 penile features (Fig. 1) were induced to various degrees in ovariectomized females by postnatal DHT (a non-aromatizable androgen), presumably via AR expressed in these developing structures (see Table 2). These observations validate the concept that the presence or absence of androgens prenatally specifies penile or clitoral identity, respectively, and that differentiated penile structures emerge postnatally as a result of continued androgen action. However, at birth some capacity for ambisexual differentiation is maintained especially in females, even though complete ExG sex reversal in females is not possible by postnatal DHT treatment, since ovariectomized DHT-treated females achieved a sex differentiation index score of 5.5, while intact males score 10 when examined at day 10.
It is likely that all 10 sex differentiation features each have a specific time-dependent “window of hormonal sensitivity”. For any particular developing element the period of androgen dependency may be exclusively within pre- or postnatal periods, or may span both. Further studies are needed to better define critical time periods for development of each androgen-dependent feature and to determine when differentiation of individual male or female elements is irreversibly imprinted/determined.
The generally accepted role of androgen in cartilage and bone development is substantiated by our study. As previously described, development of bone within the ExG is a multifaceted process consisting of (a) formation of an embryonic mesenchymal condensation (bone precursor) and (b) formation of a small bone. Both of these events are androgen-independent insofar as both occur in XTfm/Y and X/Y AR-null males and in normal and neonatally ovariectomized females, as described in this report and previously (Murakami, 1984, 1987b). Subsequent bone and cartilage growth (c) are androgen-dependent (Murakami, 1984, 1987b). These previous conclusions are verified by our findings in which the mesenchymal condensation (bone precursor) seen in newborn males forms a large os penis in intact males and in castrated DHT-treated males. Moreover, a large os clitoris forms in ovariectomized DHT-treated females. Thus, postnatal bone growth is androgen-dependent/responsive. However, neonatally castrated oil-treated male mice had a moderately long bone despite the lack of postnatal androgens, suggesting that total bone growth may be affected by both pre- and postnatal androgen. In two parallel studies, intact female mice treated with DHT also had a marked increase in size of the os clitoris size (Glucksmann and Cherry, 1972), and administration of the anti-androgen, cyproterone acetate, to male mice starting on the day of birth inhibited growth of the os penis (Glucksmann et al., 1976). These studies indicate an involvement of androgen in bone growth and also suggest that pre- and postnatal androgens are at play. The presence of AR in the periosteum and in the hyaline cartilage growth plate (see Table 2 for references) associated with the proximal end of the os penis is consistent with this interpretation.
Our results also implicate a role of androgens in chondrogenesis. AR are localized in the MUMP fibrocartilage in males, and in the corresponding distal mesenchymal condensation in females (Table 2). AR was also seen in the perichondrium surrounding the proximal hyaline cartilage. Castrated oil-treated males completely lacked MUMP fibrocartilage at day 10. Indeed, only a rudimentary mesenchymal condensation was present without evidence of cartilage differentiation in castrated oil-treated mice even at three months, when the distal fibrocartilage is easily identifiable as the MUMP (Rodriguez et al., 2011; Yang et al., 2010). DHT-treated castrated males developed MUMP fibrocartilage, and had prominent hyaline cartilage associated with the proximal aspect of the os penis. DHT-treated ovariectomized females also developed a prominent distal cartilagenous element (comparable to the MUMP cartilage), and a robust proximal hyaline cartilage growth plate. This indicates that androgen is essential for cartilage development in ExG. These results are congruent with prior literature (Glucksmann et al., 1976; Howard, 1959), and are supported by the distribution pattern of AR (Table 2).
DES at pharmacologic doses elicited a variety of teratogenic changes in neonatally castrated males and females, which correlates well with the expression of ERα and/or ERβ in developing ExG (Table 2). In males, teratogenic sensitivity to estrogen was previously described in rat and mouse studies, in which exogenous estrogen administered perinatally elicited penile dysmorphogenesis, including hypospadias, abnormal penile muscles and bone formation, and decreased penile length, diameter and weight (Goyal et al., 2007; Kim et al., 2004; Vilela et al., 2007; Willingham and Baskin, 2007). We identified two additional morphologic changes attributable to estrogen action in males. Specifically, DES completely arrested development of penile spines and proximal hyaline cartilage. Penile spine formation was also inhibited by tamoxifen in neonatally treated mice, and these changes were specifically attributed to tamoxifen since other anti-estrogens did not inhibit spine formation (Iguchi et al., 1990). These studies are difficult to interpret inasmuch as tamoxifen can have both estrogenic and anti-estrogenic activities in mouse tissues.
In females, DES caused teratogenic changes to the urethra and os clitoris. The os clitoris was absent in 100% of ovariectomized DES-treated, but not in oil-treated ovariectomized females. This finding underscores the effect of estrogen on osteogenesis (Matsumoto et al., 2006; Rochira et al., 2001). Likewise, urethral development was perturbed in 100% of DES-treated gonadectomized females resulting in common urogenital sinus/urethral-vaginal fistula as demonstrated previously in non-gonadectomized neonatally DES-treated female mice (Miyagawa et al., 2002).
The complete elimination of penile spines in castrated DES-treated male mice may be mediated via ERβ and not ERα insofar as ERβ was the predominant estrogen receptor expressed in the preputial lamina and rudimentary penile spines. DES can bind to either ERα or ERβ (Barkhem et al., 1998; Kirigaya et al., 2009). Likewise, the induction of common urogenital sinus/urethral-vaginal fistula in castrated DES-treated female mice could be due to signaling through either ERα or ERβ as both receptors are present in the epithelium within this region (central epithelial and dorsal and ventral seam epithelium (Fig. 7)).
Neonatal mouse ExG express AR, ERα and ERβ. Both testosterone and estrogen are present in serum of male and female neonatal rodents (Pang et al., 1979), and we have defined response of neonatal mouse ExG to exogenous DHT and DES. It should be emphasized that newborn gonadectomy eliminates both serum androgen and estrogen. Hence, the effects of neonatal gonadectomy seen in our study could be due to the absence of androgen, estrogen or both hormones. While the dominant hormonal condition affecting sex differentiation of ExG is the presence or absence of androgens, further studies will be required to elucidate the role of endogenous estrogen in normal development of male and female ExG. In summary, we identified specific morphogenetic events in male and female mouse ExG sex differentiation that can be affected by androgens and estrogens. These findings advance the field of mouse ExG development, and provide an important springboard towards understanding molecular mechanisms.
Finally, development of the penis and clitoris involves a constellation of morphogenetic and differentiation events that for the most part are sensitive to or dependent upon hormonal conditions during various stages of development. The present study demonstrates that specification of penile versus clitoral identity occurs prenatally as a result of the presence or absence of androgen signaling (Fig. 8A). We have defined four developmental events (Fig. 8B) triggered by androgens prenatally in males and not requiring postnatal androgen for their expression (proximal hyaline cartilage, circumferential epithelium, non-tethering, dorsal preputial cleft, and urethra completely within organ). Five additional developmental events (Fig. 8D) require postnatal androgen in males for expression (organ width, defined erectile bodies, MUMP cartilage, epithelial spines and bone length), and all five of these developmental events (along with a few more) are inducible by postnatal androgen in females (Fig. 8C). Finally, the dorsal (males) versus ventral (females) preputial cleft appears to be specified by an unknown mechanism (Fig. 8E).
Fig. 8.
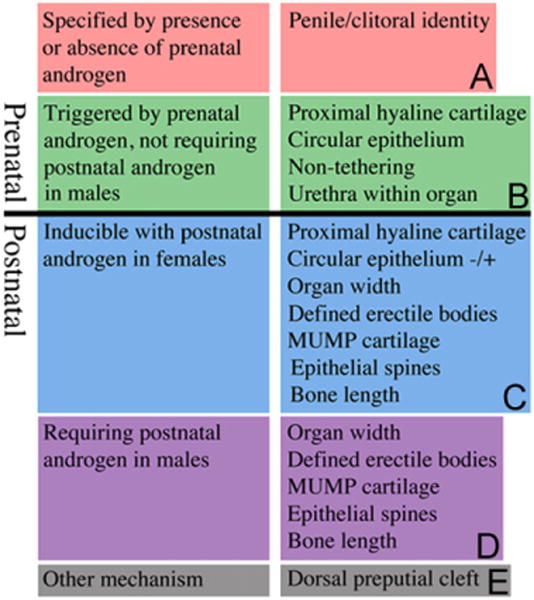
Chart of developmental events in ExG development. (A) Penile/clitoral identity specified by the presence or absence of prenatal androgen. (B) Development events triggered prenatally by androgen and not requiring continued postnatal androgen in males for expression. (C) Development events inducible with postnatal androgen in females. (D) Development events requiring postnatal androgen in males. (E) Development events regulated by an unknown mechanism.
Acknowledgments
This work was supported by the following grants: NSF Grant IOS-0920793, NIH grant RO1 DK0581050 and NH&MRC grant APP1002733.
Abbreviations
- ExG
External genitalia
- GT
genital tubercle
- DHT
dihydrotestosterone
- DES
diethylstilbestrol
- AR
androgen receptor
- ERα
estrogen receptor alpha
- ERβ
estrogen receptor beta
- MUMP
male urogenital mating protuberance
- Tfm
testicular feminization
Footnotes
Disclosure Statement
The authors have nothing to disclose.
References
- Agras K, Willingham E, Liu B, Baskin LS. Ontogeny of androgen receptor and disruption of its mRNA expression by exogenous estrogens during morphogenesis of the genital tubercle. The Journal of Urology. 2006;176:1883–1888. doi: 10.1016/S0022-5347(06)00613-6. [DOI] [PubMed] [Google Scholar]
- Agras K, Willingham E, Shiroyanagi Y, Minasi P, Baskin LS. Estrogen receptor-alpha and beta are differentially distributed, expressed and activated in the fetal genital tubercle. The Journal of Urology. 2007;177:2386–2392. doi: 10.1016/j.juro.2007.01.111. [DOI] [PubMed] [Google Scholar]
- Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S. Differential response of estrogen receptor alpha and estrogen receptor beta to partial estrogen agonists/antagonists. Molecular Pharmacology. 1998;54:105–112. doi: 10.1124/mol.54.1.105. [DOI] [PubMed] [Google Scholar]
- Bentvelsen FM, McPhaul MJ, Wilson JD, George FW. The androgen receptor of the urogenital tract of the fetal rat is regulated by androgen. Molecular and Cellular Endocrinology. 1994;105:21–26. doi: 10.1016/0303-7207(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Buchanan DL, Kurita T, Taylor JA, Lubahn DL, Cunha GR, Cooke PS. Role of stromal and epithelial estrogen receptors in vaginal epithelial proliferation, stratification and cornification. Endocrinology. 1998;139:4345–4352. doi: 10.1210/endo.139.10.6241. [DOI] [PubMed] [Google Scholar]
- Crescioli C, Maggi M, Vannelli GB, Ferruzzi P, Granchi S, Mancina R, Muratori M, Forti G, Serio M, Luconi M. Expression of functional estrogen receptors in human fetal male external genitalia. The Journal of Clinical Endocrinology and Metabolism. 2003;88:1815–1824. doi: 10.1210/jc.2002-021085. [DOI] [PubMed] [Google Scholar]
- Dietrich W, Haitel A, Huber JC, Reiter WJ. Expression of estrogen receptors in human corpus cavernosum and male urethra. The Journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society. 2004;52:355–360. doi: 10.1177/002215540405200306. [DOI] [PubMed] [Google Scholar]
- Glucksmann A, Cherry CP. The hormonal induction of an os clitoridis in the neonatal and adult rat. Journal of Anatomy. 1972;112:223–231. [PMC free article] [PubMed] [Google Scholar]
- Glucksmann A, Ooka-Souda S, Miura-Yasugi E, Mizuno T. The effect of neonatal treatment of male mice with antiandrogens and of females with androgens on the development of the os penis and os clitoridis. Journal of Anatomy. 1976;121:363–370. [PMC free article] [PubMed] [Google Scholar]
- Goyal HO, Braden TD, Williams CS, Dalvi P, Mansour MM, Mansour M, Williams JW, Bartol FF, Wiley AA, Birch L, Prins GS. Abnormal morphology of the penis in male rats exposed neonatally to diethylstilbestrol is associated with altered profile of estrogen receptor-alpha protein, but not of androgen receptor protein: a developmental and immunocytochemical study. Biology of Reproduction. 2004;70:1504–1517. doi: 10.1095/biolreprod.103.026328. [DOI] [PubMed] [Google Scholar]
- Goyal HO, Braden TD, Williams CS, Williams JW. Role of estrogen in induction of penile dysmorphogenesis: a review. Reproduction (Cambridge, England) 2007;134:199–208. doi: 10.1530/REP-07-0207. [DOI] [PubMed] [Google Scholar]
- Howard E. A complementary action of corticosterone and dehydroepian-drosterone on the mouse adrenal, with observations on the reactivity of reproductive tract structures to dehydroepiandrosterone and 11-hydroxy-androstenedione. Endocrinology. 1959;65:785–801. doi: 10.1210/endo-65-5-785. [DOI] [PubMed] [Google Scholar]
- Iguchi T, Irisawa S, Uesugi Y, Kusunoki S, Takasugi N. Abnormal development of the os penis in male mice treated neonatally with tamoxifen. Acta Anatomica. 1990;139:201–208. doi: 10.1159/000146998. [DOI] [PubMed] [Google Scholar]
- Jesmin S, Mowa CN, Matsuda N, Salah-Eldin AE, Togashi H, Sakuma I, Hattori Y, Kitabatake A. Evidence for a potential role of estrogen in the penis: detection of estrogen receptor-alpha and -beta messenger ribonucleic acid and protein. Endocrinology. 2002;143:4764–4774. doi: 10.1210/en.2002-220628. [DOI] [PubMed] [Google Scholar]
- Jesmin S, Mowa CN, Sakuma I, Matsuda N, Togashi H, Yoshioka M, Hattori Y, Kitabatake A. Aromatase is abundantly expressed by neonatal rat penis but downregulated in adulthood. Journal of Molecular Endocrinology. 2004;33:343–359. doi: 10.1677/jme.1.01548. [DOI] [PubMed] [Google Scholar]
- Jost A. Problems of fetal endocrinology: the gonadal and hypophyseal hormones. Recent Progress in Hormone Research. 1953;8:379–418. doi: 10.1016/b978-1-4831-9825-5.50017-8. [DOI] [PubMed] [Google Scholar]
- Jost A. Gonadal hormones in the sex differentiation of the mammalian fetus. In: Urpsrung RL, DeHaan H, editors. Organogenesis. Holt, Rinehart and Winston; New York: 1965. pp. 611–628. [Google Scholar]
- Kalloo NB, Gearhart JP, Barrack ER. Sexually dimorphic expression of estrogen receptors, but not of androgen receptors in human fetal external genitalia. Journal of Clinical Endocrinology and Metabolism. 1993;77:692–698. doi: 10.1210/jcem.77.3.8370691. [DOI] [PubMed] [Google Scholar]
- Kim KS, Liu W, Cunha GR, Russell DW, Huang H, Shapiro E, Baskin LS. Expression of the androgen receptor and 5 alpha-reductase type 2 in the developing human fetal penis and urethra. Cell and Tissue Research. 2002;307:145–153. doi: 10.1007/s004410100464. [DOI] [PubMed] [Google Scholar]
- Kim KS, Torres CR, Jr, Yucel S, Raimondo K, Cunha GR, Baskin LS. Induction of hypospadias in a murine model by maternal exposure to synthetic estrogens. Environmental Research. 2004;94:267–275. doi: 10.1016/S0013-9351(03)00085-9. [DOI] [PubMed] [Google Scholar]
- Kirigaya A, Kim H, Hayashi S, Chambon P, Watanabe H, Lguchi T, Sato T. Involvement of estrogen receptor beta in the induction of polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol. Zoological Science. 2009;26:704–712. doi: 10.2108/zsj.26.704. [DOI] [PubMed] [Google Scholar]
- Lim P, Allan CM, Notini AJ, Axell AM, Spaliviero J, Jimenez M, Davey R, McManus J, MacLean HE, Zajac JD, Handelsman DJ. Oestradiol-induced spermatogenesis requires a functional androgen receptor. Reproduction, Fertility, and Development. 2008;20:861–870. doi: 10.1071/rd08144. [DOI] [PubMed] [Google Scholar]
- Martin-Alguacil N, Pfaff DW, Kow LM, Schober JM. Oestrogen receptors and their relation to neural receptive tissue of the labia minora. BJU International. 2008;101:1401–1406. doi: 10.1111/j.1464-410X.2008.07626.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto C, Inada M, Toda K, Miyaura C. Estrogen and androgen play distinct roles in bone turnover in male mice before and after reaching sexual maturity. Bone. 2006;38:220–226. doi: 10.1016/j.bone.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Miyagawa S, Buchanan DL, Sato T, Ohta Y, Nishina Y, Iguchi T. Characterization of diethylstilbestrol-induced hypospadias in female mice. The Anatomical Record. 2002;266:43–50. doi: 10.1002/ar.10033. [DOI] [PubMed] [Google Scholar]
- Murakami R. Histogenesis of the os penis and os clitoridis in rats. Development, Growth and Differentiation. 1984;26:419–426. doi: 10.1111/j.1440-169X.1984.00419.x. [DOI] [PubMed] [Google Scholar]
- Murakami R. Autoradiographic studies of the localisation of androgen-binding cells in the genital tubercles of fetal rats. Journal of Anatomy. 1987a;151:209–219. [PMC free article] [PubMed] [Google Scholar]
- Murakami R. A histological study of the development of the penis of wildtype and androgen-insensitive mice. Journal of Anatomy. 1987b;153:223–231. [PMC free article] [PubMed] [Google Scholar]
- Pang SF, Caggiula AR, Gay VL, Goodman RL, Pang CS. Serum concentrations of testosterone, oestrogens, luteinizing hormone and follicle-stimulating hormone in male and female rats during the critical period of neural sexual differentiation. The Journal of Endocrinology. 1979;80:103–110. doi: 10.1677/joe.0.0800103. [DOI] [PubMed] [Google Scholar]
- Qiao L, Rodriguez E, Jr, Weiss DA, Ferretti M, Risbridger G, Cunha GR, Baskin LS. Expression of estrogen receptor alpha and beta is decreased in hypospadias. The Journal of Urology. 2012;187:1427–1433. doi: 10.1016/j.juro.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochira V, Balestrieri A, Faustini-Fustini M, Carani C. Role of estrogen on bone in the human male: insights from the natural models of congenital estrogen deficiency. Molecular and Cellular Endocrinology. 2001;178:215–220. doi: 10.1016/s0303-7207(01)00446-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez E, Jr, Weiss DA, Yang JH, Menshenina J, Ferretti M, Cunha TJ, Barcellos D, Chan LY, Risbridger G, Cunha GR, Baskin LS. New insights on the morphology of adult mouse penis. Biology of Reproduction. 2011;85:1216–11221. doi: 10.1095/biolreprod.111.091504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Ogino Y, Murakami R, Satoh Y, Bachiller D, Yamada G. Embryonic development of mouse external genitalia: insights into a unique mode of organogenesis. Evolution and Development. 2002;4:133–141. doi: 10.1046/j.1525-142x.2002.01061.x. [DOI] [PubMed] [Google Scholar]
- Vilela ML, Willingham E, Buckley J, Liu BC, Agras K, Shiroyanagi Y, Baskin LS. Endocrine disruptors and hypospadias: role of genistein and the fungicide vinclozolin. Urology. 2007;70:618–621. doi: 10.1016/j.urology.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Weiss DA, Rodriguez E, Jr, Cunha T, Menshenina J, Barcellos D, Chan LY, Risbridger G, Baskin L, Cunha G. Morphology of the external genitalia of the adult male and female mice as an endpoint of sex differentiation. Molecular and Cellular Endocrinology. 2012;354:94–102. doi: 10.1016/j.mce.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham E, Baskin LS. Candidate genes and their response to environmental agents in the etiology of hypospadias. Nature Clinical Practice Urology. 2007;4:270–279. doi: 10.1038/ncpuro0783. [DOI] [PubMed] [Google Scholar]
- Yang JH, Menshenina J, Cunha GR, Place N, Baskin LS. Morphology of mouse external genitalia: implications for a role of estrogen in sexual dimorphism of the mouse genital tubercle. The Journal of Urology. 2010;184:1604–1609. doi: 10.1016/j.juro.2010.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa T, Higashi M, Yoshioka K, Mutoh K. Distribution of aromatase and sex steroid receptors in the baculum during the rat life cycle: effects of estrogen during the early development of the baculum. Biology of Reproduction. 2011;85:105–112. doi: 10.1095/biolreprod.110.089508. [DOI] [PubMed] [Google Scholar]


